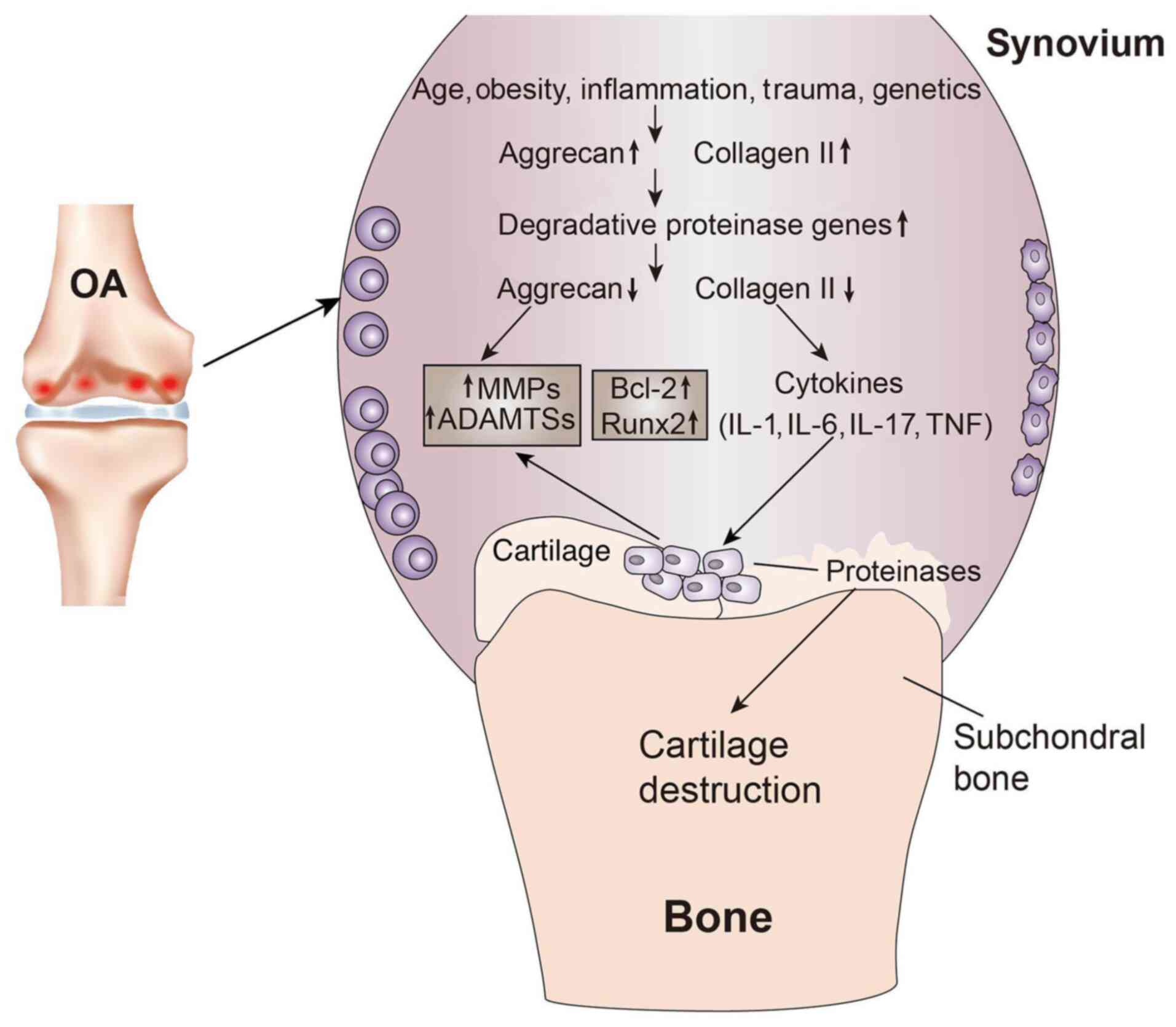
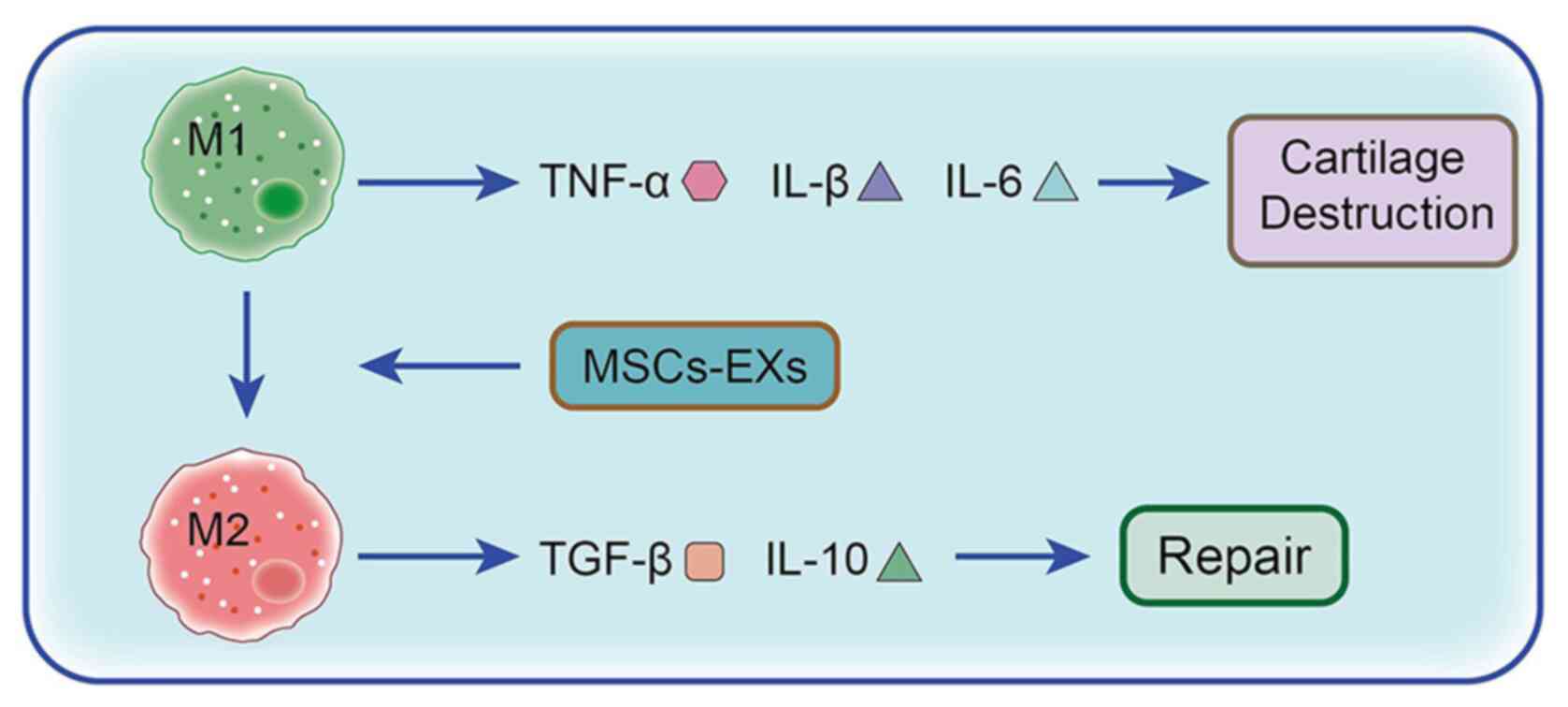
|
1
|
Abramoff B and Caldera FE: Osteoarthritis:
Pathology, diagnosis, and treatment options. Med Clin North Am.
