Introduction
Ovarian cancer has been the greatest challenge in
gynecological oncology, constituting a group of heterogeneous and
rapidly progressing neoplasm with high mortality, the pathogenesis
of which has remained to be fully elucidated (1,2).
The major subtypes of ovarian cancer are high-grade serous
carcinoma, endometrioid carcinoma, clear cell carcinoma, low-grade
serous carcinoma and mucoid carcinoma.
Immunological response disorders have an important
role in the course of such malignancies, involving the so far
poorly recognized lectins called galectins (3,4).
Galectins have been the focus of multiple studies, basically due to
their substantial role in numerous physiological processes as well
as pathologies, e.g. apoptosis, angiogenesis, adhesion,
immunological and inflammatory response, as well as the formation
and progression of certain neoplastic lesions (5,6).
Various studies also indicated that certain galectins may
potentially serve as diagnostic and prognostic markers, rendering
them targets of anticancer therapies (7–10).
Therefore, the major objective of the present review was to discuss
the role of selected galectins and their possible clinical
applications in ovarian cancer.
The following galectins have been observed to take a
significant role in the formation and progression of ovarian
cancer: Galectin-1, 3, 7, 8 and 9.
Galectin-1
Galectin-1, encoded by the LGALS1 gene located on
chromosome 22q12, is a 14 kDa monomer or a non-covalent homodimer
with one conserved carbohydrate-recognition-binding domain (CRD)
per subunit. The presence of more than one CRD in the homodimer
makes it suitable for mediation of cell adhesion, triggering the
intracellular signaling and forming multivalent lattices with the
cell surface glycoconjugates. In the extracellular matrix (ECM),
homodimers are able to link several membrane receptors, therefore
facilitating cell signaling and cell-cell interactions, which
allows for homotypic and heterotypic aggregation (11).
Galectin-1 takes a substantial part in both
physiological and pathological processes. Expression of galectin
takes place in multiple tissues, usually in the skeletal muscle
cells, the thymus gland, lymph nodes, neurons, the kidneys, the
skin and the placenta, where it is present both intracellularly and
extracellularly in the stroma of such organs. The extracellular
effect of that protein is closely associated with the CRD activity
through their effect on neutrophil surface receptors, as well as
components of the extracellular matter, such as integrin, laminin,
cancer fetal antigen, fibropontin, osteopontin, thrombospondin and
vitronectin (12–14). Extracellular galectin-1 has a
molecular weight (~15 kDa) that is slightly higher than the 14 kDa
form found in cell lysates, suggesting that the secreted galectin-1
undergoes further post-transitional modifications prior to or after
secretion (15). It was
demonstrated that extracellular 15 kDa galectin-1 was able to bind
cell surfaces at specific locations. Since galectin-1 lacks the
required signaling peptide for secretion, it was suggested that
post-translational modifications, resulting in a high molecular
mass of galectin-1, were required for galectin-1 to be exported to
the extracellular compartment (15). The intra cellular protein is found
in the nucleus, cytoplasm and the inner leaflet of the cytoplasmic
membrane and, similar to other members of this family, secreted
into the extracellular space despite the lack of the signaling
sequences required for secretion via the standard endoplasmic
reticulum/Golgi pathway (16).
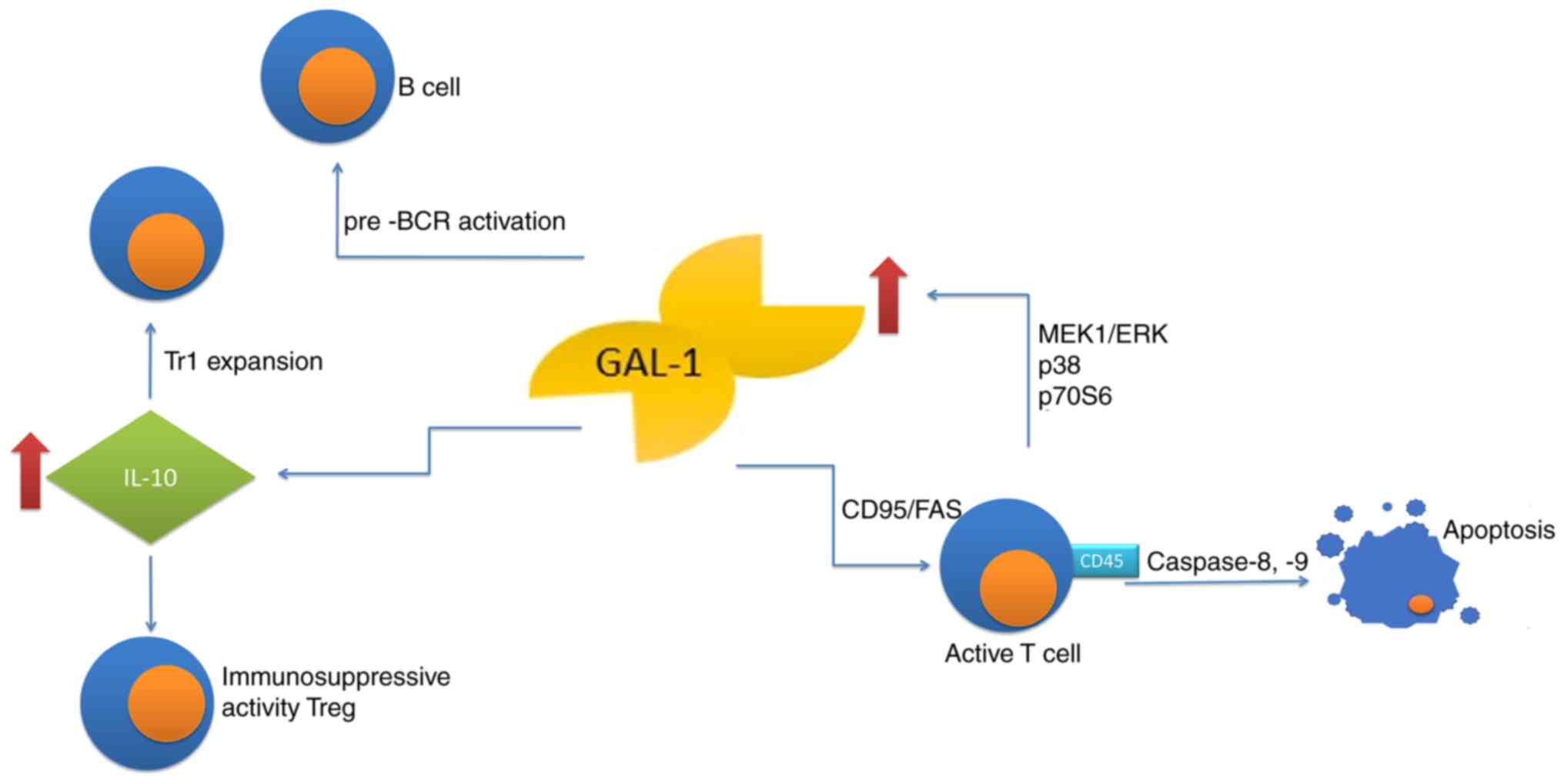
Galectin-1 is an anti-inflammatory protein,
inhibiting the synthesis of pro-inflammatory cytokines, affecting
migration and function of neutrophils, eosinophils, macrophages and
dendritic cells as well as inhibiting the degranulation of mast
cells. Likewise, the lectin controls T-cell and B-cell compartments
by modulation of the receptor clustering and signaling, therefore
taking the role of a negative-regulatory checkpoint to reprogram
the cellular activation, differentiation and survival (17). Under physiological conditions,
that protein promotes apoptosis of the activated, yet not resting
immune cells (18,19), with a notable exception of resting
T cells, which are sensitized to CD95/Fas-mediated cell death
(20). Galectin-1 also induces
phosphatidyl-serine externalization with no associated apoptosis
(21,22). Furthermore, galectins may
cross-link glycosylated proteins, leading to signal transduction
and direct cell death or activation of other signals regulating
cell fate (13,23). Apoptosis of activated
(antigen-primed) T-cells is induced by galectin-1 in a
CD45-dependent manner, while T-cell homeostasis may be regulated by
galectin-1 through inhibition of clonal expansion and induction of
apoptosis. Activated T-cell apoptosis induced by galectin-1 is
caspase-8- and −9-dependent (24). Of note, the activated T-cells
alone may produce galectin-1 through the MEK1/ERK, p38 MAP kinase
and p70S6 kinase signaling pathways, suggesting an autocrine
suicide mechanism used to terminate the effector immune response
(25). This glycan-binding
protein inhibits the immune effector functions by shifting the
balance towards a type 2 T-helper (Th2) cytokine profile (26,27), through selective deletion of Th1
and Th17 cells (28) and by
promotion of differentiation of tolerogenic dendritic cells
(29–31). Furthermore, the protein
facilitates the expansion of IL-10-producing T regulatory type-1
cells (32) and contributes to
immunosuppressive activity of
CD4+CD25+FoxP3+ T regulatory
(Treg) cells (33,34). Galectin-1 also affects the humoral
response, promoting maturation and differentiation of B lymphocytes
in the bone marrow and stimulating the secretion of antibodies
(13). In such cases, galectin-1
secreted by the bone marrow stromal cells, supports B-cell
differentiation through pre-B cell receptor (BCR) activation and
signaling (35,36). Furthermore, using an in
vitro model of lipopolysaccharide-activated B cells, Tsai et
al (37) determined that
splenic plasma blasts expressed galectin-1 in a Blimp-1 dependent
manner. They also indicated that ectopic expression of galectin-1
in mature B cells increased the immunoglobulin transcripts and
secretion (Fig. 1).
Role of galectin-1 in ovarian cancer
Chen et al (38) indicated a relation between the
increased expression of galectin-1 and elevated migration of cancer
cells and tumor invasiveness in epithelial ovarian cancer (EOC).
