Introduction
Colorectal cancer is prevalent and a leading cause
of death worldwide (1). The
mortality rate of colorectal cancer has been declining over the
past number of decades due to early diagnosis using improved
screening and treatment strategies. However, the incidence remains
high (2). Over the past several
decades, in the United States, the incidence and mortality rates of
colorectal cancer have been steadily decreasing among those aged
>50 years, but the number of those aged between 20 and 49 years
is increasing. It is estimated that the incidence and mortality
rates of colorectal cancer according to these age groups increase
uniformly with economic development due to environmental changes,
such as lifestyle, increased obesity and overall lifespan
extension, and the consumption of processed foods, alcohol and meat
(1,2). To date, colorectal cancer treatment
involves radiotherapy and traditional therapies, including surgery
and chemotherapy (3). However,
these treatments are limited by toxicity, adverse events and drug
resistance (3). A number of
studies have previously reported that colorectal and colon cancer
is negatively associated with dietary factors, including plants,
seaweeds, vegetables and fruits, which contain a variety of
phytochemicals (4–6). These phytochemicals have been
demonstrated to protect cells from damage that leads to cancer
(7–13).
The halophyte Calystegia soldanella
(Linnaeus) Roem. et Schult (Convolvulaceae) is a perennial herb
that grows on coastal sand dunes worldwide (14). This plant has been extensively
used in traditional medicine for general consumption and as a type
of herbal treatment, since it is considered to confer bioactive
effects against rheumatic arthritis, sore throat, dropsy, scurvy,
fever and diarrhea (15–17). In particular, fractions of C.
soldanella have been reported to exhibit anti-inflammatory
(18–20), antifungal (21), antiviral (22–25), anticancer (26,27) and analgesic effects (28). Although the various bioactivities
of C. soldanella have been assessed, its effects on colon
cancer have not been explored.
In a previous study, the viability of numerous
cancer cell lines was assessed, including the hepatocarcinoma
HepG2, gastric cancer AGS, colorectal cancer HT-29 and the breast
cancer cell line MCF-7, following treatment with the C.
soldanella crude extract (27). Similar effects, including a
decrease in cell viability, were observed in HT-29 and HepG2 cells
(27). Therefore, the aim of the
present study was to evaluate the mechanism underlying any changes
in HT-29 cell physiology after treatment with C. soldanella
extract fractions.
Materials and methods
Sample collection and preparation
Whole-plant C. soldanella samples were
collected from Gijang, Busan, Korea. A voucher specimen was
deposited at the Herbarium of the Division of Marine Environment
and Bioscience, Korea Maritime and Ocean University (Busan, Korea).
The entire plant samples were briefly air-dried at room temperature
for 1 month, ground into a fine powder using a blender and stored
at −20°C.
Extraction and fractionation
The crude extract of the plant samples (500 g) was
eluted in 99% ethanol for 3 h at room temperature before being
filtered and concentrated three times. The concentrated crude
extracts were evaporated under reduced pressure at 40°C using
rotary vacuum evaporator and partitioned between
H2O-methanol (9:1) and n-hexane (4.3 g). The organic
layer was further partitioned into dichloromethane (DCM; 15.2 g)
and ethyl acetate (2.3 g). The aqueous layer was also fractionated
into n-butanol (14.6 g) and water (16.4 g). Each fraction used was
completely removed using a reflux condenser and was subsequently
freeze-dried for use in experiments. All solvent reagents used for
extraction were of analytical grade. The DCM fraction was diluted
to a final concentration of 0.2% DMSO so as not to induce toxicity.
For the control, an equivalent volume of 0.2% DMSO was added to the
culture medium.
Ultra-performance liquid
chromatography coupled with electrospray ionization quadrupole
time-of-flight mass spectrometry (UPLC-ESI-Q-TOF-MS) analysis
The DCM fractions were analyzed using
UPLC-ESI-Q-TOF-MS. The UPLC system (Agilent infinity 1260 series;
Agilent Technologies Deutschland GmbH), with an incorporated
photodiode array detector (DAD) and Impact II Q-TOF mass
spectrometer (Bruker Corporation), was equipped with an ESI source
that operated on the negative ion mode. A reverse phase Kintex
core-shell C-18 column (100×2.1 mm, 1.7 µm, Phenomenex) was used at
a flow rate of 0.5 ml/min. The mobile phase consisted of water
containing 0.1% TFA (A) and 0.1% TFA containing acetonitrile (B)
using the following gradient conditions: 0–1 min, 10% B; 1–4 min,
10–20% B; 4–6 min, 20–25% B; 6–8 min, 25% B; 8–9 min, 25–30% B;
9–11 min, 30% B; 11–12 min, 30–50% B; 12–14 min, 50–60% B; 14–15
min, 60–80% B; and 15–17 min, 80% B. The injection volume was 2 µl.
Mass spectra in positive-ion or negative-ion mode were recorded
within 20 min. The UPLC profiles of the extracts were measured
using a DAD. The analyses were conducted in the negative ion mode
in a mass range from m/z 50 to 1,000. The ESI source parameters
were: Capillary voltage, 4.5 KV; nebulizing gas pressure, 1.5 bar;
drying gas temperature, 200°C, drying gas flow, 9.0 l/min; Funnel
1RF 250.0 Vpp; transfer time, 50.0 µs; and prepulse storage, 2.0
µs. The MS data were analyzed using Data Analysis 4.2 software
(Bruker Corporation).
Cell culture
The human colorectal HT-29 cell line (cat. no.
30038) was purchased from the Korean Cell Line Bank, Korean Cell
Line Research Foundation. The STR profile of the HT-29 cell line
was as follows: D3S1358, 15/17; von Willebrand factor type A,
17/19; fibrinogen α chain, 20/22; amelogenin, X; tyrosine
hydroxylase 1, 6/9; thyroid peroxidase, 8/9; CSF1P0, 11/12; D5S818,
11/12; D13S317, 11/12; and D7S820, 10. The cells were cultured at
37°C with 5% CO2 in RPMI-1640 medium (Welgene, Inc.)
supplemented with 10% FBS (Welgene, Inc.) containing 100 U/ml
penicillin and 100 µg/ml streptomycin (cat no. CA005-10; GenDEPOT,
LLC). The culture medium was refreshed every 2 days and the cells
were subcultured for use in subsequent experiments.
Cell viability assays
Cell viability was analyzed using a EZ-Cytox Kit
(cat. no. EZ-1000; DoGenBio Co., Ltd.) according to the
manufacturer's protocol. Cells were seeded into 96-well plates at
4×104 cells/well and allowed to attach for 24 h. First,
cell viability was examined for each fraction (hexane, DCM, ethyl
acetate, butanol, water) at concentrations of 0, 25, 50 or 100
µg/ml for 24 h. Next, attached cells were treated with 0, 10, 20,
40, 60, 80 or 100 µg/ml of the DCM fraction in serum-free medium
for 24 or 48 h. Subsequently, cells were incubated with the
EZ-Cytox solution (100 µl/well) for 30 min at 37°C before
absorbance at 450 nm was quantified using the FilterMAX F5
microplate reader (Molecular Devices LLC.). In addition,
morphological cell changes were subsequently observed using a light
microscope (magnification, ×200; Eclipse TS100-F; Nikon
Corporation).
Apoptosis assay
Apoptosis was assessed using the Muse®
Annexin V and Dead Cell Kit (cat. no. MCH100105; Luminex
Corporation) according to the manufacturer's protocol. Cells were
seeded into six-well plates at 1×105 cells/well and
treated with 20, 40 or 80 µg/ml concentrations of the DCM fraction
for 20 h. The cells were then harvested at a density of
5×104 cells/well and washed twice with PBS and stained
with FITC-Annexin V and dead cell reagent for 20 min at room
temperature in the dark. The percentage of apoptotic cells was
determined using the Guava® Muse® Cell
Analyzer (2012model; Luminex Corporation).
Assessment of mitochondrial membrane
potential (MMP)
The MMP was assessed using the Muse®
MitoPotential Kit (cat. no. MCH100110; Luminex Corporation.)
according to the manufacturer's protocol. Cells were seeded into
six-well plates at 1×105 cells/well and treated with 0,
20, 40 or 80 µg/ml concentrations of the DCM fraction for 20 h. The
cells were harvested at a density of 5×104 cells/well,
washed twice with PBS, stained with MitoPotential working solution
containing MitoPotential dye and incubated for 20 min in a 37°C
CO2 incubator. The MMP was determined using the Guava
Muse Cell Analyzer (2012 model).