104:293–311. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goldring MB and Goldring SR: Articular
cartilage and subchondral bone in the pathogenesis of
osteoarthritis. Ann NY Acad Sci. 1192:230–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xia B, Di Chen, Zhang J, Hu S, Jin H and
Tong P: Osteoarthritis pathogenesis: A review of molecular
mechanisms. Calcif Tissue Int. 95:495–505. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang Q, Ji Q, Wang X, Kang L, Fu Y, Yin
Y, Li Z, Liu Y, Xu X and Wang Y: SOX9 is a regulator of
ADAMTSs-induced cartilage degeneration at the early stage of human
osteoarthritis. Osteoarthritis Cartilage. 23:2259–2268. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tortorella MD and Malfait AM: Will the
real aggrecanase(s) step up: Evaluating the criteria that define
aggrecanase activity in osteoarthritis. Curr Pharm Biotechnol.
9:16–23. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matsuo M, Nishida K, Yoshida A, Murakami T
and Inoue H: Expression of caspase-3 and −9 relevant to cartilage
destruction and chondrocyte apoptosis in human osteoarthritic
cartilage. Acta Med Okayama. 55:333–340. 2001.PubMed/NCBI
|
|
7
|
Abdel-Sayed P and Pioletti DP: Strategies
for improving the repair of focal cartilage defects. Nanomedicine
(Lond). 10:2893–2905. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Medvedeva EV, Grebenik EA, Gornostaeva SN,
Telpuhov VI, Lychagin AV, Timashev PS and Chagin AS: Repair of
damaged articular cartilage: Current approaches and future
directions. Int J Mol Sci. 19:23662018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hunter DJ and Bierma-Zeinstra S:
Osteoarthritis. Lancet. 393:1745–1759. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rhee SM, You HJ and Han SK: Injectable
tissue-engineered soft tissue for tissue augmentation. J Korean Med
Sci. 29 (Suppl 3):S170–S175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schott EM, Farnsworth CW, Grier A, Lillis
JA, Soniwala S, Dadourian GH, Bell RD, Doolittle ML, Villani DA,
Awad H, et al: Targeting the gut microbiome to treat the
osteoarthritis of obesity. JCI Insight. 3:e959972018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kuo SJ, Yang WH, Liu SC, Tsai CH, Hsu HC
and Tang CH: Transforming growth factor β1 enhances heme oxygenase
1 expression in human synovial fibroblasts by inhibiting microRNA
519b synthesis. PLoS One. 12:e01760522017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang T and He C: Pro-inflammatory
cytokines: The link between obesity and osteoarthritis. Cytokine
Growth Factor Rev. 44:38–50. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kapoor M, Martel-Pelletier J, Lajeunesse
D, Pelletier JP and Fahmi H: Role of proinflammatory cytokines in
the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 7:33–42.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nguyen L, Sharma A, Chakraborty C, Saibaba
B, Ahn ME and Lee SS: Review of prospects of biological fluid
biomarkers in osteoarthritis. Int J Mol Sci. 18:6012017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mabey T, Honsawek S, Tanavalee A,
Yuktanandana P, Wilairatana V and Poovorawan Y: Plasma and synovial
fluid inflammatory cytokine profiles in primary knee
osteoarthritis. Biomarkers. 21:639–644. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Boehme KA and Rolauffs B: Onset and
progression of human osteoarthritis-can growth factors,
inflammatory cytokines, or differential miRNA expression
concomitantly induce proliferation, ECM Degradation, and
inflammation in articular cartilage? Int J Mol Sci. 19:22822018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goldring MB and Goldring SR:
Osteoarthritis. J Cell Physiol. 213:626–634. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sirikaew N, Chomdej S, Tangyuenyong S,
Tangjitjaroen W, Somgird C, Thitaram C and Ongchai S:
Proinflammatory cytokines and lipopolysaccharides up regulate MMP-3
and MMP-13 production in Asian elephant (Elephas maximus)
chondrocytes: Attenuation by anti-arthritic agents. BMC Vet Res.
15:4192019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ho YJ, Lu JW, Ho LJ, Lai JH, Huang HS, Lee
CC, Lin TY, Lien SB, Lin LC, Chen LW, et al: Anti-inflammatory and
anti-osteoarthritis effects of Cm-02 and Ck-02. Biochem Biophys Res
Commun. 517:155–163. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fearing BV and Van Dyke ME: In vitro
response of macrophage polarization to a keratin biomaterial. Acta
Biomater. 10:3136–3144. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dinarello CA: Overview of the
interleukin-1 family of ligands and receptors. Semin Immunol.
25:389–393. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Melchiorri C, Meliconi R, Frizziero L,
Silvestri T, Pulsatelli L, Mazzetti I, Borzì RM, Uguccioni M and
Facchini A: Enhanced and coordinated in vivo expression of
inflammatory cytokines and nitric oxide synthase by chondrocytes
from patients with osteoarthritis. Arthritis Rheum. 41:2165–2174.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Massicotte F, Lajeunesse D, Benderdour M,
Pelletier JP, Hilal G, Duval N and Martel-Pelletier J: Can altered
production of interleukin-1beta, interleukin-6, transforming growth
factor-beta and prostaglandin E(2) by isolated human subchondral
osteoblasts identify two subgroups of osteoarthritic patients.
Osteoarthritis Cartilage. 10:491–500. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Farahat MN, Yanni G, Poston R and Panayi
GS: Cytokine expression in synovial membranes of patients with
rheumatoid arthritis and osteoarthritis. Ann Rheum Dis. 52:870–875.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sohn DH, Sokolove J, Sharpe O, Erhart JC,
Chandra PE, Lahey LJ, Lindstrom TM, Hwang I, Boyer KA, Andriacchi
TP and Robinson WH: Plasma proteins present in osteoarthritic
synovial fluid can stimulate cytokine production via Toll-like
receptor 4. Arthritis Res Ther. 14:R72012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wojdasiewicz P, Poniatowski ŁA and
Szukiewicz D: The role of inflammatory and anti-inflammatory
cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm.