High expression of that protein correlated positively with the
Internal Federation of Gynecology and Obstetrics (FIGO) staging and
more frequent relapse of the disease. Galectin-1 expression is
associated with activation of transcription factor NF-κBp65,
resulting in poor prognosis in ovarian cancer. Activation of the
NF-κBp65 signaling pathway results in elevated expression of matrix
metalloproteinases (MMPs), MMP-9 and MMP-2 in particular. The
activity of such ECM-degrading MMPs enhances malignant metastasis.
However, the migratory and invasive abilities were significantly
reduced after galectin-1 knockdown in the human EOC cell line
HO8910, which was accompanied by suppressed NF-κB pathway
activation and downregulation of MMP-2 and MMP-9 (38). This suggests that lower expression
of galectin-1 inhibits activation of the NF-κBp65 signaling
pathway, reduces the expression of MMPs and also restricts the
metastatic capacity of the cancer cells (38).
Zhang et al (39) also evaluated the expression of
galectin-1 in EOC. They indicated that elevated expression of this
protein correlated positively with the cancer stage, which may
promote growth, migration and invasion of the ovarian cancer cells,
as well as their resistance to cisplatin. Galectin-1 was able to
upregulate c-Jun, MMP-9, Bcl-2 and p21 expression, possibly through
activation of H-Ras/Raf/ERK pathway. That protein also may be
considered a potential therapeutic target to delay EOC progression
and to increase sensitivity to cisplatin.
A study was performed by Kim et al (40), who evaluated the effect of
galectin-1 expression and its functional role in cell proliferation
and invasion in EOC. They observed that no expression of galectin-1
was present in normal ovarian tissues, while the protein was
expressed in EOC tissues. Furthermore, the study revealed that
galectin-1enhanced the proliferation and invasion of cancer cells.
It was also demonstrated that treatment of EOC cells with
galectin-1 short interfering (si)RNA and anginex inhibited cell
proliferation and invasion in vitro. These results suggest
that high galectin-1 expression may be a prognostic marker of EOC
and that galectin-1 may be a potential therapeutic target to
decrease tumor aggressiveness.
Furthermore, Schulz et al (41) analyzed galectin-1 expression in
females with ovarian cancer. The patients with overexpression of
galectin-1 in the cytoplasm and extracellularly had shorter overall
survival, while higher expression of galectin-1 in the cytoplasm
only was associated with higher metastatic potential of the cancer
cells. Elevated expression of galectin-1 was present in both, the
cell nucleus and cytoplasm of the ovarian cancer cells, as well as
within the tumor stroma. The clinical significance of serum
galectin-1 expression in patients with the EOC was evaluated by
Chen et al (42). The
authors reported increased galectin-1 expression in EOC,
emphasizing the clinical significance of galectin-1 expression in
sera and cancer-associated stroma, which may contribute to cancer
progression.
Galectin-1 may be a key factor in the interaction of
the tumor stroma with EOC where detection of increased serum
galectin-1 in certain patients with cancer may reflect various
aspects of the biological behavior of the tumor associated with a
metastatic phenotype. Further studies are required to determine the
clinical value of circulating galectin-1 in patients with
early-stage cancer to be used as a predictor of tumor invasion and
metastasis. The results of the previous studies suggested the role
of galectin-1 as a novel prognostic and progressive biomarker in
patients with EOC. Of note, Abdelwahab et al (43) evaluated the serum concentration of
galectin-1 and its expression in patients with different stages of
serous type ovarian cancer (SOC) in an attempt to define its value
as a diagnostic and prognostic marker. The results indicated that
the serum levels of galectin-1 were significantly associated with
the FIGO stage of SOC (P<0.001) and were higher in stage III/IV
as compared to stage I. Immunohistochemical staining indicated that
high expression of galectin-1 was more frequent in patients with
advanced stage as compared to the early stages, as it was present
in 37.5, 50, 68.8 and 83.3% of cases in stage I, II, III and IV,
respectively. These results support the usefulness of galectin-1
immunohistochemical expression in peri-tumoral cells as a
prognostic biomarker of successful treatment or possible
chemotherapeutic resistance. The results may suggest that
galectin-1 is overexpressed in serum and tumor tissues of patients
with SOC upon progression of the disease; this may support its
usefulness as a non-invasive biomarker for diagnosis and prognosis
of such patients (43).
Galectin-1 expression may be a potential prognostic
marker, the value of which may depend on cell localization.
Furthermore, evaluation of serum concentrations of this protein may
serve as a novel, non-invasive biomarker in the diagnosis and
prognosis of the therapeutic outcome.
Galectin-3
Galectin-3 is among the best recognized lectins with
a chimeric structure. The coding gene LGALS3 is localized on
chromosomes 1p13 and 14q21–22 (44,45) and the mass of this protein is
29–35 kDa. A characteristic feature is the presence of C-terminal
domain recognizing carbohydrates (CRD), composed of ~130 amino
acids and an N-terminal domain. The C-terminal domain frequently
has the following sequence: Asparagine, tryptophan, glycine,
arginine (NWGR). This chain is responsible for the anti-apoptotic
activity of galectin-3, where the structural changes prevent the
binding of galectin by the CRD domain (46). The CRD domain has a β-sandwich
structure composed of 11 β strands: β1-β11 positioned antiparallel
to each other. The β1, β3–6 and β10 strands form a sugar binding
surface, the S-face, while the remaining five, namely β2, β7–9 and
β11, form an antiparallel surface, the F-face (7). CRD contains the death domain
(anti-death motif, NWGR), conditioning the anti-apoptotic functions
of galectin-3 and BCL-2 and the effect of the protein with BCL-2
proteins. NGWR, i.e. the sequence
aspartate-tryptophan-glycine-arginine, is highly conserved, while
substitution of glycin with alanine reduces the anti-apoptotic
effect of galectin-3 (47,48).
The N-terminal domain (NTD) contains a region composed of multiple
repetitions of glycine, proline, tyrosine and alanins referred to
as a collan-like sequence, as well as a short
NH2-terminal region composed of 12 amino acids, which is
the site of phosphorylation. NTD contains sites sensitive to the
effects of MMP-2 and MMP-9, for which it is an endogenic substrate.
Structural changes in the NTD inhibit the expression of galectin-3
and the anti-apoptotic effect of the protein. The NTD structure
makes it possible for galectin-3 to form pentamers thanks to which
galectin forms networks with glycoproteins and glycolipids
(48–50).
The expression of this protein was proven in
monocytes, macrophages, neutrophils, fibroblasts, activated T
lymphocytes, eosinophils, basophils, mast cells, epithelial cells
of the respiratory system, the kidneys, the alimentary tract as
well as cancer cells, where the level of expression of this protein
was associated with progression, invasion and the metastatic
ability of the cells (51,52).
The presence of galectin-3 is both intracellular-in the cytoplasm,
mitochondria, the cell membrane and nucleus, as well as
extracellular, which depends on its biological function (53). Acting together with its ligands,
galectin-3 has different biological roles, such as cell growth
regulation, pre-mRNA folding, effect on differentiation,
transformation and cell motility, transduction of the cell signal,
inhibition or induction of apoptosis, as well as modulation of the
body's immunological response (41,54). It has a role in the formation of
tumors and metastasis through upregulation of proliferation, cell
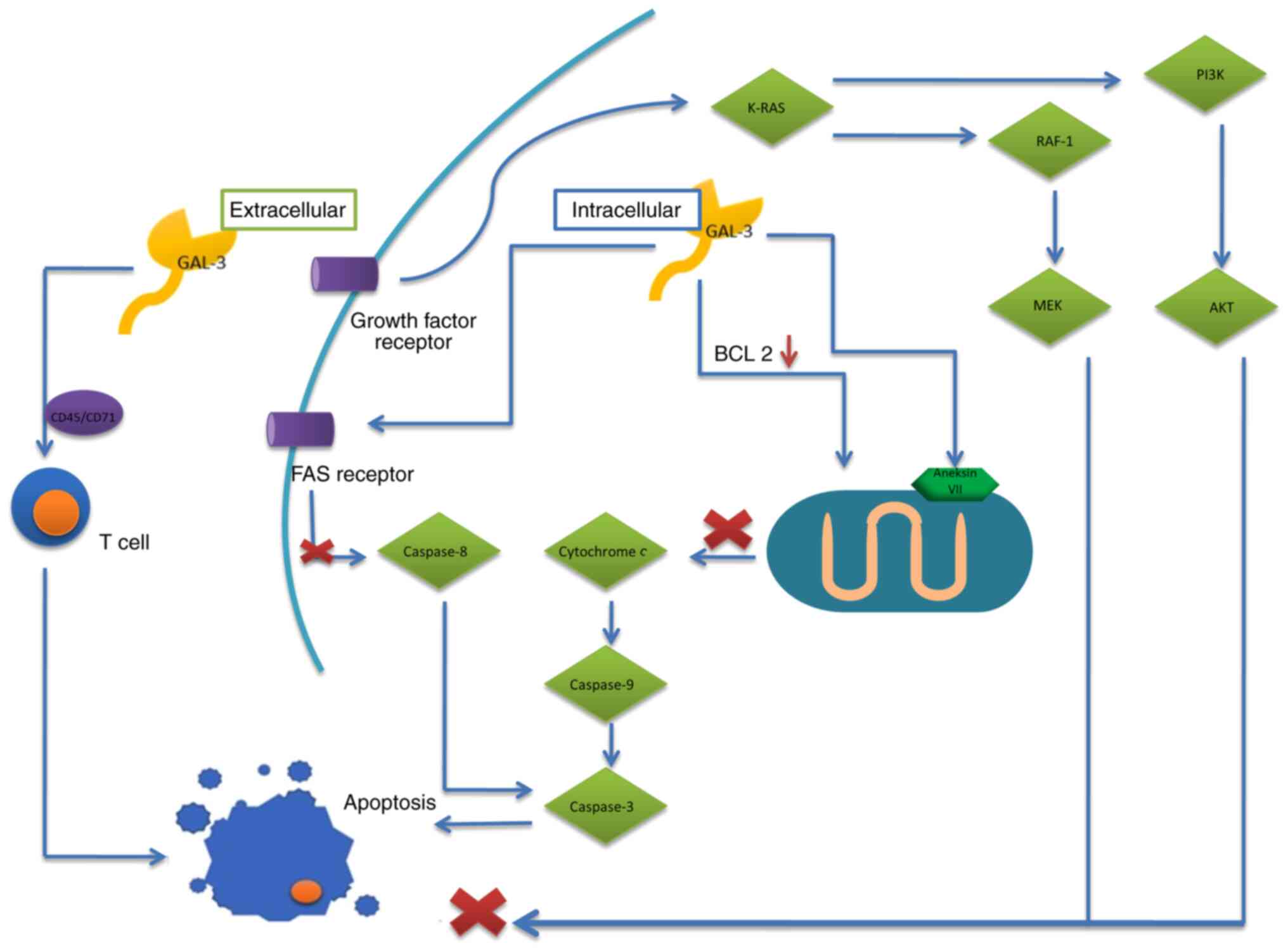
migration, angiogenesis, apoptosis and adhesion (49,53). Galectin-3 has diverse effects on
apoptosis, depending on the localization of this protein: In the
cytoplasm the effect is anti-apoptotic, while if located
extracellularly, it exerts apro-apoptotic effect. The
anti-apoptotic effect of this galectin results, among others, from
phosphorylation and dephosphorylation at theSer6
position and the presence of NWGR (7,47).
