Intrahepatic cholangiocarcinoma (ICC) is a malignant
tumour originating from the intrahepatic bile duct epithelium that
accounts for ~10-15% of primary liver cancer cases (1,2),
and its morbidity and mortality rates are increasing. At present,
the molecular mechanism of ICC is not clear. Previous studies have
shown that various cytokines produced during chronic inflammation
cause abnormalities in oncogenes, DNA mismatch repair
genes/proteins, and tumour suppressor genes. Genetic and epigenetic
changes in cholangiocytes may promote proto-oncogene activation and
tumour suppressor gene inactivation. These cumulative effects
eventually lead to malignant transformation. In addition, cytokines
also play important roles in promoting cell growth, inhibiting cell
apoptosis, increasing cell invasiveness and promoting tumour
angiogenesis (3,4). Currently, adjuvant treatments such
as radiotherapy and chemotherapy have not significantly improved
the overall survival (OS) rate of patients with ICC (5–7).
Surgery is the only effective treatment for ICC. However, ICC is
characterized by atypical clinical symptoms and early metastasis,
leading to the diagnosis of advanced cancer and a lost opportunity
for surgery. It is also prone to recurrence after surgery, and the
overall 5-year survival rate after surgery is only 14–40% (6). With further research on the
pathogenesis of ICC, an increasing number of molecular targets have
been discovered. As the convergence point of numerous oncogenic
signalling pathways, signal transducer and activator of
transcription 3 (STAT3) plays a prominent role in regulating
antitumour immune responses. In the tumour ecosystem, STAT3 is
extensively overactivated in tumour cells to promote tumor growth.
Moreover, STAT3 is also extensively overactivated in non-tumour
cells to suppress the expression of key regulators of immune cell
activation and promote the production of immunosuppressive factors
(8). Therefore, drugs targeting
the STAT3 signalling pathway have become a promising therapeutic
strategy. Multiple studies (9–11)
have shown that STAT3 expression is associated with several
clinicopathological features, including tumour size, pathological
satellites, vascular invasion, undifferentiated histology, lymph
node metastasis and TNM stage. Patients with high STAT3 levels have
a poor prognosis in terms of OS and disease-free survival (DFS). A
multivariate survival analysis showed that STAT3 was an independent
prognostic factor for OS and DFS. Furthermore, it was observed that
STAT3 overexpression promoted the invasion, metastasis and
proliferation of ICC cells in vitro and in vivo and
promoted STAT3 phosphorylation (12,13). STAT3 expression may become a new
target for the treatment of patients with ICC.
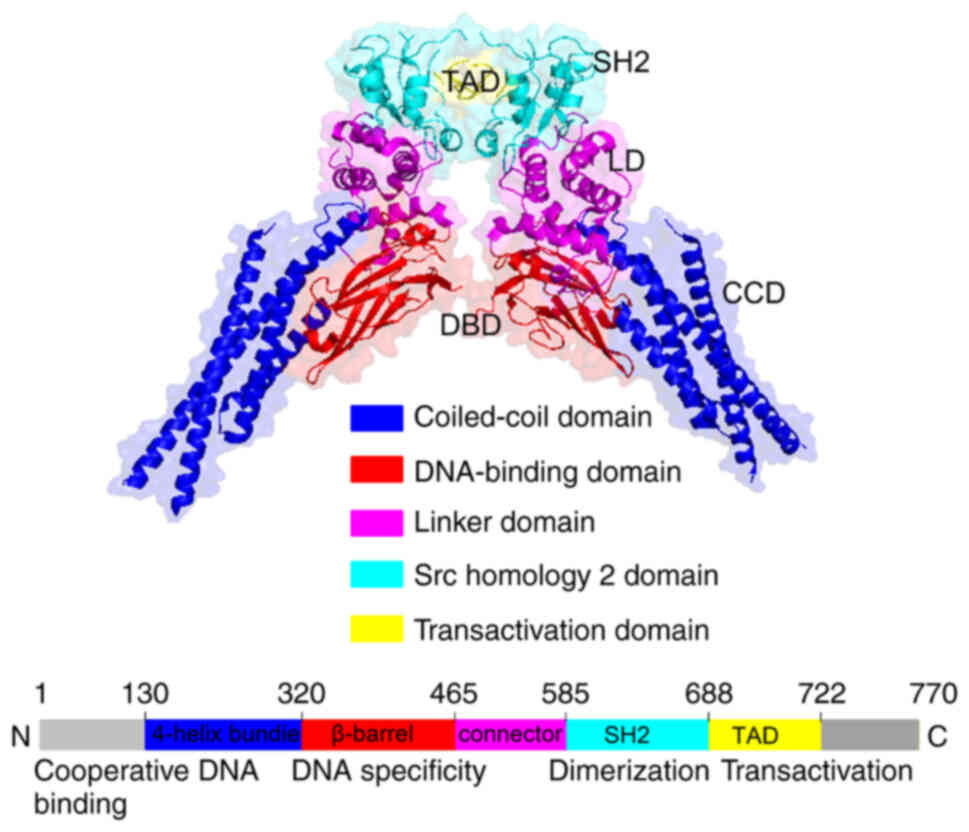
STATs are DNA-binding proteins consisting of 750–850
amino acids, and their molecular weight is 84–113 kDa. STATs play a
key role in cytokine signal transduction. The STAT family members
expressed in mammalian cells mainly include STAT1, STAT2, STAT3,
STAT4, STAT5 and STAT6, which are encoded by different genes. As
one of the earliest discovered oncogenes, STAT3 has become an
important gene that must not be ignored in tumour research and is
involved in regulating cell proliferation, differentiation,
apoptosis as well as other processes (14). STAT3 is a highly conserved protein
consisting of ~770 amino acids (only one amino acid difference
exists between mouse and human STAT3), that is expressed as three
isoforms: STAT3 ‘alpha’ ‘beta’ and STAT3 gamma. It contains an
amino terminal domain, a DNA binding domain and a C-terminal
transcription activation domain. The amino terminal domain forms a
coil structure. The structure of the DNA binding domain is an Src
homology 2 (SH2) domain. The C-terminal domain adopts the
transcription activation domain structure and is located between
the two aforementioned domains. The SH2 domain plays an important
role in signal transduction and specifically identifies
phosphorylated tyrosine residues. It is activated by
phosphorylation (15). The key
tyrosine associated with dimer formation is located in the SH2
domain. STAT3 activity is regulated by the phosphorylation of
serine 727, and phosphorylated STAT3 quickly enters the nucleus in
the form of monomers. Homodimers or heterodimers of transcription
factors are activated and interact with the promoters of their
transcriptional target genes (Fig.
