Introduction
Hepatocellular carcinoma (HCC) is a common malignant
tumor (1). HCC occurrence is
closely related to fibrosis and liver cirrhosis, which are mainly
caused by nonalcoholic fatty liver, drinking or viral infection.
After repeated exposure to risk factors, the liver experiences a
series of changes in cell proliferation and abnormal proliferation
and finally develops a malignant phenotype forming HCC (1,2).
High metastasis is an important biological characteristic of HCC
(1). With the continuous
improvement in treatment methods, the survival period of patients
has been improved to a certain degree. However, tumor invasion,
metastasis and postoperative recurrence are the main causes of
mortality of patients with HCC and exert substantial pressure and
burden on patients and their families (2). Therefore, in-depth study of the
mechanism of HCC metastasis and recurrence is very important for
the treatment of HCC.
The invasion and metastasis of HCC are closely
related to the signaling pathways that regulate the malignant
development of tumors (3).
Dysfunction of microRNAs (miRNAs) in HCC cells initiates the
related signaling pathways that promote tumor invasion and
metastasis (4). When miRNA is
loaded into an RNA-induced silencing complex (RISC), the miRNA
recognizes a binding in the target gene mRNA 3′-untranslated region
(UTR) through its seed sequence (nucleotides 2–8 from the 5′ end)
and thus, RISC plays a role in the regulation of gene expression
(5). There are three main effects
of miRNAs: Transcription inhibition, mRNA cleavage or degradation
(6). The function of miRNAs
mainly relies on the aforementioned methods to inhibit downstream
gene expression and weaken or eliminate the function of downstream
genes (7). Studies have shown
that the function of a signaling pathway is mainly exerted by
effectors and miRNAs can change the expression of key effectors in
a signaling pathway through the regulation of target genes, thereby
affecting the normal function of the signaling pathway (7,8).
This type of intracellular miRNA regulation of target genes is very
important for the function of tumor cell metastasis-related
signaling pathways and its mechanism is worthy of in-depth
study.
A previous study has demonstrated that miR-374c-5p
is widely involved in the malignant biological behavior of tumors
and plays an important role in tumor invasion and metastasis
(9). There are few studies on
miR-374c-5p with regard to tumors; while a few studies have
reported its biological function, a lack of in-depth research
remains on its specific mechanism, particularly in HCC. Its role in
HCC remains unknown. The present study aimed to clarify the
biological role of miR-374c-5p in HCC and to analyze its specific
mechanism. The results of the present study provided a scientific
and objective basis for understanding the mechanism of miR-374c-5p
in HCC.
Materials and methods
Tissue samples and related clinical
data
HCC tissue samples and matched normal adjacent
tissue samples from 100 patients were collected between May 2014
and November 2016 at the Department of Hepatobiliary Surgery,
Affiliated Hospital of North Sichuan Medical College (Nanchong,
China), and during surgical treatment (the etiology of HCC cases in
the present study is HBV, HCV and alcohol). The present study was
performed in accordance with the principles outlined in the
Declaration of Helsinki. The study was approved (approval no.
2014032) by the Human Trial Ethics Committee of the Affiliated
Hospital of North Sichuan Medical College (Nanchong, China).
Written informed consent was obtained from the patients who
provided the specimens. Each tumor tissue sample was snap-frozen in
liquid nitrogen and then stored at −80°C.
The detailed inclusion criteria were as follows: i)
HCC was confirmed by postoperative pathology and the
histopathological diagnosis was clear. Clinicopathological staging
was performed according to the 7th edition of tumor node metastasis
(TNM staging) system developed by the International Anticancer
Alliance and the American Cancer Society; ii) patients were
diagnosed with HCC for the first time without distant metastasis;
iii) patients had not received any treatment before surgery; iv)
complete follow-up information; v) no other serious self-malignant
disease; and vi) with clinical, pathological and surgical data.
The exclusion criteria were as follows: i) Those who
could not communicate normally; ii) patients with organic
dysfunction of the heart, brain, lung or kidney; iii) patients who
were diagnosed with HCC for the first time with distant metastasis;
iv) patients with incomplete clinical and pathological data; v)
patients with systemic diseases; and vi) patients with autoimmune
dysfunction.
Cell culture and transfection
HCC cell lines Huh-7 (cat. no. 1101HUM-PUMC000679),
MHCC-97H (cat. no. 4201PAT-CCTCC00404), MHCC-97L (cat. no.
CL-0497), Hep3B (cat. no. HB-8064) and normal hepatocyte THLE-2
(cat. no. CRL-2706) were purchased from the American Type Culture
Collection, Cell bank of China Typical Culture Preservation Center
(cat. no. MHCC-97H), Cell Resource Center, Institute of Basic
Medicine, Chinese Academy of Medical Sciences (cat. no. Huh-7) and
Procell Life Science & Technology Co., Ltd. (cat. no. MHCC-97L)
and cultured in high-glucose DMEM (Gibco; Thermo Fisher Scientific,
Inc.) containing 10% fetal bovine serum (FBS) (Gibco; Thermo Fisher
Scientific, Inc.) and 1% cyanidin/streptomycin double antibiotic at
37°C and 5% CO2.
miR-374c-5p mimic, miRNA negative control mimic
(miR-NC), pre-miR-374c-5p, control lentivirus were purchased from
Shanghai GeneChem, Inc. The lentivirus-mediated pre-miR-374c-5p in
Huh-7 was performed according to the manufacturer's protocol: 4 µl
of lentivirus titer (1×108 TU/ml) with pre-miR-374c-5p
was added to 1×106 Huh-7 cells at 37°C for 12 h and
three infection gradients (MOI=10, MOI=20, MOI=30). Then, the cells
were cultured in DMEM supplemented with 10% FBS, 1%
penicillin-streptomycin and 0.5 µg/ml puromycin to obtain stable
cell lines). miR-374c-5p inhibitor, miRNA-NC inhibitor, PTTG1
overexpression plasmid (pcDNA3.1-PTTG1) and empty pcDNA3.1 plasmid
were designed and synthesized by Shanghai GenePharma Co., Ltd. The
sequences were as follows: miR-374c-5p mimics,
5′-AUAAUACAACCUGCUAAGUGCU-3′; miR-NC, 5′-UUCUCCGAACGUGUCACGUTT-3′;
miR-374c-5p inhibitor, 5′-AGCACUUAGCAGGUUGUAUUAU-3′; and
NC-inhibitor, 5′-CAGUACUUUUGUGUAGUACAA-3′. HCC cells were seeded
into six-well plates (2×105 cells/well) and allowed to
settle overnight prior to transfection. miR-NC (50 nM) or
miR-374c-5p mimics (50 nM) or miR-374c-5p inhibitors (50 nM) were
transfected for 48 h at 37°C and Lipofectamine® 3000
(Thermo Fisher Scientific, Inc.) was used for transient
transfection. Cells were screened with 2 µg/ml puromycin. The
duration of each screening was 48 h at 37°C and was repeated 2
times. Then, cells were harvested for subsequent reverse
transcription-quantitative PCR (RT-qPCR) and western blot
analysis.
RT-qPCR
Total RNA including miRNAs was extracted from HCC
tissues, matched normal adjacent tissue and cell lines using
miRNeasy Mini kit (Qiagen China Co., Ltd.) according to the
manufacturer's protocol. Reverse transcription of cDNA was
performed using PrimeScript RT Reagent kit (Takara Bio, Inc.)
according to the manufacturer's protocol. In addition, qPCR was
performed with SYBR Premix Ex Taq II (Takara Bio, Inc.) and a Light
Cycler system (Roche Diagnostics GmbH). The following thermocycling
conditions for qPCR were used: Initial denaturation at 97°C for 10
min; and 40 cycles of denaturation at 92°C for 20 sec, annealing at
58°C for 15 sec, and extension at 75°C for 15 sec. The results were
analyzed using the 2−ΔΔCq method (10). The experiment was repeated three
times to verify the authenticity of the experimental results (each
sample was independently tested three times to verify the
experimental results). U6 was used as an internal control for
microRNA, and GAPDH was used for mRNA. Primers were designed using
Vazyme miRNA software (v1.01) (Vazyme Biotech Co., Ltd.). The
primers were as follows: miR-374c-5p RT primer,
5′-GTCGTATCGACTGCAGGGTCCGAGGTATTCGCAGTCGATACGACAGCACT-3′;
miR-374c-5p forward, 5′-ATAATACAACCTGCTAAGTGC-3′ and reverse,
5′-ACTGCAGGGTCCGAGGTATT-3′; U6 forward,
5′-CTCGCTTCGGCAGCACATATACT-3′ and reverse,
5′-ACGCTTCACGAATTTGCGTGTC-3′; and GAPDH forward,
5′-GGGTGGTGCAAAGAGAGTCA-3′ and reverse,
5′-GCAGGAGGCATTGCTTACAAC-3′.