Cell cycle assay
The cell cycle was assessed using the
Muse® Cell Cycle Assay Kit (cat. no. MCH100106; Luminex
Corporation) according to the manufacturer's protocol. Cells were
seeded into six-well plates at 1×105 cells/well and
treated with 0, 20, 40 or 80 µg/ml concentrations of the DCM
fraction for 20 h. The cells were harvested, washed twice with PBS,
fixed in ice cold 70% ethanol and frozen at −20°C for 3 h. The
fixed cells were stained at a density of 5×104 cells/ml
with 200 µl Muse cell cycle reagent for 30 min at room temperature.
The cell cycle phase was determined using the Guava Muse Cell
Analyzer (2012 model).
Preparation of total cell lysate
HT-29 cells were treated with 0, 20, 40 or 80 µg/ml
of the DCM fraction in serum-free medium for 24 h at 37°C. The
cells were washed with PBS and lysed in M-PER Mammalian Protein
Extraction Reagent (cat. no. 78501; Thermo Fisher Scientific, Inc.)
containing phosphate inhibitor cocktail (cat. no. 1862495; Thermo
Fisher Scientific, Inc.) and ProteaseArrest™ protease inhibitor
cocktail (cat. no. 786–108; G-Biosciences; Geno Technology, Inc.)
on ice for 30 min. The extracts were centrifuged at 12,000 × g for
10 min at 4°C and the supernatants were subsequently used for
western blotting. The mitochondrial and cytosolic fractions were
extracted using a Mitochondria Isolation Kit (cat. no. 89874;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocols. Protein concentrations were measured using a BCA Protein
Assay Kit (cat. no. 23225; Thermo Fisher Scientific, Inc.).
Western blotting
Total protein (20–40 µg protein/lane) was
electrophoresed via SDS-PAGE on a 8–15% acrylamide gel and
transferred onto polyvinylidene fluoride immobilon-P membranes
(cat. no. MLP.IPVH00010; MilliporeSigma). The membranes were
blocked with 1% bovine serum albumin (BSA; cat. no. A0100;
GenDEPOT, LLC) in TBS with 0.1% Tween-20 (TBST; 5 mM Tris, 20 mM
sodium chloride, pH 7.4) and incubated with primary antibodies
(1:1,000) in 1% BSA/TBST with gentle agitation at 4°C overnight.
The membranes were then washed twice for 15 min in TBST each and
incubated with the corresponding HRP-conjugated secondary
antibodies (1:10,000) for 2 h at room temperature, before being
washed again using TBST. Immunoreactive bands were detected using
the WesternBright® ECL HRP Substrate (cat. no. K12045;
Advansta, Inc.) and visualized using the GeneGnome 5 (model 75000;
Syngene). Differences in protein levels were determined by
semi-quantifying the western blotting band densities using ImageJ
software version IJ.146r (National Institutes of Health).
The antibodies used were as follows: Anti-Fas (cat.
no. sc-7886; rabbit; Santa Cruz Biotechnology, Inc.),
anti-caspase-8 (cat. no. sc-7890; rabbit; Santa Cruz Biotechnology,
Inc.), anti-Bcl-2 (cat. no. sc-7382; mouse; Santa Cruz
Biotechnology, Inc.), anti-Bcl-extra-large (xL; cat. no. sc-7195;
rabbit; Santa Cruz Biotechnology, Inc.), anti-Bad (cat. no.
sc-8044; mouse; Santa Cruz Biotechnology, Inc.), anti-Bax (cat. no.
sc-7480; mouse; Santa Cruz Biotechnology, Inc.), anti-caspase-9
(cat. no. sc-7885; rabbit; Santa Cruz Biotechnology, Inc.),
anti-caspase-7 (cat. no. sc-6138; rabbit; Santa Cruz Biotechnology,
Inc.), anti-caspase-3 (cat. no. sc-7148; rabbit; Santa Cruz
Biotechnology, Inc.), anti-X-linked inhibitor of apoptosis protein
(XIAP; cat. no. 2045; rabbit; Cell Signaling Technology, Inc.),
anti-cellular inhibitor of apoptosis protein (cIAP)-1 (cat. no.
7065; rabbit; Cell Signaling Technology, Inc.), anti-cIAP-2 (cat.
no. 3130; rabbit; Cell Signaling Technology, Inc.), anti-cytochrome
c (cat. no. 4272; rabbit; Cell Signaling Technology, Inc.),
anti-cytochrome c oxidase subunit IV (COX IV; cat. no. 4844;
rabbit; Cell Signaling Technology, Inc.), anti-cyclin A (cat. no.
BS-0571R; rabbit; BIOSS), anti-CDK2 (cat. no. sc-163; rabbit; Santa
Cruz Biotechnology, Inc.), anti-cell division cycle 25 A (Cdc25A;
cat. no. sc-7389; mouse; Santa Cruz Biotechnology, Inc.) and
anti-cyclin dependent kinase inhibitor 1 (p21; cat. no. sc-271532;
rabbit; Santa Cruz Biotechnology, Inc.). Anti-β-actin (cat. no.
sc-47778; mouse; Santa Cruz Biotechnology, Inc.) antibody was used
as the loading control. The secondary antibodies used were
HRP-conjugated anti-mouse IgG (cat. no. 7076; Cell Signaling
Technology, Inc.) and anti-rabbit IgG (cat. no. 7074; Cell
Signaling Technology, Inc.).
Statistical analysis
All data are presented as the mean ± SD of three
independent experiments. Means between >2 groups were compared
using one-way or two-way ANOVA followed by Bonferroni's multiple
comparison test using GraphPad Prism version 7 software (GraphPad
Software, Inc). P<0.05 was considered to indicate a
statistically significant difference.
Results
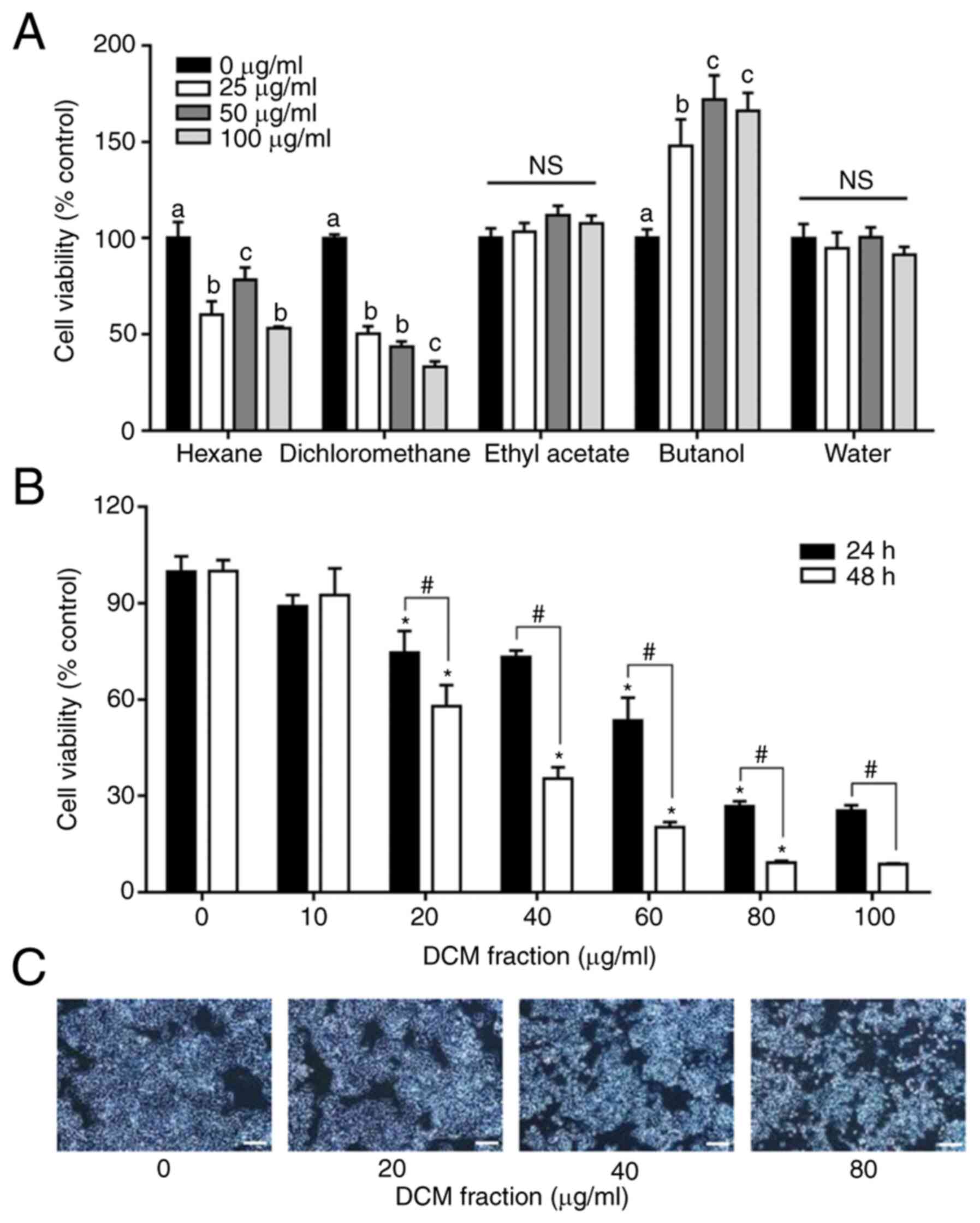
Solvent fractions of C. soldanella
reduce HT-29 cell viability
To determine the effects of the C. soldanella
fractions on cell viability, HT-29 cells were treated with each of
the five solvent fractions (n-hexane, DCM, ethyl acetate, n-butanol
and water) at different concentrations (0, 25, 50 and 100 µg/ml)
for 24 h, after which cell viability was examined. Cell viability
was significantly decreased following treatment with the DCM
fraction compared with that in the 0 µg/ml group (Fig. 1A). Therefore, the DCM fraction
with the highest dose-dependent effect was selected for further
study.