The galectin directly affects the anti-apoptotic proteins, members
of the BCL-2 family, the activation of which prevents the
expression of cytochrome c and leads to inhibition of
apoptosis. It has also been observed that reduced cell expression
of galectin-3 resulted in lower concentrations of BCL-2, therefore
increasing the pro-apoptotic potential (48,55). In addition, the effect of
galectin-3 on annexin VII, a protein reversibly binding calcium
ions and phospholipids, leads to inhibition of the release of
cytochrome c from the mitochondrion due to the transport of
nuclear galectin-3 into the region of perinuclear mitochondria
(7). Another mechanism to inhibit
apoptosis is the binding of cytoplasmic galectin-3 with CD95/Fas
receptor, leading to inhibition of caspase-8 activity and the
pro-apoptotic cascade. That protein also binds to activated K-RAS,
enabling activation of the anti-apoptotic survival pathways
K-RAS/PI3K/Akt and K-RAS/Raf-1/MEK (47,55). On the other hand, the
pro-apoptotic effect of galectin-3 includes, among others, the
influence on T lymphocytes. Extracellular galectin-3 affects
receptors CD45 and CD71, inducing apoptosis in human T lymphocytes.
When present within T lymphocytes, that protein has an
anti-apoptotic effect, promoting cell growth and enhancing TCR
signaling (56). Galectin-3
influences cell adhesion through binding of glycoproteins occurring
on the cell surface, e.g. integrins and cadherins. Initiation of a
signaling pathway enables adhesion of cancer cells to the stroma
(Fig. 2) (48). Galectin-3 also facilitates
neo-angiogenesis, primarily through VEGF and basic fibroblast
growth factor, as well as modified N-glycans on integrin αvβ3. By
activating the signaling pathways dependent on cytoplasmic tyrosine
kinase, focal adhesion kinase, it modulates the migration of the
endothelial cells (55).
Role of galectin-3 in ovarian cancer
The results obtained so far when evaluating the
expression of galectin-3 in ovarian cancer are contradictory,
indicating both elevated or reduced expression of the protein, as
compared to its expression in normal ovarian tissue. Lee et
al (57) evaluated the
expression of galectin-3 in ovarian cancer. Their results indicated
that the expression of galectin-3 was higher in clear cell
carcinoma as well as serous and mucous tumors than in endometrial
and transient ones. However, no differences in galectin-3
expression among benign, borderline, malignant mucous and serous
tumors were obtained. Various noteworthy observations were also
made by Kim et al (58),
who evaluated the clinical role of galectin-3 expression in
patients with EOC and its functional role in the proliferation of
an ovarian cancer cell line. The results pointed to an increased
expression of galectin-3 in the EOC with the absence of expression
of this protein in normal ovarian tissues. Furthermore, elevated
expression of galectin-3 was associated with shorter
progression-free survival (PFS). The studies suggested that
galectin-3 expression maybe a prognostic factor for PFS and may be
involved in the regulation of the response to paclitaxel
(PTX)-based chemotherapy in the treatment of EOC. Similarly,
Brustman (59) analyzed the
expression of the epidermal growth factor receptor (EGFR),
galectin-3 and cyclin D1 in a cohort of patients with ovarian
serous carcinomas with regard to outcome and clinicopathological
parameters. Evaluation of EGFR and cytoplasmic galectin-3
immunohistochemically indicated that testing for multiple markers
maybe an adjunct in the identification of high-risk ovarian serous
cancers.
A study by Lu et al (60) revealed that inhibitors of the
NF-κB pathway did not affect galectin-3 expression levels in
ovarian cancer cells. That protein was able to regulate the
migratory and invasive capabilities of cancer cells, as well as
chemosensitivity to carboplatin in EOC. The results indicated that
galectin-3 may be a potential novel therapeutic target in different
types of ovarian cancer and may have a role in chemosensitivity to
common chemotherapeutic drugs.
Kang et al (61) reported that overexpression of the
gene LGALS3 increased the ovarian cancer cell invasion, migration
and proliferation, while silencing of that protein with specific
siRNA reversed these biological effects. The Notch signaling
pathway was strongly activated by galectin-3 overexpression in
A2780 cells. Silencing of galectin-3 reduced the levels of cleaved
NICD1 and expression of the Notch target genes, Hes1 and Hey1.
Thus, galectin-3 may be a potent target for regulating Notch1
signaling as a therapeutic strategy for ovarian cancer. In an
endeavor to develop innovative and efficient therapies, Mirandola
et al (62) generated a
truncated, dominant-negative form of galectin-3, namely galectin-3C
and applied it to ovarian cancer cell lines; furthermore, primary
cells were established from patients with ovarian cancer. The
results indicated that galectin-3C significantly reduced the
growth, motility, invasion and the angiogenic potential of the
cultured ovarian cancer cell lines and primary cells established
from patients with ovarian cancer. Eliaz (63) presented a case of stage IV ovarian
cancer for whom underlying pro-inflammatory comorbidities and the
concentration of galectin-3 were monitored throughout the therapy.
Initially, the patient's inflammatory condition was treated with an
intensive integrative anti-inflammatory protocol using a
combination of oral and intravenous nutrients and botanicals, along
with pharmaceutical intervention. This was followed by a standard
course of chemotherapy supported by an individualized integrative
protocol. Galectin-3 levels as well as other inflammatory and tumor
markers were monitored throughout the course of treatment. This
case report was the first to demonstrate the clinical use of
galectin-3 and its potential ability to reflect changes in both the
cancer status and the inflammatory state of the patient. For the
first time, galectin-3 was used to assess and monitor the patient's
progress. However, the literature available fails to provide any
explicit evidence of the role of galectin-3 as a marker and
promotor of an inflammatory condition or cancer progression. The
author emphasized the requirement of further studies, which should
encourage clinicians to include the evaluation of the galectin-3
concentration in their therapy monitoring schemes. This may provide
a valuable marker with significant prognostic potential that may be
used to monitor the inflammatory condition, the tumor progression
and response to treatment.
Hossein et al (64) sought to determine the role of
galectin-3 in the chemo-resistance of the human ovarian cancer cell
line SKOV-3 to PTX, where recombinant human (rh)galectin-3 and
PectaSol-C modified citrus pectin (Pect-MCP) as a specific
competitive inhibitor of galectin-3. The results indicated a 41%
increase in cell proliferation, a 36% decrease in caspase-3
activity and a 33.6% increase in substrate-dependent adhesion in
the presence of rhgalectin-3, as compared to the control case
(P<0.001). The treatment of cells with a non-effective dose of
PTX (100 nM) and 0.1% Pect-MCP in combination revealed a
synergistic cytotoxic effect with cell viability reduced by 75% and
a subsequent 3.9-fold increase in caspase-3 activity. Thus,
inhibition of galectin-3 appears to be a useful therapeutic tool
for combined therapy of ovarian cancer. In their subsequent study,
Hossein et al (65)
attempted to determine the relationship between STAT3 activity and
galectin-3 and investigated the cytotoxic effect of Pect-MCP as a
specific competitive inhibitor of galectin-3 in combination with
PTX to kill SKOV-3 ovarian cancer cell multicellular tumor
spheroids (MCTS). That study was the first to demonstrate enhanced
STAT3 phosphorylation upon addition of exogenous galectin-3 to
ovarian cancer cells. Furthermore, the study revealed that both the
increased level of galectin-3 expression and STAT3 phosphorylation
were associated with MCTS size. The authors also proved that
galectin-3-mediated STAT3 phosphorylation was abrogated or reduced
in the presence of PTX + Pect-MCP in an MCTS size-dependent manner.