Western blotting
Total protein from HCC tissues, matched normal
adjacent tissue and cell lines (1×106 cells/well) was
extracted using RIPA lysis buffer (Beyotime Institute of
Biotechnology). Protein was quantified using a Bradford protein
assay (Bio-Rad Laboratories, Inc.) and a Nanodrop
spectrophotometer. An equal amount (25 µg) of protein was added to
each well of 10% gels and resolved via SDS-PAGE. Proteins were
transferred to PVDF membranes and were blocked by incubation for 1
h at 37°C with 5% non-fat powdered milk. The proteins on PVDF
membranes were incubated with the following primary antibodies:
PTTG1 (1:1,000; product code ab26273), N-cadherin (1:5,000; product
code ab76011), vimentin (1:2,000; product code ab92547), Ki-67
(1:5,000; product code ab16667), E-cadherin (1:10,000; product code
ab1416) and GAPDH (1:5,000; product code ab8245; all from Abcam)
overnight at 4°C. Then, the membranes were incubated with the
appropriate HRP-conjugated secondary antibodies (1:5,000; cat. no.
ab6721; Abcam and 1:5,000; cat. no. ab205719; Abcam) at room
temperature for 1 h. Finally, immunoreactive protein bands were
visualized using an enhanced chemiluminescence solution
(MilliporeSigma) and a ChemiDoc Imaging system (Bio-Rad
Laboratories, Inc.). Protein expression was semi-quantified using
Quantity One version 4.6 software (Bio-Rad Laboratories, Inc.).
Immunohistochemistry
The sample was fixed in 10% formalin for 12 h at
room temperature. The sample (4 µm) was dehydrated with xylene and
a descending series of ethanol solutions (100, 100, 95 and 80%).
Antigen retrieval was performed in 10 mmol/l sodium citrate
solution (pH 6) at 100°C for 15 min and the samples were cooled for
30 min. All slides were incubated with 5% goat serum (OriGene
Technologies, Inc.) for 15 min at room temperature to block
non-specific binding at room temperature. The slides were incubated
with antibodies against Ki-67 (1:500; product code ab92742; Abcam)
at 4°C overnight. Subsequently, the samples were incubated with
biotinylated secondary antibody (1:100; cat. no. SAP-9100; OriGene
Technologies, Inc.) at 37°C for 30 min then stained with
3,3-diaminobenzidine (DAB; cat. no. K5007; Dako; Agilent
Technologies, Inc.) at room temperature for 10 sec and Mayer's
hematoxylin. The membranes were then washed with phosphate-buffered
saline (PBS) and stained with DAB for 30 sec at room temperature.
Slides were visualized using a light microscope (magnification,
×400; Zeiss AG).
miRNA prediction
TargetScan (version 7.1; http://www.targetscan.org), Oncomir (http://www.oncomir.org/) and miRWalk (http://mirwalk.umm.uni-heidelberg.de/),
were used to predict target genes and conserved sites bound by
miR-374c-5p.
Luciferase reporter assay
TargetScan was used to identify downstream target
genes of miR-374c-5p. The wild-type (WT) PTTG1 3′-UTR and mutant
(MUT) PTTG1 3′-UTR oligonucleotides containing the putative binding
site for miR-374c-5p were cloned into the firefly
luciferase-expressing pMIR-REPORT vector (Obio Technology Corp.,
Ltd.). These constructs were co-transfected using
Lipofectamine® 3000 (Thermo Fisher Scientific, Inc.)
with miR-374c-5p mimics, miR-NC mimics, miR-374c-5p inhibitor and
miRNA-NC inhibitor into Huh-7 cells. After 48 h of transfection,
luciferase activity was determined using a Dual-Luciferase Reporter
Assay kit (Promega Corporation) according to the manufacturer's
protocol. The ratio of Renilla luciferase activity to
firefly luciferase activity was calculated. The firefly luciferase
activity was normalized to the Renilla luciferase
activity.
Cell Counting Kit 8 (CCK-8)
Huh-7 and Hep3B cells were seeded into 96-well
plates (1×103 cells/well) and incubated at 37°C for 24,
48 or 72 h. Then, CCK-8 reagent (10 µl; Dojindo Molecular
Technologies, Inc.) was added to each well and plates were
incubated at 37°C for 2 h. The absorbance was measured at 450 nm by
a spectrophotometer (Bio-Rad Laboratories, Inc.).
5-Ethynyl-2′-deoxyuridine (EdU)
assay
Huh-7 and Hep3B cells were seeded into 96-well
plates (2.5×104 cells/well) and incubated at 37°C until
the cells reached 30% confluence. Next, the cells were incubated
with EdU (50 µM) for 2 h, 0.5% Triton X-100 and ApolloR reaction
cocktail (100 µl) for 30 min and 100 µl Hoechst 33342 for 30 min,
sequentially. Cell proliferation was analyzed by assessing the
percentage of EdU-positive cells in all cells in each sample using
a fluorescence microscope (Lionheart; BioTek Instruments, Inc.;
magnification, ×100) and ImageJ software (version 1.8.0; National
Institutes of Health) was used for cell counting.
Wound healing assay
Cell migration was examined using wound healing
assays. Briefly, Huh-7 and Hep3B cells (5×105
cells/well) were cultured in six-well plates. When the cells
reached confluence (90–100%), any non-adherent cells were washed
away with PBS. The cell monolayer was scratched with a pipette tip
(10 µl) to generate 3 scratch wounds and then rinsed with PBS to
remove non-adherent cells, and the medium was replaced with fresh
DMEM high glucose medium without FBS. After 0 and 48 h, the
distance between the wound edges was measured. The wells were
imaged using an inverted microscope (Olympus Corporation) at ×100
magnification. ImageJ v1.8.0 (National Institutes of Health)
software was used for analysis.
Matrigel invasion assays
For the invasion assay, Huh-7 and Hep3B cells were
resuspended in high-glucose DMEM containing 1% FBS and seeded into
the upper Transwell chamber at a density of 1×105
cells/well. Transwell inserts (8 µm pore size) (Corning, Inc.) were
precoated with Matrigel (1 mg/ml) at 37°C for 30 min. A total of
500 µl of high-glucose DMEM containing 10% FBS was added to the
matched lower chamber. After 36 h of incubation, the Transwell
chambers were fixed with 4% paraformaldehyde at room temperature
for 30 min and stained with 0.5% crystal violet at room temperature
for 20 min. The number of stained cells randomly selected from six
fields was counted and images were captured under a light
microscope (magnification, ×200; Olympus Corporation).
Flow cytometric analysis of cell
apoptosis
Huh-7 and Hep3B cells (1×105 cells/well)
were seeded into six-well plates. Once they reached confluence
(70–80%), the cells were collected (300 × g for 3 min at room
temperature) and incubated with Annexin V-FITC (5 µl) and propidium
iodide solution (5 µl; Biogot Technology Co., Ltd.) at room
temperature for 15 min according to the manufacturer's
instructions. Cells were subsequently suspended in 500 µl binding
buffer. Cell apoptosis progression was analyzed using flow
cytometry (FACSAria; BD Biosciences). All data were analyzed with
ModFit version 4.0 (Verity Software House, Inc.)
Tumorigenesis assay
The 5-week-old male BALB/c-nu mice (total number,
10; n=5 in each group; age, 5 weeks; weight, 20–25 g) were
purchased from the Shanghai Experimental Animal Center (Shanghai,
China) and were kept at the Animal Center of Affiliated Hospital of
North Sichuan Medical College at 25°C and 40–70% humidity, with a
12-h light/dark cycle and free access to food and water. All animal
experiments were approved by the Animal Care Ethics Review
Committee of Affiliated Hospital of North Sichuan Medical College,
approval no 20201026) and the method of euthanasia was cervical
dislocation (when the heart stopped completely, the mouse was
determined as dead). Body weight loss >20% was assumed to be a
humane endpoint for euthanasia. For the in vivo tumor growth
assay, 1×106 Huh-7 cells were resuspended with sterile
PBS, infected with the pre-miR-374c-5p or control lentivirus and
were subcutaneously injected into the flank of each nude mouse.
Tumor sizes (the length and width of tumor nodules) were measured
every 5 days. At 30 days post-injection, the mice were
anaesthetized with pentobarbital sodium (100 mg/kg body weight) and
sacrificed by cervical dislocation. The tumor volume calculation
formula was as follows: Volume=(length × width2)/2
(11).
Statistical analyses
All quantitative data are presented as the mean ± SD
of at least three independent experiments. Statistical data were
analyzed using SPSS version 20.0 software (IBM Corp.) and GraphPad
Prism version 6.0 software (GraphPad Software, Inc.). Differences
between groups were analyzed using unpaired Student's t-test or
one-way ANOVA followed by Tukey's post hoc test. The Kaplan-Meier
method was used to assess overall survival (OS) and
disease-specific survival (DSS) and log-rank test was used to
analyze differences between the curves. Associations between
clinicopathological parameters and miR-374c-5p expression were
analyzed using Pearson's chi-squared test. P<0.05 was considered
to indicate a statistically significant difference.