2014:5614592014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Burrage PS, Mix KS and Brinckerhoff CE:
Matrix metalloproteinases: Role in arthritis. Front Biosci.
11:529–543. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Verma P and Dalal K: ADAMTS-4 and
ADAMTS-5: Key enzymes in osteoarthritis. J Cell Biochem.
112:3507–3514. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Koshy PJ, Lundy CJ, Rowan AD, Porter S,
Edwards DR, Hogan A, Clark IM and Cawston TE: The modulation of
matrix metalloproteinase and ADAM gene expression in human
chondrocytes by interleukin-1 and oncostatin M: A time-course study
using real-time quantitative reverse transcription-polymerase chain
reaction. Arthritis Rheum. 46:961–967. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
El Mansouri FE, Chabane N, Zayed N, Kapoor
M, Benderdour M, Martel-Pelletier J, Pelletier JP, Duval N and
Fahmi H: Contribution of H3K4 methylation by SET-1A to
interleukin-1-induced cyclooxygenase 2 and inducible nitric oxide
synthase expression in human osteoarthritis chondrocytes. Arthritis
Rheum. 63:168–179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gilman SC, Chang J, Zeigler PR, Uhl J and
Mochan E: Interleukin-1 activates phospholipase A2 in human
synovial cells. Arthritis Rheum. 31:126–130. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hardy MM, Seibert K, Manning PT, Currie
MG, Woerner BM, Edwards D, Koki A and Tripp CS: Cyclooxygenase
2-dependent prostaglandin E2 modulates cartilage proteoglycan
degradation in human osteoarthritis explants. Arthritis Rheum.
46:1789–1803. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lotz M: The role of nitric oxide in
articular cartilage damage. Rheum Dis Clin North Am. 25:269–282.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Haynes MK, Hume EL and Smith JB:
Phenotypic characterization of inflammatory cells from
osteoarthritic synovium and synovial fluids. Clin Immunol.
105:315–325. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Afonso V, Champy R, Mitrovic D, Collin P
and Lomri A: Reactive oxygen species and superoxide dismutases:
Role in joint diseases. Joint Bone Spine. 74:324–329. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bodmer JL, Schneider P and Tschopp J: The
molecular architecture of the TNF superfamily. Trends Biochem Sci.
27:19–26. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hosseinzadeh A, Kamrava SK, Joghataei MT,
Darabi R, Shakeri-Zadeh A, Shahriari M, Reiter RJ, Ghaznavi H and
Mehrzadi S: Ap MMR-21332-279469 optosis signaling pathways in
osteoarthritis and possible protective role of melatonin. J Pineal
Res. 61:411–425. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
MacEwan DJ: TNF receptor subtype
signalling: Differences and cellular consequences. Cell Signal.
14:477–492. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Grell M, Douni E, Wajant H, Löhden M,
Clauss M, Maxeiner B, Georgopoulos S, Lesslauer W, Kollias G,
Pfizenmaier K and Scheurich P: The transmembrane form of tumor
necrosis factor is the prime activating ligand of the 80 kDa tumor
necrosis factor receptor. Cell. 83:793–802. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hsu H, Xiong J and Goeddel DV: The TNF
receptor 1-associated protein TRADD signals cell death and
NF-kappaB activation. Cell. 81:495–504. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hsu H, Huang J, Shu HB, Baichwal V and
Goeddel DV: TNF-dependent recruitment of the protein kinase RIP to
the TNF receptor-1 signaling complex. Immunity. 4:387–396. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Varfolomeev E, Goncharov T, Fedorova AV,
Dynek JN, Zobel K, Deshayes K, Fairbrother WJ and Vucic D: c-IAP1
and c-IAP2 are critical mediators of tumor necrosis factor alpha
(TNFalpha)-induced NF-kappaB activation. J Biol Chem.
283:24295–24299. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
O'Donnell MA, Legarda-Addison D, Skountzos
P, Yeh WC and Ting AT: Ubiquitination of RIP1 Regulates an
NF-kappaB-Independent cell-death switch in TNF signaling. Curr
Biol. 17:418–424. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ea CK, Deng L, Xia ZP, Pineda G and Chen
ZJ: Activation of IKK by TNF alpha requires site-specific
ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell.
22:245–257. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bunning RA and Russell RG: The effect of
tumor necrosis factor alpha and gamma-interferon on the resorption
of human articular cartilage and on the production of prostaglandin
E and of caseinase activity by human articular chondrocytes.
Arthritis Rheum. 32:780–784. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Campbell IK, Piccoli DS, Roberts MJ,
Muirden KD and Hamilton JA: Effects of tumor necrosis factor and on
resorption of human articular cartilage and production of
plasminogen activator by human articular chondrocytes. Arthritis
Rheum. 33:542–552. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lefebvre V, Peeters-Joris C and Vaes G:
Modulation by interleukin 1 and tumor necrosis factor of production
of collagenase, tissue inhibitor of metalloproteinases and collagen
types in differentiated and dedifferentiated articular
chondrocytes. Biochim Biophys Acta. 1052:366–378. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Meyer FA, Yaron I and Yaron M:
Synergistic, additive, and antagonistic effects of interleukin-1
beta, tumor necrosis factor alpha, and gamma-interferon on
prostaglandin E, hyaluronic acid, and collagenase production by
cultured synovial fibroblasts. Arthritis Rheum. 33:1518–1525. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Saklatvala J: Tumour necrosis factor alpha
stimulates resorption and inhibits synthesis of proteoglycan in
cartilage. Nature. 322:547–549. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
van den Berg WB: Anti-cytokine therapy in
chronic destructive arthritis. Arthritis Res. 3:18–26. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Brennan FM, Chantry D, Jackson AM, Maini
RN and Feldmann M: Cytokine production in culture by cells isolated
from the synovial membrane. J Autoimmun. 2 (Suppl 1):S177–S186.
1989. View Article : Google Scholar
|
|
53
|
O'Byrne E, Blancuzzi V, Wilson DE, Wong M
and Jeng AY: Elevated substance P and accelerated cartilage
degradation in rabbit knees injected with interleukin-1 and tumor
necrosis factor. Arthritis Rheum. 33:1023–1028. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Pettipher ER, Higgs GA and Henderson B:
Interleukin 1 induces leukocyte infiltration and cartilage
proteoglycan degradation in the synovial joint. Proc Natl Acad Sci
USA. 83:8749–8753. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
van Beuningen HM, Arntz OJ and van den
Berg WB: In vivo effects of interleukin-1 on articular cartilage.
Prolongation of proteoglycan metabolic disturbances in old mice.
