Introduction
Cancer represents a significant threat to human
health worldwide and its burden of morbidity and mortality
continues to rise. According to 2020 estimates from the World
Health Organization, cancer is the first or second leading cause of
premature death (death between the ages of 30 and 69 years) in 91
out of 172 countries, and the third or fourth leading cause in
another 22 countries (1).
Opioids serve a role in controlling the sensation of
pain in the central and peripheral nervous systems; they also
regulate cellular and humoral immune responses, as well as the
expression of chemokines and chemokine receptors (2). Thus, the clinical application of
opioids is extensive and includes perioperative analgesia and
sedation, as well as pain reduction for patients with cancers
(3), including pancreatic cancer
(4), lung cancer (5), colorectal cancer (6), breast cancer (7) and head and neck cancer (8). Several experimental studies have
shown that opioids promote proliferation, migration, invasion and
angiogenesis in cancer cell culture and in vivo animal
cancer models, such as morphine in the regulation of colorectal
cancer cells (9) and human clear
cell renal cell carcinoma cells (10), meanwhile morphine stimulates
angiogenesis in mouse breast cancer models (11), which suggests that they may be
harmful to patients with cancer. Preclinical studies have shown
that opioids may be associated with cancer progression and
recurrence, increased risk of infection and decreased overall
survival (12,13). However, other studies have found
the opposite effect, namely that opioids may exhibit an inhibitory
effect on a range of cancer cells and that use of opioids in in
vivo animal cancer models does not promote tumor growth. For
example, fentanyl inhibits the cell viability and invasion of lung
(14), gastric (15) and colorectal (16) cancer. A study in which melanoma
cells were injected into mice led to hyperalgesia at the injection
site. Treatment with morphine can reduce local tumor growth and
lung metastasis (17).
To date, an increasing number of studies (18) have shown that opioids and opioid
receptors and peptides are widely distributed in various types of
tumor cell and their expression levels differ depending on the
tumor tissue type, such as breast cancer (19), colon cancer (20), endometrial cancer (21) and lung cancer (22). The upregulation of µ-opioid
receptor (MOR) in lung cancer samples with metastasis was found to
be significantly higher than that in lung cancer samples without
metastasis (23). MOR
overexpression in human bronchoalveolar lung carcinoma cells led to
increased tumor growth and lung metastases in nude mice compared
with vector transfected cells (24). In addition, the stimulatory or
inhibitory effects of opioids differ in different tumor cell types
and are associated with growth, metastasis and prognosis of cancer.
As MOR agonists, such as morphine and fentanyl, are the leading
analgesics in clinical treatment of moderate and severe pain
(25), MOR has greater clinical
relevance than the κ-opioid receptor (KOR) and a relatively high
number of studies on the association between MOR and cancer have
been published (26–28). However, such opioids can cause
side effects, such as euphoria, tolerance and respiratory
depression, and have a high risk of addiction, drug abuse and death
(29,30).
Exogenous and endogenous opioid peptides regulate
multiple functions of the body via ORs, such as food intake and
weight control (31), response to
pain (32), and regulation of
cardiac function and the immune system (33,34). KOR is one of the primary targets
of opioids, with a potent analgesic effect and few side effects
compared with MOR (35). KOR is a
member of the G-protein-coupled receptor family and its natural
endogenous ligand is dynorphin, which decreases synaptic
transmission by inhibiting adenylate cyclase and voltage-gated
calcium channels and activating voltage-gated potassium channels,
resulting in decreased neuronal action potential production and
neurotransmitter release (36).
KOR was first cloned in 1993 (37) and is detectable in different
tissues in rats, mice, guinea pigs, cattle and other animals. The
understanding of ORs is increasing. KOR can be divided into
different subtypes (38,39). KOR is distributed in the brain,
spinal cord and pain-sensing nerves (40,41), thus it is most widely used in
clinical analgesia during the perioperative period, such as using
drugs that target KOR (e.g., oxycodone) (42,43). Moreover, KOR is also expressed in
the heart, lung, colon, liver and other organs, and is associated
with the regulation of organ development, respiration, emotions,
and motor, cardiovascular, neuroendocrine and cognitive function
(44–48).
The structure and distribution of KOR provide an
important basis for its participation in regulating various
pathophysiological functions of the body (49,50). Current evidence suggests that KOR
serves a key role in the progression of tumors (51,52). For example, clinical drugs
targeting KOR, such as oxycodone and butorphanol, are given to
patients with cancer to help eliminate or minimize the harmful
effects of pain and stress on cancer progression (53). As commonly known, psychological
states can affect the outcome of human disease, for example pain
has a profoundly negative impact on the mood, social activities,
day to day life, sleep and cognitive function of patients with
cancer, which can even cause negative emotions such as stress and
depression in caregivers too (54). Stress can promote tumor
progression by inhibition of the expression of class-I and class-II
major histocompatibility complex molecules and by reducing natural
killer (NK) cell activity (55).
KOR agonists acting on peripheral and spinal sites block
tumor-induced bone pain (56). In
addition, KOR is primarily expressed in the cell membrane,
cytoplasm and nucleus. The expression levels of KOR in different
subcellular locations may predict the prognosis of patients with
tumors (57). In vitro
experiments have demonstrated that KOR has different effects on
different cancer cell types. For example, KOR inhibits
proliferation and promotes apoptosis of nasopharyngeal carcinoma
cells (58), and its
overexpression may promote activation and invasion of breast cancer
cells; these effects all involve activation of downstream signaling
pathways (59).
Previous studies have shown that KOR is upregulated
in various types of solid tumor, such as liver and non-small cell
lung cancer and other malignant tumors, and KOR expression is
associated with cancer growth and poor prognosis (60,61). However, additional experiments are
required to confirm the specific underlying mechanism. These
observations confirm that KOR serves an important role in cancer
development, including potentially promoting or inhibiting growth
and metastasis of tumors and affecting patient prognosis.
Furthermore, inflammation promotes carcinogenic
mutations that trigger changes in cytokine levels, stimulate
angiogenesis and promote tumor immune evasion (2,53).
KOR expression has also been detected in immune cells, such as
myeloid and CD4+ and CD8+ T cells (2,62).
Moreover, KOR mediates immunosuppressive effects, including
decreased antibody production and inhibition of cytokine and
chemokine expression (2).
Discovery, structure and typing of KOR
The existence of ORs was discovered in mammalian
brain tissue using radioactive ligand binding in 1973 (63). To date, five opioid receptors have
been identified: MOR, KOR, δ- and ζ-OR and nociception receptor
(36,64). Numerous researchers have
investigated the structure, typing, localization and function of
ORs, which has provided novels routes for opioid drug research and
development and laid a foundation for drug development and use
(65,66). KOR was first cloned from mouse
brain in 1993 by Minami et al (37). The KOR endogenous ligand,
dynorphin, was later identified as a 17-amino acid peptide derived
from prodynorphin that is distributed in the central nervous system
(67). However, they bind to ORs
on the cell membrane, thereby regulating neuronal excitation via
intracellular signaling pathways, which affect learning, cognition,
nociception and endocrine function (68). Exogenous opioid peptides, such as
morphine, heroin and fentanyl, also act by binding to ORs (69).
KOR belongs to the G protein-coupled receptor family
of proteins, which share the same basic structure: An extracellular
N-terminal region, seven transmembrane domains and intracellular
C-terminal and caudal regions (70). Hydrophobicity analysis has shown
that the structure of KOR consists of three intracellular and three
cytoplasmic rings, two extracellular glycosylation sites at the
N-terminus and one intracellular phosphorylation site at the
C-terminus containing a disulfide bond (45,46). Genetic analysis has suggested that
OR genes are located on different human chromosomes (45,71). As their exon/intron sequence is
similar, it was originally hypothesized that the MOR, DOR and KOR
may be derived from the same ancestral gene (72). However, different ORs have
different pharmacological properties and are expressed in different
anatomical locations (70).
Terenius (69) isolated the
full-length cDNA encoding human KOR using cDNA cloning technology.
The KOR gene is located at q11-12 of human chromosome 8, with a
coding region length of ~1,143 bases, translating into a protein of
380 amino acid residues. In addition, KOR mRNA transcripts are
detectable in different tissues in rats, mice, guinea pigs and
other animals, and have high homology with the sequence of the
human KOR gene. This indicates that KOR gene sequences are highly
conserved between different species (37,73). However, the role of KOR splice
variants at different sites is not fully understood.
Receptor-ligand binding and competitive inhibition
experiments have demonstrated that KOR has two different binding
sites (74,75). Accordingly, KOR is divided into
two different subtypes. The κ1 subtype is sensitive to
[D-Ala2, D-Leu5]-enkephalin (DADLE), but not
to U50488H, whereas the κ2 subtype is sensitive to U50488H, but not
to DADLE. κ1 ORs can also be divided into κ1A
and κ1B sub-subtypes. Similarly, κ2-subtype ORs can be
divided into κ2A and κ2B sub-subtypes. In
addition, a third subtype, subtype κ3, has also been proposed. The
receptor-binding characteristics of the κ3- and κ2-subtype opioid
receptors are similar (38,39). Thus, KOR is still largely divided
into only two subtypes, κ1 and κ2. Since most of the aforementioned
KOR types are based on radioligand receptor binding experiments,
further studies are needed to confirm their classification. Given
the heterogeneity of the effects of KOR, additional preclinical
studies are needed to gain insight into the specific mechanisms of
action.
Expression and physiological function of
KOR
KOR was originally considered to be expressed
exclusively in the central nervous system (37). In situ hybridization
experiments in rats revealed that KOR mRNA is present in the
dentate gyrus of the hippocampus, hypothalamus, certain thalamic
nuclei, descending conduction pathway of the cerebral cortex,
caudate nucleus, olfactory bulb, nucleus accumbens, brainstem,
spinal cord and other parts (46). Previous studies have observed KOR
mRNA expression in the heart, kidney, adrenal medulla, digestive
tract, peripheral vascular, uterus, placenta, T cells and
macrophages of humans and animals. Nevertheless, the density of KOR
in central nervous tissue is higher than that in peripheral tissue
(44–48). This suggests that KOR is widely
distributed, which provides a basis for its potential involvement
in regulating various physiological functions of the body.
KOR can bind to the heterotrimer G proteins Gi and
Go, which are sensitive to pertussis toxin (72). When opioid peptides or specific
receptor agonists bind to KOR on the cell membrane, KOR activation
leads to the dissociation of G proteins into Gα and Gβγ subunits,
which mediate various intracellular signaling pathways (76,77) (Fig.
1).