Results
miR-374c-5p expression is
downregulated in HCC and related cells
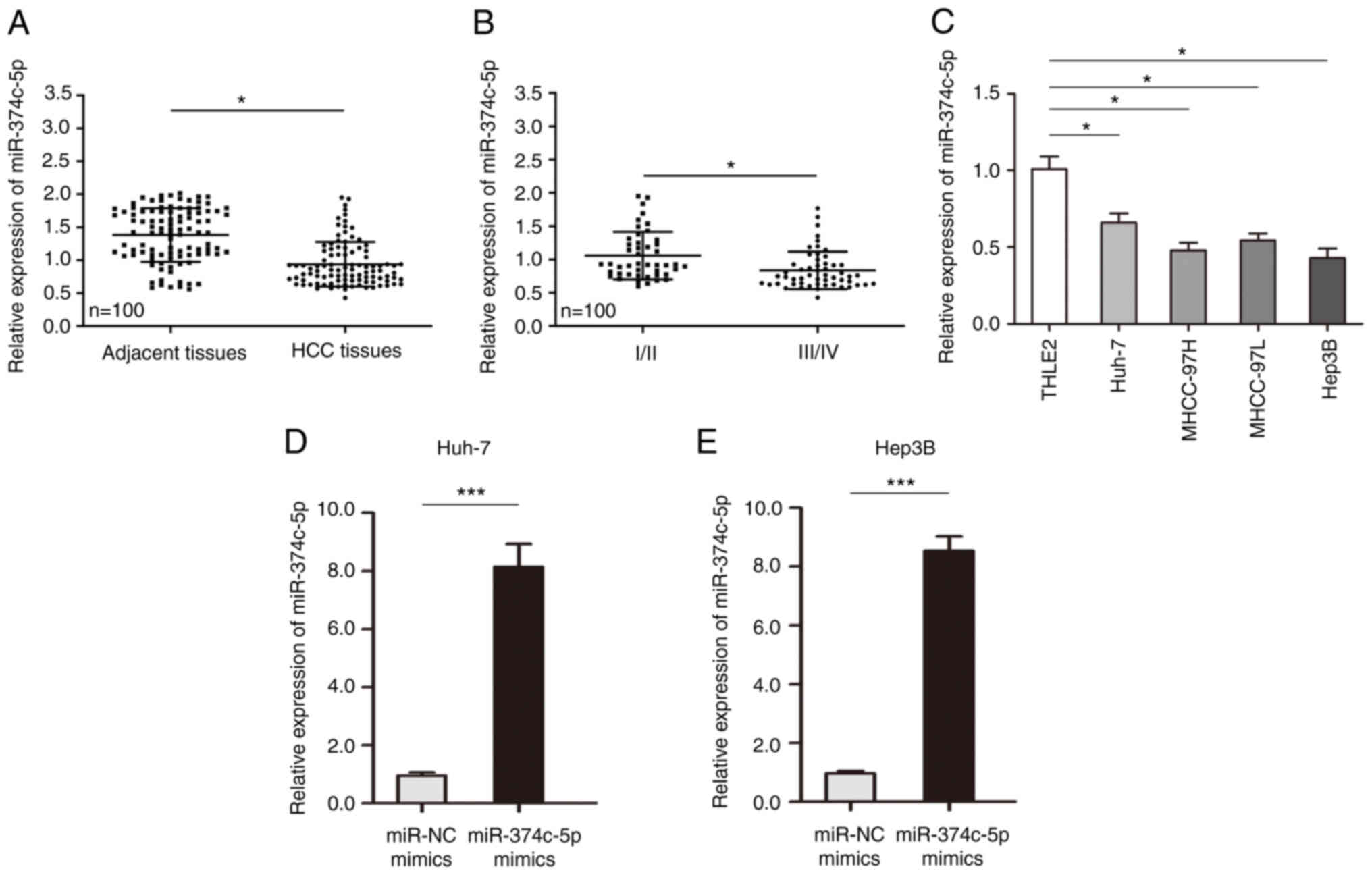
First, the expression of miR-374c-5p in HCC was
analyzed and the results revealed that the expression of
miR-374c-5p in HCC was lower than in matched adjacent tissue
samples (Fig. 1A; P<0.05). The
expression of miR-374c-5p was lower in patients with later stage
HCC (Fig. 1B; P<0.05). In HCC
cell lines, the expression of miR-374c-5p was significantly lower
than that in normal liver cell line THLE-2 (Fig. 1C; P<0.05). To evaluate whether
miR-374c-5p is involved in the malignant biological behavior of
HCC, two HCC cell lines (Huh-7 and Hep3B) were transfected with
miR-374c-5p mimic and the expression level of miR-374c-5p in Huh-7
and Hep3B cells was successfully increased (Fig. 1D and E; P<0.001).
miR-374c-5p is associated with poor
prognosis in HCC
The average value of relative miR-374c-5p expression
was used to divide the patients into ‘high’ or ‘low’ groups.
miR-374c-5p expression was low in 69 out of 100 HCC samples (69%)
and high in 31 out of 100 HCC samples (31%) and miR-374c-5p
expression was positively associated to TNM stage (P=0.040) and
multiplicity (P=0.041) and miR-374c-5p was not found to be
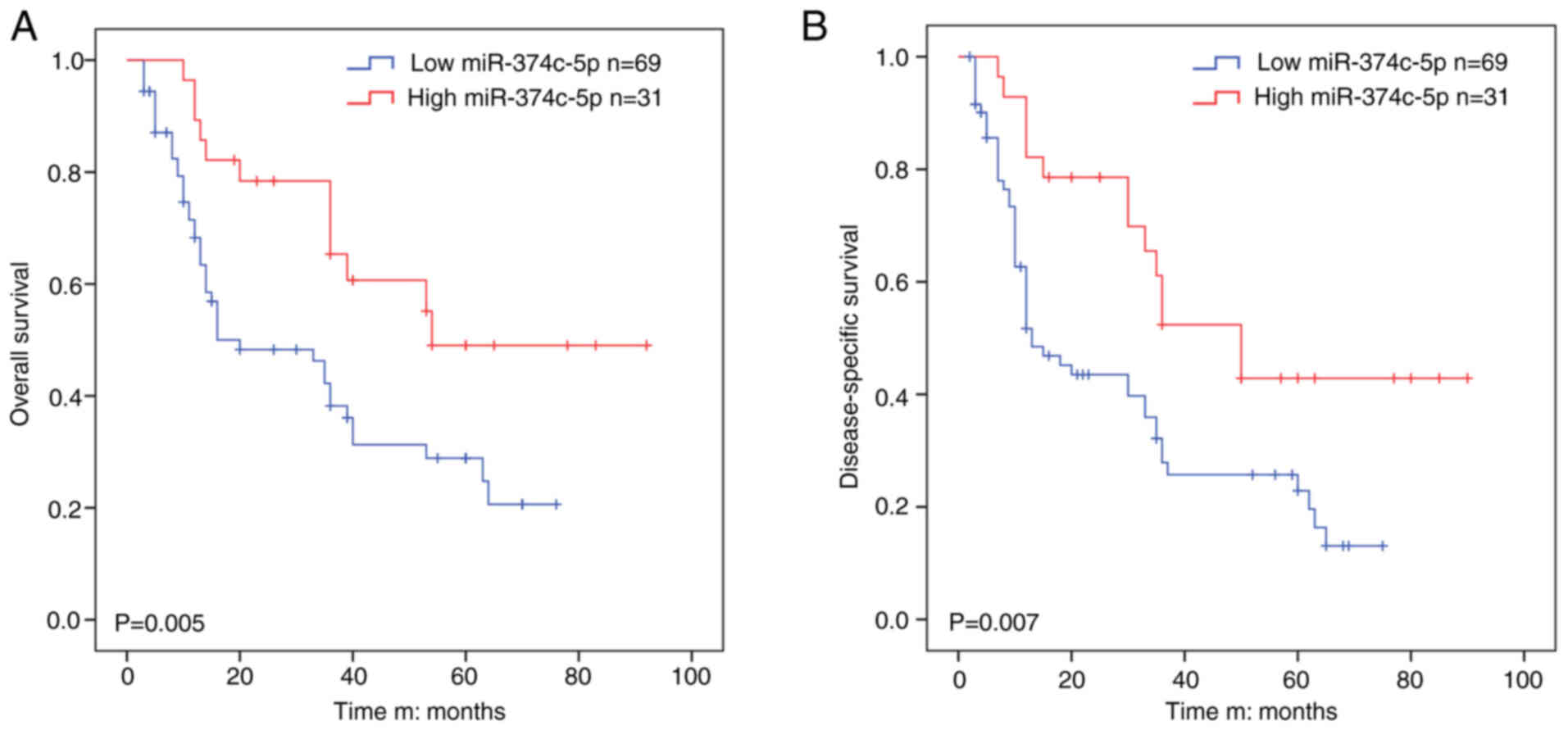
associated with liver cirrhosis (Table I). Kaplan-Meier survival analysis
revealed that HCC patients with low miR-374c-5p expression had
significantly shorter OS than those with high miR-374c-5p
expression in (Fig. 2A; P=0.005).