1). STAT3 is widely expressed in certain types of cells and
tissues. STAT3 plays an indispensable role in early embryonic
development and bone marrow cell differentiation in a
STAT3-deficient mouse model (16). Under normal circumstances, STAT3,
the main regulator that balances cell proliferation and apoptosis,
participates in maintaining the growth and development of embryonic
stem cells. Concurrently, it also participates in processes such as
antigen tolerance. STAT3 activation is strictly regulated by a
negative feedback mechanism, and it is inactivated and transported
to the cytoplasm after transducing specific signals. However, upon
stimulation with carcinogenic signals, STAT3 is continuously
activated, exists in the nucleus in a constant activation state,
and continuously activates target genes to promote tumour
progression (17).
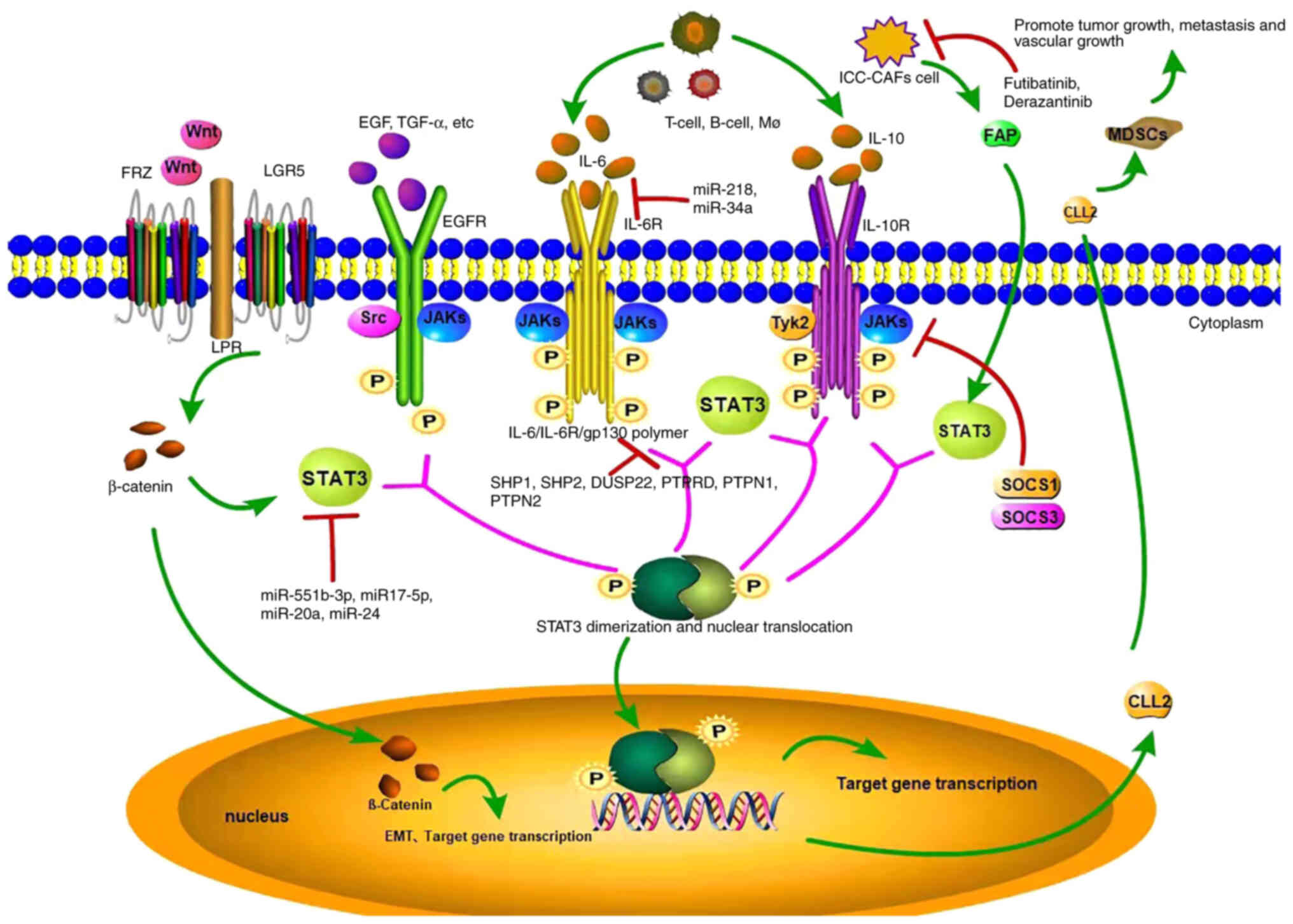
The JAK/STAT pathway is closely related to
inflammatory factors. IL-6 binds to the soluble IL-6 receptor
(SIL-6R) to activate the IL-6/JAK2/STAT3 signalling pathway
(18). Briefly, SIL-6R recognizes
and binds to IL-6 to form the SIL-6R/IL-6 complex, and activates
glycoprotein 130 (GP130) on the surface of the cell membrane.
Activated GP130 activates receptor tyrosine kinase and binds to the
STAT3 protein, which phosphorylates and activates the nuclear
transcription factor (19). STAT3
enters the nucleus and regulates the expression of inflammatory
cytokines. The IL-6 family mainly includes IL-6, IL-11, ciliary
neurotrophic factor, leukaemia inhibitory factor, oncostatin M
(OSM), cardiac trophic factor 1, cardiac trophic protein-like
cytokine and cardiac trophic protein 2 (20). For example, IL-6 expressed in T
cells, B cells or macrophages further promotes STAT3
phosphorylation and activation by activating JAK1, and STAT3
subsequently enters the nucleus to initiate downstream gene
transcription (8), participating
in the malignant process of ICC. Due to the continuous stimulation
of upstream molecules, the abnormally and continuously activated
IL-6/JAK/STAT3 pathway leads to resistance to apoptosis and further
promotes tumour development. A study has shown that almost all
cytokines in the IL-6 family activate the STAT3 protein. STAT3 is
also considered the most important transcription factor mediating
IL-6 function (8).