Reductions in the viability of HT-29 cells was
confirmed following treatment with different concentrations (0–100
µg/ml) of DCM fraction for 24 and 48 h (Fig. 1B). HT-29 cell viability appeared
to be decreased following DCM fraction treatment in a time- and
dose-dependent manner compared with those in the 0 µg/ml group.
After treatment with 0, 10, 20, 40, 60, 80 and 100 µg/ml DCM, cell
viability was 100±4.7, 89.0±3.5, 74.6±6.7, 73.2±2.0, 53.5±7.1,
26.7±1.6 and 25.4±1.7% at 24 h, respectively, whereas it was
100±3.4, 92.6±8.3, 57.9±6.6, 35.4±3.6, 20.2±1.6, 9.3±0.5 and
8.7±0.2% at 48 h, respectively (Fig.
1B). In addition, it was observed that the morphological
changes confirmed via microscope were reduced in the same way as
the results of the cell viability assay (Fig. 1C).
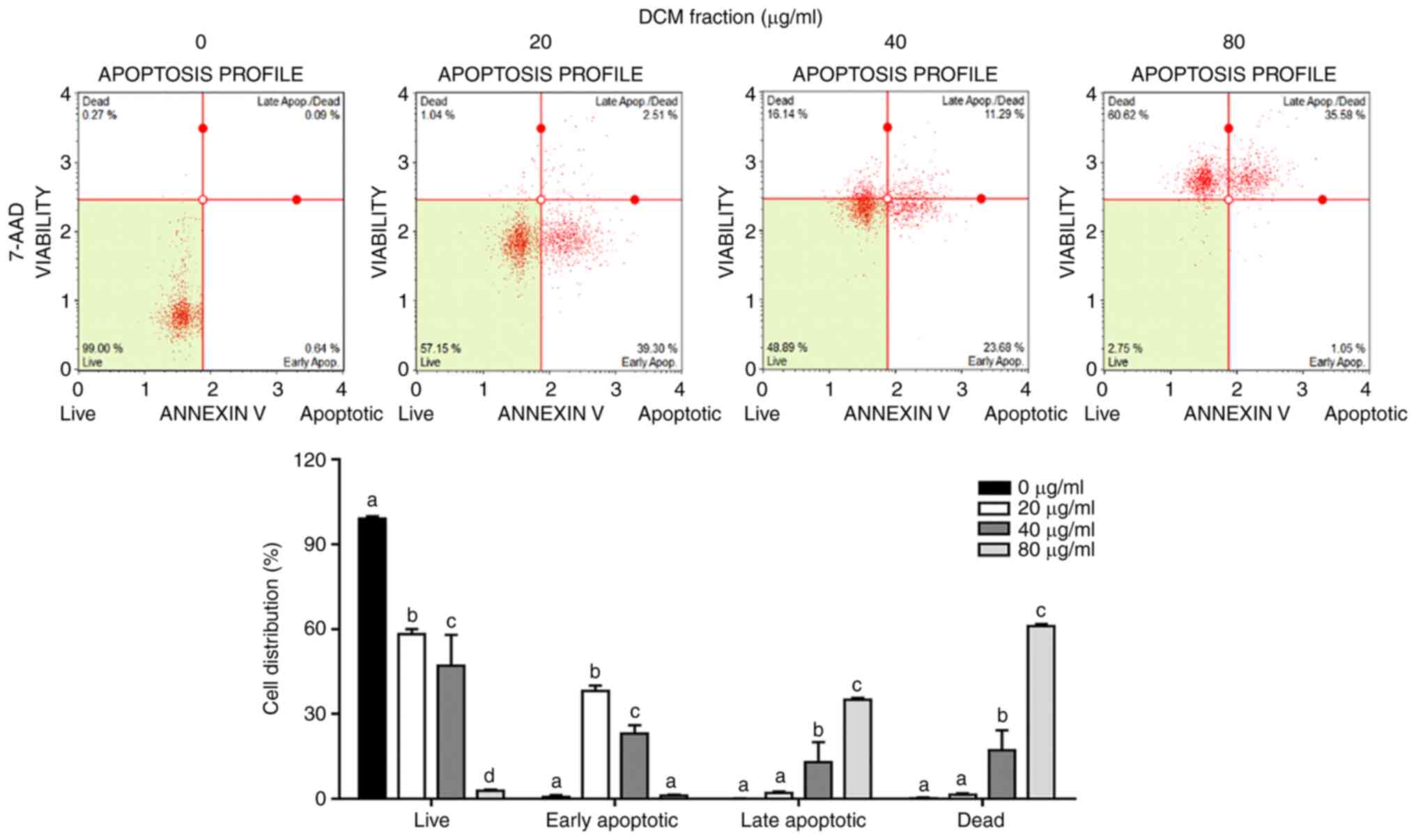
DCM fraction from C. soldanella
induces apoptosis in HT-29 cells
The Annexin V and Dead Cell Kit was used to
determine whether this decrease in cell viability induced by DCM
fraction treatment resulted from apoptosis. The rates of early and
late apoptosis were significantly increased in a dose-dependent
manner following treatment with the DCM fraction compared with
those in the 0 µg/ml group (Fig.
2). The proportions of early apoptotic cells were 0.7±0.67,
38.2±1.81, 23.0±3.07 and 1.0±0.38%, whilst those of late apoptotic
cells were 0±0.05, 2.1±0.40, 12.9±7.12 and 35.1±0.58%, following
DCM fraction treatment at concentrations of 0, 20, 40 and 80 µg/ml,
respectively.
Regarding the protein expression levels of
apoptosis-related proteins, DCM fraction treatment at 40 and 80
µg/ml significantly increased the protein expression levels of Fas
protein whilst significantly decreasing those of pro-caspase-8, an
extrinsic signaling pathway-related protein (Fig. 3), compared with those in the 0
µg/ml group. Other intrinsic apoptosis signaling pathway-related
proteins that also showed significantly decreased protein
expression levels after 40 and 80 µg/ml DMC treatment were Bcl-2
and Bcl-xL, whilst those that showed significantly increased
protein expression levels following 40 and 80 µg/ml DCM fraction
treatment were Bad and Bax (Fig.
3). Consequently, the Bax/Bcl-2 ratio was significantly
increased in the 40 and 80 µg/ml DCM fraction treatment groups
compared with that in the 0 µg/ml group. In addition, 40 and 80
µg/ml DCM fraction treatment also significantly decreased the
expression of pro-caspase-9, pro-caspase-7 and pro-caspase-3 levels
compared with those in the 0 µg/ml group. The protein expression
levels of XIAP, cIAP-1 and cIAP-2, caspase inhibitors involved in
apoptosis inhibition, were significantly decreased by 40 and 80
µg/ml DCM treatment compared with those in the 0 µg/ml group. These
results suggest that the treatment of HT-29 cells with DCM from
C. soldanella may induce apoptosis by regulating the
expression of pro-apoptotic, pre-apoptotic and caspase inhibitor
proteins.