Univariate analysis showed that TNM stage (HR, 1.361; P=0.021) and
miR-374c-5p expression (HR, 1.336; P=0.011) were significantly
associated with OS in HCC (Table
II). Multivariate analysis demonstrated that TNM stage (HR,
1.482; P=0.020) and miR-374c-5p expression (HR, 1.450; P=0.015)
were independent prognostic factors for OS in HCC (Table II).
 | Table I.Associations between miR-374c-5p and
clinicopathological features of patients with hepatocellular
carcinoma. |
Table I.
Associations between miR-374c-5p and
clinicopathological features of patients with hepatocellular
carcinoma.
|
|
| Expression level of
miR-374c-5p |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
characteristics | Cases | Low (n=69) | High (n=31) | P-value |
|---|
| Sex |
|
|
| 0.875 |
|
Male | 56 | 39 | 17 |
|
|
Female | 44 | 30 | 14 |
|
| Age, years |
|
|
| 0.628 |
|
<50 | 48 | 32 | 16 |
|
|
≥50 | 52 | 37 | 15 |
|
| AFP (ng/ml) |
|
|
| 0.728 |
|
≤20 | 38 | 27 | 11 |
|
|
>20 | 62 | 42 | 20 |
|
| HBsAg |
|
|
| 0.878 |
|
Positive | 72 | 50 | 22 |
|
|
Negative | 28 | 19 | 9 |
|
| TNM stage |
|
|
| 0.04 |
|
I/II | 46 | 27 | 19 |
|
|
III/IV | 54 | 42 | 12 |
|
| Tumor size
(cm) |
|
|
| 0.146 |
| ≤5 | 43 | 33 | 10 |
|
|
>5 | 57 | 36 | 21 |
|
| Multiplicity |
|
|
| 0.041 |
|
Single | 43 | 25 | 18 |
|
|
Multiple (≥2) | 57 | 44 | 13 |
|
| Intrahepatic
metastasis |
|
|
| 0.935 |
|
Presence | 49 | 34 | 15 |
|
|
Absence | 51 | 35 | 16 |
|
| Child-Pugh |
|
|
| 0.621 |
| A | 45 | 30 | 15 |
|
| B | 39 | 29 | 10 |
|
| C | 16 | 10 | 6 |
|
 | Table II.Univariate and multivariate analysis
of different prognostic variables influencing overall survival in
patients with hepatocellular carcinoma. |
Table II.
Univariate and multivariate analysis
of different prognostic variables influencing overall survival in
patients with hepatocellular carcinoma.
|
|
| Univariate
analysis | Multivariate
analysis model |
|---|
|
|
|
|
|
|---|
| Clinicopathological
characteristics | n | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Sex |
| 0.63
(0.563-1.398) | 0.607 |
|
|
|
Male | 56 |
|
|
|
|
|
Female | 44 |
|
|
|
|
| Age, years |
| 0.73
(0.483-1.032) | 0.481 |
|
|
|
<50 | 48 |
|
|
|
|
|
≥50 | 52 |
|
|
|
|
| AFP (ng/ml) |
| 1.203
(1.318-3.601) | 0.63 |
|
|
|
≤20 | 38 |
|
|
|
|
|
>20 | 62 |
|
|
|
|
| HBsAg |
| 1.218
(1.003-2.984) | 0.505 |
|
|
|
Positive | 72 |
|
|
|
|
|
Negative | 28 |
|
|
|
|
| TNM stage |
| 1.361
(0.946-2.036) | 0.021 | 1.482
(1.003-3.458) | 0.02 |
|
I/II | 46 |
|
|
|
|
|
III/IV | 54 |
|
|
|
|
| Tumor size
(cm) |
|
|
|
|
|
| ≤5 | 43 |
|
|
|
|
|
>5 | 57 |
|
|
|
|
| Multiplicity |
| 0.609
(0.637-1.669) | 0.463 |
|
|
|
Single | 43 |
|
|
|
|
|
Multiple (≥2) | 57 |
|
|
|
|
| Intrahepatic
metastasis |
| 0.366
(0.691-1.238) | 0.556 |
|
|
|
Presence | 49 |
|
|
|
|
|
Absence | 51 |
|
|
|
|
| Child-Pugh |
| 0.861
(0.567-1.3309) | 0.433 |
|
|
| A | 45 |
|
|
|
|
| B | 39 |
|
|
|
|
| C | 16 |
|
|
|
|
| miR-374c-5p
expression |
| 1.336
(0.894-1.739) | 0.011 | 1.45
(0.963-2.003) | 0.015 |
|
Low | 69 |
|
|
|
|
|
High | 31 |
|
|
|
|
Kaplan-Meier survival analysis revealed that low
miR-374c-5p expression had significantly shorter DSS than those
with high miR-374c-5p expression (Fig. 2B; P=0.007). Univariate analysis
showed that TNM stage (HR, 1.421; P=0.011) and miR-374c-5p
expression (HR, 1.033; P=0.022) were significantly associated with
DSS in HCC (Table III).
Multivariate analysis revealed that TNM stage (HR, 1.506; P=0.015)
and miR-374c-5p expression (HR, 1.047; P=0.024) were independent