Lipopolysaccharide (LPS) activates the IL-6/STAT3 signalling
pathway in normal hepatic bile duct epithelial cells (21). LPS induces activation of the
IL-6/STAT3 signalling pathway by not only activating this
signalling pathway but also by increasing the expression of C-MYC
and MCL-1, suggesting that the IL-6/STAT3 signalling pathway may be
an important hub mediating inflammation and ICC (22). Both OSM and IL-11 are IL-6 family
cytokines expressed in inflammatory and cancer processes.
Tumour-associated neutrophils (TANs) and tumour-associated
macrophages (TAMs) produce higher levels of OSM and IL-11 in
coculture, respectively (23).
Both of these cytokines activate the STAT3 signalling pathway in
ICC cells. STAT3 knockout eliminates the tumour-promoting effects
of TANs and TAMs on ICC, and increased levels of TANs and TAMs are
related to the increased levels of p-STAT3 in tumour samples from
patients with ICC (24).
Researchers concluded that the effects of TANs and TAMs on ICC
mainly depend on OSM- and IL-11-mediated activation of the STAT3
signalling pathway (Fig. 2).
IL-10 is a cytokine encoded by the IL-10 gene. In
humans, IL-10 is produced mainly by immune cells, including
monocytes, type 2 T helper cells and regulatory T cells (Tregs).
IL-10 may play a role by regulating the JAK2/STAT3 signalling
pathway and the extracellular signal-regulated kinase 1/2 pathway
to alter the expression of downstream genes (25,26). The role of the JAK1/STAT3 pathway
in tumours has attracted increasing attention. The JAK1/STAT3
pathway is an important pathway mediating cytokine signal
transduction and is involved in various cell functions, such as
differentiation, survival, proliferation and apoptosis, as well as
pathological immune and inflammatory processes (27). According to previous studies
(28,29), IL-10 induces STAT3 phosphorylation
in Tregs. Although STAT3-deficient Tregs inhibit the proliferation
of CD4+ T cells in vitro, their number in
inflamed tissues is reduced, and their ability to inhibit the
inflammatory activity of TH17 is also reduced (30). Thus, the mechanism by which IL-10
inhibits tumour-associated inflammation may be related to STAT3
phosphorylation and its downstream effects on cytokine receptors or
subsequent gene expression. After the successful polarization of M2
macrophages in vitro, IL-10 levels in the supernatant of M2
macrophages were significantly increased compared with untreated
THP1 cells, and IL-10 was suggested to promote ICC cell migration,
invasion and epithelial transformation via the STAT3 pathway
(31) (Fig. 2).
EGFR, with a molecular weight of 170 kDa, is a
member of the epidermal growth factor receptor family. EGFR is
mainly located on the surface of human epithelial cells,
fibroblasts, glial cells and other cells, and its signal
transduction pathway plays an important role in promoting cell
growth, differentiation, as well as other physiological processes.
Loss of EGFR protein tyrosine kinase function or abnormal activity
of key factors in related signalling pathways may lead to the
development of tumours, immune deficiencies and cardiovascular
diseases. Upon binding of the ligand to its extracellular ligand
binding domain, EGFR is phosphorylated and forms either a homodimer
or heterodimer, initiating an extensive intracellular signalling
cascade (32–34). STAT3, one of the most important
downstream effectors, is phosphorylated at Tyr705 by activated EGFR
and is then translocated to the nucleus for transcriptional
regulation, contributing to cell proliferation, resistance to
apoptosis and angiogenesis (35,36). At present, EGFR overexpression or
abnormal expression has been detected in various tumours, leading
to the activation of downstream signalling pathways, particularly
the continuous activation of STAT3 that causes its nuclear
translocation and the transcription of downstream genes. Numerous
in vitro and in vivo experiments have shown that the
continuous expression and abnormal activation of the EGFR/STAT3
pathway are closely related to the occurrence and development of
ICC (37). The overactivated
EGFR/STAT3 signalling pathway is closely related to the development
of ICC, based on an immunohistochemical analysis of ICC samples.
EGFR-STAT3 overactivation promotes the growth of ICC cells
(38,39) (Fig.
2).
LGR5 is a member of the G protein-coupled receptor
subfamily, also known as HG38 and GPR49. It is a large protein
composed of 18 leucine-rich repeat units and 7 transmembrane
regions. The structure of the protein is characterized by an
extracellular region containing a signal peptide, 17 leucine-rich
repeats and a highly conserved 7 α-helix transmembrane region
(40). Previous studies (41–43) have detected increased expression
of LGR5 in gastrointestinal, ovarian, liver, basal cell carcinoma
and other tumour tissues to varying degrees (44). IκB kinase α upregulates the
expression of LGR5 by activating the STAT3 signalling pathway and
accelerates tumour progression in skin basal cell carcinoma cells
(45). LGR5 is essential for Wnt
signalling-induced activation of β-catenin, and by further
activating STAT3, it enhances CSC-like features and the EMT,
leading to aggressive tumour progression and a poor prognosis for
patients with ICC (Fig. 2).
Fibroblast activation protein (FAP) is a
membrane-bound glycoprotein that belongs to the serine protease
family. It is a dimer composed of FAPα and β subunits with a
molecular weight of 170 kDa (46). It has endopeptidase and weak
dipeptidase activity, degrades a variety of dipeptides and type I
collagen and is selectively expressed in cancer-associated
fibroblasts (CAFs) of a variety of human solid tumours (47). FAP is expressed in embryonic
cells, injured tissues and mesenchymal fibroblasts of >90% of
malignant epithelial tumours, but it is rarely expressed in benign
tumours and normal tissues; it is associated with extracellular
matrix remodelling, tumour proliferation and metabolism (48,49).
A previous study showed that FAP induces
inflammatory phenotypes and inflation-related gene expression
signatures in CAFs (50).
Inducing the expression of FAP in normal fibroblasts produces an
inflammatory phenotype similar to that of CAFFAP+ cells.