Arthritis Rheum. 34:606–615. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ding X, Zhang Y, Huang Y, Liu S, Lu H and
Sun T: Cadherin-11 involves in synovitis and increases the
migratory and invasive capacity of fibroblast-like synoviocytes of
osteoarthritis. Int Immunopharmacol. 26:153–161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liu S, Cao C, Zhang Y, Liu G, Ren W, Ye Y
and Sun T: PI3K/Akt inhibitor partly decreases TNF-α-induced
activation of fibroblast-like synoviocytes in osteoarthritis. J
Orthop Surg Res. 14:4252019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Porée B, Kypriotou M, Chadjichristos C,
Beauchef G, Renard E, Legendre F, Melin M, Gueret S, Hartmann DJ,
Malléin-Gerin F, et al: Interleukin-6 (IL-6) and/or soluble IL-6
receptor down-regulation of human type II collagen gene expression
in Articular chondrocytes requires a decrease of Sp1·Sp3 ratio and
of the binding activity of both factors to the COL2A1 promoter. J
Biol Chem. 283:4850–4865. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Rowan AD, Koshy PJ, Shingleton WD, Degnan
BA, Heath JK, Vernallis AB, Spaull JR, Life PF, Hudson K and
Cawston TE: Synergistic effects of glycoprotein 130 binding
cytokines in combination with interleukin-1 on cartilage collagen
breakdown. Arthritis Rheum. 44:1620–1632. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cawston TE, Curry VA, Summers CA, Clark
IM, Riley GP, Life PF, Spaull JR, Goldring MB, Koshy PJ, Rowan AD
and Shingleton WD: The role of oncostatin M in animal and human
connective tissue collagen turnover and its localization within the
rheumatoid joint. Arthritis Rheum. 41:1760–1771. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Scanzello CR, Umoh E, Pessler F,
Diaz-Torne C, Miles T, Dicarlo E, Potter HG, Mandl L, Marx R, Rodeo
S, et al: Local cytokine profiles in knee osteoarthritis: Elevated
synovial fluid interleukin-15 differentiates early from end-stage
disease. Osteoarthritis Cartilage. 17:1040–1048. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Honsawek S, Deepaisarnsakul B, Tanavalee
A, Yuktanandana P, Bumrungpanichthaworn P, Malila S and Saetan N:
Association of the IL-6-174G/C gene polymorphism with knee
osteoarthritis in a Thai population. Genet Mol Res. 10:1674–1680.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Attur MG, Patel RN, Abramson SB and Amin
AR: Interleukin-17 up-regulation of nitric oxide production in
human osteoarthritis cartilage. Arthritis Rheum. 40:1050–1053.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ryu JH, Yang S, Shin Y, Rhee J, Chun CH
and Chun JS: Interleukin-6 plays an essential role in
hypoxia-inducible factor 2α-induced experimental osteoarthritic
cartilage destruction in mice. Arthritis Rheum. 63:2732–2743. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kwan Tat S, Padrines M, Théoleyre S,
Heymann D and Fortun Y: IL-6, RANKL, TNF-alpha/IL-1: Interrelations
in bone resorption pathophysiology. Cytokine Growth Factor Rev.
15:49–60. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Chenoufi HL, Diamant M, Rieneck K, Lund B,
Stein GS and Lian JB: Increased mRNA expression and protein
secretion of interleukin-6 in primary human osteoblasts
differentiated in vitro from rheumatoid and osteoarthritic bone. J
Cell Biochem. 81:666–678. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Sakao K, Takahashi KA, Arai Y, Saito M,
Honjo K, Hiraoka N, Asada H, Shin-Ya M, Imanishi J, Mazda O and
Kubo T: Osteoblasts derived from osteophytes produce interleukin-6,
interleukin-8, and matrix metalloproteinase-13 in osteoarthritis. J
Bone Miner Metab. 27:412–423. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ding X, Cao Y, Xing Y, Ge S, Lin M and Li
J: TIMP-1 mediates inflammatory and immune response to IL-6 in
adult orbital xanthogranulomatous disease. Ocul Immunol Inflamm.
28:288–297. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
De Hooge ASK, van de Loo FAJ, Bennink MB,
Bennink MB, Arntz OJ, de Hooge P and van den Berg WB: Male IL-6
gene Knock Out mice developed more advanced osteoarthritis upon
aging. Osteoarthritis Cartilage. 13:66–73. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Steel JC, Waldmann TA and Morris JC:
Interleukin-15 biology and its therapeutic implications in cancer.
Trends Pharmacol Sci. 33:35–41. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Perera LP: Interleukin 15: Its role in
inflammation and immunity. Arch Immunol Ther Exp (Warsz).
48:457–464. 2000.PubMed/NCBI
|
|
72
|
Waldmann TA and Tagaya Y: The multifaceted
regulation of interleukin-15 expression and the role of this
cytokine in NK cell differentiation and host response to
intracellular pathogens. Annu Rev Immunol. 17:19–49. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Baslund B, Tvede N, Danneskiold-Samsoe B,
Larsson P, Panayi G, Petersen J, Petersen LJ, Beurskens FJ,
Schuurman J, van de Winkel JG, et al: Targeting interleukin-15 in
patients with rheumatoid arthritis: A proof-of-concept study.
Arthritis Rheum. 52:2686–2692. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Mcinnes IB, al-Mughales J, Field M, Leung
BP, Huang FP, Dixon R, Sturrock RD, Wilkinson PC and Liew FY: The
role of interleukin-15 in T-cell migration and activation in
rheumatoid arthritis. Nat Med. 2:175–182. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Tao Y, Qiu X, Xu C, Sun B and Shi C:
Expression and correlation of matrix metalloproteinase-7 and
interleukin-15 in human osteoarthritis. Int J Clin Exp Pathol.
8:9112–9118. 2015.PubMed/NCBI
|
|
76
|
Santos Savio A, Machado Diaz AC, Chico
Capote A, Miranda Navarro J, Rodríguez Alvarez Y, Bringas Pérez R,
Estévez del Toro M and Guillen Nieto GE: Differential expression of
pro-inflammatory cytokines IL-15Ralpha, IL-15, IL-6 and TNFalpha in
synovial fluid from rheumatoid arthritis patients. BMC
Musculoskelet Disord. 16:512015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Badolato R, Ponzi AN, Millesimo M,
Notarangelo LD and Musso T: Interleukin-15 (IL-15) induces IL-8 and
monocyte chemotactic protein 1 production in human monocytes.
Blood. 90:2804–2809. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Sun JM, Sun LZ, Liu J, Su BH and Shi L:
Serum interleukin-15 levels are associated with severity of pain in
patients with knee osteoarthritis. Dis Markers. 35:203–206. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Chang SH and Dong C: Signaling of
interleukin-17 family cytokines in immunity and inflammation. Cell
Signal. 23:1069–1075. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhang X, Angkasekwinai P, Dong C and Tang
H: Structure and function of interleukin-17 family cytokines.
