Introduction
Ischemia-reperfusion (I/R) injury poses a great
threat to the survival of grafts and recipients, leading to an
increase in the morbidity and mortality of patients (1). Lung I/R injury (LIRI) usually occurs
in various clinical situations, including cardiac arrest, trauma,
pulmonary thrombosis, lung transplantation and cardiopulmonary
bypass surgery (2,3). Respiration failure as a result of
LIRI may cause related acute lung injury (ALI) or acute respiratory
distress syndrome (4). At
present, the most effective therapeutic targets and the most
promising treatments for LIRI are still awaiting clinical trial
(5). Thus, the identification and
exploration of targets or drugs with high efficacy to treat LIRI is
urgently required.
Lidocaine is a local anesthetic that exerts efficacy
within 1–3 min after administration, with an anesthetic effect that
lasts for 1–3 h (6). In clinical
practice, it is used to treat arrhythmia and is the first line of
treatment for patients with ventricular tachycardia and tremor
(7,8). The current evidence has demonstrated
that treatment with lidocaine eliminates severe arrhythmia and
prevents heart death in an in vivo rat model of acute
myocardial I/R (9). Chen et
al (10) reported that the
injection of lidocaine into the hepatoduodenal ligament of rats
could protect against I/R-induced liver injury. In addition, Lei
et al (11) reported that
low-dose lidocaine protected against transient focal cerebral
ischemia in a rat model. From these studies, it is clear that
lidocaine serves a protective role in numerous types of I/R injury
in different tissues. However, the effects of lidocaine on LIRI
remain unclear and the methods for LIRI prevention and treatment
are yet to be elucidated.
Ferroptosis is a newly discovered type of cell
death, which has distinct properties and functions related to
physical conditions or various diseases (12), such as tumors, kidney injury and
IRI. It has been demonstrated that the inhibition of ferroptosis
can effectively protect against IRI (13). Previous studies have also revealed
that blocking ferroptosis greatly alleviates cardiomyopathy and
that targeting ferroptosis could effectively treat fatal
heart-related diseases (12,13). For example, targeting ferroptosis
in cardiomyocytes can alleviate sepsis-induced cardiac injury
(14). Of note, it has also been
reported that lidocaine could promote ferroptosis via the microRNA
(miR)-382-5p/cystine/glutamate transporter axis in ovarian and
breast cancer cells (15).
However, whether ferroptosis is involved in the development of LIRI
and whether ferroptosis is also regulated by lidocaine during LIRI
remain unclear.
The p38 MAPK signaling pathway participates in a
large number of different biological effects (16). For example, the p38 pathway can
respond to inflammatory stimuli to maintain cellular homeostasis in
different tissues (17). The p38
pathway also regulates apoptosis and autophagy homeostasis in
response to chemotherapeutic agents (18). Various studies have revealed that
the regulation of p38 MAPK may partially prevent the occurrence of
IRI. For instance, Rutaecarpine inhibits oxidative stress by
suppressing the JNK/p38 MAPK signaling pathway, thereby attenuating
renal IRI in rats (19). Apelin
improves IRI-induced diabetic myocardium by suppressing apoptosis
and oxidative stress through PI3K and P38-MAPK signaling pathways
(20). Fibronectin-α4β1
ameliorates hepatic cold ischemia and reperfusion injury by
regulating MMP-9 and MT1-MMP via the p38 MAPK pathway (21). In addition, lidocaine has been
verified to protect against I/R-induced brain injury by regulating
the p38 MAPK signaling pathway (22). Lidocaine also relieved LPS-induced
lung injury by blocking the p38 MAPK signaling pathway in an in
vivo rat model of ALI (23).
Thus, it was hypothesized that lidocaine may protect against LIRI
by blocking the p38 MAPK signaling pathway.
The current study aimed to determine the protective
role and underlying mechanisms of lidocaine on
hypoxia/reoxygenation (H/R)-induced LIRI.
Materials and methods
Cell culture and treatment
Human type II alveolar epithelial cells (A549; cat.
no. BNCC337696) were purchased from the BeNa Culture Collection
(Beijing Beina Chunglian Institute of Biotechnology) and cultured
in DMEM (Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.), and 1% antibiotics (100
U/ml penicillin and 100 mg/ml streptomycin; Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in a humidified atmosphere with 5%
CO2. Lidocaine (cat. no. HY-B0185; MedChemExpress) at
different doses (0.5, 1, 5 and 10 mM) was then administered to A549
cells at room temperature for 24 h. To explore the underlying
mechanism of lidocaine (10 mM), p79350 (50 µM; Invitrogen; Thermo
Fisher Scientific, Inc.), an agonist of p38, was adopted to treat
A549 cells at room temperature for 1 h.
Lung I/R injury model
construction
To simulate lung I/R injury in vitro, A549
cells were cultured under hypoxic conditions (1% O2, 5%
CO2 and 94% N2) at 37°C for 24 h. Afterwards,
cells were exposed to reoxygenation (5% CO2 and 95% air)
for 4 h (24).
MTT assay
To increase the reliability of subsequent
experiments, the effects of different concentrations of lidocaine
on the viability of A549 cells and H/R-induced A549 cells were
detected using MTT. A549 cells were inoculated into 96-well plates
at a density of 1×104 cells/well for 24 h. Cells were
then exposed to 10 µl MTT solution (Beyotime Institute of
Biotechnology) in accordance with the manufacturer's protocol, and
incubated at 37°C for an additional 4 h. Subsequently, 200 ml DMSO
was added and the absorbance was detected using a microplate at a
wavelength of 490 nm.
TUNEL assay
The effect of different concentrations of lidocaine
(0.5, 1, 5 and 10 mM) on H/R-induced A549 cell apoptosis was
assessed using a TUNEL assay (Beyotime Institute of Biotechnology)
in accordance with the manufacturer's protocol. Cells
(1×106 cells/well) were fixed with 4% paraformaldehyde
for 30 min at room temperature, and incubated with 0.3% Triton
X-100 for 5 min, followed by incubation with 50 µl TUNEL reaction
reagent at 37°C for 1 h. After staining with 0.5 µg/ml of DAPI for
10 min at room temperature, TUNEL-positive cells randomly selected
from five fields of view were imaged using a fluorescence
microscope with antifade mounting medium (Beyotime Institute of
Biotechnology; magnification, ×200).
ELISA
ELISA was employed to determine the release of
various inflammatory cytokines from the cell supernatants of
H/R-induced A549 cells (5×105 cells/well), including
IL-6 (cat. no. SQ6000B), IL-8 (cat. no. Q8000B) and TNF-α (cat. no.
STA00D; all from R&D Systems, Inc.). Optical density was
recorded at 450 nm using a microplate reader (Bio-Rad Laboratories,
Inc.).
Malondialdehyde (MDA), superoxide
dismutase (SOD) and glutathione peroxidase (GSH-Px)
measurement
The levels of MDA (cat. no. A003-1-2), SOD (cat. no.
A001-3-2) and GSH-Px (cat. no. A005-1-2) in cell suspensions were
measured using assay kits (all from Nanjing Jiancheng
Bioengineering Institute), according to the manufacturer's
specifications.
Iron and reactive oxygen species (ROS)
level measurement
The intracellular Fe2+ level and ROS
level in cells was detected using an iron colorimetric assay kit
(cat. no. K390-100; BioVision, Inc.) (25) and a fluorescent probe
2′,7′-Dichlorodihydrofluorescein diacetate (DCFH-DA; Beyotime
Institute of Biotechnology) according to the manufacturer's
protocol, respectively.