Higher expression of galectin-3 in MCTS maybe related to PTX
resistance through the increased phosphorylated (p-)STAT3 levels
and hypoxia-inducible factor-1α may further increase galectin-3
expression levels. For the first time, it was demonstrated that
Pect-MCP may be considered as a potential drug to enhance the
effect of PTX on ovarian cancer cell MCTS through inhibition of
STAT3 activity. Cai et al (66) investigated the effect of
galectin-3 on Toll-like receptor 4 (TLR4) signaling and thus PTX
resistance and identified associations between serum galectin-3
levels or TLR4 expression and a PTX resistance phenotype. In
vitro treatment with exogenous galectin-3 restored cell
survival and migration of SKOV-3 and ES-2 cells, which was
decreased by galectin-3 silencing and PTX treatment. Furthermore,
the protein suppressed the interaction between TLR4 and caveolin-1
(Cav-1) in SKOV-3 and ES-2 cells. In addition, overexpression of
Cav-1 dampened the expression of MyD88 and p-p65 stimulated by
galectin-3 and enhanced apoptosis in SKOV-3 cells under PTX
exposure. The results indicated that exogenous galectin-3 may
induce PTX resistance through TLR4 signaling activation by
inhibiting the TLR4-Cav-1 interaction (66). Wang et al (67) related the mitochondrial function
in galectin-3-mediated regulation to cisplatin resistance in
ovarian cancer OVCAR-3 cells. The study indicated that
cisplatin-promoted cytochrome c release from mitochondria,
mitochondrial reactive oxygen species and superoxide were markedly
inhibited by galectin-3 overexpression, while they were aggravated
by galectin-3 knockdown. The cisplatin-downregulated MMP was also
blocked by galectin-3 overexpression, while it was deteriorated by
galectin-3 knockdown. It was suggested that galectin-3 inhibits the
sensitivity of human EOC OVCAR-3 cells to cisplatin via inhibition
of cisplatin-mediated growth reduction, induction of apoptosis and
dysfunction of the mitochondria. This implies that galectin-3 maybe
an effective target to sensitize ovarian cancer cells to
chemotherapy.
Various noteworthy observations were made by Bieg
et al (68) who assessed
whether morin, a natural flavonoid, and a recognized NF-κB
inhibitor are able to sensitize ovarian cancer cells to cisplatin
through suppressed expression of galectin-3. They indicated that
morin exhibited antineoplastic activity towards the ovarian cancer
cell lines TOV-21G and SKOV-3 in terms of reduced cell viability
and proliferation, as well as increased induction of apoptosis.
Morin sensitized ovarian cancer cells TOV-21G and SKOV-3 to
cisplatin, which was associated with lower expression of
galectin-3. Of note, combined treatment with selected
concentrations of morin and cisplatin had a synergic effect; morin
sensitized the cells to cisplatin, which may be used to reduce the
required therapeutic dosage in the future.
Another study by Bieg et al (69) reported that miR-424-3p suppressed
galectin-3 expression and sensitized ovarian cancer cells to
cisplatin. They indicated that miR-424-3p mimics sensitized the
ovarian cancer cell lines TOV-21G and SKOV-3 to cisplatin through
decreased expression of galectin-3. The authors suggested
miR-424-3p as a useful candidate for combined treatment with
cisplatin.
The role of galectin-3 expression in different
malignancies remains controversial. However, it appears that
galectin-3 maybe a promising target to develop novel diagnostic and
therapeutic strategies in oncology.
Galectin-7
Galectin-7 is a protein with a molecular weight of
15 kDa; it is a member of the prototype galectin subgroup, encoded
by the LGALS7 gene localized on chromosome 19q13.2 (70,71). It occurs as a monomer or a
symmetric homodimer, the structure of which is stabilized by
electrostatic activity between two monomers (7). As a monomer, galectin contains one
conserved CRD and the homodimer contains two identical CRDs
(72). Contrary to galectin-1 and
galectin-3, galectin-7 has high tissue specificity. The expression
is manifested in the heart muscle cells, epithelium of the
alimentary system, fetal tissues, skin keratinocytes and other
epithelial tissues (70,73). Similar to other galectins,
galectin-7 has a role in the proliferation, adhesion, migration,
apoptosis and modulation of the immunological system response.
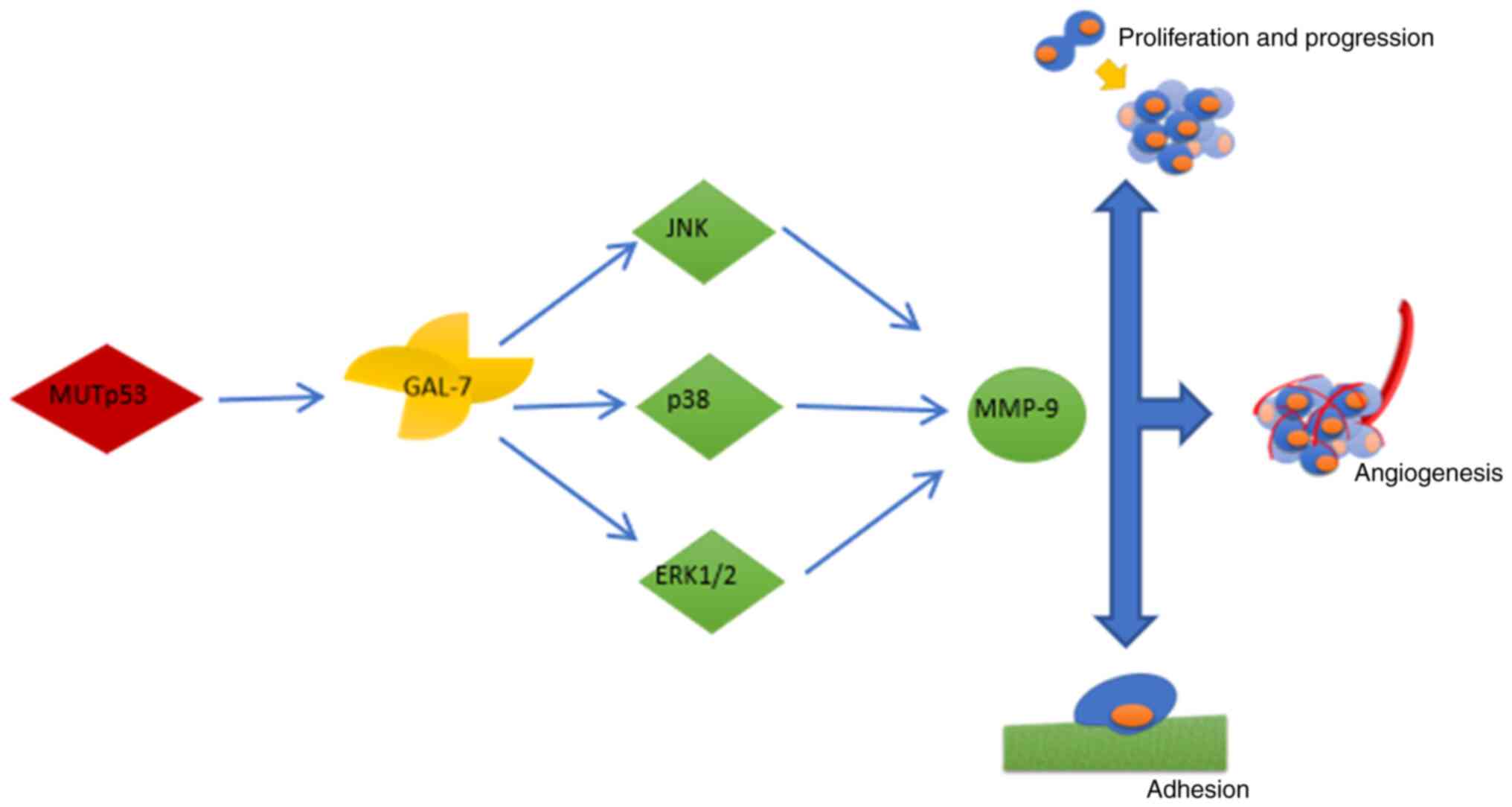
Galectin-7 has an oncogenic effect; however, in certain tumor
types, it may also have an anti-carcinogenic role. It is suggested
that increased expression of galectin-7 in cancer is induced by a
muted form of p53 suppressor protein, which is present in certain
types of tumor (41,74). Higher expression of galectin-7
might be involved in the regulation of carcinogenesis, contributing
to the induction of apoptosis in tumor infiltrating T cells, both
DC4+ and CD8+ as well as in regulation
associated with macrophages, NK cells and dendritic cells (74,75).
Galectin-7 also contributes to increased migration
of cancer cells, which is associated with reduced cell adhesion,
through enhancing the activity of MMP-2 and MMP-9; this contributes
to tumor progression and distant metastasis. Recent studies
highlight the importance of the relationship between galectin-7 and
p53 (76). The signaling pathways
affected by galectin-7 to enhance proliferation, migration and
invasiveness of cancer cells, are associated with kinases activated
by mitogens through increasing the phosphorylation of ERK1/2 and
JNK1/2 (77). The greater
capacity of cancer cells to migrate is associated
withgalectin-7-induced expression of the COL4α1 gene encoding chain
α of type IV collagen and intracellular adhesion molecule 1
(71). It also activates the
signaling pathway associated with serine-threonine kinase Akt and
its effector, PI3K. The PI3K/Akt pathway has a particular role in
neoplastic diseases, enhancing the viability and proliferation of
cancer cells (Fig. 3) (70,78).