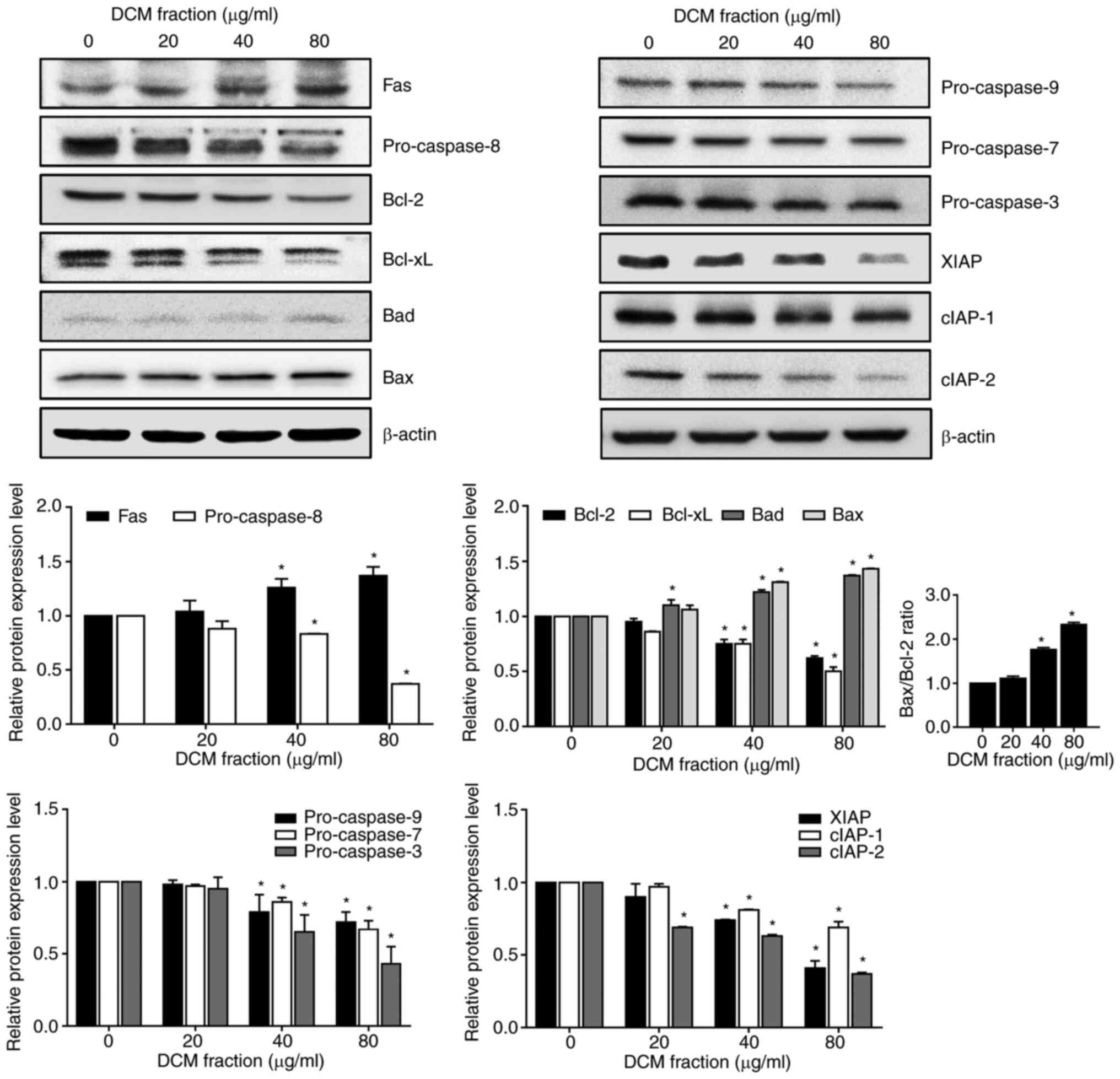 | Figure 3.Effects of DCM fraction treatment on
the expression of apoptosis-related proteins in HT-29 cells. HT-29
cells were incubated with various concentrations of the DCM
fraction for 20 h. The expression levels apoptosis-related proteins
Fas, pro-caspase-8, Bcl-2, Bcl-xL, Bad, Bax, pro-caspase-9,
pro-caspase-7, pro-caspase-3, XIAP, cIAP-1 and cIAP-2 were then
determined by western blotting. β-actin was used as the loading
control. The bands were semi-quantitatively analyzed by ImageJ
software and the relative protein expression levels are presented.
All results were normalized to the untreated control (0 µg/ml).
Data are presented as the mean ± SD of three independent
experiments. *P<0.05 vs. 0 µg/ml DCM. DCM, dichloromethane; xL,
extra-large; XIAP, X-linked inhibitor of apoptosis protein; cIAP-1,
cellular inhibitor of apoptosis protein-1; cIAP-2, cellular
inhibitor of apoptosis protein-2. |
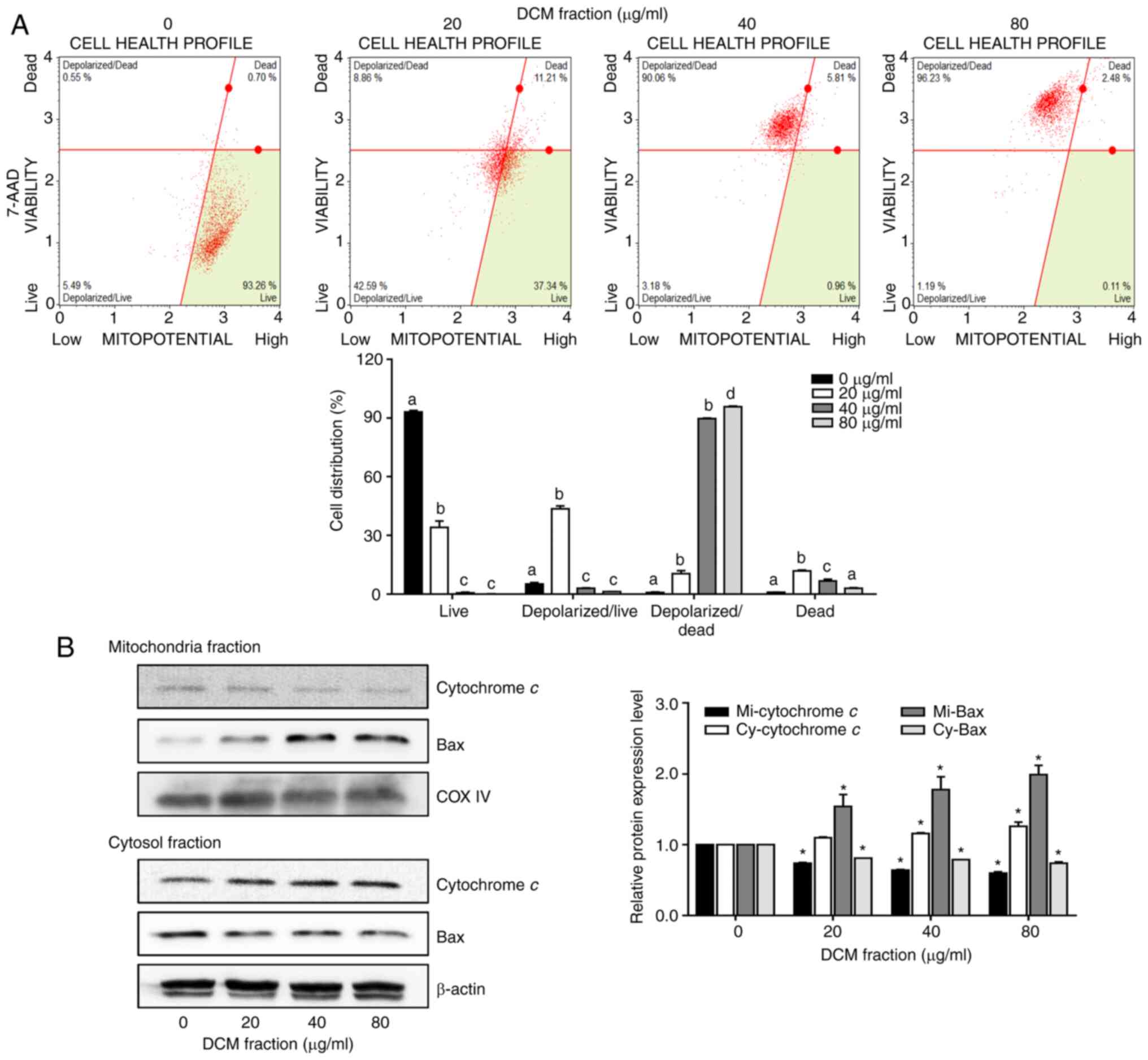
DCM fraction from C. soldanella
induces MMP changes in HT-29 cells
Since MMP changes are also associated with apoptosis
(29–31), the effects of DCM fraction
treatment on the MMP in HT-29 cells were investigated. The
proportions of live and dead cells with depolarized mitochondria
were markedly increased following DCM fraction treatment compared
with those in the 0 µg/ml group. The proportions of depolarized
live cells were 5.2±0.75, 43.6±1.49, 2.9±0.21 and 1.2±0.05%,
whereas the proportions of depolarized dead cells were 0.8±0.24,
10.5±1.48, 89.7±0.33 and 95.8±0.35%, at concentrations of 0, 20, 40
and 80 µg/ml, respectively (Fig.