Monolayer cell paracellular
permeability assay
The monolayer cell permeability assay was performed
using Transwell apparatus. H/R-induced A549 cells (5×104
cells/well) were inoculated on Transwell polyester membrane cell
culture inserts in a 24-well plate. Samples were then incubated at
37°C in a humidified atmosphere containing 5% CO2.
Within 20–21 days, A549 cells with a confluence of 80–90% were
obtained. Subsequently, 200 µl Hanks' balanced Salt solution
containing 1 mg/ml sodium fluorescein was added to the monolayer of
the upper chamber. Absorption was detected using a fluorescence
microplate reader, with an excitation and absorption wavelength of
490 and 520 nm, respectively.
Western blotting
Total protein was extracted from A549 cells using
RIPA lysis buffer (Sangon Biotech Co., Ltd.) and then quantified
using a BCA kit (Pierce; Thermo Fisher Scientific, Inc.) in
accordance with the manufacturer's instructions. After 15% SDS-PAGE
electrophoresis, samples (30 µg) were transferred to PVDF membranes
and subsequently blocked with 5% non-fat milk for 2 h at room
temperature. Membranes were then incubated with primary antibodies
against zonula occludens-1 (ZO-1; 1:1,000; cat. no. orb35080;
Biorbyt), Occludin (1:1,000; cat. no. orb11181; Biorbyt), Claudin-1
(1:1,000; cat. no. orb127883; Biorbyt), ferritin heavy chain 1
(FTH1; 1:1,000; cat. no. orb49039; Biorbyt), glutathione peroxidase
4 (GPX4; 1:1,000; cat. no. orb340797; Biorbyt), transferrin (Tf;
1:1,000; cat. no. ab277635; Abcam), phosphorylated (p)-p38
(1:1,000; cat. no. ab4822; Abcam), p38 (1:2,000; cat. no. ab170099;
Abcam) and GAPDH (1:2,500; cat. no. ab9485; Abcam) at 4°C
overnight. Next, the membranes were incubated with horseradish
peroxidase-conjugated goat anti-rabbit (1:2,000; cat. no. ab97051;
Abcam) antibodies for 2 h at room temperature. Relative protein
levels were visualized using an ECL kit (Beijing Solarbio Science
& Technology Co., Ltd.) and semi-quantified using ImageJ
software 1.52 (National Institutes of Health).
Statistical analysis
All the above experiments were conducted
independently at least 3 times. Statistical analysis was conducted
using SPSS 20.0 (IBM Corp.) and data are presented as the mean ±
SD. One-way ANOVA was used to analyze data, after which Tukey's
multiple comparison post hoc test was performed. P<0.05 was
considered to indicate a statistically significant difference.
Results
Lidocaine increases the relative
viability of H/R-induced A549 cells
To increase the reliability of subsequent
experiments, an MTT assay was employed to detect the viability of
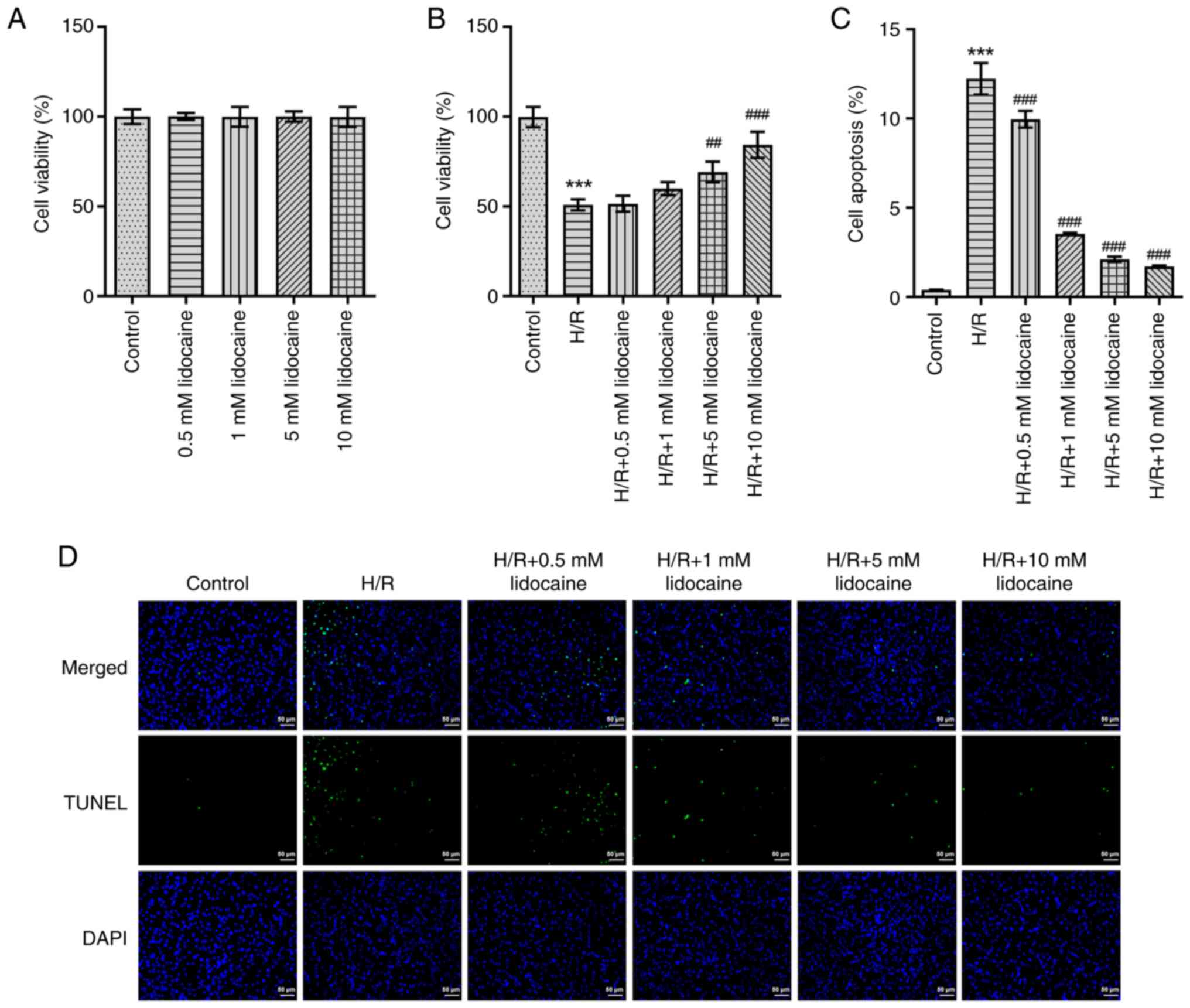
A549 and H/R-induced A549 cells. As presented in Fig. 1A, lidocaine had no marked effect
on A549 cell viability. Although cell viability was greatly
suppressed following H/R-induction, lidocaine treatment partly
reversed this effect in a concentration-dependent manner (Fig. 1B). To further elucidate the
biological role of lidocaine, H/R-induced A549 cell apoptosis was
detected by using a TUNEL assay. When compared with the H/R group,
the results revealed that lidocaine treatment significantly
decreased the apoptosis of H/R-induced A549 cells (Fig. 1C and D).