Protein Cell. 2:26–40. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Korn T, Bettelli E, Oukka M and Kuchroo
VK: IL-17 and Th17 cells. Annu Rev Immunol. 27:485–517. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Pawłowska J, Mikosik A, Soroczynska-Cybula
M, Jóźwik A, Łuczkiewicz P, Mazurkiewicz S, Lorczyński A, Witkowski
JM and Bryl E: Different distribution of CD4 and CD8 T cells in
synovial membrane and peripheral blood of rheumatoid arthritis and
osteoarthritis patients. Folia Histochem Cytobiol. 47:627–632.
2009.PubMed/NCBI
|
|
83
|
Ishii H, Tanaka H, Katoh K, Nakamura H,
Nagashima M and Yoshino S: Characterization of infiltrating T cells
and Th1/Th2-type cytokines in the synovium of patients with
osteoarthritis. Osteoarthritis Cartilage. 10:277–281. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Suurmond J, Dorjée AL, Boon MR, Knol EF,
Huizinga TW, Toes RE and Schuerwegh AJ: Mast cells are the main
interleukin 17-positive cells in anticitrullinated protein
antibody-positive and -negative rheumatoid arthritis and
osteoarthritis synovium. Arthritis Res Ther. 13:R1502011.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Liu Y, Peng H, Meng Z and Wei M:
Correlation of IL-17 Level in synovia and severity of knee
osteoarthritis. Med Sci Monit. 21:1732–1736. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Koenders MI, Marijnissen RJ, Devesa I,
Lubberts E, Joosten LA, Roth J, van Lent PL, van de Loo FA and van
den Berg WB: Tumor necrosis factor-interleukin-17 interplay induces
S100A8,interleukin-1β,and matrix metalloproteinases, and drives
irreversible cartilage destruction in murine arthritis: Rationale
for combination treatment during arthritis. Arthritis Rheum.
63:2329–2339. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Honorati MC, Cattini L and Facchini A:
VEGF production by osteoarthritic chondrocytes cultured in
micromass and stimulated by IL-17 and TNF-alpha. Connect Tissue
Res. 48:239–245. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Honorati MC, Neri S, Cattini L and
Facchini A: Interleukin-17, a regulator of angiogenic factor
release by synovial fibroblasts. Osteoarthritis Cartilage.
14:345–352. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Lubberts E, Joosten LA, van de Loo FA, van
den Gersselaar LA and van den Berg WB: Reduction of
interleukin-17-induced inhibition of chondrocyte proteoglycan
synthesis in intact murine articular cartilage by interleukin-4.
Arthritis Rheum. 43:1300–1306. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Okamura H, Tsutsui H, Komatsu T, Yutsudo
M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K,
et al: Cloning of a new cytokine that induces IFN-gamma production
by T cells. Nature. 378:88–91. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ghayur T, Banerjee S, Hugunin M, Butler D,
Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, et
al: Caspase-1 processes IFN-gamma-inducing factor and regulates
LPS-induced IFN-gamma production. Nature. 386:619–623. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Malemud CJ: Cytokines as therapeutic
targets for osteoarthritis. BioDrugs. 18:23–35. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Joosten LA, Radstake TR, Lubberts E, van
den Bersselaar LA, van Riel PL, van Lent PL, Barrera P and van den
Berg WB: Association of interleukin-18 expression with enhanced
levels of both interleukin-1beta and tumor necrosis factor alpha in
knee synovial tissue of patients with rheumatoid arthritis.
Arthritis Rheum. 48:339–347. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Joosten LAB, Smeets RL, Koenders MI, van
den Bersselaar LA, Helsen MM, Oppers-Walgreen B, Lubberts E,
Iwakura Y, van de Loo FA and van den Berg WB: Interleukin-18
promotes joint inflammation and induces interleukin-1-driven
cartilage destruction. Am J Pathol. 165:959–967. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Inoue H, Hiraoka K, Hoshino T, Okamoto M,
Iwanaga T, Zenmyo M, Shoda T, Aizawa H and Nagata K: High levels of
serum IL-18 promote cartilage loss through suppression of aggrecan
synthesis. Bone. 42:1102–1110. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
John T, Kohl B, Mobasheri A, Ertel W and
Shakibaei M: Interleukin-18 induces apoptosis in human articular
chondrocytes. Histol Histopathol. 22:469–482. 2007.PubMed/NCBI
|
|
97
|
Dai SM, Shan ZZ, Nishioka K and Yudoh K:
Implication of interleukin 18 in production of matrix
metalloproteinases in Articular chondrocytes in arthritis: Direct
effect on chondrocytes may not be pivotal. Ann Rheum Dis.
64:735–742. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Powers R, Garrett DS, March CJ, Frieden
EA, Gronenborn AM and Clore GM: The high-resolution,
three-dimensional solution structure of human interleukin-4
determined by multidimensional heteronuclear magnetic resonance
spectroscopy. Biochemistry. 32:6744–6762. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Wlodawer A, Pavlovsky A and Gustchina A:
Crystal structure of human recombinant interleukin-4 at 2.25 A
resolution. FEBS Lett. 309:59–64. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Carr C, Aykent S, Kimack NM and Levine AD:
Disulfide assignments in recombinant mouse and human interleukin 4.
Biochemistry. 30:1515–1523. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Mueller TD, Zhang JL, Sebald W and Duschl
A: Structure, binding, and antagonists in the IL-4/IL-13 receptor
system. Biochim Biophys Acta. 1592:237–250. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Schlaak JF, Pfers I, Meyer Zum
Büschenfelde KH and Märker-Hermann E: Different cytokine profiles
in the synovial fluid of patients with osteoarthritis, rheumatoid
arthritis and seronegative spondylarthropathies. Clin Exp
Rheumatol. 14:155–162. 1996.PubMed/NCBI
|
|
103
|
Brown MA and Hural J: Functions of IL-4
and control of its expression. Crit Rev Immunol. 17:1–32. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Wang P, Wu P, Siegel MI, Egan RW and
Billah MM: Interleukin (IL)-10 inhibits nuclear factor kappa B (NF
kappa B) activation in human monocytes. IL-10 and IL-4 suppress
cytokine synthesis by different mechanisms. J Biol Chem.
270:9558–9563. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
te Velde AA, Huijbens RJ, Heije K, de
Vries JE and Figdor CG: Interleukin-4 (IL-4) inhibits secretion of
IL-1beta, tumor necrosis factor alpha, and human IL-6 by human
monocytes. Blood. 76:1392–1397. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Paul WE: Interleukin-4: A prototypic
immunoregulatory lymphokine. Blood. 77:1859–1870. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Vannier E, Miller MC and Dinarello CA:
Coordinated antiinflammatory effects of interleukin 4: Interleukin
4 suppresses interleukin 1 production but up-regulates gene
expression and synthesis of interleukin 1 receptor antagonist. Proc
Natl Acad Sci USA. 89:4076–4080. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Hart PH, Vitti GF, Burgess DR, Whitty GA,
Piccoli DS and Hamilton JA: Potential anti-inflammatory effects of
interleukin 4: Suppression of human monocyte tumor necrosis factor
alpha, interleukin 1, and prostaglandin E2. Proc Natl Acad Sci USA.