4A). The protein expression levels of MMP-related proteins were
examined using western blotting. DCM fraction treatment at 40 and
80 µg/ml significantly increased the release of cytochrome c
into the cytosol from the mitochondria compared with that in the 0
µg/ml group (Fig. 4B). In
addition, DCM fraction treatment also resulted in the significantly
increased translocation of Bax into the mitochondria from the
cytosol in a dose-dependent manner compared with the 0 µg/ml
group.
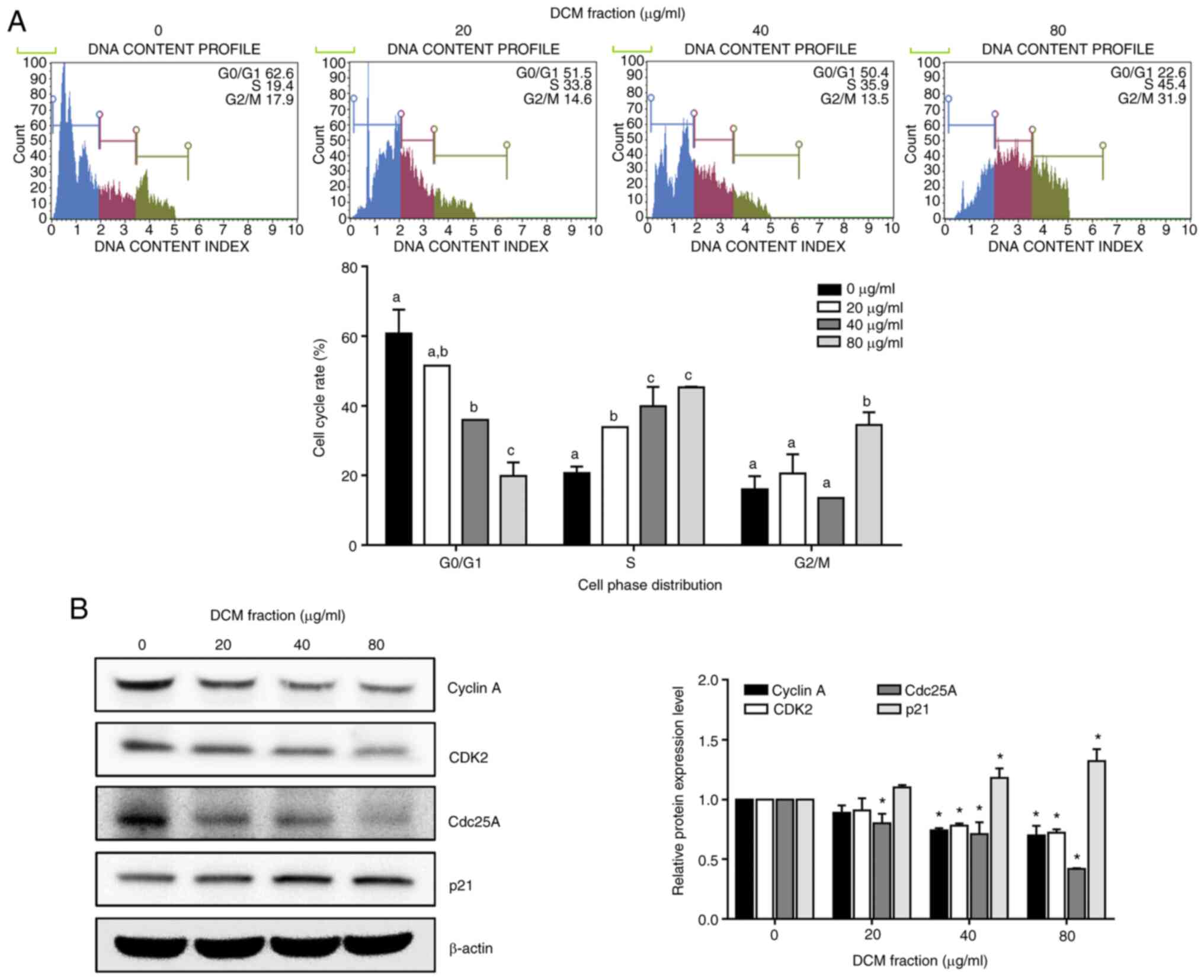
DCM from C. soldanella induces S-phase
arrest in HT-29 cells
To determine whether decreased cell viability was
associated with the cell cycle, HT-29 cell cycle progression was
analyzed using a cell cycle kit following DCM fraction treatment.
DCM significantly induced S-phase arrest in a dose-dependent manner
compared with the 0 µg/ml group, with S-phase cell proportions of
20.7±1.8, 33.8±0.2, 39.9±5.6 and 45.3±0.2% at concentrations of 0,
20, 40 and 80 µg/ml of the DCM fraction, respectively (Fig. 5A). Subsequently, the relative
protein expression levels of S-phase-related proteins were analyzed
using western blotting. DCM fraction treatment at 40 and 80 µg/ml
led to the significant downregulation of cyclin A, CDK2 and Cdc25A
protein expression and the significant upregulation of p21 protein
expression compared with those in the 0 µg/ml group (Fig. 5B).
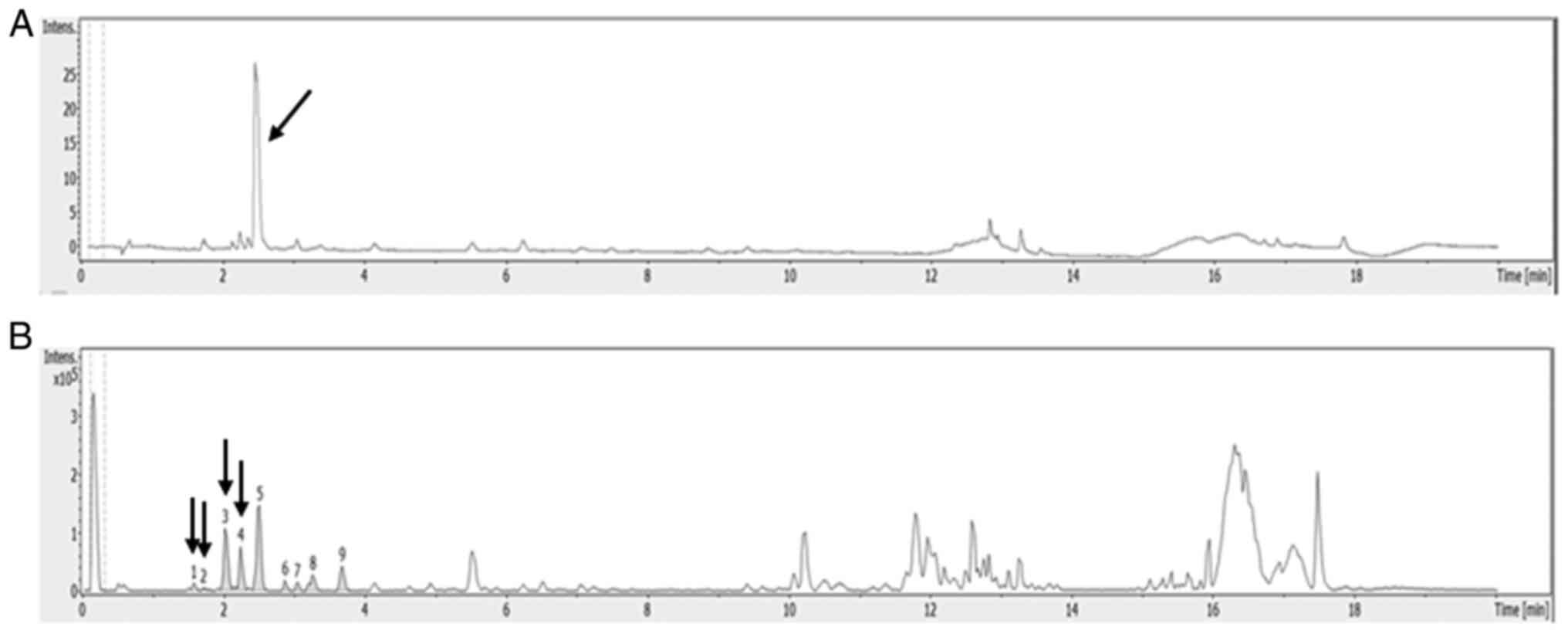
ESI-Q-TOF-MS analysis of DCM fraction
from C. soldanella
UPLC-ESI-Q-TOF-MS analysis was applied to analyze
the polyphenolic compounds in the DCM fraction of C.
soldanella to screen for any anticancer substances. The HPLC-UV
chromatogram (350 nm) and total ion current chromatogram in the DCM
fraction are presented in the Fig. 6A
and B.