Lidocaine alleviates oxidative stress
and the inflammatory response in H/R-induced A549 cells
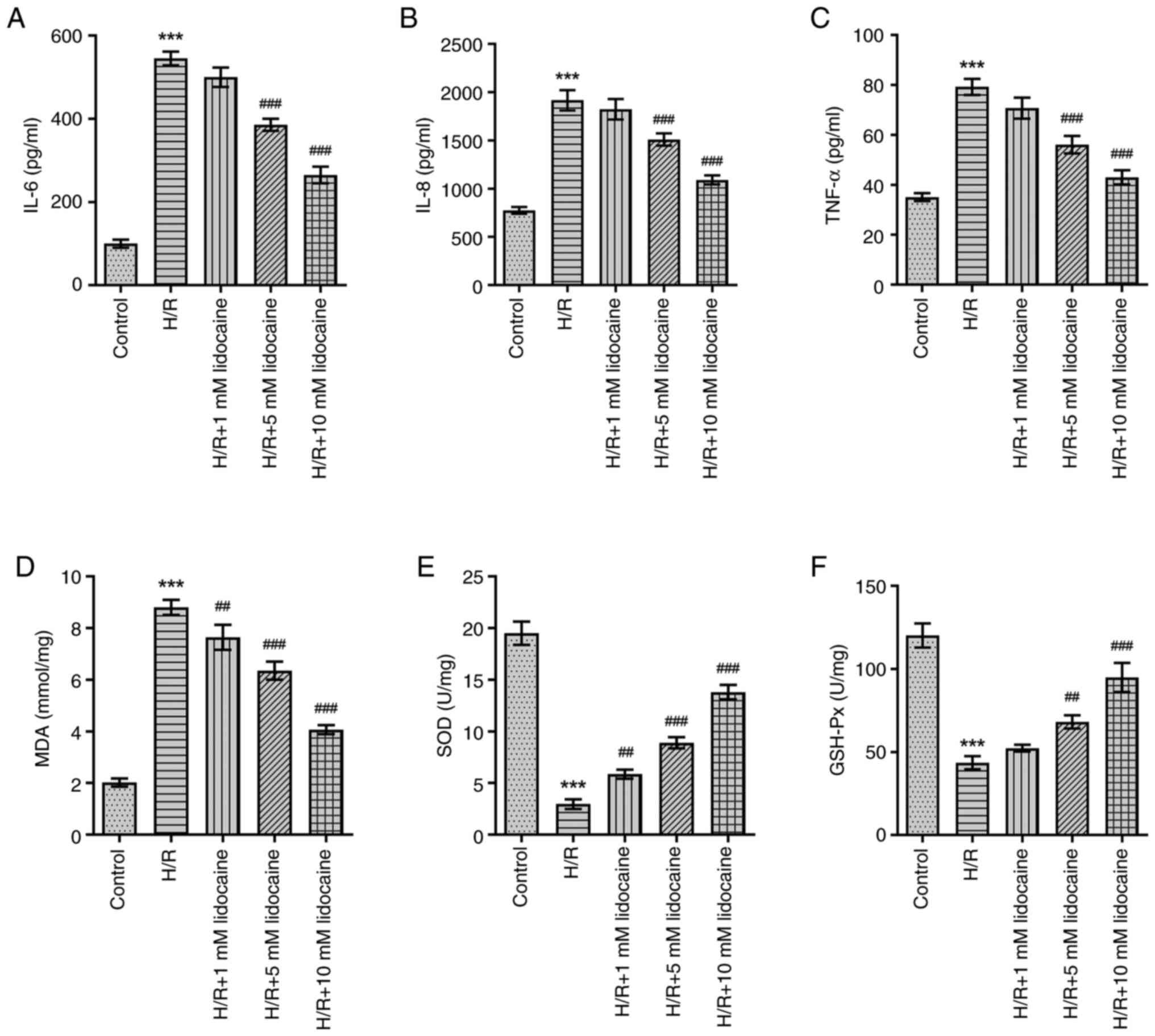
As inflammation and oxidative stress have a
significant influence on IRI, the effects of lidocaine on the
inflammatory response and oxidative stress were assessed in
H/R-induced A549 cells. In comparison with the control group, IL-6,
IL-8 and TNF-α levels were significantly increased in H/R-induced
A549 cells (Fig. 2A-C). However,
this effect was reversed following lidocaine treatment,
particularly at the 10 mM concentration, which exhibited the most
prominent inhibitory effect on the inflammatory response. H/R
significantly increased MDA levels, and significantly decreased SOD
and GSH-Px levels (Fig. 2D-F).
However, downregulated SOD and GSH-Px, and upregulated MDA levels
were reversed following lidocaine treatment in H/R-induced A549
cells. In summary, lidocaine treatment eased the inflammatory
response and reduced oxidative stress.
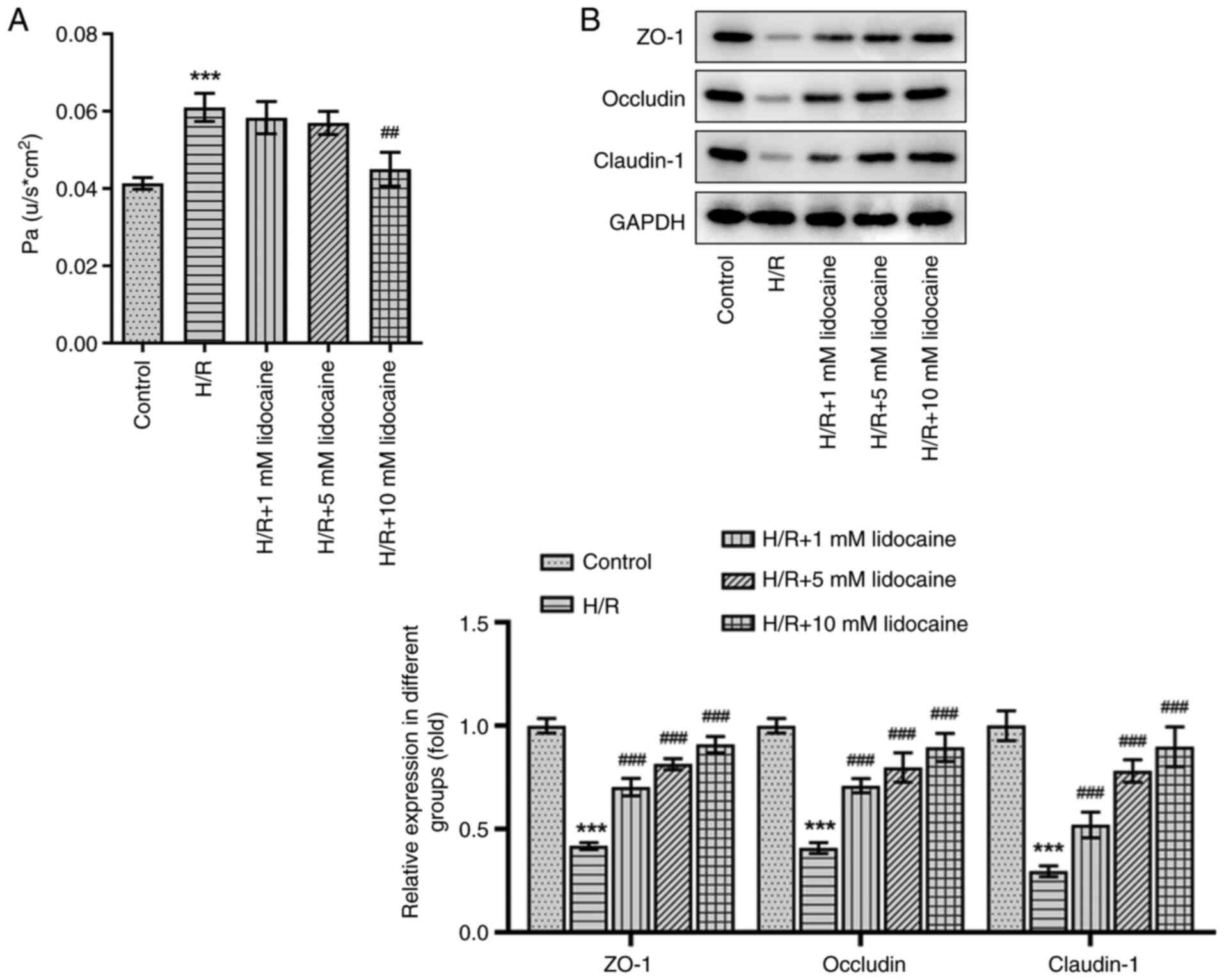
Lidocaine relieves barrier dysfunction
in H/R-induced A549 cells
A monolayer cell paracellular permeability assay was
performed to evaluate the effects of lidocaine on H/R-induced A549
cell permeability. Compared with the control group, cell
permeability was increased following H/R induction, which was an
effect that was diminished by 10 mM lidocaine treatment (Fig. 3A). The expression of tight
junction proteins was measured via western blotting. The results
revealed that the expression levels of certain proteins, including
ZO-1, Occludin and Claudin-1, were decreased in H/R-induced A549
cells (Fig. 3B). However, this
effect was significantly reversed by lidocaine treatment,
particularly at the 10 mM dosage. The results indicated that
lidocaine protected against barrier dysfunction by decreasing cell
permeability and increasing tight junction protein expression in
H/R-induced A549 cells.