86:3803–3807. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Yeh LA, Augustine AJ, Lee P, Riviere LR
and Sheldon A: Interleukin-4, an inhibitor of cartilage breakdown
in bovine articular cartilage explants. J Rheumatol. 22:1740–1746.
1995.PubMed/NCBI
|
|
110
|
Van Meegeren ME, Roosendaal G, Jansen NW,
Wenting MJ, van Wesel AC, van Roon JA and Lafeber FP: IL-4 alone
and in combination with IL-10 protects against blood-Induced
cartilage damage. Osteoarthritis Cartilage. 20:764–772. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
van Lent PL, Holthuysen AE, Slöetjes A,
Lubberts E and van den Berg WB: Local overexpression of adeno-viral
IL-4 protects cartilage from metallo proteinase-induced destruction
during immune complex-mediated arthritis by preventing activation
of pro-MMPs. Osteoarthritis Cartilage. 10:234–243. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Doi H, Nishida K, Yorimitsu M, Komiyama T,
Kadota Y, Tetsunaga T, Yoshida A, Kubota S, Takigawa M and Ozaki T:
Interleukin-4 downregulates the cyclic tensile stress-induced
matrix metalloproteinases-13 and cathepsin B expression by rat
normal chondrocytes. Acta Medica Okayama. 62:119–126.
2008.PubMed/NCBI
|
|
113
|
Yorimitsu M, Nishida K, Shimizu A, Doi H,
Miyazawa S, Komiyama T, Nasu Y, Yoshida A, Watanabe S and Ozaki T:
Intra-articular injection of interleukin-4 decreases nitric oxide
production by chondrocytes and ameliorates subsequent destruction
of cartilage in instability-induced osteoarthritis in rat knee
joints. Osteoarthritis Cartilage. 16:764–771. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
von Kaeppler EP, Wang Q, Raghu H, Bloom
MS, Wong H and Robinson WH: Interleukin 4 promotes
anti-inflammatory macrophages that clear cartilage debris and
inhibits osteoclast development to protect against osteoarthritis.
Clin Immunol. 229:1087842021. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Shah SS and Mithoefer K: Current
Applications of growth factors for knee cartilage repair and
osteoarthritis treatment. Curr Rev Musculoskelet Med. 13:641–650.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
McAlindon TE, Teale JD and Dieppe PA:
Levels of insulin related growth factor 1 in osteoarthritis of the
knee. Ann Rheum Dis. 52:229–231. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
McQuillan DJ, Handley CJ, Campbell MA,
Bolis S, Milway VE and Herington AC: Stimulation of proteoglycan
biosynthesis by serum and insulin-like growth factor-I in cultured
bovine articular cartilage. Biochem J. 240:423–430. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
van Osch GJ, van den Berg WB, Hunziker EB,
Hunziker EB and Häuselmann HJ: Differential effects of IGF-1 and
TGF beta-2 on the assembly of proteoglycans in pericellular and
territorial matrix by cultured bovine articular chondrocytes.
Osteoarthritis Cartilage. 6:187–195. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Sah RL, Chen AC, Grodzinsky AJ and Trippel
SB: Differential effects of bFGF and IGF-I on matrix metabolism in
calf and adult bovine cartilage explants. Arch Biochem Biophys.
308:137–147. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Yaeger PC, Masi TL, de Ortiz JL, Binette
F, Tubo R and McPherson JM: Synergistic action of transforming
growth factor-beta and insulin-like growth factor-I induces
expression of type II collagen and aggrecan genes in adult human
articular chondrocytes. Exp Cell Res. 237:318–325. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Madry H, Kaul G, Cucchiarini M, Stein U,
Zurakowski D, Remberger K, Menger MD, Kohn D and Trippel SB:
Enhanced repair of articular cartilage defects in vivo by
transplanted chondrocytes overexpressing insulin-like growth factor
I (IGF-I). Gene Ther. 12:1171–1179. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Fortier LA, Mohammed HO, Lust G and Nixon
AJ: Insulin-like growth factor-I enhances cell-based repair of
articular cartilage. J Bone Joint Surg Br. 84:276–288. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Davies LC, Blain EJ, Gilbert SJ, Caterson
B and Duance VC: The potential of IGF-1 and TGFbeta1 for promoting
‘adult’ articular cartilage repair: An in vitro study. Tissue Eng
Part A. 14:1251–1261. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Longobardi L, O'Rear L, Aakula S,
Johnstone B, Shimer K, Chytil A, Horton WA, Moses HL and Spagnoli
A: Effect of IGF-I in the chondrogenesis of bone marrow mesenchymal
stem cells in the presence or absence of TGF-beta signaling. J Bone
Miner Res. 21:626–636. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Morisset S, Frisbie DD, Robbins PD, Nixon
AJ and McIlwraith CW: IL-1ra/IGF-1 gene therapy modulates repair of
microfractured chondral defects. Clin Orthop Relat Res.
462:221–228. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Mushtaq T, Bijman P, Ahmed SF and
Farquharson C: Insulin-like growth factor-I augments chondrocyte
hypertrophy and reverses glucocorticoid-mediated growth retardation
in fetal mice metatarsal cultures. Endocrinology. 145:2478–2486.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Koike M, Yamanaka Y, Inoue M, Tanaka H,
Nishimura R and Seino Y: Insulin-like growth factor-1 rescues the
mutated FGF receptor 3 (G380R) expressing ATDC5 cells from
apoptosis through phosphatidylinositol 3-kinase and MAPK. J Bone
Miner Res. 18:2043–2051. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Fytili P, Giannatou E, Karachalios T,
Malizos K and Tsezou A: Interleukin-10G and interleukin-10R
microsatellite polymorphisms and osteoarthritis of the knee. Clin
Exp Rheumatol. 23:621–627. 2005.PubMed/NCBI
|
|
129
|
Jansen NWD, Roosendaal G, Hooiveld MJJ,
Bijlsma JW, van Roon JA, Theobald M and Lafeber FP: Interleukin-10
protects against blood-induced joint damage. Br J Haematol.
142:953–961. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Wang Y and Lou S: Direct protective effect
of interleukin-10 on articular chondrocytes in vitro. Chin Med J
(Engl). 114:723–725. 2001.PubMed/NCBI
|
|
131
|
Behrendt P, Preusse-Prange A, Klüter T,
Haake M, Rolauffs B, Grodzinsky AJ, Lippross S and Kurz B: IL-10
reduces apoptosis and extracellular matrix degradation after
injurious compression of mature articular cartilage. Osteoarthritis
Cartilage. 24:1981–1988. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Rudwaleit M, Yin Z, Siegert S, Grolms M,
Radbruch A, Braun J and Sieper J: Response to methotrexate in early
rheumatoid arthritis is associated with a decrease of T cell
derived tumour necrosis factor alpha, increase of interleukin 10,
and predicted by the initial concentration of interleukin 4. Ann
Rheum Dis. 59:311–314. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Zeng L, Kempf H, Murtaugh LC, Sato ME and
Lassar AB: Shh establishes an Nkx3.2/Sox9 autoregulatory loop that
is maintained by BMP signals to induce somitic chondrogenesis.