Role of galectin-7 in ovarian
cancers
The studies performed so far focused primarily on
the role of galectin-1 and −3 in ovarian cancer, while
investigations regarding the role of galectin-7 in such
malignancies have been scarce. The first to come was a study by Kim
et al (79) who evaluated
the prognostic significance of galectin-7 in patients with EOC and
its functional role in cell proliferation in an ovarian cancer cell
line. The results pointed to upregulated galectin-7 expression as
compared to normal ovarian tissues, as well as an association of
high galectin-7 expression with older age, high mortality and poor
overall survival outcome. Furthermore, the residual tumor volume
was larger in the high-expression group as compared to the low
expression group. As indicated by in vitro results,
knockdown of galectin-7 expression by using its siRNA inhibited
proliferation of the tumor cells. High galectin-7 expression was
suggested to be related to poor prognosis in EOC and that protein
may have a possible functional role in cell proliferation. Future
studies should address the potential use of galectin-7 as a useful
therapeutic target in the treatment of EOC. Labrie et al
(74) evaluated the expression of
galectin-7 in EOC cells. Immunochemical analysis of galectin-7
expression in a tissue microarray suggested no presence of that
protein in normal ovarian tissues; however, galectin-7 was present
in the epithelial cells of all histological EOC subtypes, where
expression in malignant tumors was significantly higher. The
results indicated that elevated expression of galectin-7 was
associated with tumor progression through increased invasiveness of
the cancer cells and a pro-apoptotic effect on cells of the
immunological system. The authors pointed to the clinical
significance of galectin-7 overexpression in ovarian cancer and
provided a rationale for targeting galectin-7 to improve the
clinical outcome (74).
Furthermore, Bibens-Laulan and St-Pierre (80) evaluated the expression of that
protein in ovarian and breast cancer. Their results suggested that
targeting extracellular galectin-7 with either CRD-specific
inhibitors or dimer-disrupting peptides may be more efficient for
targeting intracellular galectin-7-mediated interactions than
previously expected. The study indicated that extracellular
galectin-7 controlled the intracellular pool of galectin-7. It does
so via two distinct, yet complementary mechanisms: First, by
increasing the transcriptional activation of LGALS7 gene
transcription, and furthermore, via re-entry into the cells. Schulz
et al (41) confirmed
cytoplasmic galectin-7 expression as a negative prognostic factor
for ovarian cancer. Galectin-7 may thus be a novel, promising and
specific therapeutic target for EOC.
Galectin-8
Galectin-8 is a ‘tandem-repeat’-type galectin, which
contains two carbohydrate recognition domains connected by a linker
peptide. The complexity of galectin-8 is related to the alternative
splicing of its mRNA precursor, known to generate isoforms.
Regarding its carbohydrate-binding specificity, galectin-8 has a
unique feature among the galectins: Its C-terminal domain has a
higher affinity for N-glycan-type branched oligosaccharides, while
the N-terminal domain has a strong affinity for α2-3-sialylated or
3′-sulfated β-galactosides (81).
The LGALS8 gene covers 33 kbp of the genomic DNA. It is localized
on chromosome 1 (1q42.11) and contains 11 exons. Through
alternative splicing, the gene produces 14 different transcripts,
altogether encoding 6 proteins (82). That protein is expressed both in
the cytoplasm and the nuclei of the vascular endothelial cells of
normal and tumor-associated blood vessels, as well as in lymphatic
endothelial cells (83). This
galectin has both pro- and anti-adhesive functions, depending on
its subcellular localization (84–86). Galectin-8 acts as an ECM protein,
positively or negatively regulating cell adhesion, depending on the
extracellular context, as well as on the cell surface
counter-receptors, such as the integrins (82,84,85).
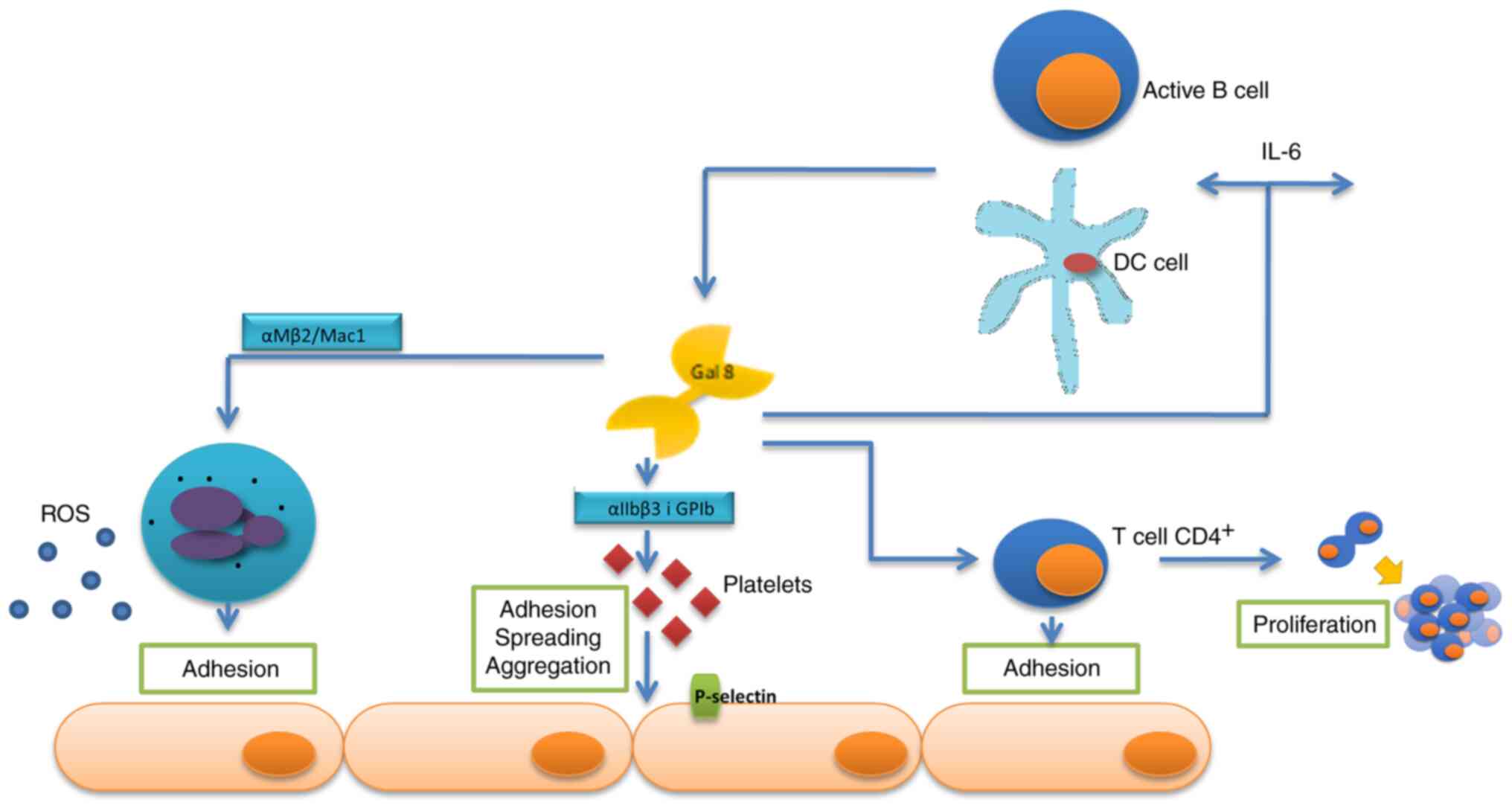
The impact of galectin-8 on the inflammatory system
is highly complex. Under inflammatory stimulation, galectin-8 is
secreted by the endothelium and exposed on its surface. Galectin-8
itself may activate endothelial cells by increasing their
permeability, releasing inflammatory molecules and inducing
adhesion of resting platelets. The interaction of galectin-8 with
integrin αIIbβ3 and glycoprotein Ib (GPIb) at the platelet surface
triggers adhesion, spreading, aggregation, release of granules
content and P-selectin exposure. Furthermore, galectin-8 interacts
with integrin αMβ2/Mac1in order to induce firm adhesion of
neutrophils and the subsequent transendothelial migration and to
trigger the production of superoxide. Another source of galectin-8
may be activated dendritic cells and B cells, which are able to be
stimulated by lectin to produce proinflammatory cytokines. However,
promotes antigen-independent proliferation of CD4+ T
cells, as well as their adhesion to the endothelium (Fig. 4) (87).
Galectin-8 has a role in autoimmune diseases, such
as rheumatoid arthritis or lupus erythematosus, and modulates tumor
progression (86).
It has been indicated that following secretion,
galectin-8 acts as a matrix protein, equipotent to fibronectin in
the promotion of cell adhesion, by ligating and inducing clustering
of cell surface receptors. Immobilized galectin-8 dose-dependently
enhanced cell adhesion of different cancer cell types, such as
H1299 human non-small cell lung carcinoma cells, and promoted U373
glioblastoma cell migration. Furthermore, soluble galectin-8
induces migration of U87 glioblastoma cells (88).
Galectin-9
Galectin-9 is a protein with a 36 kDa molecular
weight, a member of the tandem-repeat galectins and encoded by the
gene LGAL9, localized on chromosome 17p11.2. Its structure contains
two different CRD groups linked by a peptide with a sequence of ~70
amino acids. The protein may occur as a monomer but also a dimer or
a multimer (7,53,89). Galectin-9 has high tissue
specificity. Its expression is present mostly in the epithelial
cells of the skin and the digestive system (53).