prognostic factors for DSS in HCC (Table III).
 | Table III.Univariate and multivariate analysis
of different prognostic variables influencing disease-specific
survival in patients with hepatocellular carcinoma. |
Table III.
Univariate and multivariate analysis
of different prognostic variables influencing disease-specific
survival in patients with hepatocellular carcinoma.
|
|
| Univariate
analysis | Multivariate
analysis model |
|---|
|
|
|
|
|
|---|
| Clinicopathological
characteristics | n | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Sex |
| 0.438
(0.601-1.266) | 0.552 |
|
|
|
Male | 56 |
|
|
|
|
|
Female | 44 |
|
|
|
|
| Age, years |
| 0.579
(0.683-1.531) | 0.773 |
|
|
|
<50 | 48 |
|
|
|
|
|
≥50 | 52 |
|
|
|
|
| AFP (ng/ml) |
| 1.396
(1.435-3.669) | 0.707 |
|
|
|
≤20 | 38 |
|
|
|
|
|
>20 | 62 |
|
|
|
|
| HBsAg |
| 1.007
(1.047-3.607) | 0.633 |
|
|
|
Positive | 72 |
|
|
|
|
|
Negative | 28 |
|
|
|
|
| TNM stage |
| 1.421
(0.681-2.614) | 0.011 | 1.506
(0.763-2.964) | 0.015 |
|
I/II | 46 |
|
|
|
|
|
III/IV | 54 |
|
|
|
|
| Tumor size
(cm) |
|
|
|
|
|
| ≤5 | 43 |
|
|
|
|
|
>5 | 57 |
|
|
|
|
| Multiplicity |
| 1.223
(0.537-1.336) | 0.72 |
|
|
|
Single | 43 |
|
|
|
|
|
Multiple (≥2) | 57 |
|
|
|
|
| Intrahepatic
metastasis |
| 0.743
(0.631-1.576) | 0.607 |
|
|
|
Presence | 49 |
|
|
|
|
|
Absence | 51 |
|
|
|
|
| Child-Pugh |
| 0.438
(0.364-1.483) |
|
|
|
| A | 45 |
|
|
|
|
| B | 39 |
|
|
|
|
| C | 16 |
|
|
|
|
| miR-374c-5p
expression |
| 1.033
(0.537-1.209) | 0.022 | 1.047
(0.673-1.557) | 0.024 |
|
Low | 69 |
|
|
|
|
|
High | 31 |
|
|
|
|
Increasing the expression of
miR-374c-5p inhibits the proliferation, invasion, migration and
promotes the apoptosis of HCC in vitro
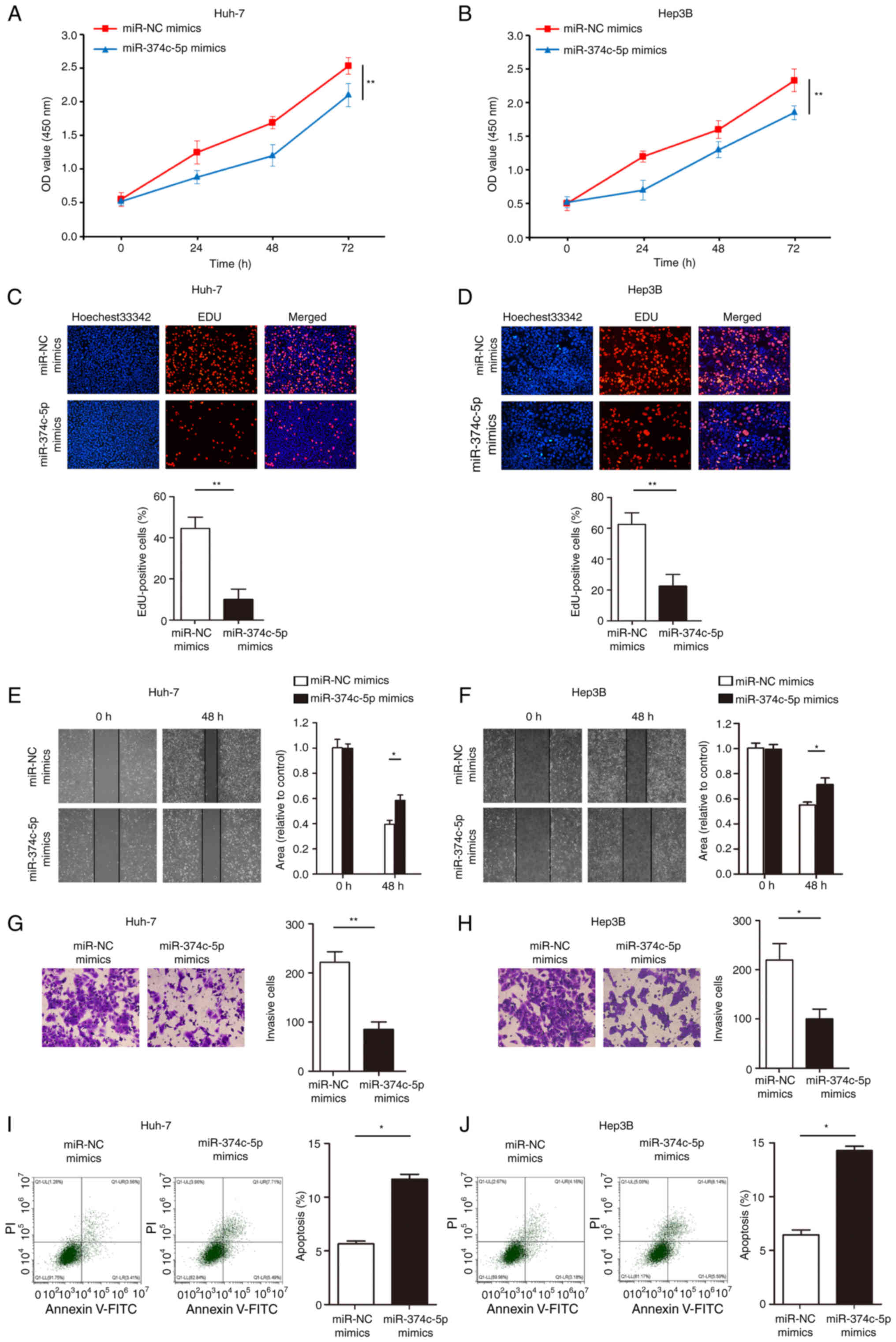
Cell proliferation ability was then assessed with
CCK-8 assays and the results revealed that overexpression of
miR-374c-5p significantly inhibited Huh-7 and Hep3B cell
proliferation (Fig. 3A and B;
P<0.01). The number of Huh-7 and Hep3B EdU-positive cells in the
miR-374c-5p overexpression group was less than in the control group
(Fig. 3C and D; P<0.01).
Furthermore, overexpression of miR-374c-5p significantly inhibited
Huh-7 and Hep3B cell migration and invasion (Fig. 3E-H; P<0.05). In addition,
transfection with miR-374c-5p mimics significantly promoted Huh-7
and Hep3B cell apoptosis (Fig. 3I and
J; P<0.05).
PTTG1 is a direct target gene of
miR-374c-5p in HCC
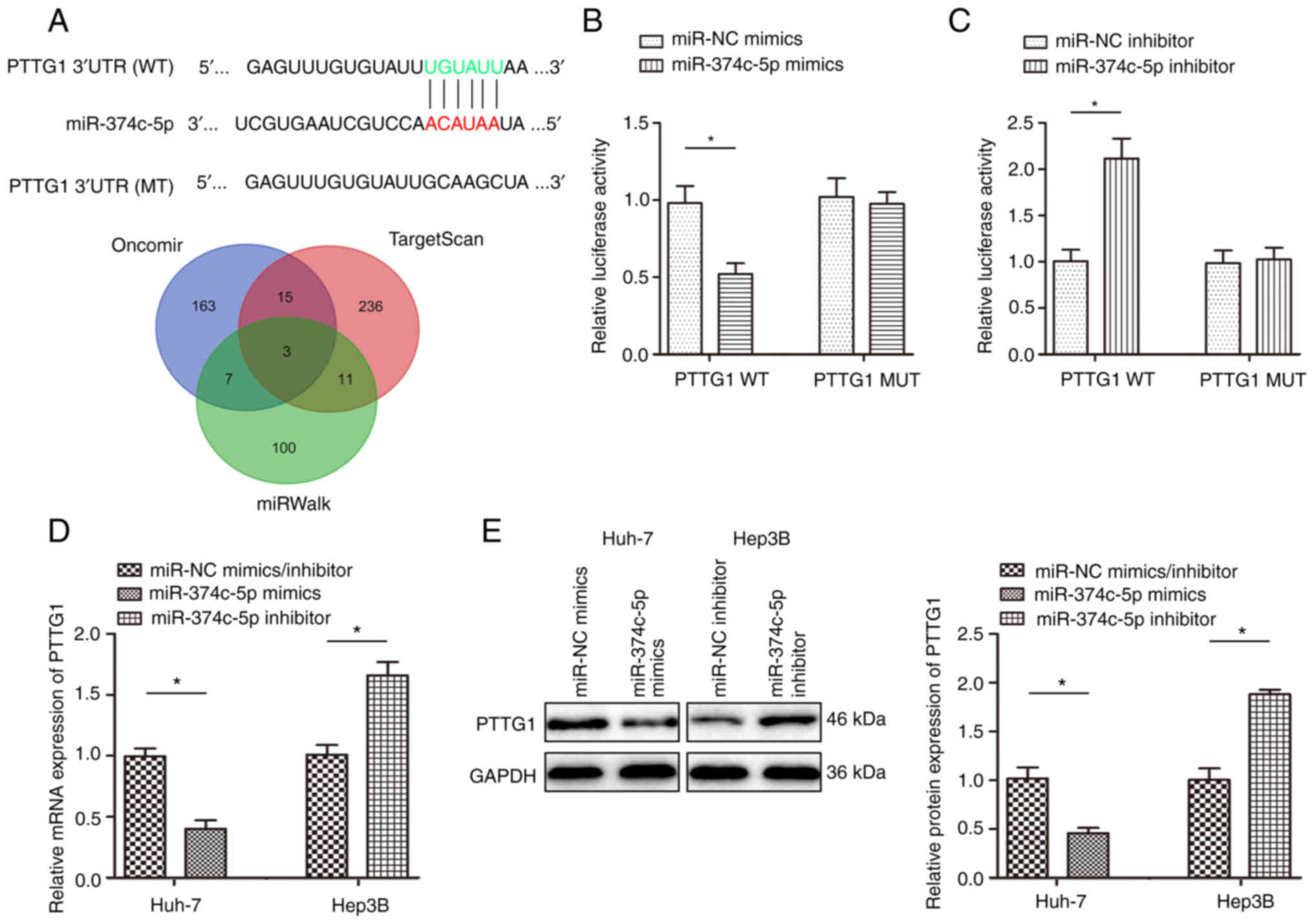
A total of 3 miRNA databases identified PTTG1 as a
potential target of miR-374c-5p and the complementary sequence of
miR-374c-5p was found in the 3′-UTR of PTTG1 mRNA (Fig. 4A). Luciferase reporter vectors
containing the WT or MUT PTTG1 3′-UTR sequence were used to reveal
the interaction of miR-374c-5p with PTTG1. The results demonstrated
that co-transfection with miR-374c-5p mimics significantly
inhibited luciferase activity in cells transfected with WT PTTG1
3′-UTR (Fig. 4B; P<0.05).
Additionally, co-transfection with miR-374c-5p inhibitor
significantly promoted luciferase activity in cells transfected
with WT PTTG1 3′-UTR (Fig. 4C;
P<0.05). Huh-7 and Hep3B cells were transfected with miR-374c-5p
inhibitor and the expression level of miR-374c-5p was successfully
decreased in Huh-7 and Hep3B (Fig.
S1; P<0.05).
RT-qPCR and western blot assays revealed that
miR-374c-5p overexpression significantly reduced PTTG1 mRNA and
protein expression and inhibiting the expression of miR-374c-5p
promoted PTTG1 mRNA and protein expression (Fig. 4D and E; P<0.05).