Molecular ion mass, MS/MS fragment ion mass and
MS-based compound analysis data are all provided in Table I. UPLC-ESI-Q-TOF MS analysis
demonstrated that the major compounds in the DCM fraction were
hydroxybenzoic acid, hydrosinapinic acid and coumaric acid, which
are phenolic acid derivatives, and quercetrin, which is a flavonoid
quercetin derivative.
 | Table I.Identified compounds in the DCM
fraction. |
Table I.
Identified compounds in the DCM
fraction.
| Peak no. | Compound | Retention time,
min | Mass | m/z | MS fragment |
|---|
| 1 | Hydroxybenzoic
acid | 1.6 | 138.12 | 137.02 | 112.98 |
| 2 | Hydrosinapinic
acid | 2.0 | 226.08 | 225.11 | 181.12, 121.02 |
| 3 | Coumaric acid | 2.2 | 164.04 | 163.03 | 119.05 |
| 4 | Quercetrin | 3.0 | 448.38 | 447.09 | 301.03, 146.93 |
Discussion
The ultimate aim of discovering novel
chemotherapeutic strategies is to overcome drug resistance
(32). A number of studies have
previously investigated the potential anticancer activity of
natural compounds/products and suggested them to be promising
sources of new anticancer drugs (4–13).
For example, the natural compound S-adenosylmethionine has been
found to exert anti-tumor properties, including reduction of cell
proliferation, induction of apoptosis, autophagy and inhibition of
invasion and metastasis, in various cancer cell types, such as
human hepatocellular carcinoma, human breast cancer and head and
neck squamous cancer cells, in previous in vitro and in
vivo studies (33–36).
To date, anticancer-associated studies related to
C. soldanella have revealed cytotoxic effects of methanol
and chloroform extracts on the lung cancer A545 and colon cancer
Col2 cell lines (26) and the
anticancer effects of an 85% aqueous methanol fraction on the liver
cancer cell line HepG2 (27).
However, only a few studies have investigated the anticancer
effects of C. soldanella.
In the present study, solvent fractions were
obtained from C. soldanella, which have been previously
reported to exert anticancer activity in human liver cancer cell
line HepG2 (27), before an
effective fraction that can exhibit anticancer effects on the
colorectal cancer cell line HT-29 was selected. Following the crude
extraction of C. soldanella with ethanol, the crude extracts
were fractionated into n-hexane, DCM, ethyl acetate, n-butanol and
water. The effects of these five fractions on HT-29 cell viability
were examined before the DCM fraction was selected due to its
significant time- and dose-dependent effects.
Apoptosis serves a critical role in the regulation
of cell development and proliferation (37,38). This process has become a target of
cancer treatments due to its association with a number of different
types of cancer (39,40). Two major pathways of apoptosis
have been identified: i) The extrinsic death receptor pathway; and
ii) the endoplasmic reticulum stress pathway and intrinsic
mitochondrial apoptosis (41). To
determine the effect of DCM fraction treatment on cell viability
and apoptosis, apoptosis and apoptosis-related protein expression
levels in HT-29 cells that were treated with the DCM fraction were
investigated. The results demonstrated significantly increased
apoptotic rates and marked changes in the expression levels of
apoptosis-related proteins in both the extrinsic and intrinsic
signaling pathways. Treatment with DCM fraction increased Fas and
decreased pro-caspase-8, which corresponds to the extrinsic
signaling pathway. In addition, treatment with DCM fraction
increased Bad and Bax, and decreased Bcl-2, Bcl-xL, pro-caspase-9,
pro-caspase-7 and pro-caspase-3, which corresponds to the intrinsic
signaling pathway.
The mitochondrial apoptosis pathway involves a
number of components, including pre-apoptotic proteins Bcl-2 and
Bcl-xL and pro-apoptotic proteins Bax and Bak (42–44). The ratio of Bax/Bcl-2 determines
the direction of apoptosis regulation. An increased Bax/Bcl-2 ratio
can lead to the loss of mitochondrial membrane potentials, which is
an important process in the initiation of apoptosis (45). Furthermore, it has been reported
that an increased Bax/Bcl-2 ratio can activate caspase-3 to in turn
activate apoptosis (46,47). In the present study, caspase-3 was
also markedly activated following DCM fraction treatment. Since the
Bax/Bcl-2 ratio was also markedly increased with DCM fraction
treatment, it was hypothesized that DCM may activate apoptosis by
altering the mitochondrial membrane potential.
Apoptosis involves the regulation of a series of
proteins mainly in the mitochondrial signaling pathway (48). The mitochondria maintain the
cellular energy balance and regulate cell death processes (49). Cellular energy generated during
mitochondrial respiration is stored as an electrochemical gradient
across the mitochondrial membrane, which allows the mitochondria to
induce ATP synthesis (49).
Changes in the MMP are associated with apoptosis, necrosis and
caspase-independent cell death processes in addition to the opening
of mitochondrial transition pores, to release cytochrome c
into the cytosol and initiate apoptotic and caspase cascades
(31,49). In the present study, MMP changes
following DCM fraction treatment led to matrix condensation and
exposure of cytochrome c to the intermembrane space, which
may have activated apoptosis (Fig.
4).
Numerous proteins associated with cell cycle
regulation are known to be involved in apoptosis (50). In the present study, cell cycle
assay was performed and the protein expression levels of several
cell cycle-related proteins were investigated to determine whether
apoptosis induced by DCM fraction treatment was due to cell cycle
regulation in HT-29 cells. The results demonstrated that DCM
fraction treatment significantly increased the proportion of cells
in the S-phase. Cyclins and cyclin-dependent kinases serve
important roles in cell cycle regulation, where changes in the
composition of cyclin/CDK complexes can either increase or decrease
cell proliferation and/or differentiation through apoptosis
(51,52). Since cyclin A, CDK2 and Cdc25A
serve critical roles in S-phase regulation (53,54), their protein expression levels
following DCM fraction treatment were examined. The results showed
marked downregulation of cyclin A, CDK2 and Cdc25A expression
following DCM treatment. CDK1, p21 (Waf1/Cip1) have been previously
shown to induce cell cycle arrest by inhibiting CDK activity
(55,56). The results of the present study
demonstrated a dose-dependent increase in p21 protein expression
levels in response to DCM, which may have inhibited Cyclin A-CDK2
complex formation and contributed to cell cycle arrest between the
S and G2/M phases.
It has been frequently reported that numerous
compounds can exert anticancer activity independent of p53
(57–60). The tumor suppressor p53 has been
found to be dysfunctional in ≥50% colorectal cancer cases (58,59). Colorectal cancer cells and tumors
with p53 mutation are reported to be more aggressive and resistant
to chemotherapy (60). In the
present study, the HT-29 cell line is a p53 mutant cell line
(genotype R273H), to which DCM exerted anticancer effects in the
absence of p53. Although not conducted in the present study,
anticancer effects following DCM fraction treatment are also
hypothesized to be present in p53 wild-type cell lines and warrants
further study.
Previous studies have reported that C.
soldanella contains a variety of polyphenolic compounds,
including flavonoids, flavonoid glycosides and phenolic acid
derivatives (56,61).
Molecular ion mass, MS/MS fragment ion mass and
MS-based compound analysis data are all provided in Table I. UPLC-ESI-Q-TOF MS analysis
demonstrated that the major compounds in the DCM fraction were
hydroxybenzoic acid, hydrosinapinic acid and coumaric acid, which
are phenolic acid derivatives, and quercetrin, which is a flavonoid
quercetin derivative. Various phenolic acids, such as coumaric
acid, caffeic acid and ferulic acid, function as secondary plant
metabolites (62). In particular,
cinnamic acid-based phenoic acid compounds, such as coumaric acid,
caffeic acid, ferulic acid and sinapinic acid, have been reported
to exert anticancer activity in various colon cancer cell lines
(63). Coumaric acid is reported
to induce apoptosis in the colon cancer HCT-115 cell line and
induce G1/S arrest in the colon cancer cell line Caco-2
(64–66). These aforementioned phenolic acids
also have inhibitory activities on the proliferation of various
colorectal/colon cancer cell lines (67–69).
In conclusion, to the best of our knowledge, the
present study was the first to report the anticancer effects of DCM
from C. soldanella on HT-29 colorectal cancer cells, which
possibly occurred by MMP alteration, S-phase arrest and induction
of apoptosis through the intrinsic/extrinsic signaling pathways.