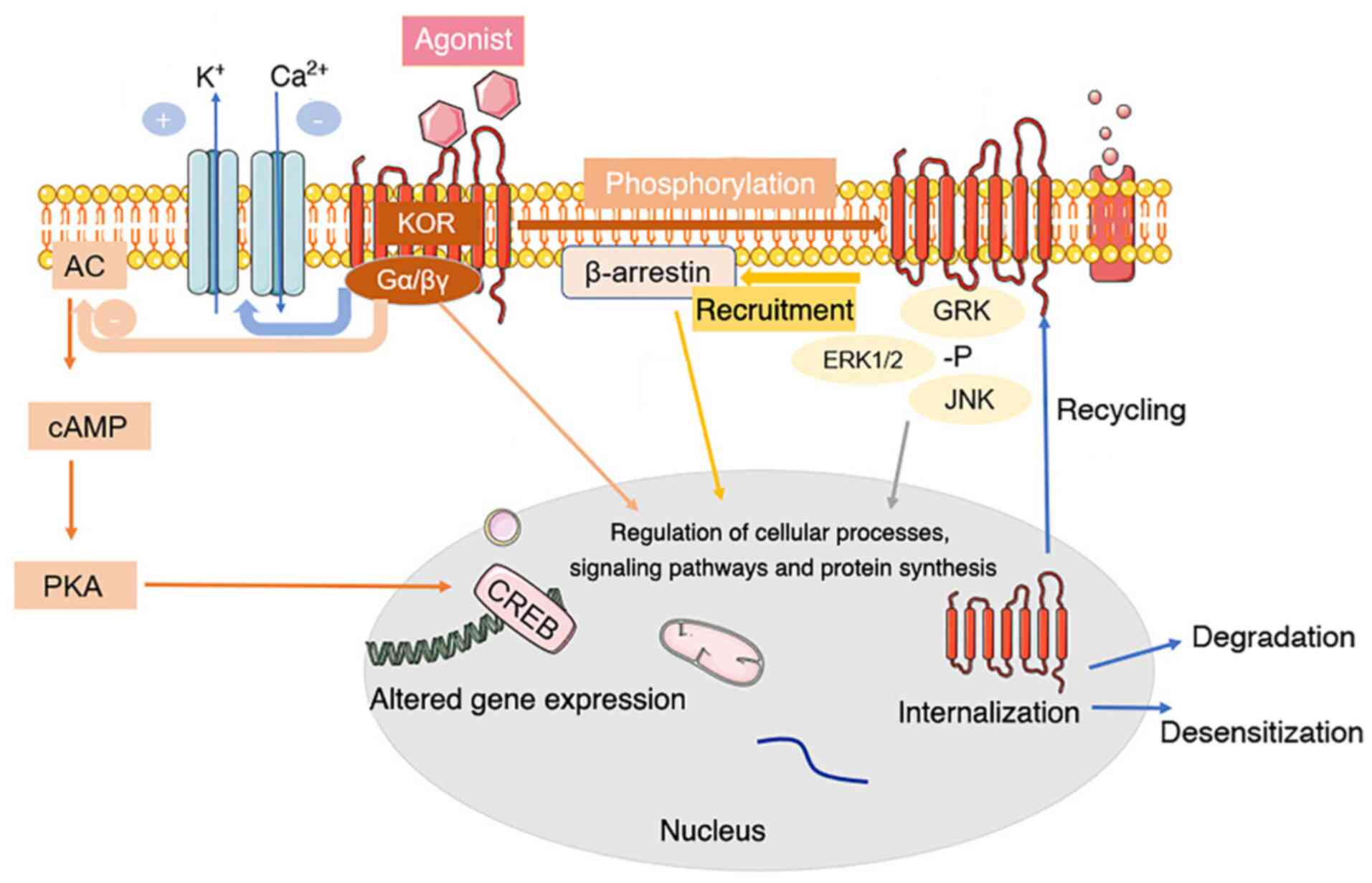 | Figure 1.Common signal transduction and
regulation of KOR. Activation of KOR leads to conformational
changes and dissociation of the pertussis toxin-sensitive G-protein
subunits, activating G-protein-gated inwardly rectifying potassium
channels and inhibiting voltage-gated calcium ion channels. The α
subunit binds GTP and dissociates from Gβγ. The GTP-binding protein
α subunit inhibits the classical adenylyl cyclase/cyclic AMP/PKA
pathway. Both Gα-GTP and free Gβγ can regulate secondary cascade
activation. KOR activation also activates a β-arrestin-dependent
signaling cascade. This interaction with scaffolding partners, such
as β-arrestin, can be dependent or independent of receptor
phosphorylation. KOR is phosphorylated in response to agonist
occupation by multiple kinases, each of which has multiple
isoforms. Phosphorylation by a particular kinase dictates secondary
cascade interactions or subsequent receptor fate. Phosphorylation
of KOR leads to internalization of the receptor, contributing to
KOR agonist tolerance, response to agonist occupancy and subsequent
signaling pathway activation, desensitization or degradation of the
receptor. +, activation; -, blockade or inhibition; KOR, κ-opioid
receptor; AC, adenylyl cyclase; cAMP, cyclic AMP; CREB, cAMP
responsive element binding protein; PKA, protein kinase A; Gα/βγ, G
protein α/βγ subunit; GRK, G protein-coupled receptor kinase 1; -P,
pyrophosphate. |
KOR also interact with various helper proteins and
alter the effectiveness of agonist-mediated cell signaling
pathways, such as activating the extracellular signal-regulated
kinase (ERK1/2) (78), c-Jun
amino-terminal kinase (79),
JAK2/STAT3 and interferon regulatory factor 2 signaling (80), which determine the signals
produced and influence receptor transport, targeting, fine-tuning
and intracellular localization by providing scaffolds that connect
receptors and cytoskeletal networks (77,81).
Pain is a common initial signal for a patient to
seek medical attention and 30–50% of patients with cancer
experience moderate to severe pain that has a notable negative
impact on their quality of life (82). Opioids are often used to relieve
cancer-associated pain in patients and improve quality of life
(83,84). KOR has long been a research target
for novel analgesics because activating KOR relieves pain
effectively without causing side effects such as addiction,
respiratory depression and constipation (85).
Moreover, KOR is essential for emotional regulation.
Studies have shown that the endogenous opioid peptide dynorphin
activates KOR in the nucleus accumbens and KOR agonists inhibit
dopamine (DA) transmission (86).
By contrast, KOR antagonists increase the release of basal DA and
may produce antidepressant-like effects (35). The role of KOR in behavioral
despair, stress and other depression models has been studied and
activation of KOR can regulate anxiety-like behavior (87–89). Buprenorphine is a KOR antagonist
and a MOR partial agonist. In animal models, buprenorphine
significantly decreased forced swimming immobility time (90) and clinical trials have shown
potential for the treatment of depression in patients who do not
respond to traditional antidepressants (91,92). It has been reported that the KOR
and MOR agonist (−)-3-N-Ethylaminothiazolo
[5,4-b]-N-cyclopropylmethylmorphinan hydrochloride serves an
antidepressant and anti-anxiety role (93), however, the underlying mechanism
remains to be explored.
KOR activation also stimulates hippocampal
cholinergic neurons, thus alleviating scopolamine-induced memory
impairment and decreasing cognitive impairment caused by ischemic
hippocampal nerve injury (94,95). Moreover, activation of KOR
decreases brain tissue damage, improves brain function and promotes
brain function recovery in numerous animal models of cerebral
ischemia (96,97).
A variety of ORs are found in the heart, among which
KOR is dominant. The heart secretes endogenous or paracrine opioid
peptides and regulates cardiac function by acting on ORs on the
myocardium; this serves a critical role in fighting oxidative
stress and myocardial ischemia/reperfusion injury (44,98). The myocardial protective effects
of selective KOR agonist U50488H and butorphanol tartrate, which
primarily acts on KOR-based analgesic agents, have been
demonstrated (99–101).
Lastly, KOR agonists can be used for the treatment
of refractory pruritus. Nalfurafine (TRK-820), a highly selective
KOR agonist is used to treat pruritus in patients with uremia and
chronic pain liver disease and those receiving peritoneal dialysis
(102). In addition, KOR is also
involved in regulating respiration, immunity, exercise, addiction,
feeding, diuresis and other functions (103).
Expression, function and significance of KOR
in various types of cancer
Hepatocellular carcinoma (HCC)
Liver cancer is predicted to be the sixth most
commonly diagnosed cancer and the fourth leading cause of cancer
death worldwide in 2018, accounting for ~841,000 new cases and
782,000 deaths annually (104).
HCC is the most common subtype of liver cancer, accounting for
75–85% of primary liver cancer (104). The main risk factors for HCC
include chronic infection with hepatitis B virus or hepatitis C
virus, aflatoxin-contaminated food, heavy alcohol intake and type 2
diabetes (105).
Chen et al (60) used reverse transcription (RT)
fluorescence quantitative (q)PCR to detect the expression of KOR
mRNA in liver cancer and adjacent tissue samples and found that the
expression of KOR mRNA in liver cancer tissue was significantly
lower than that in adjacent tissue samples (Table I). Subsequent immunohistochemical
detection of 174 cases of liver cancer showed that the expression
of KOR protein in liver cancer tissue was significantly
downregulated (60). Further
analysis showed that low expression of KOR at both the mRNA and
protein levels was significantly associated with invasive
clinicopathological features (such as tumor size, vascular
invasion, differentiation and TNM stage) of patients with HCC.
Moreover, Kaplan-Meier survival analysis suggested that
downregulation of KOR in HCC predicted a poor prognosis for
patients with HCC (60). Patients
with decreased KOR expression had a lower survival rate and
increased recurrence (60).
Therefore, KOR may inhibit the progression of HCC and could
represent a potential therapeutic target for the treatment of HCC.
These results indicate that down-regulation of KOR in HCC tumor
tissues has a strong association with poor prognosis and KOR might
be a potential tumor suppressor. However, the localization of KOR
in HCC cells remain unclear, and the association between KOR and
HCC remains to be fully elucidated. Future studies should examine
the role of KOR in the occurrence and development of HCC and other
types of liver cancer, as well as the underlying regulatory
mechanisms.
 | Table I.Regulation of KOR in various types of
cancer. |
Table I.
Regulation of KOR in various types of
cancer.
| Type of cancer | Regulation of
KOR | Function | Prognosis | (Refs.) |
|---|
| Hepatocellular
carcinoma | Downregulated | Promotes growth,
invasion and angiogenesis; inhibits differentiation | Poor | (60) |
| Esophageal squamous
cell carcinoma | Upregulated | Promotes metastasis
and growth | Poor | (57) |
| Non-small cell
lung | Upregulated | Promotes
chemosensitivity; inhibits proliferation and growth | Good | (108) |
| Breast | Upregulated | Promotes
chemosensitivity; inhibits growth and proliferation | Good | (117–119) |
| Prostate | Upregulated | Inhibits
proliferation | Unknown | (126,127) |
| Kidney | Upregulated | Promotes
proliferation | Unknown | (10) |
| Nasopharyngeal
carcinoma | Upregulated | Promotes
apoptosis | Unknown | (58) |
| Glioma | Upregulated | Promotes
proliferation and DNA synthesis | Unknown | (132) |
Non-small cell lung cancer
(NSCLC)
At present, the incidence and mortality of lung
cancer rank among the highest across all cancer types globally, and
most cases are NSCLC (106). A
research analysis (61) indicated
that opioid receptors are more highly expressed in various human
solid cancers, and KOR expression was found to be increased at the
mRNA level in adenocarcinomas of the lung and pancreas, prostate
carcinoma and myxoid/round cell liposarcoma, compared with healthy
control tissues (Table I).