In addition, FAP continuously activates STAT3 in fibroblasts in
mouse liver tumour models, and CAFFAP+ is the main
source of CCL2. STAT3-CCL2 signalling increases the recruitment of
myeloid-derived suppressor cells (MDSCs) and thus promotes tumour
growth from CAFFAP+ cells. Moreover, FAP, p-STAT3, and
CCL2 levels are positively correlated with adverse pathological
features of ICC, and increased FAP levels predict low survival
rates. Recently, accumulating evidence has shown that CAFs
participate in the progression of ICC by affecting tumour cells
(51–54). Additionally, a previous study has
shown that CAFFAP+ is the main source of CCL2 in the ICC
microenvironment (55). In
addition, the tumorigenic function of FAP mediated by CCL2 in ICC
depends on its intracellular activation of STAT3 signalling in
CAFs. FAP recruits MDSCs in a CCL2-dependent manner in ICC. In
addition to mediating immunosuppression, MDSCs promote tumour
progression by enhancing angiogenesis through a paracrine pathway,
suggesting that approaches specifically targeting
CAFFAP+ may be a more effective and safer treatment
strategy for ICC (Fig. 2).
Approximately 70% of malignant tumours present
abnormally increased STAT3 activity, including acute myeloid
leukaemia, multiple myeloma, bladder, breast and colon cancer and
ICC (56–64). Phosphorylated STAT3 levels have
been revealed to be associated with poor clinical outcomes in
patients with these cancers. Therefore, extensive effort has been
devoted to identifying and developing STAT3 inhibitors for cancer
treatment. However, given the wide range of intracellular functions
of STAT3, possible inhibitors have been difficult to develop.
However, numerous phase I, phase II, and even phase III trials of
drugs targeting STAT3 have been conducted. A number of these
treatments are only used as research tools due to their
shortcomings, such as limited bio-absorption, utilization, drug
resistance, and poor stability, but other drugs achieve favourable
effects through oral bio-absorption and by binding to the STAT3 SH2
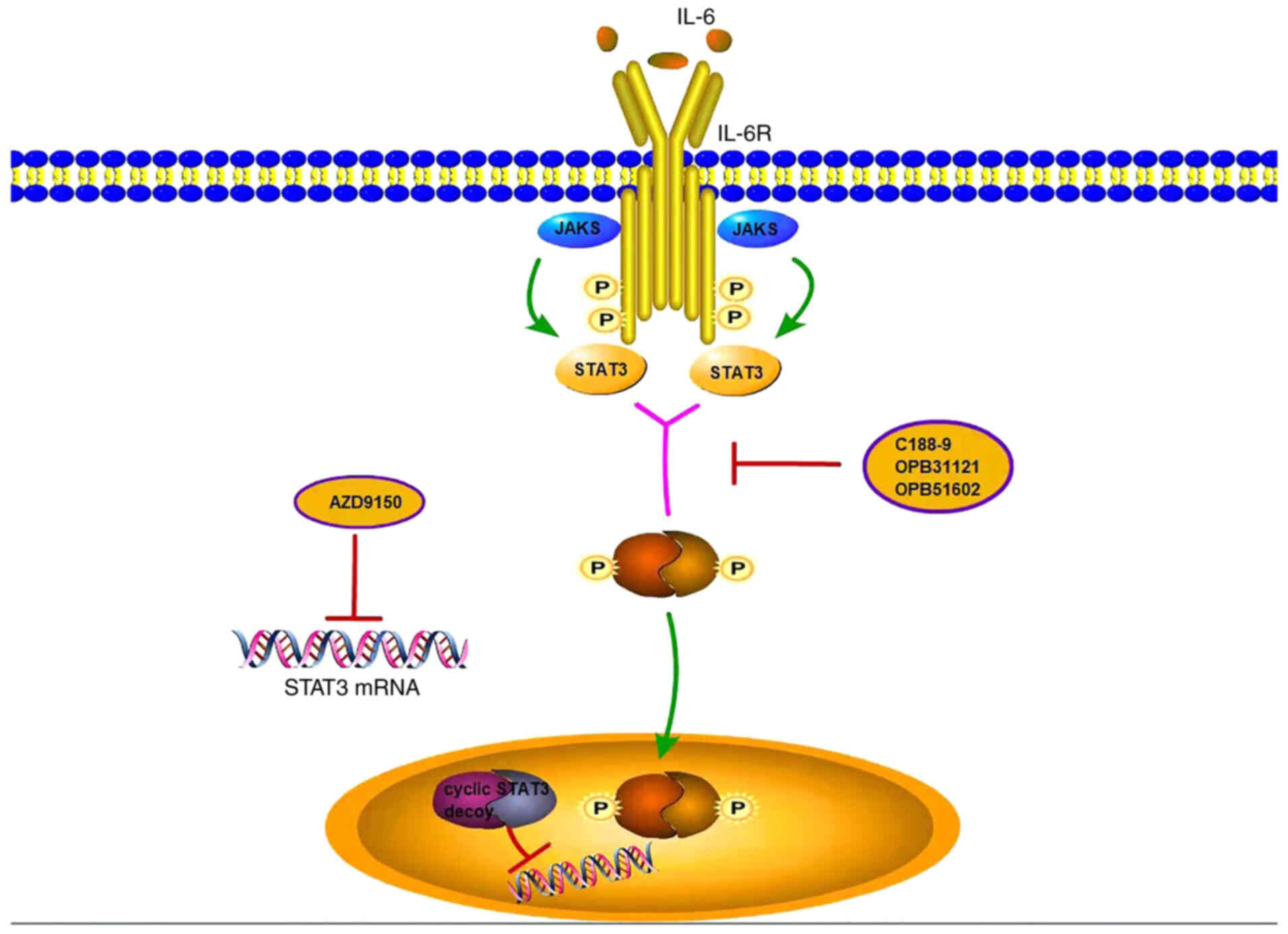
domain (65–68). Numerous nonpeptide SH2 domain
inhibitors have also been identified and shown to inhibit STAT3
activity, including STA-21, IL-6, STAT, TIC, c188-9,
OPB31121/51602, WP1066, S3I-201, BP-1-102, STX-0119 and HJC0123
(69–76). The application of these agents in
ICC requires further confirmation. In addition to the function of
STAT3 inhibitors, another method to inhibit STAT3 activity is to
inhibit the interaction of STAT3 with target gene promoter
elements. AZD9150 is the second generation of previous iterations
optimized by merger, 2′, 4′ constraints of ethyl STAT3 antisense
oligonucleotide-modified residues, which have been shown to prevent
STAT3 from binding DNA in a variety of tumours after intravenous
injections to inhibit tumour growth (77–83) (Fig.