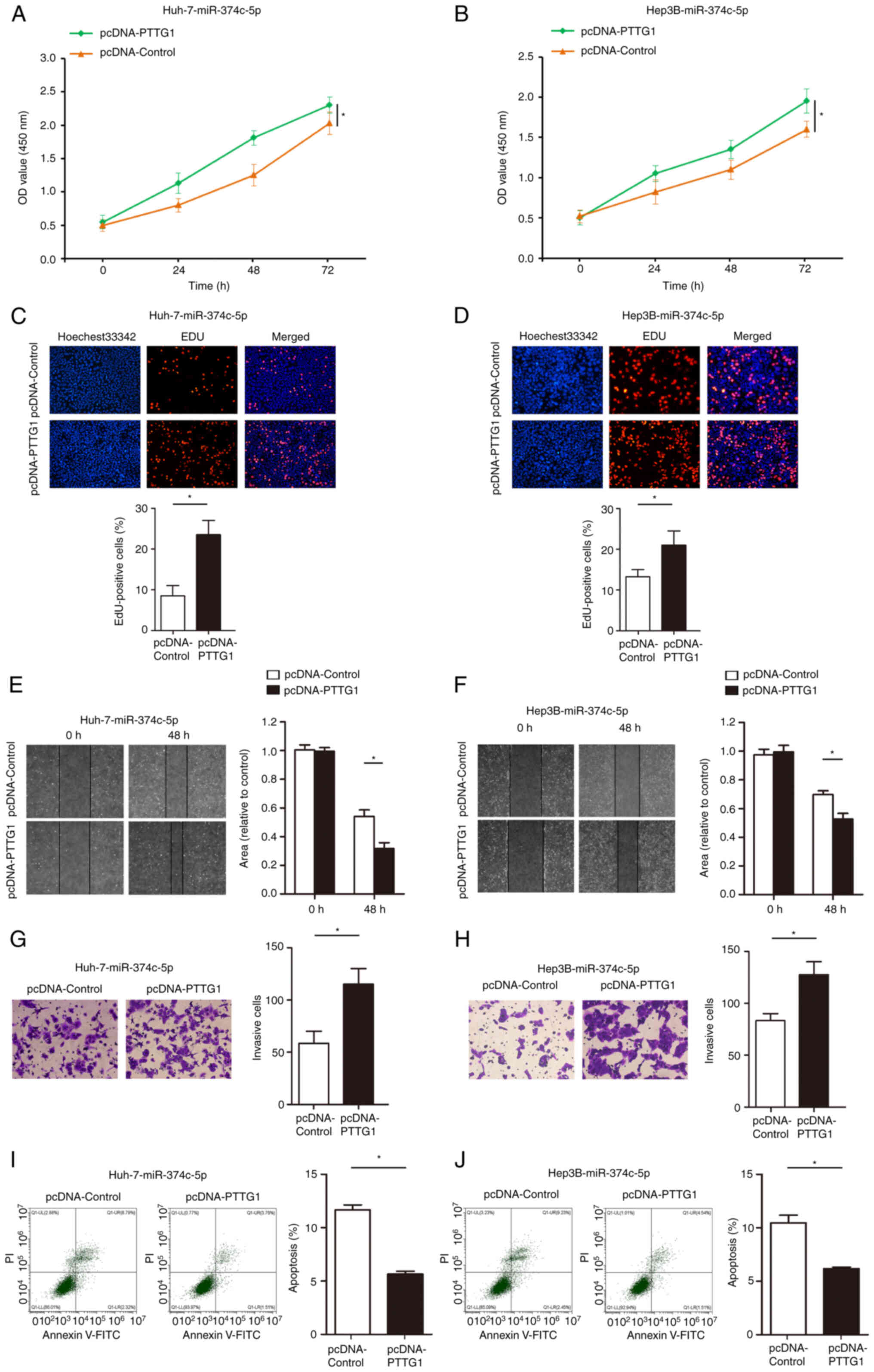
PTTG1 abolishes the inhibitory effect
of miR-374c-5p on HCC proliferation, migration, invasion and its
promoting effect on apoptosis
To determine whether PTTG1 is a direct target gene
of miR-374c-5p, a loss-of-function experiment was performed on
pre-miR-374c-5p-Huh-7 and pre-miR-374c-5p-Hep3B cells. CCK-8 assays
revealed that restoration of PTTG1 expression significantly
abolished the inhibitory effects of miR-374c-5p on proliferation
(Fig. 5A and B; P<0.05). As
demonstrated in Fig. 5C and D,
restoration of PTTG1 expression significantly abolished the
inhibitory effects of miR-374c-5p on EdU incorporation.
Furthermore, restoration of PTTG1 expression significantly
abolished the inhibitory effects of miR-374c-5p on migration and
invasion (Fig. 5E-H; P<0.05).
In addition, restoration of PTTG1 expression significantly
abolished the promotion of Huh-7 and Hep3B cell apoptosis (Fig. 5I and J; P<0.05).
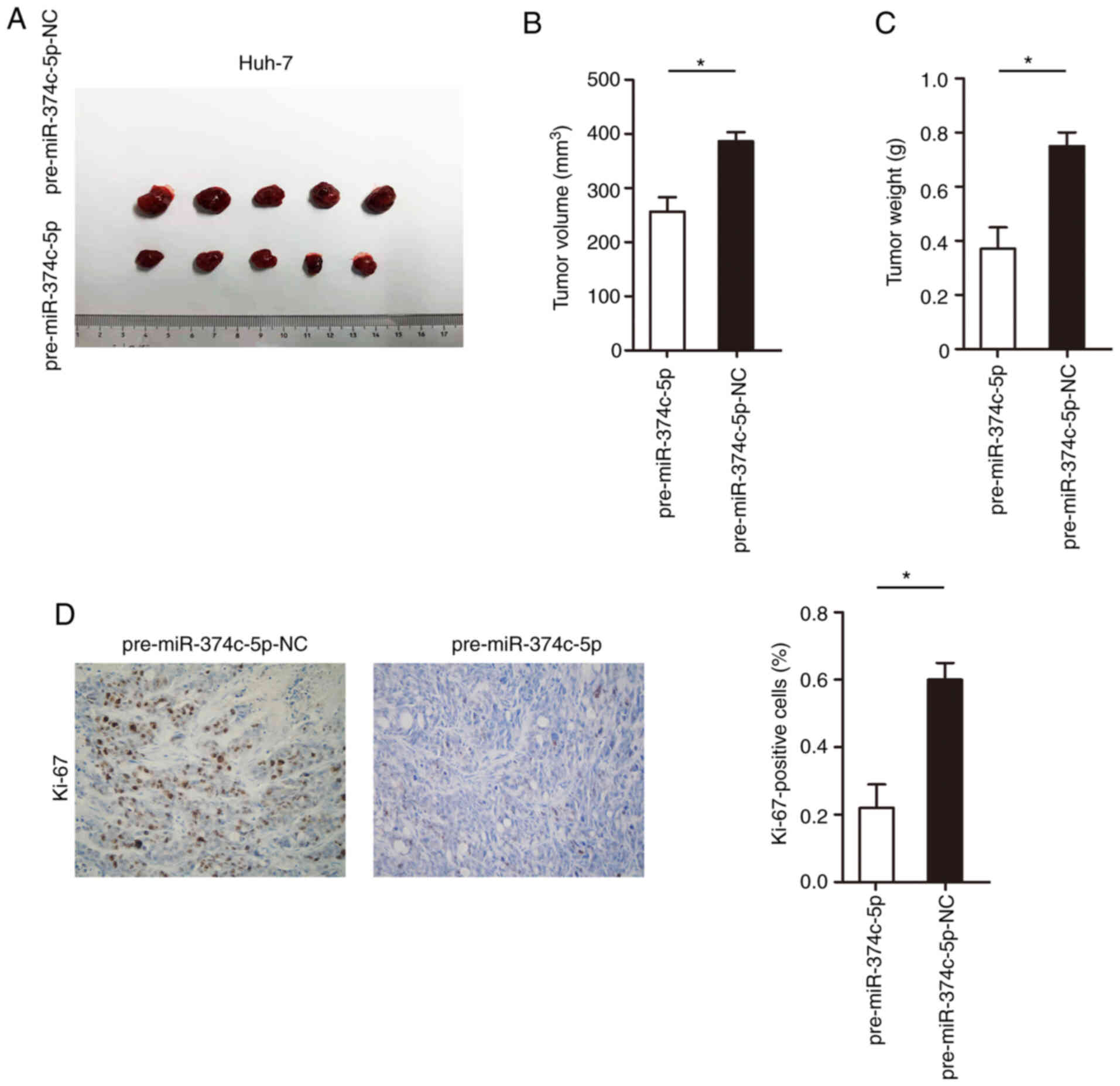
Increasing the expression of
miR-374c-5p significantly inhibits the proliferation of HCC in
vitro
The influence of miR-374c-5p on proliferation in
vivo was further identified; Huh-7 cells were stably
transfected with miR-374c-5p overexpression vector and then
injected into the flanks of nude mice to form subcutaneous ectopic
tumors. Huh-7 cells were transfected with pre-miR-374c-5p and the
expression level of miR-374c-5p was successfully increased in Huh-7
cells (Fig. S2; P<0.001). As
revealed in Fig. 6A-C, increasing
the expression of miR-374c-5p significantly decreased the tumor
volume and weight (P<0.05). In addition, increasing the
expression of miR-374c-5p significantly inhibited the expression of
Ki-67 in tumor tissues (Fig. 6D;
P<0.05).