As early as 1990, KOR agonists were shown to inhibit
the proliferation of H157 NSCLC cells (107). A follow-up study (108) has reported that KOR is highly
expressed in two NSCLC cell lines (HCC827 and H1975) and treating
these cells with the selective KOR agonist U50488H decreases their
viability and proliferation in a concentration-dependent manner and
the selective KOR antagonist norbinaltorphimine reverses this
effect. Gefitinib is an oral epidermal growth factor
receptor-tyrosine kinase (EGFR-TK) inhibitor (109). Inhibition of EGFR-TK inhibits
tumor growth, metastasis and angiogenesis, and increases cancer
cell apoptosis (110,111).
The inhibition of tumor cell viability and
proliferation by gefitinib in HCC827 cells can be further enhanced
by co-treatment with the selective KOR agonist U50488H (108). Glycogen synthase kinase (GSK)3-β
is a multifunctional serine/threonine kinase involved in regulating
the function of several metabolism and signaling pathways, as well
as proteins and transcription factors (112). GSK3β drives oncogenic
progression either by its inhibition or its activation, depending
on the cell type. Inactivation of GSK3β has been reported in lung
cancer (113) and higher level
of inactivated of GSK3β (pSer9GSK3β) observed (114). Phosphorylation of GSK3-β leads
to inactivation of the tumor suppressor gene p53, which is key to
the progression of several types of cancer (115,116). Following treatment with KOR
agonist U50488H, phosphorylation of GSK-3β is decreased in H1975
lung cancer cells (108).
Activation of KOR may decrease GSK-3β phosphorylation by inhibiting
the cAMP/protein kinase A pathway or activating the JNK pathway,
thus inhibiting NSCLC growth (108). These findings suggest that KOR
may serve a role in the prevention and treatment of NSCLC and KOR
may augment the effect of anti-tumor drugs, decreasing drug
resistance and activating key signaling pathways and molecules to
inhibit lung cancer cell proliferation and promote apoptosis
(108).
Breast cancer
KOR is also expressed in primary breast cancer and
different breast cancer cell lines, such as MCF7 and T47D (117,118). It has been reported that opioids
inhibit the growth of the human T47D breast cancer cell line via
KOR in a dose-dependent manner (118). In a follow-up study, it was also
found that the chemotherapeutic drug paclitaxel bound to KOR, which
exhibited an anti-tumor effect in breast cancer, suggesting that
KOR directly enhanced the effect of this drug and modulated cancer
cell viability and proliferation (118). A retrospective analysis of
triple-negative breast cancer found that intraoperative use of
opioids was associated with decreased risk of tumor recurrence
(119). KOR is upregulated and
Toll-like receptor 4 is downregulated in breast tumor tissue
compared with normal breast tissue (119). Kaplan-Meier survival analysis
has suggested that low expression of KOR in patients with breast
cancer is associated with shorter overall and disease-free survival
(52). KOR is also upregulated in
breast cancer compared with normal human mammary epithelial cells
(59). In addition,
downregulation of KOR inhibits survival and migration of breast
cancer cells and decreases expression of proteins and genes
associated with epithelial-to-mesenchymal transition, such as
N-cadherin, Snail and vimentin, while increasing the expression of
E-cadherin (59). KOR knockdown
also promotes inactivation of the PI3K/AKT signaling pathway, which
decreases cell viability and promotes cell death (59). KOR may be a potential tumor
suppressor, which may be associated with epithelial-mesenchymal
transformation and regulation of the PI3K/AKT signaling pathway
(59). Altogether, these findings
suggest that analgesics that target KOR activation may be suitable
for patients with breast cancer.
Esophageal squamous cell carcinoma
(ESCC)
Esophageal cancer is the sixth leading cause of
cancer-associated mortality in the world. According to statistics
from the International Agency for Research on Cancer, >80% of
esophageal cancer cases are ESCC (120). The geographical distribution of
ESCC is heterogeneous and China has a high incidence of ESCC
(120), where it is the fourth
most common malignancy.
Zhang et al (57) found that KOR is highly expressed
in the KYSE180 and EC109 ESCC cell lines. Immunohistochemical
staining results of patients with ESCC suggested that KOR was
highly expressed in esophageal cancer tissue. In addition, KOR
protein was highly expressed on the membrane of cancer cells and
significantly upregulated in the nucleus and cytoplasm. Follow-up
analysis of clinicopathological features of 256 patients with ESCC
showed that high nuclear expression of KOR was significantly
associated with lymph node metastasis. Therefore, overexpression of
KOR in ESCC may have functional significance, and nuclear KOR
expression may be a risk factor for lymph node metastasis. However,
there is no clear evidence that KOR is as a tumor marker of
ESCC.
Urological and prostate tumors
Previous retrospective studies have shown that
patients undergoing prostate or bladder cancer surgery have higher
disease-specific and disease-free survival, as well as increased
tumor recurrence rates are likely to increase with increased
perioperative opioid use (12,121,122).
In 1988, dynorphin was shown to promote
proliferation of the DU145 prostate cancer cell line by activating
KOR. Naloxone, a classical OR antagonist, increased cell viability
and proliferation by 25% when used alone but paradoxically inhibits
the effects of dynorphin on cell viability and proliferation when
the two drugs are used in combination (123). Kampa et al (124) demonstrated that KOR is expressed
in the PC3 and DU145 androgen-independent prostate cancer cell
lines. Subsequently, a novel opiate-active peptide
(Tyr-Ile-Phe-Asn-Leu) was found to bind to KOR and exhibit an
effective, dose-dependent and reversible anti-proliferation effect
on PC3 and DU145 prostate cancer cells (125). Yamashita et al (126) detected KOR mRNA expression in
the LNCaP and VCaP cell lines using RT-qPCR analysis. Androgens may
affect the proliferation and viability of prostate cancer cells
partly by regulating OR expression and other mechanisms (126,127).
The selective KOR opioid agonist U50488 induces
proliferation of 768-O and RLC-310 renal carcinoma cells; this may
be mediated by the anti-apoptotic protein surviving (10).
The aforementioned individual reports revealed the
association between KOR, androgens and anti-apoptotic proteins and
may have important implications for understanding urological tumor
formation and treatment. To the best of our knowledge, however,
studies on the association between KOR and tumors of the urinary
system are still scarce, and further studies are needed to confirm
the aforementioned preliminary findings.
Glioma
Glioma is the most common tumor of the central
nervous system, accounting for 80% of malignant brain tumors
(128). The rat C6 glioma cell
line has been used as a model to study various mechanisms of opioid
action (129–131). Using RT-PCR analysis and
radioligand studies, it has been demonstrated that C6 cells express
KOR (129,130). Bohn et al (132) found that the KOR selective
agonist U-69,593 stimulates proliferation of C6 cells by activating
phospholipase C (PLC), PKC and ERK (132). In this regard, the function of
KOR seems to be the opposite of that of MOR; activation of MOR in
C6 glioma cells attenuates KOR-induced DNA synthesis and tumor
proliferation, suggesting that MOR may be a negative regulator of
KOR in glioma (133).
Other types of cancer
Pheochromocytoma results in production of opioid
peptides, such as dynorphins (134). The KOR binding site is the most
common opioid binding site in surgically resected pheochromocytoma
and the Kat45 human pheochromocytoma cell line (134). Activation of KOR inhibits
biosynthesis and release of catecholamine in pheochromocytoma
(134). In addition, the
expression of KOR may be associated with paracrine regulation of
the proliferation of pheochromocytoma cells (134,135). The KOR agonist U-69,593 inhibits
the expression of EGF on pheochromocytoma cells in PC12 rats
(136). However, the
upregulation of the KOR ligand in human pheochromocytoma compared
with normal tissue has not been demonstrated to be statistically
significant, and it is unclear whether KOR agonists activate other
molecules and downstream signaling pathways via KOR.
In the endometrium, KOR is the most common opioid
binding site (21), and its
endogenous ligand dynorphin is produced in also this tissue
(137). TGFβ1 is a primary
endometrial growth factor that affects proliferation of normal and
tumor human endometrial epithelial and stromal cells and promotes
apoptosis of normal endometrial stromal cells (138,139). Chatzaki et al (137) found that treatment with
U-69,593, a specific KOR activator, inhibits production of TGFβ1 in
normal, epithelial, stromal and Ishikawa endometrial cancer
cells.
It has been reported that the presence of KOR in the
CNE-2 human nasopharyngeal carcinoma cell line promotes tumor cell
apoptosis by activating the PLC pathway (58).
KOR is located in the excitatory and inhibitory
motor neurons of the intermuscular nerve of the gastrointestinal
tract and human colon (140).
KOR inhibits excitatory transmission of the neuromuscular and
attenuates gastrointestinal peristalsis (140). The expression of KOR is
increased in primary gastric and duodenal neuroendocrine tumors
compared with paracancerous tissue. In a prospective study, KOR
expression was associated with distant liver metastasis of small
intestinal and pancreatic neuroendocrine tumors (141). These results all suggest that
KOR serves an essential role in cancer.