3). AZD9150 is expected to achieve favourable efficacy in ICC
treatment.
According to previous studies, tumour proliferation,
invasion and metastasis, angiogenesis, drug resistance and
prognosis are all related to tobacco, alcohol, diet, stress,
infection, and chronic inflammation (84,85). Inflammatory factors such as
intrahepatic bile duct stones with chronic cholangitis, a high
incidence of viral hepatitis B, and biliary parasite infection are
considered high-risk factors for ICC (86). STAT3 is located at the
intersection of multiple oncogenic signalling pathways and is
abnormally activated in malignant tumour tissues, including ICC.
STAT3 is mainly activated by various kinases through
phosphorylation (87). Activated
STAT3 transduces signals from various cytokines and growth factors
into the nucleus and participates in regulating the transcription
of corresponding target genes, thereby participating in modulating
cell survival, proliferation, angiogenesis as well as other
processes (88). This
inflammatory cascade activates STAT3, leading to the overproduction
of bile duct epithelium growth factor, thus promoting CCA
initiation. Due to the role of STAT3 in inflammation and cancer
development, targeting STAT3 is a rational treatment strategy for
ICC. Numerous studies have shown that STAT3 activation is closely
related to the prognosis of patients with multiple myeloma, gastric
cancer, hepatocellular carcinoma, lung and laryngeal cancer and ICC
(89–92). STAT3 is associated with the
development of malignant tumours mainly through STAT3-mediated
expression of key target genes that regulate cell proliferation,
apoptosis inhibition and the hypoxia response (93). Activated STAT3 also induces the
expression of VEGF, which promotes invasive and metastatic
angiogenesis (94). In addition,
STAT3 binds to the IL-6 promoter, creating a positive feedback loop
that leads to increased IL-6 expression. VEGF and IL-6 also exert
immunosuppressive effects that may promote the immune escape of
tumour cells following STAT3 overactivation, thus forming a vicious
cycle of the occurrence, metastasis, and invasion of ICC (95). STAT3 is often used as an important
indicator to distinguish ICC from extrahepatic cholangiocarcinoma.
Studies (96–98) have shown significantly higher
STAT3 expression in ICC than in extrahepatic cholangiocarcinoma.
Downregulated STAT3 expression was revealed to significantly reduce
the proliferation of ICC cell lines, such as RBE and ICC-9810
cells, and significantly increase the apoptotic rate of RBE and
ICC-9810 cells. However, when STAT3 expression was upregulated, the
opposite results were obtained. STAT3 promoted the proliferation
and inhibited the apoptosis of intrahepatic bile duct cancer cells
(99).
STAT3 expression and activation are currently known
to be regulated by various mechanisms. Certain cytokines and growth
factors activate STAT3 by binding to specific receptors and
participate in the pathophysiological process of diseases. Under
physiological conditions, STAT3 activation is rapid and transient,
lasting only minutes to hours. In the tumour microenvironment,
dysregulation of growth factors, cytokines, and co-stimulators
leads to continued phosphorylation of STAT3 tyrosine residues.
Excessive or constitutive activation of STAT3 alters cell
proliferation and apoptosis, promotes invasion and metastasis, and
exacerbates immunosuppression in the microenvironment, directly
affecting the prognosis and quality of life of patients (100).
Considering the important association between the
high STAT3 expression and the malignancy and prognosis of ICC,
STAT3 is expected to become a molecular marker for clinical disease
staging and may become a new therapeutic target. Based on certain
preclinical studies that have identified the potential therapeutic
effects of drugs targeting the STAT signal transduction pathway,
the development of highly effective and well-tolerated drugs is
anticipated in the future. The molecular mechanism of ICC requires
further exploration (101),
which will facilitate the application of specific genes and
signalling pathways to the classification of ICC molecular subtypes
and the development of targeted therapeutic drugs. Further studies
are required to take advantage of multidisciplinary comprehensive
treatment, including surgery, chemotherapy and targeted therapy,
according to the molecular characteristics of ICC in order to
improve the quality of life and prolong the survival time of
patients.
Not applicable.
The present study was supported by grants from the following
organizations: The Hunan Provincial Natural Science Foundation of
China (grant no. 2020JJ5610), the Hunan Natural Science Fund for
Excellent Young Scholars (grant no. 2021JJ20003), the Youth Talent
of Hunan (grant no. 2020RC3066), the China Postdoctoral Science
Foundation (grant no. 2020M68115/2021T140197) and the Natural
Science Foundation of Changsha (grant no. kq2007023/kq2004115).
All data generated or analyzed during this study are
included in this published article.
RY and YS contributed to the analysis and manuscript
preparation. KS revised the review. CP, WY and SL contributed to
the conception of the study. YS and SL helped perform the analysis
and participated in constructive discussions. All authors have read
and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Razumilava N and Gores GJ:
Cholangiocarcinoma. Lancet. 383:2168–2179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Saha SK, Zhu AX, Fuchs CS and Brooks GA:
Forty-year trends in cholangiocarcinoma incidence in the U.S.:
Intrahepatic disease on the rise. Oncologist. 21:594–599. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cucchetti A, Cappelli A, Mosconi C, Zhong
JH, Cescon M, Pinna AD and Golfieri R: Improving patient selection
for selective internal radiation therapy of intra-hepatic
cholangiocarcinoma: A meta-regression study. Liver Int.
37:1056–1064. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang XW, Yuan JM, Chen JY, Yang J, Gao QG,
Yan XZ, Zhang BH, Feng S and Wu MC: The prognostic importance of
jaundice in surgical resection with curative intent for gallbladder
cancer. BMC Cancer. 14:6522014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Esnaola NF, Meyer JE, Karachristos A,
Maranki JL, Camp ER and Denlinger CS: Evaluation and management of
intrahepatic and extrahepatic cholangiocarcinoma. Cancer.