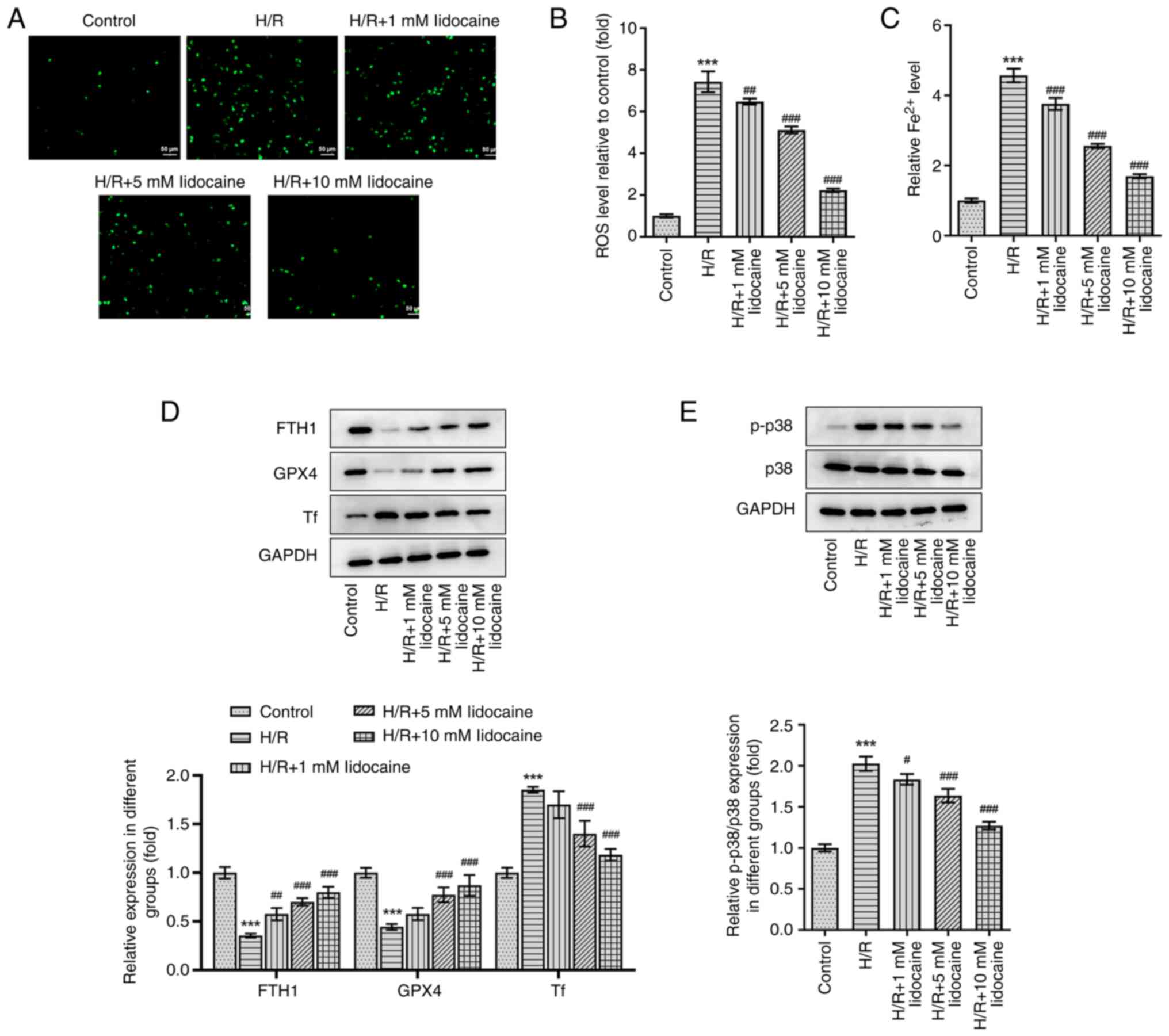
Lidocaine inhibits p38 MAPK-mediated
ferroptosis in H/R-induced A549 cells
To explore the mechanism of lidocaine in
ferroptosis, ROS level and iron level was first evaluated. As shown
in Fig. 4A-C, ROS and iron levels
were significantly elevated by H/R induction, but were then
significantly reduced by lidocaine treatment in a
concentration-dependent manner. Subsequently, western blotting was
performed and the expression levels of p38 MAPK pathway-related and
ferroptosis-related proteins were assessed, including FTH1, GPX4
and Tf. Compared with the control group, the expression levels of
FTH1 and GPX4 were significantly decreased by H/R induction;
however, the expression of Tf was significantly increased under the
same conditions. Following lidocaine treatment at various
concentrations, the H/R-induced decrease of FTH1 and GPX4, and the
H/R-induced increase of Tf, was significantly reversed (Fig. 4D). Furthermore, the H/R-induced
increase in p-p38, a p38 MAPK-related protein, in A549 cells was
significantly downregulated following lidocaine treatment (Fig. 4E). However, p38 expression
remained unchanged after lidocaine treatment and H/R induction. To
conclude, lidocaine inhibited ferroptosis in H/R-induced A549 cells
by regulating the p38 MAPK signaling pathway.
Lidocaine suppresses oxidative stress
and the inflammatory response in H/R-induced A549 cells by blocking
the p38 MAPK signaling pathway
To investigate the underlying mechanism of lidocaine
on the inflammatory response and oxidative stress, p79350, an
agonist of p38, was used to pre-treat A549 cells. Lidocaine at a
concentration of 10 mM was selected for further experiments.