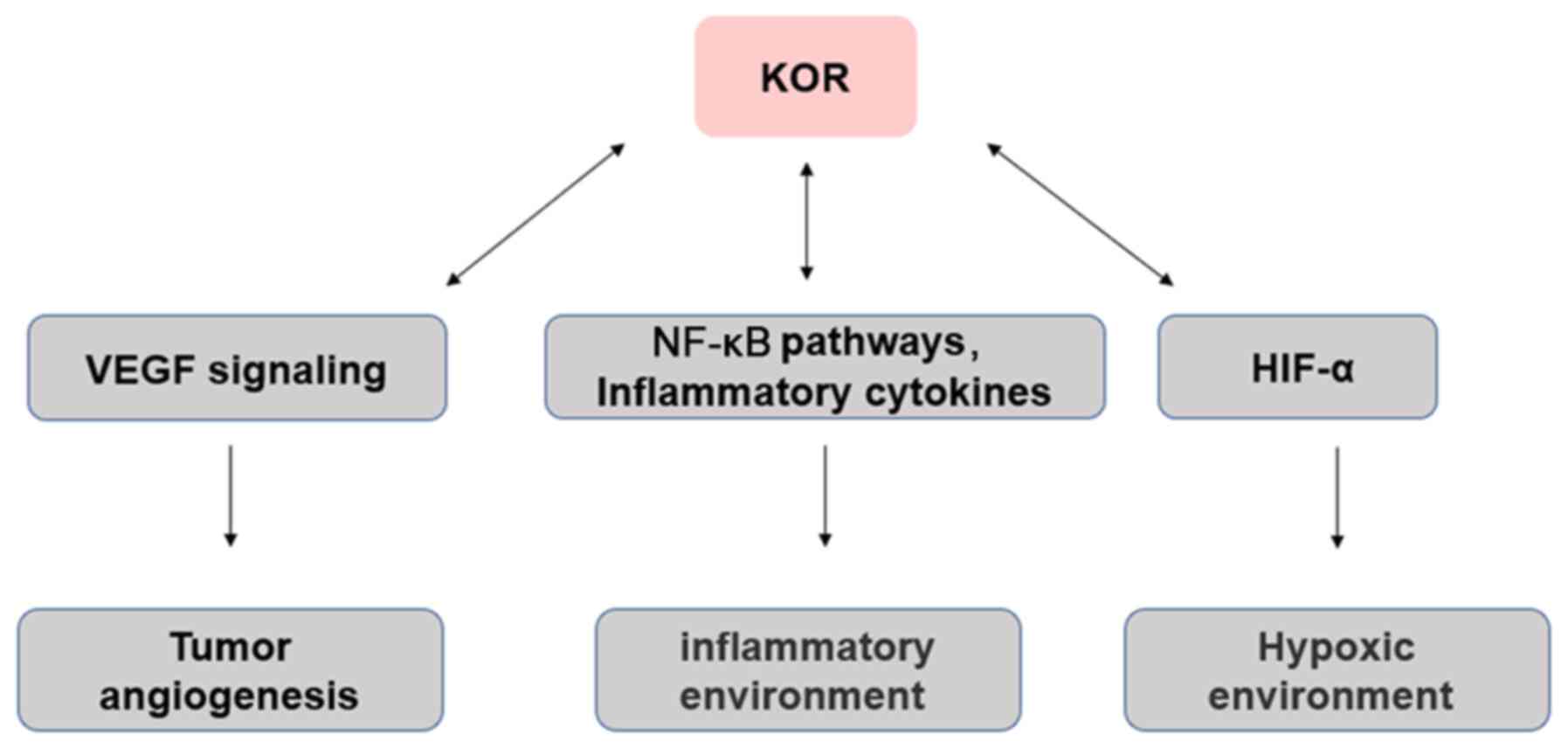
Potential roles of KOR in cancer
Angiogenesis
Tumor angiogenesis is necessary for tumor
progression, the provision of nutrients and oxygen, as well as the
removal of metabolic waste and carbon dioxide (142). Neovascularization in tumor
tissue is a complex process of imbalance between pro- and
antiangiogenic factors in the tumor microenvironment (142). Anti-angiogenesis therapy has
achieved promising results in gastric and colorectal cancer, as
well as NSCLC and other types of malignant tumor (143,144). Vascular growth factors such as
vascular endothelial growth factor (VEGF) are highly expressed in
tumors and induce tumor angiogenesis (142,145). In the clinic, angiogenesis
inhibitors targeting VEGF signaling are used as anticancer drugs
(146), such as Avastin
(bevacizumab) is a monoclonal antibody against VEGF in ovarian
(147), colorectal (148), kidney (149) and breast (150) cancer. Studies by Yamamizu et
al (51,151,152) have suggested that activation of
KOR inhibits differentiation and tissue angiogenesis of embryonic
stem cell-derived Flk1+ vascular endothelial progenitor
cells. KOR agonists U50488H and TRK820 inhibit vascular endothelial
cell migration and angiogenesis by inhibiting VEGF receptor
expression, although continued use of higher doses of TRK820 have
no significant effect on tumor growth. In addition, Lewis lung
cancer (LLC) or B16 melanoma has been transplanted subcutaneously
into KOR knockout mice. At 19 days post-transplantation, LLC and
B16 tumors in KOR knockout mice were significantly larger in size
and weight than those in control mice and showed greater
proliferation and tumor angiogenesis. These results suggest that
KOR activation inhibits tumor angiogenesis (51,151,152). Although a number of individual
angiogenesis inhibitors have demonstrated their ability to inhibit
tumor progression and metastasis in a variety of cancer models, the
effects of tumor regression vary by cancer type when the same
angiogenesis inhibitors are used, suggesting that the future
direction of anti-angiogenesis gene therapy is to identify
prognostic biomarkers to help determine the most effective
angiogenesis inhibitor genes for each cancer, which will largely
depend on further understanding of the biological mechanisms of
tumor angiogenesis. In view of the anti-angiogenic effects of KOR,
the possibility of developing novel targeted drugs in combination
with chemotherapy, targeted therapy and immunomodulatory drugs in
the treatment of various types of cancer should be explored
(Fig. 2).
Inflammation
Inflammation is usually associated with the
development and progression of cancer (153). The cells that cause
cancer-related inflammation are genetically stable, so drug
resistance does not appear quickly; therefore, targeting
inflammation is an attractive strategy for cancer prevention and
treatment (153). Inflammation
promotes the occurrence and development of certain types of tumor,
but inhibits others, including bacterial and viral infections,
autoimmune diseases, obesity, smoking, asbestos exposure and
excessive alcohol consumption, all of which increase the risk of
cancer and stimulate malignant progression (153,154). In addition, chronic inflammation
promotes carcinogenic mutations, as well as changes in
pro-inflammatory cytokines that stimulate angiogenesis and promote
tumor immune evasion (2,53).
Previous studies (48,62,155) have shown that ORs are
distributed outside the central nervous system, such as on the
surface of various types of immune cell, including monocytes,
macrophages and neutrophils, as well as T and B lymphocytes.
ORs serve an essential role in immune regulation,
which can lead to neuroinflammation of the central nervous system
and immune suppression of the peripheral immune system (48,62). There is evidence that KOR-specific
activators exert different anti-inflammatory effects. For example,
KOR-specific activators inhibit phagocytosis of macrophages,
aggregation of neutrophils, release of TNF-α, IL-10, IL-1 and IL-6,
production of inducible nitric oxide synthase, nitric oxide release
and nuclear translocation of NF-κB/p65 induced by
lipopolysaccharide (156,157).
Morphine affects the inflammatory response in the tumor
microenvironment (158).
Immunosuppression has been reported as the downregulation of NK
cell activity, responses of T and B cells to mitogens, antibody
formation in vivo and in vitro, reduction of
phagocytic and microbicidal activity of neutrophils and macrophages
(154,158), cytokine and chemokine production
by macrophages (159), microglia
and astrocytes, sensitization to various infections using animal
models, and the enhanced replication of human immunodeficiency
virus in vitro (158).
Moreover, MOR and KOR may have opposite effects, with the former
associated with induction of pro-inflammatory activity and the
latter with anti-inflammatory activity (159). Therefore, KOR may indirectly
affect the occurrence and development of tumors via its effects on
inflammation (Fig. 2).
Hypoxia
Hypoxia serves an important role in the tumor
microenvironment. In solid tumors, cancerous cells adapt to a
hypoxic environment through a variety of cellular mechanisms
(160). Hypoxic tumor cells
secrete VEGF and lactate, as well as a number of cytokines that
modulate the tumor microenvironment, which increases their
viability (160,161). The expression levels of KOR and
hypoxia-inducible factor 1α (HIF-1α) are significantly increased in
live human neurons following 24-h hypoxia (162). HIF-1α knockdown decreases KOR
expression (162). Thus, during
hypoxia, KOR promotes cell viability and its expression may be
regulated by HIF-1α (163). In
addition, the induction of hypoxia in neuroblastoma cells leads to
KOR internalization, which is inhibited by selective KOR
antagonists or dynein inhibitors and reversed by reoxygenation
(162). Thus, regulation of KOR
during hypoxia is mediated by its activation via a dynein-dependent
mechanism Further investigations are required to determine whether
the mechanism underlying the association between hypoxia influences
cancer onset (Fig. 2).
Effects of opioids on tumors
Archaeologists speculate that as early as the
Neolithic period, human ancestors found poppies in the mountains of
the Mediterranean (164).
Following the invention of the hypodermic syringe and hollow needle
in the 1850s, morphine began to be used in minor surgery, both for
postoperative and chronic pain and as an adjunct to general
anesthetic (164).
The World Health Organization-based three-step
medication principle (165) has
been used to treat cancer-associated pain. Pain potentially
decreases survival (166), one
primary mechanism for the potential effects of opioids on survival
is through immune effects (167), so it remains vital that pain is
effectively managed. Adequate control of cancer-associated pain
using opioids has been shown to improve quality of life, compliance
with cancer treatment and decrease emotional stress, and thus may
have a positive impact on patient survival (168,169). Cancer treatment primarily
includes surgical resection (170,171), postoperative radiotherapy
(172), chemotherapy and
targeted therapy (173,174), although certain patients
experience recurrence and metastasis following treatment. However,
several studies have shown that peri- and postoperative opioid
analgesics affect the metastasis and recurrence of cancer, although
the reported effects are inconsistent (175–177).
Clinical drugs targeting KOR, such as oxycodone
(178) and butorphanol (179), are KOR/MOR mixed agonists.
Similar to morphine, oxycodone inhibits proliferation and migration
of human lung adenocarcinoma cells and induces apoptosis (180). In addition, oxycodone has
different effects on the proliferation, migration and apoptosis of
cancer cells and weakens or enhances the efficacy of chemotherapy,
depending on the type of cancer cells and the expression levels of
EGFR in these cells (181).
Transmembrane protein with EGF-like and two
follistatin like domains 1 (TMEFF1) serves a vital role in the
development of various types of tumor, including brain (182), endometrial carcinoma (183) and ovarian cancer (184). Butorphanol significantly
inhibits the malignant biological behavior of ES-2 and SKOV3
ovarian cancer cells and the expression of TMEFF1 is significantly
downregulated in ovarian cancer cells (185).
Conclusion and future prospects
Opioids are often considered to have a negative
impact on cancer prognosis (169), the effects of opioid receptors
observed in patients with cancer are different, so the relationship
between opioid receptors and cancer has attracted attention
(186). Several studies have
shown that targeting KOR may be applied to treat a variety of
diseases (87–89,94,95,187). Despite the availability of
several types of treatment, cancer remains a significant threat to
human health (188). The opioid
system is associated with cancer progression and cell proliferation
and tumor prognosis (51,53). ORs affect patients with cancer
differently (23,27,51); therefore the association between
ORs and cancer is important. In recent years, KOR has been found to
be associated with several types of cancer and may influence its
progression and prognosis (52,127,189). In addition to its impact on
analgesia and the immune, endocrine, nervous and cardiovascular
systems (103), KOR also has a
significant impact on certain types of solid tumors and cancer
cells and may affect the prognosis of patients with cancer
(57,59,60). Most OR agonists bind to KOR and
inhibit cell proliferation (124). Thus, KOR may be a potential
therapeutic target for cancer therapy. This may facilitate the
development of novel drugs and a strategic shift in the treatment
of intra- and postoperative, as well as cancer-associated,
pain.