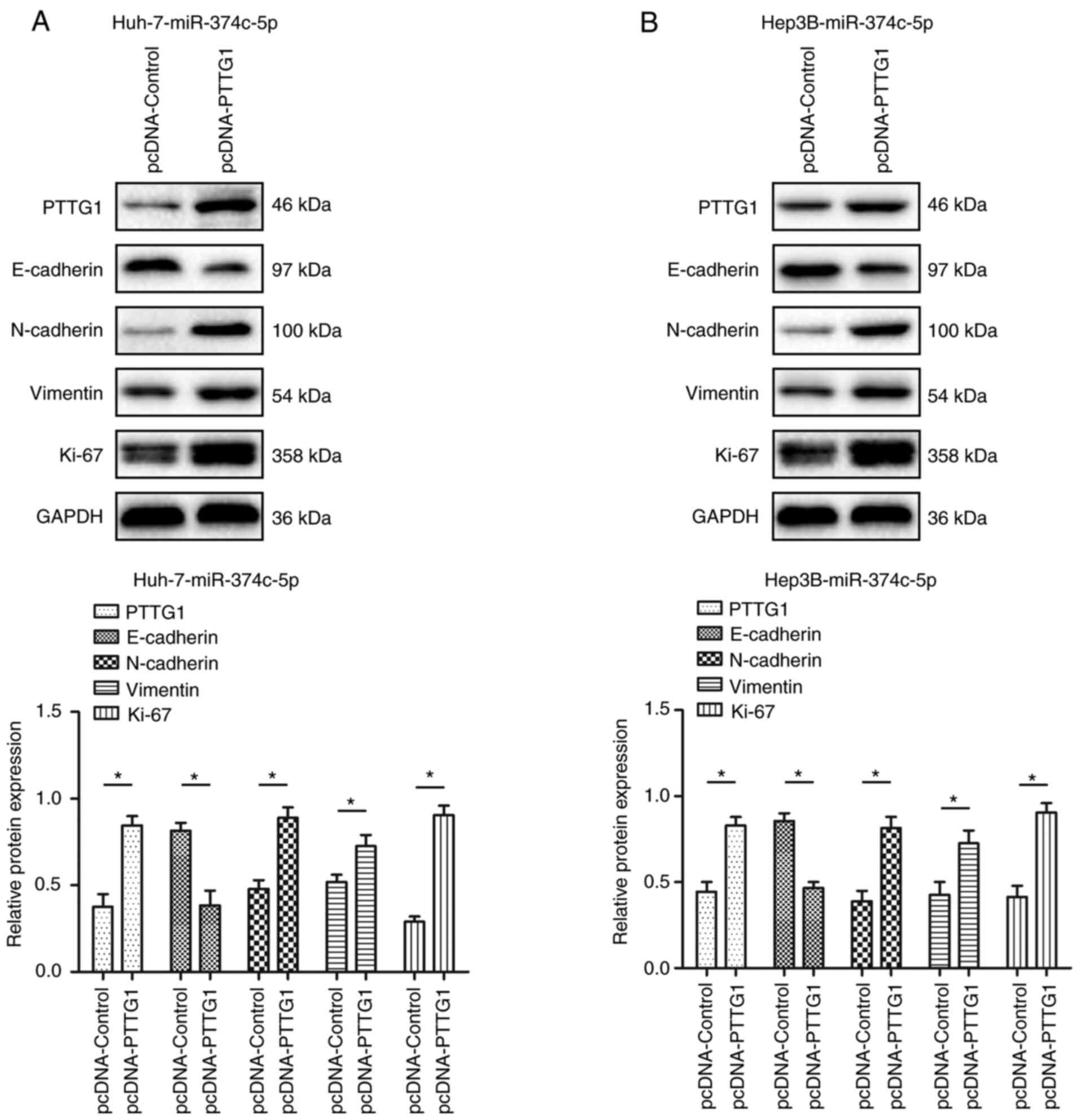
miR-374c-5p regulates
epithelial-mesenchymal transition (EMT) through PTTG1
After restoring the expression of PTTG1, the
expression of N-cadherin, vimentin and Ki-67 was restored to a
certain extent. The expression of E-cadherin, was inhibited to a
certain extent (Fig. 7;
P<0.05).
Discussion
Cancer is a complex disease involving multiple genes
and pathways (12). Therefore,
cancer treatment targeting a single gene is not very useful. One
miRNA can regulate the expression of hundreds of genes involved in
different cell functions, making miRNA a promising therapeutic
target (13). Since the first
report of the abnormal regulation of miRNAs in cancer in 2002
(14), numerous studies have
found that miRNAs play a critical role in tumorigenesis, including
tumor suppressor miRNAs (ts-miRs) and oncogene miRNAs (onco-miRs)
(14,15). Upregulated onco-miRs and
downregulated ts-miRNAs lead to the occurrence and development of
tumors (15). These miRNAs have
attracted widespread attention as biomarkers and therapeutic
targets.
miRNAs are small noncoding RNAs involved in the
regulation of gene expression and currently there are 1,917 human
genome miRNA entries (16). The
biosynthesis of miRNAs is a rapid process. Studies on
Drosophila have shown that the biosynthesis of miRNAs is the
fastest among transcripts (16,17). Similar to genes encoding proteins,
miRNAs may have independent transcriptional regulatory units, but
they are often located within introns of host genes, suggesting
coregulation of transcription (18). However, the expression of miRNAs
is not necessarily related to the level of their host genes.
Compared with their host genes, numerous intronic miRNAs have
independent transcription start sites and are controlled by
different promoters and/or other regulatory sequences (19). For example, super-enhancers
involved in cell characteristics can drive both the transcription
and processing of miRNAs (20).
Interestingly, super-enhancers are also dysregulated in human
cancers, and may at least partially explain the abnormal expression
of certain oncogenic miRNAs (20).
miR-374c-5p has been reported to be involved in the
malignant progression of a variety of tumors. For example, the
expression of miR-374c-5p was significantly low in cervical cancer
and increasing miR-374c-5p expression significantly improved the
invasion and migration abilities of cervical cancer cells (21). In addition, miR-374c-5p inhibited
Snail by directly targeting FOXC1 and inhibiting FOXC1 expression
(21). In breast cancer, low
miR-374c-5p expression promoted the proliferation, migration and
EMT of breast cancer cells (22).
MiR-374c-5p interacted with TAF7 and downregulated its expression
(22). In addition, miR-374c-5p
regulated DEP domain containing 1 (DEPDC1) by mediating TAF7.
Finally, miR-374c-5p inhibited breast cancer development through
TAF7-mediated DEPDC1 transcriptional regulation (22). Based on literature analysis, it
was found that miR-374c-5p can significantly regulate downstream
target genes and related signaling pathways in HCC and regulate the
malignant progression of HCC by affecting factors related to tumor
proliferation and metastasis. The effect of miR-374c-5p on the
prognosis of HCC may involve the expression of gene transcription
factors and N6-methyladenosine modification of genes. By regulating
the key factors in these signaling pathways, the EMT process that
regulates the malignant progression of HCC cells is altered and the
invasion of HCC cells is promoted (21–23). Based on the analysis of previous
studies and the summary of the present results, it was determined
that the molecular mechanism of overexpression of miR-374c-5p
inhibiting the occurrence and metastasis of HCC may be similar to
that in other tumors, such as regulating the expression of EMT key
proteins, the FOXC1/Snail pathway and N6 methyladenosine (21,23). Of course, further experiments are
required focusing on the aforementioned molecular mechanism to
further verify the effect of miR-374c-5p on the occurrence and
metastasis of HCC. In agreement with these data, it was revealed
that the expression of miR-374c-5p was lower in HCC than in normal
adjacent tissues. Patients with low miR-374c-5p expression had poor
prognosis and short survival time. MiR-374c-5p expression was an
independent prognostic factor for OS and DSS in HCC. Furthermore,
overexpression of miR-374c-5p inhibited HCC cell proliferation,
migration and invasion in vitro and in vivo.
EMT is a process in which epithelial cells transform
into mesenchymal cells under specific physiological or pathological
conditions (24). In previous
years, EMT has been demonstrated to play an important role in tumor
metastasis. EMT occurs at the initial stage of tumor metastasis and
can lead to the loss of the expression of E-cadherin, claudin,
occludin and other junction molecules in epithelial cells. It can
also destroy cell polarity and increase the expression of lysozymes
involved in degradation of the extracellular matrix and basement
membrane, such as matrix metalloproteinases, thus destroying the
histological barrier to tumor cell invasion. Concurrently, EMT can
promote the production of cancer stem cells (CSCs) (25,26). In HCC, cancer-promoting molecules
induce EMT by activating the Notch and Wnt signaling pathways
upstream of EMT or by regulating the activities of the EMT-related
transcription factors ZEB2 and Snail II (27). Concurrently, it was found that EMT
can also cause HCC cells to obtain stem cell characteristics, thus
promoting the production of HCC CSCs and accelerating the invasion
and metastasis of tumor cells (28). In the present study, it was
demonstrated that miR-374c-5p directly targeted the 3′-UTR of PTTG1
and regulated the expression of PTTG1. Additionally, miR-374c-5p
altered EMT by affecting PTTG1 expression thereby affecting the
malignant biological behavior of HCC.