The present study also demonstrated that phenolic acid derivatives
are the main components of the DCM fraction, which exerted
inhibitory activities on HT-29 colorectal cancer cell by apoptosis
and cell cycle arrest.
Acknowledgements
Not applicable.
Funding
The present study was part of the Future Fisheries Food Research
Center Project, funded by the Ministry of Oceans and Fisheries
(grant no. 201803932).
Availability of data and materials
The datasets used and/or analyzed in the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
IHK, TE and TJN conceived and designed the
experiments. IHK, JYP and HJK performed the experiments. IHK, TE
and HJK analyzed and interpreted the results and wrote and revised
the manuscript. IHK, TE and TJN confirm the authenticity of all the
raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rawla P, Sunkara T and Barsouk A:
Epidemiology of colorectal cancer: Incidence, mortality, survival,
and risk factors. Prz Gastroenterol. 14:89–103. 2019.PubMed/NCBI
|
|
2
|
Cancer Trends Progress Report; Colorectal
Cancer Treatment. National Cancer Institute; 2020
|
|
3
|
Xie YH, Chen YX and Fang JY: Comprehensive
review of targeted therapy for colorectal cancer. Signal Transduct
Target Ther. 5:222020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pandey R, Singh PK and Shrivastava AK:
Seaweeds: Potential candidates in human colon cancer therapy. Colon
Cancer Diagnosis Ther. Jun 5–2021.(Epub ahead of print). doi:
10.1007/978-3-030-64668-4_13. View Article : Google Scholar
|
|
5
|
Zhao Y and Jiang Q: Roles of the
polyphenol-gut microbiota interaction in alleviating colitis and
preventing colitis-associated colorectal cancer. Adv Nutr.
12:546–565. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
García-Lafuente A, Guillamón E, Villares
A, Rostagno MA and Martínez JA: Flavonoids as anti-inflammatory
agents: Implications in cancer and cardiovascular disease. Inflamm
Res. 58:537–552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Akiyama Y, Kimura Y, Enatsu R, Mikami T,
Wanibuchi M and Mikuni N: Advantages and disadvantages of combined
chemotherapy with carmustine wafer and bevacizumab in patients with
newly diagnosed glioblastoma: A single-institutional experience.
World Neurosurg. 113:e508–e514. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bar-Shalom R, Bergman M, Graossman S,
Azzam N, Sharvit L and Fares F: Inula viscosa extract inhibits
growth of colorectal cancer cells in vitro and in vivo through
induction of apoptosis. Front Oncol. 9:2272019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bravo L: Polyphenols: Chemistry dietary
sources metabolism nutritional significance. Nutr Rev. 56:317–333.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moreira H, Slezak A, Szyjka A, Oszmianski
J and Gasiorowski K: Antioxidant and cancer chemopreventive
activities of cistus and pomegranate polyphenols. Acta Pol Pharm.
74:688–698. 2017.PubMed/NCBI
|
|
11
|
del Mar Blanquer-Rosselló M,
Hernández-López R, Roca P, Oliver J and Valle A: Resveratrol
induces mitochondrial respiration and apoptosis in SW620 colon
cancer cells. Biochim Biophys Acta Gen Sugj. 1861:431–440. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim IH and Nam TJ: Fucoidan downregulates
insulin-like growth factor-I receptor levels in HT-29 human colon
cancer cells. Oncol Rep. 39:1516–1522. 2018.PubMed/NCBI
|
|
13
|
Kim IH, Kwon MJ and Nam TJ: Differences in
cell death and cell cycle following fucoidan treatment in
high-density HT-29 colon cancer cells. Mol Med Rep. 15:4116–4122.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bae CY, Hwang JS, Bae JJ, Choi SC, Lim SH,
Choi DG, Kim JG and Choo YS: Physiological responses of
Calystegia soldanella under drought stress. J Ecol Environ.
36:255–265. 2013. View Article : Google Scholar
|
|
15
|
Bae KH: The Medicinal Plants of Korea.
Korea, Kyo-Hak publishing. (Seoul). 2000.
|
|
16
|
Lee YS, Kwak CG and Kim NW: Nutritional
characteristics of Calystegia japonica. Korean J Food
Preserv. 19:619–625. 2012. View Article : Google Scholar
|
|
17
|
Takagi S, Yamaki M, Masuda K and Kubota M:
Studies on the purgative drugs. IV. On the constituents of
Calystegia japonica Choisy (author's transl). Yakugaku
Zasshi. 97:1369–1371. 1977.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim Y, Min HY, Park HJ, Lee EJ, Park EJ,
Hwang HJ, Jin C, Lee YS and Lee SK: Suppressive effects of nitric
oxide production and inducible nitric oxide synthase (iNOS) gene
expression by Calystegia soldanella methanol extract on
lipopolysaccharide-activated RAW 264.7 cells. Eur J Cancer Prev.
13:419–424. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang Z and Feng C: Experimental study on
anti-inflammatory and analgesic effects of water extracts of
Calystegia soldanella. Chin Arch Tradit Chin Med.
6:72010.
|
|
20
|
Lee JI, Kim IH, Choi YH, Kim EY and Nam
TJ: PTP1B inhibitory effect of alkyl p-coumarates from
Calystegia soldanella. Nat Prod Commun. 9:1585–1588.
2014.PubMed/NCBI
|
|
21
|
Nidiry ES, Ganeshan G and Lokesha AN:
Antifungal activity and isomerization of octadecyl p-coumarates
from Ipomoea carnea subsp. fistulosa. Nat Prod Commun.
6:1889–1892. 2011.PubMed/NCBI
|
|
22
|
Ono M, Kanemaru Y, Yasuda S, Okawa M,
Kinjo J, Miyashita H, Yokomizo K, Yoshimitsu H and Nohara T: A new
resin glycoside from Calystegia soldanella and its antiviral
activity towards herpes. Nat Prod Res. 31:2660–2664. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ono M, Takigawa A, Kanemaru Y, Kawakami G,
Kabata K, Okawa M, Kinjo J, Yokomizo K, Yoshimitsu H and Nohara T:
Calysolins V–IX, resin glycosides from Calystegia soldanella
and their antiviral activity toward herpes. Chem Pharm Bull
(Tokyo). 62:97–105. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ono M, Kawakami G, Takigawa A, Kabata K,
Okawa M, Kinjo J, Yokomizo K, Yoshimitsu H and Nohara T: Calysolins
X–XIII, resin glycosides from Calystegia soldanella, and
their antiviral activity toward herpes simplex virus. Chem Pharm
Bull (Tokyo). 62:839–844. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ono M, Takigawa A, Muto H, Kabata K, Okawa
M, Kinjo J, Yokomizo K, Yoshimitsu H and Nohara T: Antiviral
activity of four new resin glycosides calysolins XIV–XVII from
Calystegia soldanella against Herpes Simplex Virus. Chem
Pharm Bull (Tokyo). 63:641–648. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Min HY, Kim Y, Lee EJ, Hwang HJ, Park EJ
and Lee SK: Cytotoxic activities of indigenous plant extracts in
cultured human cancer cells. Nat Prod Sci. 8:170–172. 2002.
|
|
27
|
Lee JI, Kim IH and Nam TJ: Crude extract
and solvent fractions of Calystegia soldanella induce G1 and
S phase arrest of the cell cycle in HepG2 cells. Int J Oncol.
50:414–420. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ly JD, Grubb DR and Lawen A: The
mitochondrial membrane potential (deltapsi(m)) in apoptosis; an
update. Apoptosis. 8:115–128. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gottlieb E, Armour SM, Harris MH and
Thompson CB: Mitochondrial membrane potential regulates matrix
configuration and cytochrome c release during apoptosis. Cell Death
Differ. 10:709–717. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang J, Cao L, Li Y, Liu H, Zhang M, Ma H,
Wang B, Yuan X and Li Q: Gracillin isolated from Reineckia
carnea induces apoptosis of A549 cells via the mitochondrial
pathway. Drug Des Devel Ther. 15:233–243. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Christensen ME, Jansen ES, Sanchez W and
Waterhouse NJ: Flow cytometry-based assays for the measurement of
apoptosis-associated mitochondrial membrane depolarisation and
cytochrome c release. Methods. 61:138–145. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mosca L, Pagano M, Pecoraro A,
Borzacchiello L, Mele L, Cacciapuoti G, Porcelli M, Russo G and
Russo A: S-Adenosyl-L-methionine overcomes uL3-mediated drug
resistance in p53 deleted colon cancer cells. Int J Mol Sci.