The role of galectin-9 in cancer progression has
remained largely elusive; however, the effect of galectin-9 upon
adhesion, migration and the immunological system suggests its
significant role in tumor development. The protein was indicated to
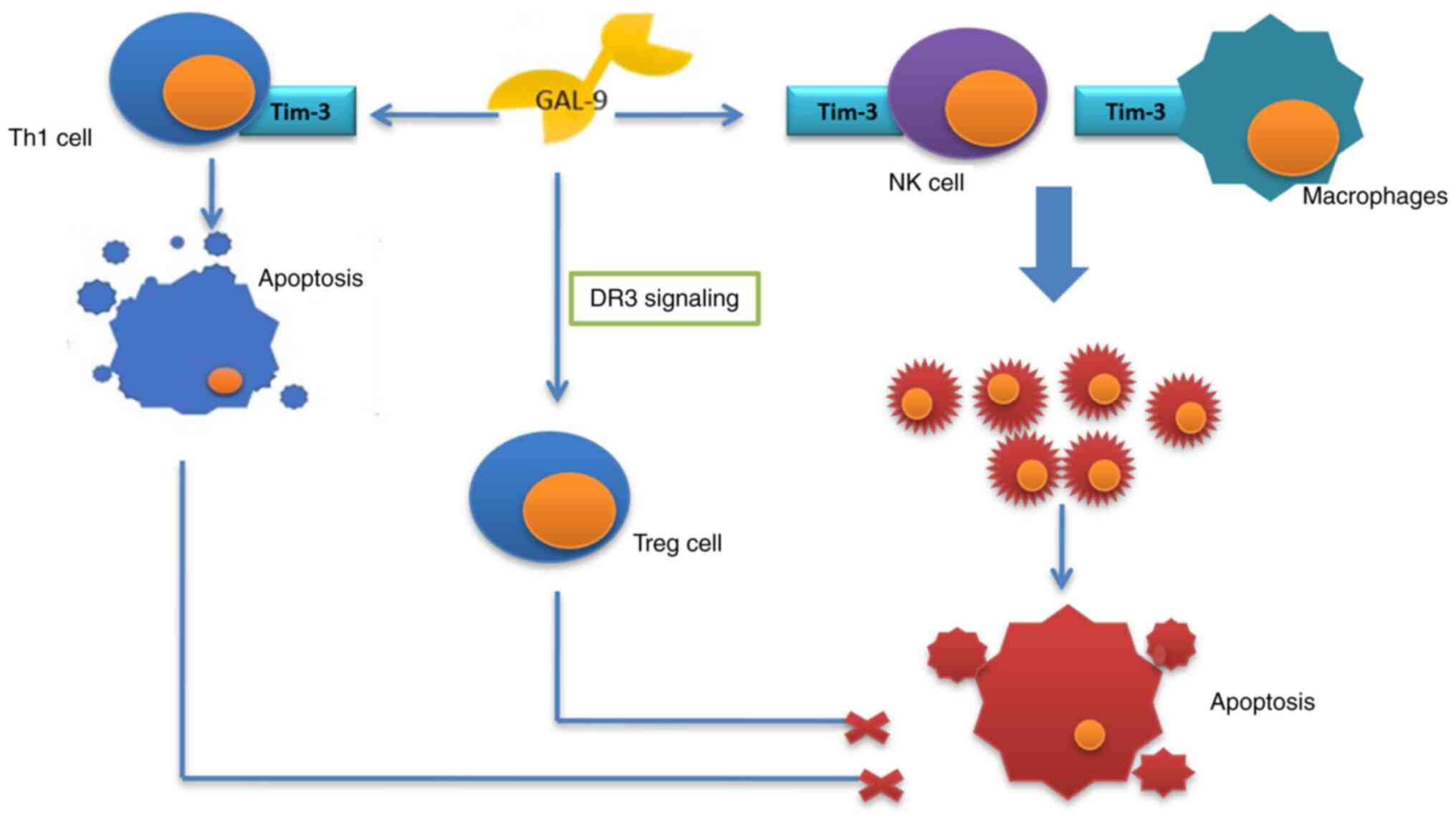
impair the anti-tumor response of the immunological system and also
to stimulate the process (54). A
growing body of evidence proves the anti-tumor function of
galectin-9. Enhancing adhesion of the tumor cells, it prevents
their migration and infiltration of the tissues. It also has a role
to increase apoptosis and suppress proliferation of cancer cells
and supports the cytostatic function of the natural killer cells,
acting together with Tim-3 receptor and stimulating the macrophages
(90,91). The protein may, however, have a
contrary effect, through activation of death receptor 3 (DR3)
signaling, promoting activity of Treg lymphocytes and also,
together with Tim-3 receptor, inducing apoptosis of Th1 lymphocytes
(Fig. 5) (90).
Galectin-8 and −9 in ovarian cancer
Studies evaluating the role of galectin-8 and −9 in
ovarian cancer are scarce. The original study to assess the
expression of these proteins in cancer was performed by Lahm et
al (92). The authors
assessed the expression of the genes of all the currently known
human galectins in tumor cell lines of different histogenetic
origin (galectinomics). The presence of human galectin-1–4 and −7–9
mRNA was monitored by reverse transcription PCR analyses in a panel
of 61 human tumor cell lines derived from a variety of cancer
types, not only ovarian but also breast, colon, lung, brain, skin,
kidney, urogenital system and hematopoietic system cancers. The
results indicated that the most abundantly expressed member of this
lectin family was galectin-8, with 59 positive cell lines. Signals
for galectin-9 appeared in colorectal carcinoma cell lines with a
frequency similar to that of galectin-4 but with certain inter-cell
line differences. Its expression was restricted to cell lines of
this tumor type-the tested ovarian carcinoma and hematopoietic
malignancies (92).
The expression of galectin-8 and −9 in ovarian
cancer was also evaluated by Schulz et al (41) using immunohistochemistry and the
association of these results with clinical patient characteristics
were determined. The impact of the levels of different types of
galectin on disease-free survival and the overall survival was also
assessed. Galectin-8 and −9 staining was detected in the cytoplasm
of the ovarian cancer cells. The predominant staining intensity (0=
negative, 1= low, 2= moderate and 3= strong) and percentage of the
stained cells (0=0%, 1=1-10, 2=11-50. 3=51–80 and 4= 81–100% of the
stained cells) were evaluated and scores were multiplied to obtain
the Remmele immunoreactive score (IRS). In the survival analysis
for galectin-8, patients were classified into low-expression IRS ≤1
and high-expression (IRS>1) groups. In terms of galectin-9,
patients were divided into negative expression (IRS=0), moderate
expression (1≥ IRS ≥6) and high-expression groups. The results
indicated that galectin-8 expression was a positive prognostic
factor for overall and disease-free survival of patients with
ovarian cancer, while galectin-9 expression influenced overall and
disease-free survival in two different ways: Moderate galectin-9
expression correlated with reduced survival as compared to
galectin-9-negative cases, while patients with high galectin-9
expression had the best outcome (41).
Conclusions
The results of the studies performed so far point to
complex roles of galectins-1, −3 and −7–9 in the carcinogenesis of
ovarian cancer. Elucidation of the phenomenon may contribute to the
development of novel therapies targeting these proteins. In
particular, it appears important to recognize the reasons for
changes in the expression of these galectins. Galectins may also
become a useful diagnostic and prognostic tool to evaluate tumor
progression or the clinical outcome in patients with ovarian
cancer; this, however, requires further study.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the current
study.
Authors' contributions
Conceptualization was by AMP; investigation by AMP
and DW. Literature search and selection was by AMP and MSK.
Original draft preparation was by AMP, AJ, PKD and MSK and
reviewing and editing by AMP, AE and ZKA. Project administration
was by AMP and DW. All authors have read and agreed to the
published version of the manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kossai M, Leary A, Scoazec JY and Genestie
C: Ovarian cancer: A heterogeneous disease. Pathobiology. 85:41–49.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kroeger PT and Drapkin R: Pathogenesis and
heterogeneity in ovarian cancer. Curr Opin Obstet Gynecol.
29:26–34. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Labrie M, De Araujo LOF, Communal L,
Mes-Masson AM and St-Pierre Y: Tissue and plasma levels of
galectins in patients with high grade serous ovarian carcinoma as
new predictive biomarkers. Sci Rep. 7:13242017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dubé-Delarosbil C and St-Pierre Y: The
emerging role of galectins in high-fatality cancers. Cell Mol Life
Sci. 75:1215–1226. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Compagno D, Gentilini LD, Jaworski FM,
Pérez IG, Contrufo G and Laderach DJ: Glycans and galectins in
prostate cancer biology, angiogenesis and metastasis. Glycobiology.
24:899–906. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thijssen VL, Rabinovich GA and Griffioen
AW: Vascular galectins: Regulators of tumor progression and targets
for cancer therapy. Cytokine Growth Factor Rev. 24:547–558. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dings RP, Miller MC, Griffin RJ and Mayo
KH: Galectins as molecular targets for therapeutic intervention.
Int J Mol Sci. 19:905–927. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wdowiak K, Francuz T, Gallego-Colon E,
Ruiz-Agamez N, Kubeczko M, Grochoła I and Wojnar J: Galectin
targeted therapy in oncology: Current knowledge and perspectives.
Int J Mol Sci. 19:210–231. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Balan V, Nangia-Makker P and Raz A:
Galectins as cancer biomarkers. Cancers (Basel). 2:592–610. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rabinovich GA, Baum LG, Tinari N,
Paganelli R, Natoli C, Liu FT and Iacobelli S: Galectins and their
ligands: Amplifiers, silencers or tuners of the inflammatory
response? Trends Immunol. 23:313–320. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Camby I, Le Mercier M, Lefranc F and Kiss
R: Galectin-1: A small protein with major functions. Glycobiology.
16:137R–157R. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu MH, Hong TM, Cheng HW, Pan SH, Liang
YR, Hong HC, Chiang WF, Wong TY, Shieh DB, Shiau AL, et al:
Galectin-1-mediated tumor invasion and metastasis, up-regulated
matrix metalloproteinase expression, and reorganized actin
cytoskeletons. Mol Cancer Res. 7:311–318. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Anginot A, Espeli M, Chasson L, Mancini SJ
and Schiff C: Galectin1 modulates plasma cell homeostasis and
regulates the humoral immune response. J Immunol. 190:5526–5533.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sandberg TP, Oosting J, van Pelt G, Mesker
WE, Tollenaar RAEM and Morreau H: Molecular profiling of colorectal
tumors stratified by the histological tumor-stroma ratio-increased
expression of galectin-1 in tumors with high stromal content.