Nevertheless, the notion that KOR can be a
potential therapeutic target needs further research. At present,
preliminary basic studies on the effect of KOR on tumors are not
comprehensive, and most are in vitro experiments (10,58,59). Most of the available clinical
studies are retrospective in design and the conclusions are not
consistent (52,186). Therefore, when treating patients
with cancer, the dosage and treatment duration of opioids should be
weighed (186,190). To design opioid analgesics
without side effects, it is important to understand the molecular
mechanisms, signaling pathways and effects of KOR in combination
with chemotherapeutic drugs (191). For example, by decreasing entry
of opioids into the central nervous system, selective targeting of
KOR to the peripheral nervous system and inflammatory tissues is
biased towards activation of analgesia-associated intracellular
signaling pathways. Additionally, clinical trials are needed to
test the efficacy of KOR in cancer therapy and to identify its
potential benefits in decreasing cancer morbidity and mortality, as
well as improving quality of life. Further investigation of the
roles of KOR may facilitate development of novel treatment for
cancer and other types of disease.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Clinical Medical
Technology Innovation Project of Hunan (grant nos. 2020SK51903 and
2018SK51702).
Availability of data and materials
Not applicable.
Authors' contributions
QZ and ZZ conceived the study. SL and WL performed
the literature search. QZ drafted the manuscript. BW and NL
critically revised the manuscript. Data authentication is not
applicable. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Colombet M, Soerjomataram I,
Parkin DM, Piñeros M, Znaor A and Bray F: Cancer statistics for the
year 2020: An overview. Int J Cancer. April 5–2021.(Epub ahead of
print). View Article : Google Scholar
|
|
2
|
Rogers TJ: Kappa opioid receptor
expression and function in cells of the immune system. Handb Exp
Pharmacol. 271:419–433. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Trescot AM: Review of the role of opioids
in cancer pain. J Natl Compr Cancer Netw. 8:1087–1094. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lohse I and Brothers SP: Pathogenesis and
treatment of pancreatic cancer related pain. Anticancer Res.
40:1789–1796. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mercadante S and Vitrano V: Pain in
patients with lung cancer: Pathophysiology and treatment. Lung
Cancer. 68:10–15. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Szczepaniak A, Fichna J and Zielińska M:
Opioids in cancer development, progression and metastasis: Focus on
colorectal cancer. Curr Treat Options Oncol. 21:62020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Satija A, Ahmed SM, Gupta R, Ahmed A, Rana
SPS, Singh SP, Mishra S and Bhatnagar S: Breast cancer pain
management-a review of current & novel therapies. Indian J Med
Res. 139:216–225. 2014.PubMed/NCBI
|
|
8
|
Ing JW: Head and neck cancer pain.
Otolaryngol Clin North Am. 50:793–806. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu H, Zhang H, Weng ML, Zhang J, Jiang N,
Cata JP, Ma D, Chen WK and Miao CH: Morphine promotes tumorigenesis
and cetuximab resistance via EGFR signaling activation in human
colorectal cancer. J Cell Physiol. 236:4445–4454. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma Y, Ren Z, Ma S, Yan W, He M, Wang D and
Ding P: Morphine enhances renal cell carcinoma aggressiveness
through promotes survivin level. Ren Fail. 39:258–264. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ustun F, Durmus-Altun G, Altaner S,
Tuncbilek N, Uzal C and Berkarda S: Evaluation of morphine effect
on tumour angiogenesis in mouse breast tumour model, EATC. Med
Oncol. 28:1264–1272. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guerrero Orriach JL, Raigon Ponferrada A,
Malo Manso A, Herrera Imbroda B, Escalona Belmonte JJ, Ramirez
Aliaga M, Ramirez Fernandez A, Crespo JD, Soriano Perez AM,
Fontaneda Heredia A, et al: Anesthesia in combination with propofol
increases disease-free survival in bladder cancer patients who
undergo radical tumor cystectomy as compared to inhalational
anesthetics and opiate-based analgesia. Oncology. 98:161–167. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hasegawa T, Oguri T, Osawa T, Sawa T,
Osaga S, Okuyama T, Uchida M, Maeno K, Fukuda S, Nishie H, et al:
Opioid dose and survival of patients with incurable nonsmall cell
lung cancer: A prospective cohort study. J Palliat Med.
21:1436–1441. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gong S, Ying L, Fan Y and Sun Z: Fentanyl
inhibits lung cancer viability and invasion via upregulation of
miR-331-3p and repression of HDAC5. Onco Targets Ther.
13:13131–13141. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li C, Qin Y, Zhong Y, Qin Y, Wei Y, Li L
and Xie Y: Fentanyl inhibits the progression of gastric cancer
through the suppression of MMP-9 via the PI3K/Akt signaling
pathway. Ann Transl Med. 8:1182020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang XL, Chen ML and Zhou SL: Fentanyl
inhibits proliferation and invasion of colorectal cancer via
β-catenin. Int J Clin Exp Pathol. 8:227–235. 2015.PubMed/NCBI
|
|
17
|
Sasamura T, Nakamura S, Iida Y, Fujii H,
Murata J, Saiki I, Nojima H and Kuraishi Y: Morphine analgesia
suppresses tumor growth and metastasis in a mouse model of cancer
pain produced by orthotopic tumor inoculation. Eur J Pharmacol.
441:185–191. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fichna J and Janecka A: Opioid peptides in
cancer. Cancer Metastasis Rev. 23:351–366. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chatikhine VA, Chevrier A, Chauzy C, Duval
C, d'Anjou J, Girard N and Delpech B: Expression of opioid peptides
in cells and stroma of human breast cancer and adenofibromas.
Cancer Lett. 77:51–56. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zagon IS, Hytrek SD and McLaughlin PJ:
Opioid growth factor tonically inhibits human colon cancer cell
proliferation in tissue culture. Am J Physiol. 271:R511–R518.
1996.PubMed/NCBI
|
|
21
|
Hatzoglou A, Gravanis A, Margioris AN,
Zoumakis E and Castanas E: Identification and characterization of
opioid-binding sites present in the Ishikawa human endometrial
adenocarcinoma cell line. J Clin Endocrinol Metab. 80:418–423.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Roth KA and Barchas JD: Small cell
carcinoma cell lines contain opioid peptides and receptors. Cancer.
57:769–773. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Singleton PA, Mirzapoiazova T, Hasina R,
Salgia R and Moss J: Increased µ-opioid receptor expression in
metastatic lung cancer. Br J Anaesth. 113 (Suppl 1):i103–i108.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lennon FE, Mirzapoiazova T, Mambetsariev
B, Salgia R, Moss J and Singleton PA: Overexpression of the
µ-opioid receptor in human non-small cell lung cancer promotes Akt
and mTOR activation, tumor growth, and metastasis. Anesthesiology.
116:857–867. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wolff RF, Aune D, Truyers C, Hernandez AV,
Misso K, Riemsma R and Kleijnen J: Systematic review of efficacy
and safety of buprenorphine versus fentanyl or morphine in patients
with chronic moderate to severe pain. Curr Med Res Opin.
28:833–845. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cieślińska A, Sienkiewicz-Szłapka E,
Kostyra E, Fiedorowicz E, Snarska J, Wroński K, Tenderenda M,
Jarmołowska B and Matysiewicz M: µ-Opioid receptor gene (OPRM1)
polymorphism in patients with breast cancer. Tumour Biol.
36:4655–4660. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Singleton PA, Moss J, Karp DD, Atkins JT
and Janku F: The mu opioid receptor: A new target for cancer
therapy? Cancer. 121:2681–2688. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Steele GL, Dudek AZ, Gilmore GE, Richter
SA, Olson DA, Eklund JP and Zylla DM: Impact of pain, opioids, and
the mu-opioid receptor on progression and survival in patients with
newly diagnosed stage IV pancreatic cancer. Am J Clin Oncol.
43:591–597. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim J, Ham S, Hong H, Moon C and Im HI:
Brain reward circuits in morphine addiction. Mol Cells. 39:645–653.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Benyamin R, Trescot AM, Datta S,
Buenaventura R, Adlaka R, Sehgal N, Glaser SE and Vallejo R: Opioid
complications and side effects. Pain Physician. 11 (Suppl
2):S105–S120. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bodnar RJ: Endogenous opioid modulation of
food intake and body weight: Implications for opioid influences
upon motivation and addiction. Peptides. 116:42–62. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bagley EE and Ingram SL: Endogenous opioid
peptides in the descending pain modulatory circuit.
Neuropharmacology. 173:1081312020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Barron BA: Opioid peptides and the heart.
Cardiovasc Res. 43:13–16. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gein SV and Baeva TA: Endogenous opioid
peptides in regulation of innate immunity cell functions.
Biochemistry (Mosc). 76:309–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mercadante S and Romualdi P: The
therapeutic potential of novel kappa opioid receptor-based
treatments. Curr Med Chem. 27:2012–2020. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Waldhoer M, Bartlett SE and Whistler JL:
Opioid receptors. Annu Rev Biochem. 73:953–990. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Minami M, Toya T, Katao Y, Maekawa K,
Nakamura S, Onogi T, Kaneko S and Satoh M: Cloning and expression
of a cDNA for the rat kappa-opioid receptor. FEBS Lett.
329:291–295. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schoffelmeer AN, Hogenboom F and Mulder
AH: Kappa1- and kappa2-opioid receptors mediating presynaptic
inhibition of dopamine and acetylcholine release in rat
neostriatum. Br J Pharmacol. 122:520–524. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Brooks AI, Standifer KM, Rossi GC, Mathis
JP and Pasternak GW: Characterizing kappa3 opioid receptors with a
selective monoclonal antibody. Synapse. 22:247–252. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cahill CM, Taylor AM, Cook C, Ong E, Morón
JA and Evans CJ: Does the kappa opioid receptor system contribute
to pain aversion? Front Pharmacol. 5:2532014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Crowley NA and Kash TL: Kappa opioid
receptor signaling in the brain: Circuitry and implications for
treatment. Prog Neuropsychopharmacol Biol Psychiatry. 62:51–60.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ruan X, Mancuso KF and Kaye AD: Revisiting
oxycodone analgesia: A review and hypothesis. Anesthesiol Clin.
35:e163–e174. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Schmidt-Hansen M, Bennett MI, Arnold S,
Bromham N and Hilgart JS: Oxycodone for cancer-related pain.