PTTG1 plays an important role in a variety of
malignant tumors (29–31). A recent study revealed that PTTG1
acts as a marker of tumor stem cells and regulates tumor stem
cells, germline and stem cell-related genes and the malignant
progression of ovarian tumors by affecting the self-renewal and
epithelial-mesenchymal transformation ability of tumor stem cells
(32). In addition, PTTG1 protein
expression was found to be significantly higher in colorectal
cancer tissues and cell lines (33). The overexpression of PTTG1 was
positively correlated with the clinical stage, TNM stage and
differentiation of tumors (33).
In vitro, knockout of PTTG1 inhibited the growth and
metastasis of colorectal cancer (33). Univariate and multivariate
analysis showed that PTTG1 overexpression was an independent
adverse prognostic factor in patients with colorectal cancer
(33). In breast cancer, PTTG1
was associated with a low survival rate and could affect cell cycle
arrest in breast cancer cells (34). A recent study revealed that the
level of PTTG1 was correlated with that of estrogen and was
positively correlated with tamoxifen resistance (34).
In conclusion, the present study provided new
evidence that low miR-374c-5p expression promotes HCC growth and
metastasis by regulating PTTG1 and can significantly promote EMT.
The results suggested that miR-374c-5p can serve as an important
target for effective prediction and intervention of malignant
progression of HCC. Of course, our research still has certain
limitations. In future research, more specimens of clinical
patients with HCC will be collected, for further study and analysis
of the molecular mechanism of miR-374c-5p in HCC, to provide a
scientific and reliable experimental basis for the clinical
treatment of HCC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Sichuan Provincial Department
of Education, (grant no. 16TDD00025), the Popularization and
Application Project of Sichuan Health Commission (grant no.
20PJ149), the Scientific Research Project of Affiliated Hospital of
North Sichuan Medical College (grant no. 2020ZD001), the Scientific
Research Project of Affiliated Hospital of North Sichuan Medical
College (grant no. 2020JC035), the Cooperation Project Between
Nanchong City and North Sichuan Medical College (grant nos.
19SXHZ0290 and 20SXQT0026), the Free Exploring Basic Research
Project of Science and Technology of Sichuan province (grant no.
2020YJ0186) and the National Natural Science Foundation of China
(grant no. 82003147).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
GY and JL conceived and designed the experiments.
YX, GW, WL, TT and JS conducted all the experiments. GY and JL
acquired and analyzed the data. GY and JL wrote and revised the
manuscript. GY, YX, GW, WL, TT, JS and JL confirm the authenticity
of all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no.
2014032) by the Ethics Committee of Affiliated Hospital of North
Sichuan Medical College (Nanchong, China). The present study was
performed in accordance with the principles outlined in the
Declaration of Helsinki. All patients provided written informed
consent. All animal experiments were approved by the Animal Care
Ethics Review Committee of Affiliated Hospital of North Sichuan
Medical College, approval no. 20201026.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McGlynn KA, Petrick JL and El-Serag HB:
Epidemiology of hepatocellular carcinoma. Hepatology. 73 (Suppl
1):S4–S13. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Llovet JM, Kelley RK, Villanueva A, Singal
AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J and
Finn RS: Hepatocellular carcinoma. Nat Rev Dis Primers. 7:62021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Morishita A, Oura K, Tadokoro T, Fujita K,
Tani J and Masaki T: MicroRNAs in the pathogenesis of
hepatocellular carcinoma: A review. Cancers (Basel). 13:5142021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hobert O: Gene regulation by transcription
factors and microRNAs. Science. 319:1785–1786. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cohen A, Burgos-Aceves MA and Smith Y:
Estrogen repression of microRNA as a potential cause of cancer.
Biomed Pharmacother. 78:234–238. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dong LI, Zheng Y, Gao L and Luo X: lncRNA
NEAT1 prompts autophagy and apoptosis in MPTP-induced Parkinson's
disease by impairing miR-374c-5p. Acta Biochim Biophys Sin
(Shanghai). 53:870–882. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fu H, Zhang Y, Chen Y, Chen J and Chen P:
CSN1 facilitates proliferation and migration of hepatocellular
carcinoma cells by upregulating cyclin A2 expression. Mol Med Rep.
23:462021.PubMed/NCBI
|
|
12
|
Mullard A: Addressing cancer's grand
challenges. Nat Rev Drug Discov. 19:825–826. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Puik JR, Meijer LL, Le Large TY, Prado MM,
Frampton AE, Kazemier G and Giovannetti E: miRNA profiling for
diagnosis, prognosis and stratification of cancer treatment in
cholangiocarcinoma. Pharmacogenomics. 18:1343–1358. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang M, Matyunina LV, Walker LD, Chen W,
Xiao H, Benigno BB, Wu R and McDonald JF: Evidence for the
importance of post-transcriptional regulatory changes in ovarian
cancer progression and the contribution of miRNAs. Sci Rep.
7:81712017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Perron MP and Provost P: Protein
interactions and complexes in human microRNA biogenesis and
function. Front Biosci. 13:2537–2547. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oliveto S, Mancino M, Manfrini N and Biffo
S: Role of microRNAs in translation regulation and cancer. World J
Biol Chem. 8:45–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He C, Li Z, Chen P, Huang H, Hurst LD and
Chen J: Young intragenic miRNAs are less coexpressed with host
genes than old ones: Implications of miRNA-host gene coevolution.
Nucleic Acids Res. 40:4002–4012. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao X, Qiao Y, Han D, Zhang Y and Ma N:
Enemy or partner: Relationship between intronic micrornas and their
host genes. IUBMB Life. 64:835–840. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hinske LC, Galante PA, Kuo WP and
Ohno-Machado L: A potential role for intragenic miRNAs on their
hosts' interactome. BMC Genomics. 11:5332010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang Y, Huang H, Li M, Zhang X, Liu Y and
Wang Y: MicroRNA-374c-5p regulates the invasion and migration of
cervical cancer by acting on the Foxc1/snail pathway. Biomed
Pharmacother. 94:1038–1047. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hao S, Tian W, Chen Y, Wang L, Jiang Y,
Gao B and Luo D: MicroRNA-374c-5p inhibits the development of
breast cancer through TATA-box binding protein associated factor
7-mediated transcriptional regulation of DEP domain containing 1. J
Cell Biochem. 120:15360–15368. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yue Y, Deng P, Xiao H, Tan M, Wang H, Tian
L, Xie J, Chen M, Luo Y, Wang L, et al: N6-methyladenosine-mediated
downregulation of miR-374c-5p promotes cadmium-induced cell
proliferation and metastasis by targeting GRM3 in breast cancer
cells. Ecotoxicol Environ Saf. 229:1130852022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: EMT: 2016. Cell. 166:21–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pastushenko I and Blanpain C: EMT
transition states during tumor progression and metastasis. Trends
Cell Biol. 29:212–226. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jayachandran A, Dhungel B and Steel JC:
Epithelial-to-mesenchymal plasticity of cancer stem cells:
Therapeutic targets in hepatocellular carcinoma. J Hematol Oncol.
9:742016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Giannelli G, Koudelkova P, Dituri F and
Mikulits W: Role of epithelial to mesenchymal transition in
hepatocellular carcinoma. J Hepatol. 65:798–808. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Georgakopoulos-Soares I, Chartoumpekis DV,
Kyriazopoulou V and Zaravinos A: EMT factors and metabolic pathways
in cancer. Front Oncol. 10:4992020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guo XC, Li L, Gao ZH, Zhou HW, Li J and
Wang QQ: The long non-coding RNA PTTG3P promotes growth and
metastasis of cervical cancer through PTTG1. Aging (Albany NY).
11:1333–1341. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Horning AM, Wang Y, Lin CK, Louie AD,
Jadhav RR, Hung CN, Wang CM, Lin CL, Kirma NB, Liss MA, et al:
Single-Cell RNA-seq reveals a subpopulation of prostate cancer
cells with enhanced cell-cycle-related transcription and attenuated
androgen response. Cancer Res. 78:853–864. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qiu M, Li G, Wang P, Li X, Lai F, Luo R,
Liu B and Lin J: aarF domain containing kinase 5 gene promotes
invasion and migration of lung cancer cells through
ADCK5-SOX9-PTTG1 pathway. Exp Cell Res. 392:1120022020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Parte S, Virant-Klun I, Patankar M, Batra
SK, Straughn A and Kakar SS: PTTG1: A unique regulator of
stem/cancer stem cells in the ovary and ovarian cancer. Stem Cell
Rev Rep. 15:866–879. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ren Q and Jin B: The clinical value and
biological function of PTTG1 in colorectal cancer. Biomed
Pharmacother. 89:108–115. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Meng C, Zou Y, Hong W, Bao C and Jia X:
Estrogen-regulated PTTG1 promotes breast cancer progression by
regulating cyclin kinase expression. Mol Med. 26:332020. View Article : Google Scholar : PubMed/NCBI
|