Oncotarget. 9:31502–31515. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Satelli A, Rao PS, Gupta PK, Lockman PR,
Srivenugopal KS and Rao US: Varied expression and localization of
multiple galectins in different cancer cell lines. Oncol Rep.
19:587–594. 2008.PubMed/NCBI
|
|
16
|
Hughes RC: Secretion of the galectin
family of mammalian carbohydrate-binding proteins. Biochim Biophys
Acta. 1473:172–185. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sundblad V, Morosi LG, Geffner JR and
Rabinovich GA: Galectin-1: A Jack-of-All-Trades in the resolution
of acute and chronic inflammation. J Immunol. 199:3721–3730. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Perillo NL, Pace KE, Seilhamer JJ and Baum
LG: Apoptosis of T cells mediated by galectin-1. Nature.
378:736–739. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rabinovich GA, Iglesias MM, Modesti NM,
Castagna LF, Wolfenstein-Todel C, Riera CM and Sotomayor CE:
Activated rat macrophages produce a galectin-1-like protein that
induces apoptosis of T cells: Biochemical and functional
characterization. J Immunol. 160:4831–4840. 1998.PubMed/NCBI
|
|
20
|
Matarrese P, Tinari A, Mormone E, Bianco
GA, Toscano MA, Ascione B, Rabinovich GA and Malorni W: Galectin-1
sensitizes resting human T lymphocytes to Fas (CD95)-mediated cell
death via mitochondrial hyperpolarization, budding, and fission. J
Biol Chem. 280:6969–6985. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dias-Baruffi M, Zhu H, Cho M, Karmakar S,
McEver RP and Cummings RD: Dimeric galectin-1 induces surface
exposure of phosphatidylserine and phagocytic recognition of
leukocytes without inducing apoptosis. J Biol Chem.
278:41282–41293. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stowell SR, Karmakar S, Stowell CJ,
Dias-Baruffi M, McEver RP and Cummings RD: Human galectins-1, −2,
and −4 induce surface exposure of phosphatidylserine in activated
human neutrophils but not in activated T cells. Blood. 109:219–227.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hernandez JD and Baum LG: Ah, sweet
mystery of death! Galectins and control of cell fate. Glycobiology.
12:127–136. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sturm A, Lensch M and Andre S: Human
galectin-2: Novel inducer of T cell apoptosis with distinct profile
of caspase activation. J Immunol. 173:3825–3837. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fuertes MB, Molinero LL, Toscano MA,
Ilarregui JM, Rubinstein N, Fainboim L, Zwirner NW and Rabinovich
GA: Regulated expression of galectin-1 during T-cell activation
involves Lck and Fyn kinases and signaling through MEK1/ERK, p38
MAP kinase and p70S6 kinase. Mol Cell Biochem. 267:177–185. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rubinstein N, Alvarez M, Zwirner NW,
Toscano MA, Ilarregui JM, Bravo A, Mordoh J, Fainboim L, Podhajcer
OL and Rabinovich GA: Targeted inhibition of galectin-1 gene
expression in tumor cells results in heightened T cell-mediated
rejection: A potential mechanism of tumor-immune privilege. Cancer
Cell. 5:241–251. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Toscano MA, Commodaro AG, Ilarregui JM,
Bianco GA, Liberman A, Serra HM, Hirabayashi J, Rizzo LV and
Rabinovich GA: Galectin-1 suppresses autoimmune retinal disease by
promoting concomitant Th2- and T regulatory-mediated
anti-inflammatory responses. J Immunol. 176:6323–6332. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Toscano MA, Bianco GA, Ilarregui JM, Croci
DO, Correale J, Hernandez JD, Zwirner NW, Poirier F, Riley EM, Baum
LG and Rabinovich GA: Differential glycosylation of TH1, TH2 and
TH-17 effector cells selectively regulates susceptibility to cell
death. Nat Immunol. 8:825–834. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kuo PL, Huang MS, Cheng DE, Hung JY, Yang
CJ and Chou SH: Lung cancer-derived galectin-1 enhances tumorigenic
potentiation of tumor-associated dendritic cells by expressing
heparin-binding EGF-like growth factor. J Biol Chem. 287:9753–9764.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Soldati R, Berger E, Zenclussen AC, Jorch
G, Lode HN, Salatino M, Rabinovich GA and Fest S: Neuroblastoma
triggers an immunoevasive program involving galectin-1-dependent
modulation of T cell and dendritic cell compartments. Int J Cancer.
131:1131–1141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ilarregui JM, Croci DO, Bianco GA, Toscano
MA, Salatino M, Vermeulen ME, Geffner JR and Rabinovich GA:
Tolerogenic signals delivered by dendritic cells to T cells through
a galectin-1-driven immunoregulatory circuit involving interleukin
27 and interleukin 10. Nat Immunol. 10:981–991. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cedeno-Laurent F, Opperman M, Barthel SR,
Kuchroo VK and Dimitroff CJ: Galectin-1 triggers an
immunoregulatory signature in Th cells functionally defined by
IL-10 expression. J Immunol. 188:3127–3137. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Baatar D, Olkhanud PB, Wells V, Indig FE,
Mallucci L and Biragyn A: Tregs utilize beta-galactoside-binding
protein to transiently inhibit PI3K/p21ras activity of human
CD8+ T cells to block their TCR-mediated ERK activity
and proliferation. Brain Behav Immun. 23:1028–1037. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Garín MI, Chu CC, Golshayan D,
Cernuda-Morollón E, Wait R and Lechler RI: Galectin-1: A key
effector of regulation mediated by CD4+CD25+
T cells. Blood. 109:2058–2065. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Espeli M, Mancini SJ, Breton C, Poirier F
and Schiff C: Impaired B-cell development at the pre-BII-cell stage
in galectin-1-deficient mice due to inefficient pre-BII/stromal
cell interactions. Blood. 113:5878–5886. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mourcin F, Breton C, Tellier J, Narang P,
Chasson L, Jorquera A, Coles M, Schiff C and Mancini SJ:
Galectin-1-expressing stromal cells constitute a specific niche for
pre-BII cell development in mouse bone marrow. Blood.
117:6552–6561. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tsai CM, Chiu YK, Hsu TL, Lin IY, Hsieh SL
and Lin KI: Galectin-1 promotes immunoglobulin production during
plasma cell differentiation. J Immunol. 181:4570–4579. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen L, Yao Y, Sun L and Tang J:
Galectin-1 promotes tumor progression via NF-κB signaling pathway
in epithelial ovarian cancer. J Cancer. 8:3733–3741. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang P, Shi B, Zhou M, Jiang H, Zhang H,
Pan X, Gao H, Sun H and Li Z: Galectin-1 overexpression promotes
progression and chemoresistance to cisplatin in epithelial ovarian
cancer. Cell Death Dis. 5:e9912014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim HJ, Jeon HK, Cho YJ, Park YA, Choi JJ,
Do IG, Song SY, Lee YY, Choi CH, Kim TJ, et al: High galectin-1
expression correlates with poor prognosis and is involved in
epithelial ovarian cancer proliferation and invasion. Eur J Cancer.
48:1914–1921. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schulz H, Schmoeckel E, Kuhn C, Hofmann S,
Mayr D, Mahner S and Jeschke U: Galectins-1, −3, and −7 are
prognostic markers for survival of ovarian cancer patients. Int J
Mol Sci. 18:12302017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen L, Yao Y, Sun L, Zhou J, Liu J, Wang
J, Li J and Tang J: Clinical implication of the serum galectin-1
expression in epithelial ovarian cancer patients. J Ovarian Res.
8:782015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Abdelwahab M, Ebian H, Ibrahim T, Badr M,
Lashin M, Yassin M, Ismail A and Obaya A: Clinical significance of
serum galectin-1 and its tissue immunohistochemical expression in
serous ovarian carcinoma patients. J Obstet Gynecol. 9:937–953.
2019.
|
|
44
|
Argüeso P and Panjwani N: Focus on
molecules: Galectin-3. Exp Eye Res. 9:2–3. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pinnelli V, Sirsiker M and Silvia WD:
Galectin-3: A novel biomarker. Int J Chem Pharm Res. 2:81–94.
2013.
|
|
46
|
Lityńska A and Pokrywka M: Structure and
biological functions of galectin-3 Część Part I. Post Biol Kom.
37:677–684. 2010.
|
|
47
|
Pokrywka M and Lityńska A: Structure and
biological functions of galectin-3 Part II. Post Biol Kom.
37:685–697. 2010.
|
|
48
|
Ruvolo P: Galectin-3 as a guardian of the
tumor microenvironment. Biochim Biophys Acta. 1863:427–437. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gudowska M and Chrostek L: Diagnostic role
of galectin-3. Pol Merkur Lekarski. 222:408–412. 2014.(In Polish).
PubMed/NCBI
|
|
50
|
Li M, Feng YM and Fang SQ: Overexpression
of ezrin and galectin-3 as predictors of poor prognosis of cervical
cancer. Braz J Med Biol Res. 50:5356–5365. 2017. View Article : Google Scholar
|
|
51
|
Wdowiak K, Spychałowicz W, Fajkis M and
Wojnar J: Galectins in hematological malignancies-role, functions
and potential therapeutic targets. Postepy Hig Med Dosw (Online.
70:95–103. 2016.(In Polish). View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Punt S, Thijssen VL, Vrolijk J, de Kroon
CD, Gorter A and Jordanova E: Galectin-1, −3 and −9 expression and
clinical significance in squamous cervical cancer. PLoS One.
10:e01291192015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Vladoiu MC, Labrie M and St-Pierre Y:
Intracellular galectins in cancer cells: Potential new targets for
therapy (Review). Int J Oncol. 44:1001–10014. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chetry M, Thapa S, Hu X, Song Y, Zhang J,
Zhu H and Zhu X: The role of galectins in tumor progression,
treatment and prognosis of gynecological cancers. J Cancer.