Cochrane Database Syst Rev. 8:Cd0038702017.PubMed/NCBI
|
|
44
|
Jin WQ, Tai KK, Chan TK and Wong TM:
Further characterization of [3H]U69593 binding sites in the rat
heart. J Mol Cell Cardiol. 27:1507–1511. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Simonin F, Gavériaux-Ruff C, Befort K,
Matthes H, Lannes B, Micheletti G, Mattéi MG, Charron G, Bloch B
and Kieffer B: kappa-Opioid receptor in humans: cDNA and genomic
cloning, chromosomal assignment, functional expression,
pharmacology, and expression pattern in the central nervous system.
Proc Natl Acad Sci USA. 92:7006–7010. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gavériaux-Ruff C, Peluso J, Befort K,
Simonin F, Zilliox C and Kieffer BL: Detection of opioid receptor
mRNA by RT-PCR reveals alternative splicing for the delta- and
kappa-opioid receptors. Brain Res Mol Brain Res. 48:298–304. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang YJ, Rasakham K, Huang P, Chudnovskaya
D, Cowan A and Liu-Chen LY: Sex difference in κ-opioid receptor
(KOPR)-mediated behaviors, brain region KOPR level and
KOPR-mediated guanosine 5′-O-(3-[35S]thiotriphosphate) binding in
the guinea pig. J Pharmacol Exp Ther. 339:438–450. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Machelska H and Celik M: Opioid receptors
in immune and glial cells-implications for pain control. Front
Immunol. 11:3002020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Spasov AA, Grechko O and Shtareva DM:
Kappa-opioid receptor: Molecular structure and function. Eksp klin
Farmakol. 77:27–35. 2014.(In Russian).
|
|
50
|
Carroll FI and Carlezon WA Jr: Development
of κ opioid receptor antagonists. J Med Chem. 56:2178–2195. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yamamizu K, Hamada Y and Narita M: κ
Opioid receptor ligands regulate angiogenesis in development and in
tumours. Br J Pharmacol. 172:268–276. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shi Y, Luo J, Tian J, Zou Q and Wang X:
The kappa opioid receptor may be a potential tumor suppressor by
regulating angiogenesis in breast cancer. Med Hypotheses.
150:1105682021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wigmore T and Farquhar-Smith P: Opioids
and cancer: Friend or foe? Curr Opin Support Palliat Care.
10:109–118. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Strang P: Cancer pain-a provoker of
emotional, social and existential distress. Acta Oncol. 37:641–644.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Reiche EM, Nunes SO and Morimoto HK:
Stress, depression, the immune system, and cancer. Lancet Oncol.
5:617–625. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Edwards KA, Havelin JJ, McIntosh MI,
Ciccone HA, Pangilinan K, Imbert I, Largent-Milnes TM, King T,
Vanderah TW and Streicher JM: A kappa opioid receptor agonist
blocks bone cancer pain without altering bone loss, tumor size, or
cancer cell proliferation in a mouse model of cancer-induced bone
pain. J Pain. 19:612–625. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhang YF, Xu QX, Liao LD, Xu XE, Wu JY,
Shen J, Wu ZY, Shen JH, Li EM and Xu LY: κ-Opioid receptor in the
nucleus is a novel prognostic factor of esophageal squamous cell
carcinoma. Hum Pathol. 44:1756–1765. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Diao CT, Li L, Lau SY, Wong TM and Wong
NS: kappa-Opioid receptor potentiates apoptosis via a phospholipase
C pathway in the CNE2 human epithelial tumor cell line. Biochim
Biophys Acta. 1499:49–62. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li H, Ma Z and Lei Y: The expression of
kappa-opioid receptor promotes the migration of breast cancer cells
in vitro. BMC Anesthesiol. 21:2102021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chen D, Chen Y, Yan Y, Pan J, Xing W, Li Q
and Zeng W: Down-regulation of the tumour suppressor κ-opioid
receptor predicts poor prognosis in hepatocellular carcinoma
patients. BMC Cancer. 17:5532017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Tripolt S, Neubauer HA, Knab VM, Elmer DP,
Aberger F, Moriggl R and Fux DA: Opioids drive breast cancer
metastasis through the δ-opioid receptor and oncogenic STAT3.
Neoplasia. 23:270–279. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Brejchova J, Holan V and Svoboda P:
Expression of opioid receptors in cells of the immune system. Int J
Mol Sci. 22:3152020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Pert CB and Snyder SH: Opiate receptor:
Demonstration in nervous tissue. Science. 179:1011–1014. 1973.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kiguchi N, Ding H, Kishioka S and Ko MC:
Nociceptin/orphanin FQ peptide receptor-related ligands as novel
analgesics. Curr Top Med Chem. 20:2878–2888. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Dhaliwal A and Gupta M: Physiology, opioid
receptor. StatPearls (Internet). StatPearls Publishing; Treasure
Island, FL: 2021
|
|
66
|
Valentino RJ and Volkow ND: Untangling the
complexity of opioid receptor function. Neuropsychopharmacology.
43:2514–2520. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Chavkin C, James IF and Goldstein A:
Dynorphin is a specific endogenous ligand of the kappa opioid
receptor. Science. 215:413–415. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Bruchas MR, Land BB and Chavkin C: The
dynorphin/kappa opioid system as a modulator of stress-induced and
pro-addictive behaviors. Brain Res. 1314:44–55. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Terenius L: From opiate pharmacology to
opioid peptide physiology. Ups J Med Sci. 105:1–15. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Goldstein A and Naidu A: Multiple opioid
receptors: Ligand selectivity profiles and binding site signatures.
Mol Pharmacol. 36:265–272. 1989.PubMed/NCBI
|
|
71
|
Giros B, Pohl M, Rochelle JM and Seldin
MF: Chromosomal localization of opioid peptide and receptor genes
in the mouse. Life Sci. 56:PL369–PL375. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wei LN and Loh HH: Transcriptional and
epigenetic regulation of opioid receptor genes: Present and future.
Annu Rev Pharmacol Toxicol. 51:75–97. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Xie GX, Meng F, Mansour A, Thompson RC,
Hoversten MT, Goldstein A, Watson SJ and Akil H: Primary structure
and functional expression of a guinea pig kappa opioid (dynorphin)
receptor. Proc Natl Acad Sci USA. 91:3779–3783. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wu H, Wacker D, Mileni M, Katritch V, Han
GW, Vardy E, Liu W, Thompson AA, Huang XP, Carroll FI, et al:
Structure of the human κ-opioid receptor in complex with JDTic.
Nature. 485:327–332. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Akil H and Watson SJ: Cloning of kappa
opioid receptors: Functional significance and future directions.
Prog Brain Res. 100:81–86. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Law PY, Loh HH and Wei LN: Insights into
the receptor transcription and signaling: Implications in opioid
tolerance and dependence. Neuropharmacology. 47 (Suppl
1):S300–S311. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Al-Hasani R and Bruchas MR: Molecular
mechanisms of opioid receptor-dependent signaling and behavior.
Anesthesiology. 115:1363–1381. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Bruchas MR, Xu M and Chavkin C: Repeated
swim stress induces kappa opioid-mediated activation of
extracellular signal-regulated kinase 1/2. Neuroreport.
19:1417–1422. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kam AY, Chan AS and Wong YH: Kappa-opioid
receptor signals through Src and focal adhesion kinase to stimulate
c-Jun N-terminal kinases in transfected COS-7 cells and human
monocytic THP-1 cells. J Pharmacol Exp Ther. 310:301–310. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Finley MJ, Steele A, Cornwell WD and
Rogers TJ: Transcriptional regulation of the major HIV-1
coreceptor, CXCR4, by the kappa opioid receptor. J Leukoc Biol.
90:111–121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Georgoussi Z, Georganta EM and Milligan G:
The other side of opioid receptor signalling: Regulation by
protein-protein interaction. Curr Drug Targets. 13:80–102. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Fink RM and Gallagher E: Cancer pain
assessment and measurement. Semin Oncol Nurs. 35:229–234. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wiffen PJ, Cooper TE, Anderson AK, Gray
AL, Grégoire MC, Ljungman G and Zernikow B: Opioids for
cancer-related pain in children and adolescents. Cochrane Database
Syst Rev. 7:Cd0125642017.PubMed/NCBI
|
|
84
|
Wiffen PJ, Wee B, Derry S, Bell RF and
Moore RA: Opioids for cancer pain-an overview of cochrane reviews.
Cochrane Database Syst Rev. 7:Cd0125922017.PubMed/NCBI
|
|
85
|
Paton KF, Atigari DV, Kaska S, Prisinzano
T and Kivell BM: Strategies for developing κ opioid receptor
agonists for the treatment of pain with fewer side effects. J
Pharmacol Exp Ther. 375:332–348. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Escobar ADP, Casanova JP, Andrés ME and
Fuentealba JA: Crosstalk between kappa opioid and dopamine systems
in compulsive behaviors. Front Pharmacol. 11:572020. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
McLaughlin JP, Li S, Valdez J, Chavkin TA
and Chavkin C: Social defeat stress-induced behavioral responses
are mediated by the endogenous kappa opioid system.
Neuropsychopharmacology. 31:1241–1248. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Knoll AT and Carlezon WA Jr: Dynorphin,
stress, and depression. Brain Res. 1314:56–73. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Carlezon WA Jr and Krystal AD:
Kappa-Opioid antagonists for psychiatric disorders: From bench to
clinical trials. Depress Anxiety. 33:895–906. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Falcon E, Maier K, Robinson SA, Hill-Smith
TE and Lucki I: Effects of buprenorphine on behavioral tests for
antidepressant and anxiolytic drugs in mice. Psychopharmacology
(Berl). 232:907–915. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ahmadi J, Jahromi MS and Ehsaei Z: The
effectiveness of different singly administered high doses of
buprenorphine in reducing suicidal ideation in acutely depressed
people with co-morbid opiate dependence: A randomized,
double-blind, clinical trial. Trials. 19:4622018. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Serafini G, Adavastro G, Canepa G, De
Berardis D, Valchera A, Pompili M, Nasrallah H and Amore M: The
efficacy of buprenorphine in major depression, treatment-resistant
depression and suicidal behavior: A systematic review. Int J Mol
Sci. 19:24102018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wang Q, Long Y, Hang A, Zan GY, Shu XH,
Wang YJ and Liu JG: The anxiolytic- and antidepressant-like effects
of ATPM-ET, a novel κ agonist and µ partial agonist, in mice.