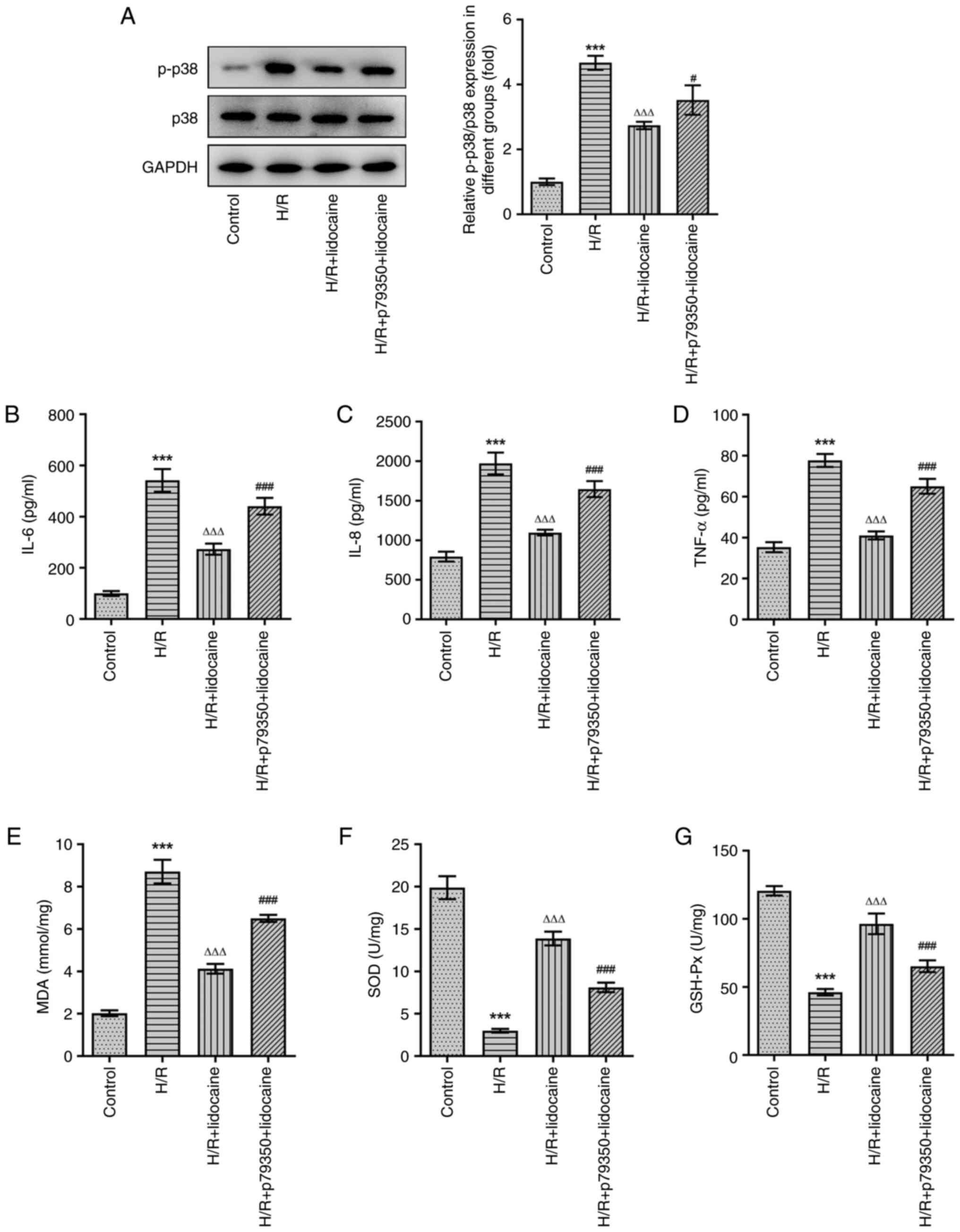
Compared with the H/R + lidocaine group, p-p38 protein expression
was upregulated in the H/R + p79350 + lidocaine group, which
demonstrated that p79350 increased the activity of p39 MAPK
signaling (Fig. 5A). The results
of ELISA suggested that H/R induction significantly stimulated the
release of IL-6, IL-8 and TNF-α, while lidocaine treatment
significantly inhibited this effect. Furthermore, when compared
with the H/R + lidocaine group, IL-6, IL-8 and TNF-α levels were
increased again in the H/R + p79350 + lidocaine group (Fig. 5B-D). Subsequently, the expression
levels of MDA, SOD and GSH-Px were assessed. As demonstrated in
Fig. 5E-G, increased MDA
expression levels induced by H/R were significantly downregulated
in the H/R + lidocaine group. Additionally, the H/R-induced
decrease in SOD and GSH-Px expression were subsequently
significantly increased in A549 cells in the H/R + lidocaine group.
However, MDA expression was increased, and SOD and GSH-Px
expression levels were decreased in the H/R + p79350 + lidocaine
group, when compared with the H/R + lidocaine group. In summary,
p79350 partially reversed the inhibitory effects of lidocaine on
the inflammatory response and oxidative stress in H/R-induced A549
cells.
Lidocaine inhibits barrier dysfunction
and ferroptosis in H/R-induced A549 cells by blocking the p38 MAPK
signaling pathway
A549 cell permeability was assessed by using a
monolayer cell paracellular permeability assay. As presented in
Fig. 6A, the H/R-induced increase
of cell permeability was significantly suppressed in the H/R +
lidocaine group; however, these inhibitory effects were partially
reversed in the H/R + p79350 + lidocaine group. The expression
levels of tight junction proteins were measured via western
blotting. In comparison with the H/R group, ZO-1, Occludin and
Claudin-1 expression levels were significantly increased in the H/R
+ lidocaine group (Fig. 6B).
However, this increase was subsequently decreased in the H/R +
p79350 + lidocaine group, indicating that it counteracted the
protective effects of lidocaine. In addition, the reduced ROS and
iron levels in the H/R + lidocaine group were partially reversed in
the H/R + p79350 + lidocaine group in A549 cells (Fig. 6C-E). Then, western blotting was
also employed to measure the expression of ferroptosis-related
proteins, including FTH1, GPX4 and Tf. The results revealed that
H/R induction decreased the expression levels of FTH1 and GPX4, but
increased the expression of Tf (Fig.
6F). FTH1 and GPX4 expression levels were significantly
upregulated in the H/R + lidocaine group, and significantly
downregulated Tf expression levels in A549 cells; however, these
effects were reversed in the H/R + p79350 + lidocaine group. To
summarize, lidocaine suppressed barrier dysfunction and ferroptosis
in H/R-induced A549 cells by blocking the p38 MAPK signaling
pathway.