22:1032020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lu SC and Mato JM: S-Adenosylmethionine in
cell growth, apoptosis and liver cancer. J Gastroenterol Hepatol.
23 (Suppl 1):S73–S77. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cave DD, Desiderio V, Mosca L, Ilisso CP,
Mele L, Caraglia M, Cacciapuoti G and Porcelli M:
S-Adenosylmethionine-mediated apoptosis is potentiated by autophagy
inhibition induced by chloroquine in human breast cancer cells. J
Cell Physiol. 233:1370–1383. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ilisso CP, Delle Cave D, Mosca L, Pagano
M, Coppola A, Mele L, Caraglia M, Cacciapuoti G and Porcelli M:
S-Adenosylmethionine regulates apoptosis and autophagy in MCF-7
breast cancer cells through the modulation of specific microRNAs.
Cancer Cell Int. 18:1972018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mosca L, Minopoli M, Pagano M, Vitiello F,
Carriero MV, Cacciapuoti G and Porcelli M: Effects of
S-adenosyl-L-methionine on the invasion and migration of head and
neck squamous cancer cells and analysis of the underlying
mechanisms. Int J Oncol. 56:1212–1224. 2020.PubMed/NCBI
|
|
37
|
D'Arcy MS: Cell death: A review of the
major forms of apoptosis, necrosis and autophagy. Cell Biol Int.
43:582–592. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pistritto G, Trisciuoglio D, Ceci C,
Garufi A and D'Orazi G: Apoptosis as anticancer mechanism: Function
and dysfunction of its modulators and targeted therapeutic
strategies. Aging (Albany NY). 8:603–619. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Goldar S, Khaniani MS, Derakhshan SM and
Baradaran B: Molecular mechanisms of apoptosis and roles in cancer
development and treatment. Asian Pac J Cancer Prev. 16:2129–2144.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pan Y, Ye C, Tian Q, Yan S, Zeng X, Xiao
C, Wang L and Wang H: miR-145 suppresses the proliferation,
invasion and migration of NSCLC cells by regulating the BAX/BCL-2
ratio and the caspase-3 cascade. Oncol Lett. 15:4337–4343.
2018.PubMed/NCBI
|
|
42
|
Knight T, Luedtke D, Edwards H, Taub JW
and Ge Y: A delicate balance-The Bcl-2 family and its role in
apoptosis, oncogenesis, and cancer therapeutics. Biochem Pharmacol.
162:250–261. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Orrenius S: Mitochondrial regulation of
apoptotic cell death. Toxicol Lett. 149:19–23. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Scorrano L and Korsmeyer SJ: Mechanisms of
cytochrome c release by proapoptotic BCL-2 family members. Biochem
Biophys Res Commun. 304:437–444. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Meeran SM and Katiyar SK: Grape seed
proanthocyanidins promote apoptosis in human epidermoid carcinoma
A431 cells through alterations in Cdki-Cdk-cyclin cascade, and
caspase-3 activation via loss of mitochondrial membrane potential.
Exp Dermatol. 16:405–415. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Siu WP, Pun PB, Latchoumycandane C and
Boelsterli UA: Bax-mediated mitochondrial outer membrane
permeabilization (MOMP), distinct from the mitochondrial
permeability transition, is a key mechanism in diclofenac-induced
hepatocyte injury: Multiple protective roles of cyclosporin A.
Toxicol Appl Pharmacol. 227:451–461. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ohtsuka T, Buchsbaum D, Oliver P, Makhija
S, Kimberly R and Zhou T: Synergistic induction of tumor cell
apoptosis by death receptor antibody and chemotherapy agent through
JNK/p38 and mitochondrial death pathway. Oncogene. 22:2034–2044.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang X, Lu X, Zhu R, Zhang K, Li S, Chen Z
and Li L: Betulinic acid induces apoptosis in differentiated PC12
cells via ROS-mediated mitochondrial pathway. Neurochem Res.
42:1130–1140. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Estaquier J, Vallette F, Vayssiere JL and
Mignotte B: The mitochondrial pathways of apoptosis. Adv Exp Med
Biol. 942:157–183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pucci B, Kasten M and Giordano A: Cell
cycle and apoptosis. Neoplasia. 2:291–299. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Canavese M, Santo L and Raje N: Cyclin
dependent kinases in cancer: Potential for therapeutic
intervention. Cancer Biol Ther. 13:451–457. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sperka T, Wang J and Rudolph KL: DNA
damage checkpoints in stem cells, ageing and cancer. Nat Rev Mol
Cell Biol. 13:579–590. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
George Rosenker KM, Paquette WD, Johnston
PA, Sharlow ER, Vogt A, Bakan A, Lazo JS and Wipf P: Synthesis and
biological evaluation of 3-aminoisoquinolin-1(2H)-one based
inhibitors of the dual-specificity phosphatase Cdc25B. Bioorg Med
Chem. 23:2810–2818. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tilaoui M, Mouse HA, Jaafari A and Zyad A:
Differential effect of artemisinin against cancer cell lines. Nat
Prod Bioprospect. 4:189–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhu H, Zhang L, Wu S, Teraishi F, Davis
JJ, Jacob D and Fang B: Induction of S-phase arrest and p21
overexpression by a small molecule 2-[[3-(2,3-dichlorophenoxy)
propyl]amino]ethanol in correlation with activation of ERK.
Oncogene. 23:4984–4992. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ahn NR, Ko JM and Cha HC: Comparison of
flavonoid profiles between leaves and stems of Calystegia
soldanella and Calystegia japonica. Am J Plant Sci.
3:1073–1076. 2012. View Article : Google Scholar
|
|
57
|
Al Aaraj L, Hayar B, Jaber Z, Saad W,
Saliba NA, Darwiche N and Ghaddar T: The effect of different ester
chain modifications of two guaianolides for inhibition of
colorectal cancer cell growth. Molecules. 26:54812021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Takayama T, Miyanishi K, Hayashi T, Sato Y
and Niitsu Y: Colorectal cancer: Genetics of development and
metastasis. J Gastroenterol. 41:185–192. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Nakayama M and Oshima M: Mutant p53 in
colon cancer. J Mol Cell Biol. 11:267–276. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Li XL, Zhou J, Chen ZR and Chng WJ: p53
mutations in colorectal cancer-molecular pathogenesis and
pharmacological reactivation. World J Gastroenterol. 21:84–93.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Murai Y, Setoguchi H, Ono E and Iwashina
T: Flavonoids and their qualitative variation in Calystegia
soldanella and related species (Convolvulaceae). Nat Prod
Commun. 10:429–432. 2015.PubMed/NCBI
|
|
62
|
Abotaleb M, Liskova A, Kubatka P and
Busselberg D: Therapeutic potential of plant phenolic acids in the
treatment of cancer. Biomolecules. 10:2212020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Anantharaju PG, Gowda PC, Vimalambike MG
and Madhunapantula SV: An overview on the role of dietary phenolics
for the treatment of cancers. Nutr J. 15:992016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Jaganathan SK, Supriyanto E and Mandal M:
Events associated with apoptotic effect of p-coumaric acid in
HCT-15 colon cancer cells. World J Gastroenterol. 19:7726–7734.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Janicke B, Hegardt C, Korgh M, Onning G,
Akesson B, Cirenajwis HM and Oredsson SM: The antiproliferative
effect of dietary fiber phenolic compounds ferulic acid and
p-coumaric acid on the cell cycle of Caco-2 cells. Nutr Cancer.
63:611–622. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Janicke B, Onning G and Oredsson SM:
Differential effects of ferulic acid and p-coumaric acid on S phase
distribution and length of S phase in the human colonic cell line
Caco-2. J Agric Food Chem. 53:6658–6665. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
García-Gutiérrez N, Maldonado-Celis ME,
Rojas-López M, Loarca-Piñaa GF and Campos-Vega R: The fermented
non-digestible fraction of spent coffee grounds induces apoptosis
in human colon cancer cells (SW480). J Funct Foods. 30:237–246.
2017. View Article : Google Scholar
|
|
68
|
Ekbatan SS, Li XQ, Ghorbani M, Azadi B and
Kubow S: Chlorogenic acid and its microbial metabolites exert
anti-proliferative effects, S-phase cell-cycle arrest and apoptosis
in human colon cancer Caco-2 cells. Int J Mol Sci. 19:7232018.
View Article : Google Scholar
|
|
69
|
Hernández-Arriaga AM, Oomah BD and
Campos-Vega R: Microbiota source impact in vitro metabolite colonic
production and anti-proliferative effect of spent coffee grounds on
human colon cancer cells (HT-29). Food Res Int. 97:191–198. 2017.
View Article : Google Scholar : PubMed/NCBI
|