Psychopharmacology (Berl). 233:2411–2418. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Kovalska M, Kovalska L, Tothova B, Mahmood
S, Adamkov M and Lehotsky J: Combination of hyperhomocysteinemia
and ischemic tolerance in experimental model of global ischemia in
rats. J Physiol Pharmacol. 66:887–897. 2015.PubMed/NCBI
|
|
95
|
Dennis TS, Beck KD, Cominski TP, Bobzean
SAM, Kuzhikandathil EV, Servatius RJ and Perrotti LI: Exposure to
morphine-associated cues increases mu opioid receptor mRNA
expression in the nucleus accumbens of Wistar Kyoto rats. Behav
Brain Res. 313:208–213. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Menyhárt Á, Makra P, Szepes BE, Tóth OM,
Hertelendy P, Bari F and Farkas E: High incidence of adverse
cerebral blood flow responses to spreading depolarization in the
aged ischemic rat brain. Neurobiol Aging. 36:3269–3277. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Chen C, Xi C, Liang X, Ma J, Su D, Abel T
and Liu R: The role of κ opioid receptor in brain ischemia. Crit
Care Med. 44:e1219–e1225. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Sobanski P, Krajnik M, Shaqura M,
Bloch-Boguslawska E, Schäfer M and Mousa SA: The presence of mu-,
delta-, and kappa-opioid receptors in human heart tissue. Heart
Vessels. 29:855–863. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Jaiswal A, Kumar S, Seth S, Dinda AK and
Maulik SK: Effect of U50,488H, a κ-opioid receptor agonist on
myocardial α-and β-myosin heavy chain expression and oxidative
stress associated with isoproterenol-induced cardiac hypertrophy in
rat. Mol Cell Biochem. 345:231–240. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Komaba H, Moriwaki K, Goto S, Yamada S,
Taniguchi M, Kakuta T, Kamae I and Fukagawa M: Cost-effectiveness
of cinacalcet hydrochloride for hemodialysis patients with severe
secondary hyperparathyroidism in Japan. Am J Kidney Dis.
60:262–271. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Wang H, Wang JL, Ren HW, He WF and Sun M:
Butorphanol protects on myocardial ischemia/reperfusion injury in
rats through MAPK signaling pathway. Eur Rev Med Pharmacol Sci.
23:10541–10548. 2019.PubMed/NCBI
|
|
102
|
Brust TF, Morgenweck J, Kim SA, Rose JH,
Locke JL, Schmid CL, Zhou L, Stahl EL, Cameron MD, Scarry SM, et
al: Biased agonists of the kappa opioid receptor suppress pain and
itch without causing sedation or dysphoria. Sci Signal.
9:ra1172016. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Feng Y, He X, Yang Y, Chao D, Lazarus LH
and Xia Y: Current research on opioid receptor function. Curr Drug
Targets. 13:230–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Llovet JM, Zucman-Rossi J, Pikarsky E,
Sangro B, Schwartz M, Sherman M and Gores G: Hepatocellular
carcinoma. Nat Rev Dis Primers. 2:160182016. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Maneckjee R and Minna JD: Opioid and
nicotine receptors affect growth regulation of human lung cancer
cell lines. Proc Natl Acad Sci USA. 87:3294–3298. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Kuzumaki N, Suzuki A, Narita M, Hosoya T,
Nagasawa A, Imai S, Yamamizu K, Morita H, Nagase H, Okada Y, et al:
Effect of κ-opioid receptor agonist on the growth of non-small cell
lung cancer (NSCLC) cells. Br J Cancer. 106:1148–1152. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Sim EH, Yang IA, Wood-Baker R, Bowman RV
and Fong KM: Gefitinib for advanced non-small cell lung cancer.
Cochrane Database Syst Rev. 1:CD0068472018.PubMed/NCBI
|
|
110
|
Bareschino MA, Schettino C, Troiani T,
Martinelli E, Morgillo F and Ciardiello F: Erlotinib in cancer
treatment. Ann Oncol. 18 (Suppl 6):vi35–vi41. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Rho JK, Choi YJ, Ryoo BY, Na III, Yang SH,
Kim CH and Lee JC: p53 enhances gefitinib-induced growth inhibition
and apoptosis by regulation of Fas in non-small cell lung cancer.
Cancer Res. 67:1163–1169. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
An WF, Germain AR, Bishop JA, Nag PP,
Metkar S, Ketterman J, Walk M, Weiwer M, Liu X, Patnaik D, et al:
Discovery of potent and highly selective inhibitors of GSK3b. Probe
Reports from the NIH Molecular Libraries Program. National Center
for Biotechnology Information (US); Bethesda (MD): 2010, PubMed/NCBI
|
|
113
|
Tian D, Zhu M, Chen WS, Li JS, Wu RL and
Wang X: Role of glycogen synthase kinase 3 in squamous
differentiation induced by cigarette smoke in porcine
tracheobronchial epithelial cells. Food Chem Toxicol. 44:1590–1596.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Zheng H, Saito H, Masuda S, Yang X and
Takano Y: Phosphorylated GSK3beta-ser9 and EGFR are good prognostic
factors for lung carcinomas. Anticancer Res. 27:3561–3569.
2007.PubMed/NCBI
|
|
115
|
Oren M: Decision making by p53: Life,
death and cancer. Cell Death Differ. 10:431–442. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Gao S, Brown J, Wang H and Feng X: The
role of glycogen synthase kinase 3-β in immunity and cell cycle:
Implications in esophageal cancer. Arch Immunol Ther Exp (Warsz).
62:131–144. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Maneckjee R, Biswas R and Vonderhaar BK:
Binding of opioids to human MCF-7 breast cancer cells and their
effects on growth. Cancer Res. 50:2234–2238. 1990.PubMed/NCBI
|
|
118
|
Hatzoglou A, Bakogeorgou E and Castanas E:
The antiproliferative effect of opioid receptor agonists on the
T47D human breast cancer cell line, is partially mediated through
opioid receptors. Eur J Pharmacol. 296:199–207. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Montagna G, Gupta HV, Hannum M, Tan KS,
Lee J, Scarpa JR, Plitas G, Irie T, McCormick PJ, Fischer GW, et
al: Intraoperative opioids are associated with improved
recurrence-free survival in triple-negative breast cancer. Br J
Anaesth. 126:367–376. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Abnet CC, Arnold M and Wei WQ:
Epidemiology of esophageal squamous cell carcinoma.
Gastroenterology. 154:360–373. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Zylla D, Gourley BL, Vang D, Jackson S,
Boatman S, Lindgren B, Kuskowski MA, Le C, Gupta K and Gupta P:
Opioid requirement, opioid receptor expression, and clinical
outcomes in patients with advanced prostate cancer. Cancer.
119:4103–4110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Harper P, Hald O, Lwaleed BA, Kyyaly A,
Johnston D, Cooper AJ and Birch B: The impact of morphine treatment
on bladder cancer cell proliferation and apoptosis: In vitro
studies. Exp Oncol. 40:190–193. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Moon TD: The effect of opiates upon
prostatic carcinoma cell growth. Biochem Biophys Res Commun.
153:722–727. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Kampa M, Bakogeorgou E, Hatzoglou A,
Damianaki A, Martin PM and Castanas E: Opioid alkaloids and
casomorphin peptides decrease the proliferation of prostatic cancer
cell lines (LNCaP, PC3 and DU145) through a partial interaction
with opioid receptors. Eur J Pharmacol. 335:255–265. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Kampa M, Loukas S, Tsapis A and Castanas
E: Receptorphin: A conserved peptide derived from the sequence of
the opioid receptor, with opioid displacement activity and potent
antiproliferative actions in tumor cells. BMC Pharmacol. 1:92001.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Yamashita H, Shuman L, Warrick JI, Raman
JD and Degraff DJ: Androgen represses opioid growth factor receptor
(OGFR) in human prostate cancer LNCaP cells and OGFR expression in
human prostate cancer tissue. Am J Clin Exp Urol. 6:164–171.
2018.PubMed/NCBI
|
|
127
|
Lec PM, Lenis AT, Golla V, Brisbane W,
Shuch, Garraway IP, Reiter RE and Chamie K: The role of opioids and
their receptors in urological malignancy: A review. J Urol.
204:1150–1159. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Goodenberger ML and Jenkins RB: Genetics
of adult glioma. Cancer Genet. 205:613–621. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Bohn LM, Belcheva MM and Coscia CJ:
Evidence for kappa- and mu-opioid receptor expression in C6 glioma
cells. J Neurochem. 70:1819–1825. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Remmers AE, Clark MJ, Mansour A, Akil H,
Woods JH and Medzihradsky F: Opioid efficacy in a C6 glioma cell
line stably expressing the human kappa opioid receptor. J Pharmacol
Exp Ther. 288:827–833. 1999.PubMed/NCBI
|
|
131
|
Giakoumettis D, Kritis A and Foroglou N:
C6 cell line: The gold standard in glioma research. Hippokratia.
22:105–112. 2018.PubMed/NCBI
|
|
132
|
Bohn LM, Belcheva MM and Coscia CJ:
Mitogenic signaling via endogenous kappa-opioid receptors in C6
glioma cells: Evidence for the involvement of protein kinase C and
the mitogen-activated protein kinase signaling cascade. J
Neurochem. 74:564–573. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Bohn LM, Belcheva MM and Coscia CJ:
Mu-opioid agonist inhibition of kappa-opioid receptor-stimulated
extracellular signal-regulated kinase phosphorylation is
dynamin-dependent in C6 glioma cells. J Neurochem. 74:574–581.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Kampa M, Margioris AN, Hatzoglou A,
Dermitzaki I, Denizot A, Henry JF, Oliver C, Gravanis A and
Castanas E: Kappa1-opioid binding sites are the dominant opioid
binding sites in surgical specimens of human pheochromocytomas and
in a human pheochromocytoma (KAT45) cell line. Eur J Pharmacol.
364:255–262. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Venihaki M, Gravanis A and Margioris AN:
Kappa opioids exert a strong antiproliferative effect on PC12 rat
pheochromocytoma cells. Peptides. 17:413–419. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Takekoshi K, Ishii K, Kawakami Y, Isobe K
and Nakai T: kappa-Opioid inhibits catecholamine biosynthesis in
PC12 rat pheochromocytoma cell. FEBS Lett. 477:273–277. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Chatzaki E, Margioris AN, Makrigiannakis
A, Castanas E, Georgoulias V and Gravanis A: Kappa opioids and
TGFbeta1 interact in human endometrial cells. Mol Hum Reprod.
6:602–609. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Li WW, Zhao WJ, Yang Y and Yang YM:
Effects of TGF-β1 on the expression of endometrial stromal
cell-related protein and mRNA. Eur Rev Med Pharmacol Sci.
24:11475–11480. 2020.PubMed/NCBI
|
|
139
|
Chatzaki E, Kouimtzoglou E, Margioris AN
and Gravanis A: Transforming growth factor beta1 exerts an
autocrine regulatory effect on human endometrial stromal cell
apoptosis, involving the FasL and Bcl-2 apoptotic pathways. Mol Hum
Reprod. 9:91–95. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Chamouard P, Klein A, Martin E, Adloff M
and Angel F: Regulatory role of enteric kappa opioid receptors in
human colonic motility. Life Sci. 53:1149–1156. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Sherman SK, Maxwell JE, Carr JC, Wang D,
Bellizzi AM, O'Dorisio MS, O'Dorisio TM and Howe JR: Gene
expression accurately distinguishes liver metastases of small bowel
and pancreas neuroendocrine tumors. Clin Exp Metastasis.