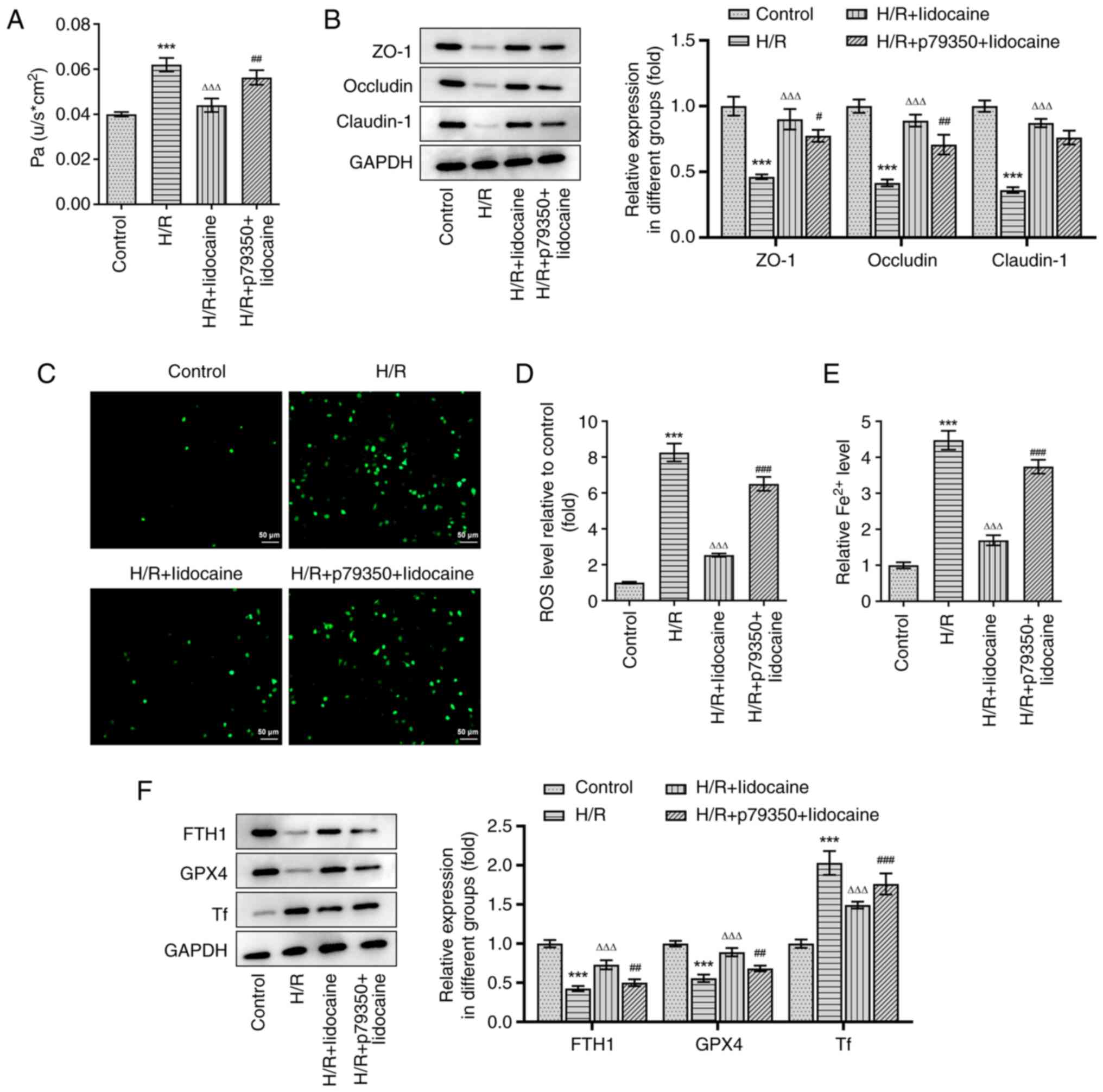 | Figure 6.Lidocaine inhibits barrier
dysfunction and ferroptosis in H/R-induced A549 cells by blocking
the p38 MAPK signaling pathway. (A) Cell permeability was detected
by performing a monolayer cell paracellular permeability assay. (B)
The expression levels of ZO-1, Occludin and Claudin-1, were
measured via western blotting. Levels of (C and D) ROS and (E) iron
were detected by using their corresponding commercial kits. (F) The
expression levels of FTH1, GPX4 and Tf were measured via western
blotting. ***P<0.001 vs. control; ΔΔΔP<0.001 vs.
H/R; #P<0.05, ##P<0.01 and
###P<0.001 vs. H/R + lidocaine. H/R,
hypoxia/reoxygenation; ZO-1, zonula occludens-1; ROS, reactive
oxygen species; FTH1, ferritin heavy chain 1; GPX4, glutathione
peroxidase 4; Tf, transferrin. |
Discussion
LIRI is a significant clinical problem that has been
evident for a number of years. It usually occurs following lung
transplantation, revascularization of pulmonary embolism,
cardiopulmonary resuscitation, pulmonary arterioplasty, shock and
various heart bypass operations (26,27). LIRI can lead to severe organ
damage and various types of cell death, resulting in an increase of
mortality (28). However,
therapeutic methods that demonstrate high efficacy have not yet
been determined (5). Thus,
investigating additional effective therapeutic measures is of great
importance. The current study therefore constructed an in
vitro model of LIRI to investigate the protective role and
underlying mechanism of lidocaine.
In the present study, lidocaine demonstrated a
protective effect against LIRI. Previous studies have demonstrated
that the constant infusion of lidocaine could protect against
myocardial I/R injury (MIRI) and prevent cardiac death (9,29).
Studies have also revealed that lidocaine administration reduced
IRI (11,30). The results of the present study
determined that lidocaine protected against LIRI in H/R-induced
A549 cells. Evidently, lidocaine can exert protective effects on IR
injury in various diseases, such as ischemic brain damage, ischemic
spinal cord and myocardial injury (31–33). Of note, it has been found that
lidocaine can inhibit the process of apoptosis under multiple
pathological states, such as cerebral I/R injury and myocardial
damage due to ischemia and reperfusion (34,35), consistent with the findings in the
present study. However, lidocaine is also reported to induce the
process of apoptosis in various cancer cell lines, thereby
inhibiting cancer development (36,37). Therefore, the effect of lidocaine
on cell apoptosis is not absolute, as lidocaine can exert
proapoptotic effects on tumor cell lines or exert antiapoptotic
effects on I/R-induced cell injury.
Inflammation serves an important role in the
mechanism of LIRI (38), such
that the regulation of inflammation has been revealed to
effectively alleviate the disease (39). Previous studies have verified that
IL-8 levels are markedly increased in lung tissue and systemic
circulation after IR (40,41).
This is problematic, as significantly higher IL-8 levels may result
in higher mortality rates following lung transplantation (42). Lan et al (39) and de Perrot et al (40) reported that elevated TNF-α and
IL-6 levels contributed to organ IR injury. In the present study,
the results of ELISA demonstrated that IL-6, IL-8 and TNF-α levels
were markedly increased in A549 cells following H/R induction.
However, the application of lidocaine inhibited these effects in a
concentration-dependent manner.
Oxidative stress is another critical factor in the
mechanism of LIRI. MDA, the end product of lipid peroxidation, can
reflect the degree of lipid peroxidation in lung tissue (43). In the present study, it was
revealed that MDA levels in H/R-induced A549 cells were increased
when compared with the control group, suggesting that cell membrane
lipid peroxidation had increased. Subsequent lidocaine treatment
decreased MDA levels, revealing that lidocaine could reduce lipid
peroxidation. SOD is the main endogenous antioxidant enzyme found
in lung tissue, the detection of which can reflect the degree of
oxidation resistance (43). The
current study determined that SOD levels were markedly decreased
following H/R induction, which was an effect that could be
partially abolished by lidocaine treatment. These results are in
line with previous findings that antioxidants may be promising
targets for LIRI (44).
As ferroptosis is a key part of LIRI, it was
investigated in the current study. Ferroptosis is a newly
discovered form of regulated cell death that depends on the
existence of iron and is triggered by lipid ROS (45). The knockout or inactivation of
GPX4 prevents cells from inhibiting lethal lipid peroxidation in
phospholipid bilayers, eventually leading to cell death (46–48). The present study revealed that the
expression levels of FTH1 and GPX4 were markedly downregulated in
H/R-induced A549 cells, indicating that ferroptosis had occurred in
LIRI, which is in line with the results of previous studies in
organs such as the kidney and intestine (49,50). The adoption of lidocaine may
therefore upregulate FTH1 and GPX4 expression levels, thus
inhibiting ferroptosis. Of note, it is interesting that the 5 mM
lidocaine concentration appeared to have similar effects to the 10
mM concentration on markers of ferroptosis and tight junction
proteins. However, the effects of 10 nM lidocaine on cell
viability, inflammatory response and oxidative stress were higher
than those of 5 mM lidocaine. The different sensitivity of
lidocaine to different cellular bioactivity may be a reason
accounting for this condition.