122:1349–1369. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ben-Menachem T: Risk factors for
cholangiocarcinoma. Eur J Gastroenterol Hepatol. 19:615–617. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang XW, Li L, Hou GJ, Yan XZ, Xu QG, Chen
L, Zhang BH and Shen F: STAT3 overexpression promotes metastasis in
intrahepatic cholangiocarcinoma and correlates negatively with
surgical outcome. Oncotarget. 8:7710–7721. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu H, Lee H, Herrmann A, Buettner R and
Jove R: Revisiting STAT3 signalling in cancer: New and unexpected
biological functions. Nat Rev Cancer. 14:736–746. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y, Shen Y, Wang S, Shen Q and Zhou X:
The role of STAT3 in leading the crosstalk between human cancers
and the immune system. Cancer Lett. 415:117–128. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
To SQ, Dmello RS, Richards AK, Ernst M and
Chand AL: STAT3 signaling in breast cancer: Multicellular actions
and therapeutic potential. Cancers (Basel). 14:4292022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu H, Kortylewski M and Pardoll D:
Crosstalk between cancer and immune cells: Role of STAT3 in the
tumour microenvironment. Nat Rev Immunol. 7:41–51. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mohassab AM, Hassan HA, Abdelhamid D,
Gouda AM, Youssif BGM, Tateishi H, Fujita M, Otsuka M and
Abdel-Aziz M: STAT3 transcription factor as target for anti-cancer
therapy. Pharmacol Rep. 72:1101–1124. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zimmers TA, Fishel ML and Bonetto A: STAT3
in the systemic inflammation of cancer cachexia. Semin Cell Dev
Biol. 54:28–41. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bromberg JF, Wrzeszczynska MH, Devgan G,
Zhao Y, Pestell RG, Albanese C and Darnell JE Jr: Stat3 as an
oncogene. Cell. 98:295–303. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin LL, Wybenga-Groot LE, Tong J, Taylor
P, Minden MD, Trudel S, McGlade CJ and Moran MF: Tyrosine
phosphorylation of the Lyn Src homology 2 (SH2) domain modulates
its binding affinity and specificity. Mol Cell Proteomics.
14:695–706. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gutiérrez M: Activating mutations of
STAT3: Impact on human growth. Mol Cell Endocrinol. 518:1109792020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang SW and Sun YM: The IL-6/JAK/STAT3
pathway: Potential therapeutic strategies in treating colorectal
cancer (Review). Int J Oncol. 44:1032–1040. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Montero P, Milara J, Roger I and Cortijo
J: Role of JAK/STAT in interstitial lung diseases; Molecular and
cellular mechanisms. Int J Mol Sci. 22:62112021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Banerjee S, Biehl A, Gadina M, Hasni S and
Schwartz DM: JAK-STAT Signaling as a target for inflammatory and
autoimmune diseases: Current and future prospects. Drugs.
77:521–546. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xin P, Xu X, Deng C, Liu S, Wang Y, Zhou
X, Ma H, Wei D and Sun S: The role of JAK/STAT signaling pathway
and its inhibitors in diseases. Int Immunopharmacol. 80:1062102020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yokoyama T, Komori A, Nakamura M, Takii Y,
Kamihira T, Shimoda S, Mori T, Fujiwara S, Koyabu M, Taniguchi K,
et al: Human intrahepatic biliary epithelial cells function in
innate immunity by producing IL-6 and IL-8 via the TLR4-NF-kappaB
and -MAPK signaling pathways. Liver Int. 26:467–476. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bode JG, Ehlting C and Häussinger D: The
macrophage response towards LPS and its control through the
p38(MAPK)-STAT3 axis. Cell Signal. 24:1185–1194. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou Z, Wang P, Sun R, Li J, Hu Z, Xin H,
Luo C, Zhou J, Fan J and Zhou S: Tumor-associated neutrophils and
macrophages interaction contributes to intrahepatic
cholangiocarcinoma progression by activating STAT3. J Immunother
Cancer. 9:e0019462021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shaul ME and Fridlender ZG:
Tumour-associated neutrophils in patients with cancer. Nat Rev Clin
Oncol. 16:601–620. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tam WY and Chi H: Bipolar/rod-shaped
microglia are proliferating microglia with distinct M1/M2
phenotypes. Sci Rep. 4:72792014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leticia P, Font-Nieves M, Van den Haute C,
Baekelandt V, Planas AM and Pozas E: IL-10 regulates adult
neurogenesis by modulating ERK and STAT3 activity. Front Cell
Neurosci. 9:572015.PubMed/NCBI
|
|
27
|
Wu X, Pan T, Quan Z, Li J, Yu Z, Wang X,
Li J, Li C, Yan M, Zhu Z, et al: IL-6 secreted by cancer-associated
fibroblasts promotes epithelial-mesenchymal transition and
metastasis of gastric cancer via JAK2/STAT3 signaling pathway.
Oncotarget. 8:20741–20750. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ju JH, Heo YJ, Cho ML, Jhun JY, Park JS,
Lee SY, Oh HJ, Moon SJ, Kwok SK, Park KS, et al: Modulation of
STAT-3 in rheumatoid synovial T cells suppresses Th17
differentiation and increases the proportion of Treg cells.
Arthritis Rheum. 64:3543–3552. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang XQ, Hu GH, Kou W, Shen Y, Kang HY and
Hong SL: Reciprocal roles of STAT3 and STAT5 in nasal polyposis. Am
J Otolaryngol. 33:741–752. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zheng Y, Wang Z, Deng L, Zhang G, Yuan X,
Huang L, Xu W and Shen L: Modulation of STAT3 and STAT5 activity
rectifies the imbalance of Th17 and Treg cells in patients with
acute coronary syndrome. Clin Immunol. 157:65–77. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yuan H, Lin Z, Liu Y, Jiang Y, Liu K, Tu
M, Yao N, Qu C and Hong J: Intrahepatic cholangiocarcinoma induced
M2-polarized Tumor-associated macrophages facilitate tumor growth
and invasiveness. Cancer Cell Int. 20:5862020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gao Y, Chen J C, ZHU Z Y, et al: Research
progress of EGFR gene mutation and its detection methods. Mol Diagn
Ther. 3:51–57. 2011.
|
|
33
|
Roskoski R Jr: ErbB/HER protein-tyrosine
kinases: Structures and small molecule inhibitors. Pharmacol Res.