Genes Dev. 16:1990–2005. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Chimal-Monroy J, Rodriguez-Leon J, Montero
JA, Gañan Y, Macias D, Merino R and Hurle JM: Analysis of the
molecular cascade responsible for mesodermal limb chondrogenesis:
Sox genes and BMP signaling. Dev Biol. 257:292–301. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Horbelt D, Denkis A and Knaus P: A
portrait of transforming growth factor β superfamily signalling:
Background matters. Int J Biochem Cell Biol. 44:469–474. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Massagué J, Blain SW and Lo RS: TGFbeta
signaling in growth control, cancer, and heritable disorders. Cell.
103:295–309. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Siegel PM and Massagué J: Cytostatic and
apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev
Cancer. 3:807–821. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Santo VE, Gomes ME, Mano JF and Reis RL:
Controlled release strategies for bone, cartilage, and
osteochondral engineering-part I: Recapitulation of native tissue
healing and variables for the design of delivery systems. Tissue
Eng Part B Rev. 19:308–326. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Cals FL, Hellingman CA, Koevoet W,
Baatenburg de Jong RJ and van Osch GJ: Effects of transforming
growth factor-β subtypes on in vitro cartilage production and
mineralization of human bone marrow stromal-derived mesenchymal
stem cells. J Tissue Eng Regen Med. 6:68–76. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Fortier LA, Barker JU, Strauss EJ,
McCarrel TM and Cole BJ: The role of growth factors in cartilage
repair. Clin Orthop Relat Res. 469:2706–2715. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Zhang RK, Li GW, Zeng C, Lin CX, Huang LS,
Huang GX, Zhao C, Feng SY and Fang H: Mechanical stress contributes
to osteoarthritis development through the activation of
transforming growth factor beta 1 (TGF-β1). Bone Joint Res.
7:587–594. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Rosen DM, Stempien SA, Thompson AY,
Brennan JE, Ellingsworth LR and Seyedin SM: Differentiation of rat
mesenchymal cells by cartilage-inducing factor. Enhanced phenotypic
expression by dihydrocytochalasin B. Exp Cell Res. 165:127–138.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Seyedin SM, Thompson AY, Bentz H, Rosen
DM, McPherson JM, Conti A, Siegel NR, Galluppi GR and Piez KA:
Cartilage-inducing factor-A. Apparent identity to transforming
growth factor-beta. J Biol Chem. 261:5693–5695. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Kato Y, Iwamoto M, Koike T, Suzuki F and
Takano Y: Terminal differentiation and calcification in rabbit
chondrocyte cultures grown in centrifuge tubes: Regulation by
transforming growth factor beta and serum factors. Proc Natl Acad
Sci USA. 85:9552–9556. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Leonard CM, Fuld HM, Frenz DA, Downie SA,
Massagué J and Newman SA: Role of transforming growth factor-beta
in chondrogenic pattern formation in the embryonic limb:
Stimulation of mesenchymal condensation and fibronectin gene
expression by exogenenous TGF-beta and evidence for endogenous
TGF-beta-like activity. Dev Biol. 145:99–109. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Xu C, Oyajobi BO, Frazer A, Kozaci LD,
Russell RG and Hollander AP: Effects of growth factors and
interleukin-1 alpha on proteoglycan and type II collagen turnover
in bovine nasal and articular chondrocyte pellet cultures.
Endocrinology. 137:3557–3565. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Ito T, Sawada R, Fujiwara Y, Seyama Y and
Tsuchiya T: FGF-2 suppresses cellular senescence of human
mesenchymal stem cells by down-regulation of TGF-beta2. Biochem
Biophys Res Commun. 359:108–114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Lin Y, Luo E, Chen X, Liu L, Qiao J, Yan
Z, Li Z, Tang W, Zheng X and Tian W: Molecular and cellular
characterization during chondrogenic differentiation of adipose
tissue-derived stromal cells in vitro and cartilage formation in
vivo. J Cell Mol Med. 9:929–939. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Bian L, Zhai DY, Tous E, Rai R, Mauck RL
and Burdick JA: Enhanced MSC chondrogenesis following delivery of
TGF-β3 from alginate microspheres within hyaluronic acid hydrogels
in-vitro and in vivo. Biomaterials. 2:6425–6434. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Bhang SH, Jeon JY, La WG, Seong JY, Hwang
JW, Ryu SE and Kim BS: Enhanced chondrogenic marker expression of
human mesenchymal stem cells by interaction with both TGF-β3 and
hyaluronic acid. Biotechnol Appl Biochem. 58:271–276. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Barry F, Boynton RE, Liu B and Murphy JM:
Chondrogenic differentiation of mesenchymal stem cells from bone
marrow: Differentiation-dependent gene expression of matrix
components. Exp Cell Res. 268:189–200. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Dave K and Gomes VG: Interactions at
scaffold interfaces: Effect of surface chemistry, structural
attributes and bioaffinity. Mater Sci Eng C Mater Biol Appl.
105:1100782019. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Huang BJ, Hu JC and Athanasiou KA:
Cell-based tissue engineering strategies used in the clinical
repair of articular cartilage. Biomaterials. 98:1–22. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Yang J, Zhang YS, Yue K and Khademhosseini
A: Cell-laden hydrogels for osteochondral and cartilage tissue
engineering. Acta Biomater. 57:1–25. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Kim YG, Choi J and Kim K: Mesenchymal stem
cell-derived exosomes for effective cartilage tissue repair and
treatment of osteoarthritis. Biotechnol J. 15:e20000822020.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Traggiai E, Volpi S, Schena F, Gattorno M,
Ferlito F, Moretta L and Martini A: Bone marrow-derived mesenchymal
stem cells induce both polyclonal expansion and differentiation of
B cells isolated from healthy donors and systemic lupus
erythematosus patients. Stem Cells. 6:562–569. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Rasmusson I, Le Blanc K, Sundberg B and
Ringdén O: Mesenchymal stem cells stimulate antibody secretion in
human B cells. Scand J Immunol. 65:336–343. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Spaggiari GM, Capobianco A, Becchetti S,
Mingari MC and Moretta L: Mesenchymal stem cell-natural killer cell
interactions: Evidence that activated NK cells are capable of
killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell
proliferation. Blood. 107:1484–1490. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Liang X, Ding Y, Zhang Y, Tse HF and Lian
Q: Paracrine mechanisms of mesenchymal stem cell-based therapy:
Current status and perspectives. Cell Transplant. 23:1045–1059.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Théry C, Witwer KW, Aikawa E, Alcaraz MJ,
Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F,
Atkin-Smith GK, et al: Minimal information for studies of
extracellular vesicles 2018 (MISEV2018): A position statement of
the international society for extracellular vesicles and update of
the MISEV2014 guidelines. J Extracell Vesicles. 7:15357502018.