Furthermore, it is reported that regulation of the
p38 MAPK signaling pathway may be an effective target for IRI, as
evidenced by a previous study that revealed MIRI could be inhibited
through the regulation of the P38 MAPK signaling pathway (51). Jiang et al (22) reported that lidocaine exerted
protective effects on cerebral IR injury by suppressing the
activation of p38 MAPK. Additionally, through the regulation of the
p38 MAPK signaling pathway, lidocaine could protect against
LPS-induced lung injury (23).
Therefore, it was hypothesized in the current study that lidocaine
treatment could relieve LIRI by blocking the p38 MAPK signaling
pathway. To test this theory, p79350, an agonist of p38, was
utilized to pre-treat A549 cells in the present study. The results
revealed that p79350 partially reversed the protective effect of
lidocaine on H/R-induced A549 cells, indicating that regulation of
the p38 MAPK signaling pathway could be an effective target
underlying the protective effects of lidocaine on LIRI.
However, there are still some limitations of the
present study. First, this study only explored the regulatory
effect of lidocaine on ferroptosis in LIRI, but whether ferroptosis
is a necessary regulatory pathway for lidocaine remains unclear.
The application of ferroptosis inhibitors is worthy of further
investigation to determine the involvement of ferroptosis in
lidocaine-treated LIRI. Furthermore, in vivo experiments
will also be conducted in our future work to further verify these
findings.
In conclusion, lidocaine could regulate
inflammation, oxidative stress and ferroptosis by blocking the p38
MAPK signaling pathway. Thus, lidocaine could act as a novel
therapeutic treatment of patients with LIRI.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NH designed the study. XM, WY and NH performed the
experiments and analyzed the data. XM and WY drafted manuscript. NH
revised the manuscript. XM and NH confirmed the authenticity of all
raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sharma AK, Charles EJ, Zhao Y, Narahari
AK, Baderdinni PK, Good ME, Lorenz UM, Kron IL, Bayliss DA,
Ravichandran KS, et al: Pannexin-1 channels on endothelial cells
mediate vascular inflammation during lung ischemia-reperfusion
injury. Am J Physiol Lung Cell Mol Physiol. 315:L301–L312. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weyker PD, Webb CA, Kiamanesh D and Flynn
BC: Lung ischemia reperfusion injury: A bench-to-bedside review.
Semin Cardiothorac Vasc Anesth. 17:28–43. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Diamond JM, Lee JC, Kawut SM, Shah RJ,
Localio AR, Bellamy SL, Lederer DJ, Cantu E, Kohl BA, Lama VN, et
al: Clinical risk factors for primary graft dysfunction after lung
transplantation. Am J Respir Crit Care Med. 187:527–534. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fei L, Jingyuan X, Fangte L, Huijun D, Liu
Y, Ren J, Jinyuan L and Linghui P: Preconditioning with rHMGB1
ameliorates lung ischemia-reperfusion injury by inhibiting alveolar
macrophage pyroptosis via the Keap1/Nrf2/HO-1 signaling pathway. J
Transl Med. 18:3012020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Laubach VE and Sharma AK: Mechanisms of
lung ischemia-reperfusion injury. Curr Opin Organ Transplant.
21:246–252. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cherobin AC and Tavares GT: Safety of
local anesthetics. An Bras Dermatol. 95:82–90. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Berk T and Silberstein SD: The use and
method of action of intravenous lidocaine and its metabolite in
headache disorders. Headache. 58:783–789. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lancaster RJ, Wren K, Hudson A, Leavitt K,
Albala M and Tischaefer D: Intravenous lidocaine for chronic
neuropathic pain a systematic review addressing nursing care. Pain
Manag Nurs. 21:194–200. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Canyon SJ and Dobson GP: Protection
against ventricular arrhythmias and cardiac death using adenosine
and lidocaine during regional ischemia in the in vivo rat. Am J
Physiol Heart Circ Physiol. 287:H1286–H1295. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen MY, Li CH, Huang ZQ, Liu JC, Zhou NX,
Huang XQ and Wang YS: Protective effects of lidocaine injected into
the hepatoduodenal ligament on warm inschemia-reperfusion injury to
the rat liver. Chin Med J (Engl). 117:275–279. 2004.PubMed/NCBI
|
|
11
|
Lei B, Cottrell JE and Kass IS:
Neuroprotective effect of low-dose lidocaine in a rat model of
transient focal cerebral ischemia. Anesthesiology. 95:445–451.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fang X, Wang H, Han D, Xie E, Yang X, Wei
J, Gu S, Gao F, Zhu N, Yin X, et al: Ferroptosis as a target for
protection against cardiomyopathy. Proc Natl Acad Sci USA.
116:2672–2680. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao M, Monian P, Quadri N, Ramasamy R and
Jiang X: Glutaminolysis and transferrin regulate ferroptosis. Mol
Cell. 59:298–308. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li N, Wang W, Zhou H, Wu Q, Duan M, Liu C,
Wu H, Deng W, Shen D and Tang Q: Ferritinophagy-mediated
ferroptosis is involved in sepsis-induced cardiac injury. Free
Radic Biol Med. 160:303–318. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun D, Li YC and Zhang XY: Lidocaine
promoted ferroptosis by targeting miR-382-5p /SLC7A11 axis in
ovarian and breast cancer. Front Pharmacol. 12:6812232021.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yong HY, Koh MS and Moon A: The p38 MAPK
inhibitors for the treatment of inflammatory diseases and cancer.
Expert Opin Investig Drugs. 18:1893–1905. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee S, Rauch J and Kolch W: Targeting MAPK
signaling in cancer: Mechanisms of drug resistance and sensitivity.
Int J Mol Sci. 21:11022020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sui XB, Kong N, Ye L, Han W, Zhou J, Zhang
Q, He C and Pan H: p38 and JNK MAPK pathways control the balance of
apoptosis and autophagy in response to chemotherapeutic agents.
Cancer Lett. 344:174–179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang CH, Hao ZY, Zhou J, Zhang L, Sun Y
and Liang C: Rutae carpine alleviates renal ischemia reperfusion
injury in rats by suppressing the JNK/p38 MAPK signaling pathway
and interfering with the oxidative stress response. Mol Med Rep.
16:922–928. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
An ST, Wang X, Shi HR, Zhang X, Meng H, Li
W, Chen D and Ge J: Apelin protects against ischemia-reperfusion
injury in diabetic myocardium via in hibiting apoptosis and
oxidative stress through P38K and p38-MAPK signaling pathways.
Aging (Albany NY). 12:25120–25137. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Duarte S, Shen X-D, Fondevila C, Busuttil
RW and Coito AJ: Fibronectin-α4β1 interactions in hepatic cold
ischemia and reperfusion injury: Regulation of MMP-9 and MT1-MMP
via the p38 MAPK pathway. Am J Transplant. 12:2689–2699. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang R, Liao J, Yang MC, Deng J, Hu YX,
Li P and Li MT: Lidocaine mediates the progression of cerebral
ischemia/reperfusion injury in rats via inhibiting the activation
of NF-kappaB p65 and p38 MAPK. Ann Transl Med. 8:5482020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen LJ, Ding YB, Ma PL, Jiang SH, Li KZ,
Li AZ, Li MC, Shi CX, Du J and Zhou HD: The protective effect of
lidocaine on lipopolysaccharide-induced acute lung injury in rats
through NF-κB and p38 MAPK signaling pathway and excessive
inflammatory responses. Eur Rev Med Pharmacol Sci. 22:2099–2108.