87:42–59. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bi WW, Zhang WH, Yin GH, Luo H, Wang SQ,
Wang H, Li C, Yan WQ and Nie DZ: Analysis of indoleamine 2–3
dioxygenase (IDO) and EGFR co-expression in breast cancer tissue by
immunohistochemistry. Asian Pac J Cancer Prev. 15:5535–5538. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao X, Sun X and Li XL: Expression and
clinical significance of STAT3, p-STAT3, and VEGF-C in small cell
lung cancer. Asian Pac J Cancer Prev. 13:2873–2877. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fang B: Genetic Interactions of STAT3 and
Anticancer Drug Development. Cancers (Basel). 6:494–525. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chan KS, Carbajal S, Kiguchi K, Clifford
J, Sano S and DiGiovanni J: Epidermal growth factor
receptor-mediated activation of Stat3 during multistage skin
carcinogenesis. Cancer Res. 64:2382–2389. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang C, Xu H, Zhou Z, Tian Y, Cao X,
Cheng G and Liu Q: Blocking of the EGFR-STAT3 signaling pathway
through afatinib treatment inhibited the intrahepatic
cholangiocarcinoma. Exp Ther Med. 15:4995–5000. 2018.PubMed/NCBI
|
|
39
|
Zhang F, Li L, Yang X, Wang B, Zhao J, Lu
S and Yu X: Expression and activation of EGFR and STAT3 during the
multistage carcinogenesis of intrahepatic cholangiocarcinoma
induced by 3′-methyl-4 dimethylaminoazobenzene in rats. J Toxicol
Pathol. 28:79–87. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kumar KK, Burgess AW and Gulbis JM:
Structure and function of LGR5: An enigmatic G-protein coupled
receptor marking stem cells. Protein Sci. 23:551–565. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Katoh M: WNT signaling in stem cell
biology and regenerative medicine. Curr Drug Targets. 9:565–570.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Katoh M and Katoh M: STAT3-induced WNT5A
signaling loop in embryonic stem cells, adult normal tissues,
chronic persistent inflammation, rheumatoid arthritis and cancer
(Review). Int J Mol Med. 19:273–278. 2007.PubMed/NCBI
|
|
43
|
Katoh M and Katoh M: WNT signaling pathway
and stem cell signaling network. Clin Cancer Res. 13:4042–4045.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gregorieff A and Clevers H: Wnt signaling
in the intestinal epithelium: From endoderm to cancer. Genes Dev.
19:877–890. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kawasaki K, Kuboki S, Furukawa K,
Takayashiki T, Takano S and Ohtsuka M: LGR5 induces β-catenin
activation and augments tumour progression by activating STAT3 in
human intrahepatic cholangiocarcinoma. Liver Int. 41:865–881. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chung KM, Hsu SC, Chu YR, Lin MY, Jiaang
WT, Chen RH and Chen X: Fibroblast activation protein (FAP) is
essential for the migration of bone marrow mesenchymal stem cells
through RhoA activation. PLoS One. 9:e887722017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Park JE, Lenter MC, Zimmermann RN,
Garin-Chesa P, Old LJ and Rettig WJ: Fibroblast activation protein,
a dual specificity serine protease expressed in reactive human
tumor stromal fibroblasts. J Biol Chem. 274:36505–36512. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hamson EJ, Keane FM, Tholen S, Schilling O
and Gorrell MD: Understanding fibroblast activation protein (FAP):
substrates, activities, expression and targeting for cancer
therapy. Proteomics Clin Appl. 8:454–463. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Huber MA, Kraut N, Park JE, Schubert RD,
Rettig WJ, Peter RU and Garin-Chesa P: Fibroblast activation
protein: Differential expression and serine protease activity in
reactive stromal fibroblasts of melanocytic skin tumors. J Invest
Dermatol. 120:182–188. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yang X, Lin Y, Shi Y, Li B, Liu W, Yin W,
Dang Y, Chu Y, Fan J and He R: FAP Promotes immunosuppression by
cancer-associated fibroblasts in the tumor microenvironment via
STAT3-CCL2 signaling. Cancer Res. 76:4124–4135. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fingas CD, Bronk SF, Werneburg NW, Mott
JL, Guicciardi ME, Cazanave SC, Mertens JC, Sirica AE and Gores GJ:
Myofibroblast-derived PDGF-BB promotes Hedgehog survival signaling
in cholangiocarcinoma cells. Hepatology. 54:2076–2088. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ohira S, Sasaki M, Harada K, Sato Y, Zen
Y, Isse K, Kozaka K, Ishikawa A, Oda K, Nimura Y and Nakanuma Y:
Possible regulation of migration of intrahepatic cholangiocarcinoma
cells by interaction of CXCR4 expressed in carcinoma cells with
tumor necrosis factor-alpha and stromal-derived factor-1released in
stroma. Am J Pathol. 168:1155–1168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Claperon A, Mergey M, Aoudjehane L,
Ho-Bouldoires TH, Wendum D, Prignon A, Merabtene F, Firrincieli D,
Desbois-Mouthon C, Scatton O, et al: Hepatic myofibroblasts promote
the progression of human cholangiocarcinoma through activation of
epidermal growth factor receptor. Hepatology. 58:2001–2011. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Claperon A, Mergey M, Nguyen Ho-Bouldoires
TH, Vignjevic D, Wendum D, Chrétien Y, Merabtene F, Frazao A,
Paradis V, Housset C, et al: EGF/EGFR axis contributes to the
progression of cholangiocarcinoma through the induction of an
epithelial-mesenchymal transition. J Hepatol. 61:325–332. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lin Y, Li B, Yang X, Cai Q, Liu W, Tian M,
Luo H, Yin W, Song Y, Shi Y and He R: Fibroblastic FAP promotes
intrahepatic cholangiocarcinoma growth via MDSCs recruitment.