31:935–944. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Li T, Kang G, Wang T and Huang H: Tumor
angiogenesis and anti-angiogenic gene therapy for cancer. Oncol
Lett. 16:687–702. 2018.PubMed/NCBI
|
|
143
|
Mody K, Baldeo C and Bekaii-Saab T:
Antiangiogenic therapy in colorectal cancer. Cancer J. 24:165–170.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Al-Husein B, Abdalla M, Trepte M, Deremer
DL and Somanath PR: Antiangiogenic therapy for cancer: An update.
Pharmacotherapy. 32:1095–1111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Carmeliet P: VEGF as a key mediator of
angiogenesis in cancer. Oncology. 69 (Suppl 3):S4–S10. 2005.
View Article : Google Scholar
|
|
146
|
Olejarz W, Kubiak-Tomaszewska G,
Chrzanowska A and Lorenc T: Exosomes in angiogenesis and
anti-angiogenic therapy in cancers. Int J Mol Sci. 21:58402020.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Pujade-Lauraine E, Hilpert F, Weber B,
Reuss A, Poveda A, Kristensen G, Sorio R, Vergote I, Witteveen P,
Bamias A, et al: Bevacizumab combined with chemotherapy for
platinum-resistant recurrent ovarian cancer: The AURELIA open-label
randomized phase III trial. J Clin Oncol. 32:1302–1308. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Heinemann V, von Weikersthal LF, Decker T,
Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller
C, Kahl C, Seipelt G, et al: FOLFIRI plus cetuximab versus FOLFIRI
plus bevacizumab as first-line treatment for patients with
metastatic colorectal cancer (FIRE-3): A randomised, open-label,
phase 3 trial. Lancet Oncol. 15:1065–1075. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Rini BI, Bellmunt J, Clancy J, Wang K,
Niethammer AG, Hariharan S and Escudier B: Randomized phase III
trial of temsirolimus and bevacizumab versus interferon alfa and
bevacizumab in metastatic renal cell carcinoma: INTORACT trial. J
Clin Oncol. 32:752–759. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Bear HD, Tang G, Rastogi P, Geyer CE Jr,
Liu Q, Robidoux A, Baez-Diaz L, Brufsky AM, Mehta RS, Fehrenbacher
L, et al: Neoadjuvant plus adjuvant bevacizumab in early breast
cancer [NSABP B-40 (NRG Oncology)]: Secondary outcomes of a phase
3, randomised controlled trial. Lancet Oncol. 16:1037–1048. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Yamamizu K, Furuta S, Hamada Y, Yamashita
A, Kuzumaki N and Narita M, Doi K, Katayama S, Nagase H, Yamashita
JK and Narita M: к Opioids inhibit tumor angiogenesis by
suppressing VEGF signaling. Sci Rep. 3:32132013. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Yamamizu K and Yamashita JK: Roles of
cyclic adenosine monophosphate signaling in endothelial cell
differentiation and arterial-venous specification during vascular
development. Circ J. 75:253–260. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Singh N, Baby D, Rajguru JP, Patil PB,
Thakkannavar SS and Pujari VB: Inflammation and cancer. Ann Afr
Med. 18:121–126. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Elinav E, Nowarski R, Thaiss CA, Hu B, Jin
C and Flavell RA: Inflammation-induced cancer: Crosstalk between
tumours, immune cells and microorganisms. Nat Rev Cancer.
13:759–771. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Chuang TK, Killam KF Jr, Chuang LF, Kung
HF, Sheng WS, Chao CC, Yu L and Chuang RY: Mu opioid receptor gene
expression in immune cells. Biochem Biophys Res Commun.
216:922–930. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Kao TK, Ou YC, Liao SL, Chen WY, Wang CC,
Chen SY, Chiang AN and Chen CJ: Opioids modulate post-ischemic
progression in a rat model of stroke. Neurochem Int. 52:1256–1265.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Zhang P, Yang M, Chen C, Liu L, Wei X and
Zeng S: Toll-Like receptor 4 (TLR4)/opioid receptor pathway
crosstalk and impact on opioid analgesia, immune function, and
gastrointestinal motility. Front Immunol. 11:14552020. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Eisenstein TK: The role of opioid
receptors in immune system function. Front Immunol. 10:29042019.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Finley MJ, Happel CM, Kaminsky DE and
Rogers TJ: Opioid and nociceptin receptors regulate cytokine and
cytokine receptor expression. Cell Immunol. 252:146–154. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Multhoff G and Vaupel P: Hypoxia
compromises anti-cancer immune responses. Adv Exp Med Biol.
1232:131–143. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Dehne N, Mora J, Namgaladze D, Weigert A
and Brüne B: Cancer cell and macrophage cross-talk in the tumor
microenvironment. Curr Opin Pharmacol. 35:12–19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Xi C, Liang X, Chen C, Babazada H, Li T
and Liu R: Hypoxia induces internalization of κ-opioid receptor.
Anesthesiology. 126:842–854. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Babcock J, Herrera A, Coricor G, Karch C,
Liu AH, Rivera-Gines A and Ko JL: Mechanism governing human
kappa-opioid receptor expression under desferrioxamine-induced
hypoxic mimic condition in neuronal NMB cells. Int J Mol Sci.
18:2112017. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Brownstein MJ: A brief history of opiates,
opioid peptides, and opioid receptors. Proc Natl Acad Sci USA.
90:5391–5393. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Meuser T, Pietruck C, Radbruch L, Stute P,
Lehmann KA and Grond S: Symptoms during cancer pain treatment
following WHO-guidelines: A longitudinal follow-up study of symptom
prevalence, severity and etiology. Pain. 93:247–257. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Zylla D, Steele G and Gupta P: A
systematic review of the impact of pain on overall survival in
patients with cancer. Support Care Cancer. 25:1687–1698. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Boland JW and Pockley AG: Influence of
opioids on immune function in patients with cancer pain: From bench
to bedside. Br J Pharmacol. 175:2726–2736. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Juneja R: Opioids and cancer recurrence.
Curr Opin Support Palliat Care. 8:91–101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Amaram-Davila J, Davis M and Reddy A:
Opioids and cancer mortality. Curr Treat Options Oncol. 21:222020.
View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Fujiwara Y, Yamamoto H and Monden M:
Molecular biology-based surgery for the future progress of cancer
treatment. Nihon Geka Gakkai Zasshi. 103:278–283. 2002.(In
Japanese). PubMed/NCBI
|
|
171
|
Hashizume M and Tsugawa K: Robotic surgery
and cancer: The present state, problems and future vision. Jpn J
Clin Oncol. 34:227–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Baskar R, Lee KA, Yeo R and Yeoh KW:
Cancer and radiation therapy: Current advances and future
directions. Int J Med Sci. 9:193–199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Lee YT, Tan YJ and Oon CE: Molecular
targeted therapy: Treating cancer with specificity. Eur J
Pharmacol. 834:188–196. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Cross D and Burmester JK: Gene therapy for
cancer treatment: Past, present and future. Clin Med Res.
4:218–227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Chen WK and Miao CH: The effect of
anesthetic technique on survival in human cancers: A meta-analysis
of retrospective and prospective studies. PLoS One. 8:e565402013.
View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Gach K, Wyrębska A, Fichna J and Janecka
A: The role of morphine in regulation of cancer cell growth. Naunyn
Schmiedebergs Arch Pharmacol. 384:221–230. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Forget P, Aguirre JA, Bencic I, Borgeat A,
Cama A, Condron C, Eintrei C, Eroles P, Gupta A, Hales TG, et al:
How anesthetic, analgesic and other non-surgical techniques during
cancer surgery might affect postoperative oncologic outcomes: A
summary of current state of evidence. Cancers (Basel). 11:5922019.
View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Liu B, Liu Y, Li N, Zhang J and Zhang X:
Oxycodone regulates incision-induced activation of neurotrophic
factors and receptors in an acute post-surgery pain rat model. J
Pain Res. 11:2663–2674. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Ji J, Lin W, Vrudhula A, Xi J, Yeliseev A,
Grothusen JR, Bu W and Liu R: Molecular interaction between
butorphanol and κ-opioid receptor. Anesth Analg. 131:935–942. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Tian M, Jin L, Li R, Zhu S, Ji M and Li W:
Comparison of oxycodone and morphine on the proliferation,
apoptosis and expression of related molecules in the A549 human
lung adenocarcinoma cell line. Exp Ther Med. 12:559–566. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Yu Y, Li D, Duan J, Xu H, Li L, Tan D and
Yan H: The pro- and anti-cancer effects of oxycodone are associated
with epithelial growth factor receptor level in cancer cells.
Biosci Rep. 40:BSR201935242020. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Gery S, Yin D, Xie D, Black KL and
Koeffler HP: TMEFF1 and brain tumors. Oncogene. 22:2723–2727. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Nie X, Gao L, Zheng M, Wang C, Wang S, Li
X, Qi Y, Zhu L, Liu J and Lin B: Overexpression of TMEFF1 in
endometrial carcinoma and the mechanism underlying its promotion of
malignant behavior in cancer cells. J Cancer. 12:5772–5788. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Nie X, Liu C, Guo Q, Zheng MJ, Gao LL, Li
X, Liu DW, Zhu LC, Liu JJ and Lin B: TMEFF1 overexpression and its
mechanism for tumor promotion in ovarian cancer. Cancer Manag Res.
11:839–855. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Wang B, Li Y, Shen Y, Xu Y and Zhang C:
Butorphanol inhibits the malignant biological behaviors of ovarian
cancer cells via down-regulating the expression of TMEFF1. Onco
Targets Ther. 13:10973–10981. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Ramirez MF, Gorur A and Cata JP: Opioids
and cancer prognosis: A summary of the clinical evidence. Neurosci
Lett. 746:1356612021. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Wang YH, Sun JF, Tao YM, Chi ZQ and Liu
JG: The role of kappa-opioid receptor activation in mediating
antinociception and addiction. Acta Pharmacol Sin. 31:1065–1070.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Torre LA, Siegel RL, Ward EM and Jemal A:
Global cancer incidence and mortality rates and trends-an update.
Cancer Epidemiol Biomarkers Prev. 25:16–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Ramirez MF, Gorur A and Cata JP: The role
of opioids in cancer progression. Int Anesthesiol Clin Spring.
58:57–63. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Rodrigue D, Winkelmann J, Price M,
Kalandranis E, Klempner L and Kapoor-Hintzen N: Opioid misuse: An
organizational response while managing cancer-related pain. Clin J
Oncol Nurs. 24:170–176. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Schmidhammer H, Erli F, Guerrieri E and
Spetea M: Development of diphenethylamines as selective kappa
opioid receptor ligands and their pharmacological activities.
Molecules. 25:50922020. View Article : Google Scholar : PubMed/NCBI
|