2018.PubMed/NCBI
|
|
24
|
Wu SY, Li MH, Ko FC, Wu GC, Huang KL and
Chu SJ: Protective effect of hypercapnic acidosis in
ischemia-reperfusion lung injury is attributable to upregulation of
heme oxygenase-1. PLoS One. 8:e747422013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li M, Wang X, Lu S, He C, Wang C, Wang L,
Wang X, Ge P and Song D: Erastin triggers autophagic death of
breast cancer cells by increasing intracellular iron levels. Oncol
Lett. 20:572020.PubMed/NCBI
|
|
26
|
Beckers PAJ, Gielis JF, Van Schil PE and
Adriaensen D: Lung ischemia reperfusion injury: The therapeutic
role of dipeptidyl peptidase 4 inhibition. Ann Transl Med.
5:1292017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kalogeris T, Baines CP, Krenz M and
Korthuis RJ: Cell biology of ischemia/reperfusion injury. Int Rev
Cell Mol Biol. 298:229–317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saito M, Chen-Yoshikawa TF, Suetsugu K,
Okabe R, Takahagi A, Masuda S and Date H: Pirfenidone alleviates
lung ischemia-reperfusion injury in a rat model. J Thorac
Cardiovasc Surg. 158:289–296. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ebel D, Lipfert P, Frassdorf J, Preckel B,
Müllenheim J, Thämer V and Schlack W: Lidocaine reduces ischaemic
but not reperfusion injury in isolated rat heart. Br J Anaesth.
86:846–852. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lei B, Popp S, Capuano-Waters C, Cottrell
JE and Kass IS: Effects of delayed administration of low-dose
lidocaine on transient focal cerebral ischemia in rats.
Anesthesiology. 97:1534–1540. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lan XY and Xu YM: Protective role of
lidocaine against cerebral ischemia-reperfusion injury: An in
vitro study. Exp Ther Med. 23:422022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu LW, Xu YF, Zhou YC, Li S and Yao J:
Regionally infused lidocaine can dose-dependently protect the
ischemic spinal cord in rabbits and may be associated with the EAA
changes. Neurosci Lett. 725:1348892020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aldakkak M, Stowe DF, Lesnefsky EJ,
Heisner JS, Chen Q and Camara AKS: Modulation of mitochondrial
bioenergetics in isolated guinea pig beating heart by potassium and
lidocaine cardioplegia: Implications for cardioprotection. J
Cardiovasc Pharmacol. 54:298–309. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kaczmarek DJ, Herzog C, Larmann J,
Gillmann HJ, Hildebrand R, Schmitz M, Westermann A, Harendza T,
Werdehausen R, Osthaus AW, et al: Lidocaine protects from
myocardial damage due to ischemia and reperfusion in mice by its
antiapoptotic effects. Anesthesiology. 110:1041–1049. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu Y, Zhang J, Zan J, Zhang F, Liu G and
Wu A: Lidocaine improves cerebral ischemia-reperfusion injury in
rats through cAMP/PKA signaling pathway. Exp Ther Med. 20:495–499.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chang YC, Hsu YC, Liu CL, Huang SY, Hu MC
and Cheng SP: Local anesthetics induce apoptosis in human thyroid
cancer cells through the mitogen-activated protein kinase pathway.
PLoS One. 9:e895632014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhu J and Han S: Lidocaine inhibits
cervical cancer cell proliferation and induces cell apoptosis by
modulating the lncRNA-MEG3/miR-421/BTG1 pathway. Am J Transl Res.
11:5404–5416. 2019.PubMed/NCBI
|
|
38
|
Chen Z, Chen Y, Zhou J, Li Y, Gong C and
Wang X: Netrin-1 reduces lung ischemia-reperfusion injury by
increasing the proportion of regulatory T cells. J Int Med Res.
48:3000605209264152020.PubMed/NCBI
|
|
39
|
Lan CC, Peng CK, Tang SE, Huang KL and Wu
CP: Carbonic anhydrase inhibitor attenuates ischemia-reperfusion
induced acute lung injury. PLoS One. 12:e01798222017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
De Perrot M, Liu M, Waddell TK and
Keshavjee S: Ischemia-reperfusion-induced lung injury. Am J Respir
Crit Care Med. 167:490–511. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
De Perrot M, Sekine Y, Fischer S, Waddell
TK, McRae K, Liu M, Wigle DA and Keshavjee S: Interleukin-8 release
during early reperfusion predicts graft function in human lung
transplantation. Am J Respir Crit Care Med. 165:211–215. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ng CS, Wan S, Arifi AA and Yim AP:
Inflammatory response to pulmonary ischemia-reperfusion injury.
Surg Today. 36:205–214. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang F, Wang F, Li F, Wang D, Li H, He X
and Zhang J: Methane attenuates lung ischemia-reperfusion injury
via regulating PI3K-AKT-NFκB signaling pathway. J Recept Signal
Transduct Res. 40:209–217. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jiang F, Zhang Y and Dusting GJ: NADPH
oxidase-mediated redox signaling: Roles in cellular stress
response, stress tolerance, and tissue repair. Pharmacol Rev.
63:218–242. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xu Y, Li X, Cheng Y, Yang M and Wang R:
Inhibition of ACSL4 attenuates ferroptotic damage after pulmonary
ischemia-reperfusion. FASEB J. 34:16262–16275. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Friedmann Angeli JP, Schneider M, Proneth
B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch
A, Eggenhofer E, et al: Inactivation of the ferroptosis regulator
Gpx4 triggers acute renal failure in mice. Nat Cell Biol.
16:1180–1191. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Matsushita M, Freigang S, Schneider C,
Conrad M, Bornkamm GW and Kopf M: T cell lipid peroxidation induces
ferroptosis and prevents immunity to infection. J Exp Med.
212:555–568. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mayr L, Grabherr F, Schwärzler J,
Reitmeier I, Sommer F, Gehmacher T, Niederreiter L, He GW, Ruder B,
Kunz KTR, et al: Dietary lipids fuel GPX4-restricted enteritis
resembling Crohn's disease. Nat Commun. 11:17752020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Muller T, Dewitz C, Schmitz J, Schröder
AS, Bräsen JH, Stockwell BR, Murphy JM, Kunzendorf U and Krautwald
S: Necroptosis and ferroptosis are alternative cell death pathways
that operate in acute kidney failure. Cell Mol Life Sci.
74:3631–3645. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li Y, Feng D, Wang Z, Zhao Y, Sun R, Tian
D, Liu D, Zhang F, Ning S, Yao J and Tian X: Ischemia-induced ACSL4
activation contributes to ferroptosis-mediated tissue injury in
intestinal ischemia/reperfusion. Cell Death Differ. 26:2284–2299.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yang ZH, Lu YJ, Gu KP, Xiang ZY and Huang
HM: Effect of ulinastatin on myocardial ischemia-reperfusion injury
through JNK and P38 MAPK signaling pathways. Eur Rev Med Pharmacol
Sci. 23:8658–8664. 2019.PubMed/NCBI
|