View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Li X, Ellman M, Muddasani P, Wang JH,
Cs-Szabo G, van Wijnen AJ and Im HJ: Prostaglandin E2 and its
cognate EP receptors control human adult articular cartilage
homeostasis and are linked to the pathophysiology of
osteoarthritis. Arthritis Rheum. 60:513–523. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Cosenza S, Ruiz M, Toupet K, Jorgensen C
and Noël D: Mesenchymal stem cells derived exosomes and
microparticles protect cartilage and bone from degradation in
osteoarthritis. Sci Rep. 7:162142017. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Song Y, Zhang TJ, Li Y and Gao Y:
Mesenchymal stem cells decrease M1/M2 ratio and alleviate
inflammation to improve limb ischemia in mice. Med Sci Monit.
26:e9232872020. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Toh WS, Zhang B, Lai RC and Lim SK: Immune
regulatory targets of mesenchymal stromal cell exosomes/small
extracellular vesicles in tissue regeneration. Cytotherapy.
20:1419–1426. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Chang YH, Wu KC, Harn HJ, Lin SZ and Ding
DC: Exosomes and stem cells in degenerative disease diagnosis and
therapy. Cell Transplant. 27:349–363. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Wang Y, Yu D, Liu Z, Zhou F, Dai J, Wu B,
Zhou J, Heng BC, Zou XH, Ouyang H and Liu H: Exosomes from
embryonic mesenchymal stem cells alleviate osteoarthritis through
balancing synthesis and degradation of cartilage extracellular
matrix. Stem Cell Res Ther. 8:1892017. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Temenoff JS and Mikos AG: Review: Tissue
engineering for regeneration of articular cartilage. Biomaterials.
21:431–440. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Hutmacher DW: Scaffolds in tissue
engineering bone and cartilage. Biomaterials. 21:2529–2543. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Zuluaga M, Gregnanin G, Cencetti C, Di Meo
C, Gueguen V, Letourneur D, Meddahi-Pellé A, Pavon-Djavid G and
Matricardi P: PVA/Dextran hydrogel patches as delivery system of
antioxidant astaxanthin: A cardiovascular approach. Biomed Mater.
13:0150202017. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Jiang T, Heng S, Huang X, Zheng L, Kai D,
Loh XJ and Zhao J: Biomimetic
poly(poly(ε-caprolactone)-Polytetrahydrofuran urethane) based
nanofibers enhanced chondrogenic differentiation and cartilage
regeneration. J Biomed Nanotechnol. 15:1005–1017. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Fasolino I, Raucci MG, Soriente A, Demitri
C, Madaghiele M, Sannino A and Ambrosio L: Osteoinductive and
anti-inflammatory properties of chitosan-based scaffolds for bone
regeneration. Mater Sci Eng C Mater Biol Appl. 105:1100462019.
View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Bhardwaj N, Singh YP and Mandal BB: Silk
fibroin scaffold-based 3D Co-culture model for modulation of
chondrogenesis without hypertrophy via reciprocal Cross-talk and
paracrine signaling. ACS Biomater Sci Eng. 5:5240–5254. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Campo GM, Avenoso A, Campo S, D'Ascola A,
Traina P and Calatroni A: Chondroitin-4-sulphate inhibits NF-kB
translocation and caspase activation in collagen-induced arthritis
in mice. Osteoarthritis Cartilage. 16:1474–1483. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Avenoso A, D'Ascola A, Scuruchi M,
Mandraffino G, Calatroni A, Saitta A, Campo S and Campo GM:
Hyaluronan in the experimental injury of the cartilage: Biochemical
action and protective effects. Inflamm Res. 67:5–20. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Chen L, Liu J, Guan M, Zhou T, Duan X and
Xiang Z: Growth factor and its polymer scaffold-based delivery
system for cartilage tissue engineering. Int J Nanomedicine.
15:6097–6111. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Park E, Hart ML, Rolauffs B, Stegemann JP
and T Annamalai R: Bioresponsive microspheres for on-demand
delivery of anti-inflammatory cytokines for articular cartilage
repair. J Biomed Mater Res A. 108:722–733. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Moutos FT, Glass KA, Compton SA, Ross AK,
Gersbach CA, Guilak F and Estes BT: Anatomically shaped
tissue-engineered cartilage with tunable and inducible anticytokine
delivery for biological joint resurfacing. Proc Natl Acad Sci USA.
113:E4513–E4522. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Levinson C, Lee M, Applegate LA and
Zenobi-Wong M: An injectable heparin-conjugated hyaluronan scaffold
for local delivery of transforming growth factor β1 promotes
successful chondrogenesis. Acta Biomater. 99:168–180. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Armiento AR, Stoddart MJ, Alini M and
Eglin D: Biomaterials for articular cartilage tissue engineering:
Learning from biology. Acta Biomater. 65:1–20. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Qasim M, Chae DS and Lee NY: Advancements
and frontiers in nano-based 3D and 4D scaffolds for bone and
cartilage tissue engineering. Int J Nanomedicine. 14:4333–4351.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Zhao Y, Li Y, Qu R, Chen X, Wang W, Qiu C,
Liu B, Pan X, Liu L, Vasilev K, et al: Cortistatin binds to TNF-α
receptors and protects against osteoarthritis. EBioMedicine.
41:556–570. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Cui Z, Crane J, Xie H, Jin X, Zhen G, Li
C, Xie L, Wang L, Bian Q, Qiu T, et al: Halofuginone attenuates
osteoarthritis by inhibition of TGF-β activity and H-type vessel
formation in subchondral bone. Ann Rheum Dis. 75:1714–1721. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Chavez RD and Serra R: Scaffoldless
tissue-engineered cartilage for studying transforming growth factor
beta-mediated cartilage formation. Biotechnol Prog. 36:e28972020.
View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Goldring MB, Otero M, Plumb DA, Dragomir
C, Favero M, El Hachem K, Hashimoto K, Roach HI, Olivotto E, Borzì
RM and Marcu KB: Roles of inflammatory and anabolic cytokines in
cartilage metabolism: Signals and multiple effectors converge upon
MMP-13 regulation in osteoarthritis. Eur Cell Mater. 21:202–220.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Contentin R, Demoor M, Concari M, Desancé
M, Audigié F, Branly T and Galéra P: Comparison of the chondrogenic
potential of mesenchymal stem cells derived from bone marrow and
umbilical cord blood intended for cartilage tissue engineering.
Stem Cell Rev Rep. 16:126–143. 2020. View Article : Google Scholar : PubMed/NCBI
|