9:4742–4755. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang L, Zhao Y, Wang Y and Wu X: The role
of galectins in cervical cancer biology and progression. Biomed Res
Int. 2018:21759272018.PubMed/NCBI
|
|
56
|
Farhad M, Rolig A and Redmond W: The role
of Galectin-3 in modulating tumor growth and immunosuppression
within the tumor microenvironment. Oncoimmunology. 7:e14344672018.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lee JH, Zhang X, Shin BK, Lee ES and Kim
I: Mac-2 binding protein and galectin-3 expression in mucinous
tumors of the ovary: An annealing control primer system and
immunohistochemical study. Pathology. 41:229–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kim MK, Sung CO, Do IG, Jeon HK, Song TJ,
Park HS, Lee YY, Kim BG, Lee JW and Bae DS: Overexpression of
galectin-3 and its clinical significance in ovarian carcinoma. Int
J Clin Oncol. 16:352–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Brustmann H: Epidermal growth factor
receptor expression in serous ovarian carcinoma: An
immunohistochemical study with galectin-3 and cyclin D1 and
outcome. Int J Gynecol Pathol. 27:380–389. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lu H, Liu Y, Wang D, Wang L, Zhou H, Xu G,
Xie L, Wu M, Lin Z and Yu Y: Galectin-3 regulates metastatic
capabilities and chemotherapy sensitivity in epithelial ovarian
carcinoma via NF-κB pathway. Tumour Biol. 37:11469–11477. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kang HG, Kim DH, Kim SJ, Cho Y, Jung J,
Jang W and Chun KH: Galectin-3 supports stemness in ovarian cancer
stem cells by activation of the Notch1 intracellular domain.
Oncotarget. 7:68229–68241. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Mirandola L, Yu Y, Cannon MJ, Jenkins MR,
Rahman RL and Nguyen DD: Galectin-3 inhibition suppresses drug
resistance, motility, invasion and angiogenic potential in ovarian
cancer. Gynecol Oncol. 135:573–579. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Eliaz I: The role of galectin-3 as a
marker of cancer and inflammation in a stage IV ovarian cancer
patient with underlying pro-inflammatory comorbidities. Case Rep
Oncol. 6:343–349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hossein G, Keshavarz M, Ahmadi S and
Naderi N: Synergistic effects of PectaSol-C modified citrus pectin
an inhibitor of galectin-3 and paclitaxel on apoptosis of human
SKOV-3 ovarian cancer cells. Asian Pac J Cancer Prev. 14:7561–7568.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hossein G, Halvaei S, Heidarian Y,
Dehghani-Ghobadi Z, Hassani M, Hosseini H, Naderi N and Sheikh
Hassani S: Pectasol-C modified citrus pectin targets
galectin-3-induced STAT3 activation and synergize paclitaxel
cytotoxic effect on ovarian cancer spheroids. Cancer Med.
8:4315–4329. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Cai G, Ma X, Chen B, Huang Y, Liu S, Yang
H and Zo W: Galectin-3 induces ovarian cancer cell survival and
chemoresistance via TLR4 signalingactivation. Tumour Biol.
37:11883–11891. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wang D, You D and Li L: Galectin-3
regulates chemotherapy sensitivity in epithelial ovarian carcinoma
via regulating mitochondrial function. J Toxicol Sci. 44:47–56.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Bieg D, Sypniewski D, Nowak E and Bednarek
I: Morin decreases galectin-3 expression and sensitizes ovarian
cancer cells to cisplatin. Arch Gynecol Obstet. 298:1181–1194.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Bieg D, Sypniewski D, Nowak E and Bednarek
I: MiR-424-3p suppresses galectin-3 expression and sensitizes
ovarian cancer cells to cisplatin. Arch Gynecol Obstet.
299:1077–1087. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Kaur M, Kaur T, Kamboj S and Singh J:
Roles of galectin-7 in cancer. Asian Pac J Cancer Prev. 17:455–461.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Menkhorst E, Griffiths M, van Sinderen M,
Rainczuk K, Niven K and Dimitriadis E: Galectin-7 is elevated in
endometrioid (type I) endometrial cancer and promotes cell
migration. Oncol Lett. 16:4721–4728. 2018.PubMed/NCBI
|
|
72
|
Chou F, Chen H, Kuo C and Sytwu H: Role of
galectins in tumors and in clinical immunotherapy. Int J Mol Sci.
19:430–441. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Johannes L, Jacob R and Leffler H:
Galectins at a glance. J Cell Sci. 131:jcs2088842018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Labrie M, Vladoiu MC, Grosset A, Gaboury L
and St-Pierre Y: Expression and functions of galectin-7 in ovarian
cancer. Oncotarget. 5:7705–7721. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Higareda-Almaraz JC, Ruiz-Moreno JS,
Klimentova J, Barbieri D, Salvador-Gallego R, Ly R,
Valtierra-Gutierrez IA, Dinsart C, Rabinovich GA, Stulik J, et al:
Systems-level effects of ectopic galectin-7 reconstitution in
cervical cancer and its microenvironment. BMC Cancer. 16:680–692.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
St-Pierre Y: Towards a better
understanding of the relationships between Galectin-7, p53 and
MMP-9 during cancer progression. Biomolecules. 11:8792021.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Guo JP and Li XG: Galectin-7 promotes the
invasiveness of human oral squamous cell carcinoma cells via
activation of ERK and JNK signaling. Oncol Lett. 13:1919–1924.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Krześlak A: Akt kinase: A key regulator of
metabolism and progression of tumors. Postepy Hig Med Dosw
(Online). 64:490–503. 2010.(In Polish). PubMed/NCBI
|
|
79
|
Kim HJ, Jeon HK, Lee JK, Sung CO, Do IG,
Choi CH, Kim TJ, Kim BG, Bae DS and Lee JW: Clinical significance
of galectin-7 in epithelial ovarian cancer. Anticancer Res.
33:1555–1561. 2013.PubMed/NCBI
|
|
80
|
Bibens-Laulan N and St-Pierre Y:
Intracellular galectin-7 expression in cancer cells results from an
autocrine transcriptional mechanism and endocytosis of
extracellular galectin-7. PLoS One. 12:e01871942017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Elola MT, Ferragut F, Cárdenas Delgado VM,
Nugnes LG, Gentilini L, Laderach D, Troncoso MF, Compagno D,
Wolfenstein-Todel C and Rabinovich GA: Expression, localization and
function of galectin-8, a tandem-repeat lectin, in human tumors.
Histol Histopathol. 29:1093–1105. 2014.PubMed/NCBI
|
|
82
|
Zick Y, Eisenstein M, Goren RA, Hadari YR,
Levy Y and Ronen D: Role of galectin-8 as a modulator of cell
adhesion and cell growth. Glycoconj J. 19:517–526. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Troncoso MF, Ferragut F, Bacigalupo ML,
Cárdenas Delgado VM, Nugnes LG, Gentilini L, Laderach D,
Wolfenstein-Todel C, Compagno D, Rabinovich GA and Elola MT:
Galectin-8: A matricellular lectin with key roles in angiogenesis.
Glycobiology. 10:907–914. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Elola MT, Wolfenstein-Todel C, Troncoso
MF, Vasta GR and Rabinovich GA: Galectins: Matricellular
glycan-binding proteins linking cell adhesion, migration, and
survival. Cell Mol Life Sci. 64:1679–1700. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Levy Y, Arbel-Goren R, Hadari YR, Eshhar
S, Ronen D, Elhanany E, Geiger B and Zick Y: Galectin-8 functions
as a matricellular modulator of cell adhesion. J Biol Chem.
276:31285–31295. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Troncoso MF, Elola MT, Croci DO and
Rabinovich GA: Integrating structure and function of
‘tandem-repeat’ galectins. Front Biosci (Schol Ed). 4:864–887.
2012.PubMed/NCBI
|
|
87
|
Tribulatti MV, Carabelli J, Prato CA and
Campetella O: Galectin-8 in the onset of the immune response and
inflammation. Glycobiology. 30:134–142. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Ferragut F, Cagnoni AJ, Colombo LL,
Sánchez Terrero C, Wolfenstein-Todel C, Troncoso MF, Vanzulli SI,
Rabinovich GA, Mariño KV and Elola MT: Dual knockdown of Galectin-8
and its glycosylated ligand, the activated leukocyte cell adhesion
molecule (ALCAM/CD166), synergistically delays in vivo breast
cancer growth. Biochim Biophys Acta Mol Cell Res. 1866:1338–1352.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Fan J, Tang X, Wang Q, Zhang Z, Wu S, Li
W, Liu S, Yao G, Chen H and Sun L: Mesenchymal stem cells alleviate
experimental autoimmune cholangitis through immunosuppression and
cytoprotective function mediated by galectin-9. Stem Cell Res Ther.
9:237–349. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zhou X, Sun L, Jing D, Xu G, Zhang J, Lin
L, Zhao J, Yao Z and Lin H: Galectin-9 expression predicts
favorable clinical outcome in solid tumors: A systematic review and
meta-analysis. Front Physiol. 9:4522018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Fujihara S, Mori H, Kobara H, Rafiq K,
Niki T, Hirashima M and Masaki T: Galectin-9 in cancer therapy.
Recent Pat Endocr Metab Immune Drug Discov. 7:130–137. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Lahm H, André S, Hoeflich A, Fischer JR,
Sordat B, Kaltner H, Wolf E and Gabius HJ: Comprehensive galectin
fingerprinting in a panel of 61 human tumor cell lines by RT-PCR
and its implications for diagnostic and therapeutic procedures. J
Cancer Res Clin Oncol. 127:375–386. 2001. View Article : Google Scholar : PubMed/NCBI
|