Neoplasia. 21:1133–1142. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chen CL, Cen L, Kohout J, Hutzen B, Chan
C, Hsieh FC, Loy A, Huang V, Cheng G and Lin J: Signal transducer
and activator of transcription 3 activation is associated with
bladder cancer cell growth and survival. Mol Cancer. 7:782008.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sonnenblick A, Shriki A, Galun E, Axelrod
JH, Daum H, Rottenberg Y, Hamburger T, Mali B and Peretz T: Tissue
microarray-based study of patients with lymph node-positive breast
cancer shows tyrosine phosphorylation of signal transducer and
activator of transcription 3 (tyrosine705-STAT3) is amarker of good
prognosis. Clin Transl Oncol. 14:232–236. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Schaefer LK, Ren Z, Fuller GN and Schaefer
TS: Constitutive activation of Stat3alpha in brain tumors:
Localization to tumor endothelial cells and activation by the
endothelial tyrosine kinase receptor (VEGFR-2). Oncogene.
21:2058–2065. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Takemoto S, Ushijima K, Kawano K,
Yamaguchi T, Terada A, Fujiyoshi N, Nishio S, Tsuda N, Ijichi M,
Kakuma T, et al: Expression of activated signal transducer and
activator of transcription-3 predicts poor prognosis in cervical
squamous-cell carcinoma. Br J Cancer. 101:967–972. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhang HF, Chen Y, Wu C, Wu ZY, Tweardy DJ,
Alshareef A, Liao LD, Xue YJ, Wu JY, Chen B, et al: The opposing
function of STAT3 as an oncoprotein and tumor suppressor is
dictated by the expression status of STAT3β in esophageal squamous
cell carcinoma. Clin Cancer Res. 22:691–703. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Geiger JL, Grandis JR and Bauman JE: The
STAT3 pathway as a therapeutic target in head and neck cancer:
Barriers and innovations. Oral Oncol. 56:84–92. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Li S, Priceman SJ, Xin H, Zhang W, Deng J,
Liu Y, Huang J, Zhu W, Chen M, Hu W, et al: Icaritin inhibits
JAK/STAT3 signaling and growth of renal cell carcinoma. PLoS One.
8:e816572013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wang Y, Qu A and Wang H: Signal transducer
and activator of transcription 4 in liver diseases. Int J Biol Sci.
11:448–455. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Suh YA, Jo SY, Lee HY and Lee C:
Inhibition of IL-6/STAT3 axis and targeting Axl and Tyro3 receptor
tyrosine kinases by apigenin circumvent taxol resistance in ovarian
cancer cells. Int J Oncol. 46:1405–1411. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Turkson J, Ryan D, Kim JS, Zhang Y, Chen
Z, Haura E, Laudano A, Sebti S, Hamilton AD and Jove R:
Phosphotyrosyl peptides block Stat3-mediated DNA binding activity,
gene regulation, and cell transformation. J Biol Chem.
276:45443–45455. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Turkson J, Kim JS, Zhang S, Yuan J, Huang
M, Glenn M, Haura E, Sebti S, Hamilton AD and Jove R: Novel
peptidomimetic inhibitors of signal transducer and activator of
transcription 3 dimerization and biological activity. Mol Cancer
Ther. 3:261–269. 2004.PubMed/NCBI
|
|
67
|
Mandal PK, Gao F, Lu Z, Ren Z, Ramesh R,
Birtwistle JS, Kaluarachchi KK, Chen X, Bast RC Jr, Liao WS and
McMurray JS: Potent and selective phosphopeptide mimetic prodrugs
targeted to the Src homology 2 (SH2) domain of signal transducer
and activator of transcription 3. J Med Chem. 54:3549–3563. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Auzenne EJ, Klostergaard J, Mandal PK,
Liao WS, Lu Z, Gao F, Bast RC Jr, Robertson FM and McMurray JS: A
phosphopeptide mimetic prodrug targeting the SH2 domain of Stat3
inhibits tumor growth and angiogenesis. J Exp TherOncol.
10:155–162. 2012.PubMed/NCBI
|
|
69
|
Hayakawa F, Sugimoto K, Harada Y,
Hashimoto N, Ohi N, Kurahashi S and Naoe T: A novel STAT inhibitor,
OPB-31121, has a significant antitumor effect on leukemia with
STAT-addictive oncokinases. Blood Cancer J. 3:e1662013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Kim MJ, Nam HJ, Kim HP, Han SW, Im SA, Kim
TY, Oh DY and Bang YJ: OPB-31121, a novel small molecular
inhibitor, disrupts the JAK2/STAT3 pathway and exhibits an
antitumor activity in gastric cancer cells. Cancer Lett.
335:145–152. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Bendell JC, Hong DS, Burris HA III, Naing
A, Jones SF, Falchook G, Bricmont P, Elekes A, Rock EP and Kurzrock
R: Phase 1, open-label, dose-escalation, and pharmacokinetic study
of STAT3 inhibitor OPB-31121 in subjects with advanced solid
tumors. Cancer Chemother Pharmacol. 74:125–130. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Oh DY, Lee SH, Han SW, Kim MJ, Kim TM, Kim
TY, Heo DS, Yuasa M, Yanagihara Y and Bang YJ: Phase I study of
OPB-31121, an oral STAT3 inhibitor, in patients with advanced solid
tumors. Cancer Res Treat. 47:607–615. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Okusaka T, Ueno H, Ikeda M, Mitsunaga S,
Ozaka M, Ishii H, Yokosuka O, Ooka Y, Yoshimoto R, Yanagihara Y and
Okita K: Phase 1 and pharmacological trial of OPB-31121, a signal
transducer and activator of transcription-3 inhibitor, in patients
with advanced hepatocellular carcinoma. Hepatol Res. 45:1283–1291.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wong AL, Soo RA, Tan DS, Lee SC, Lim JS,
Marban PC, Kong LR, Lee YJ, Wang LZ, Thuya WL, et al: Phase I and
biomarker study of OPB-51602, a novel signal transducer and
activator of transcription (STAT) 3 inhibitor, in patients with
refractory solid malignancies. Ann Oncol. 26:998–1005. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ogura M, Uchida T, Terui Y, Hayakawa F,
Kobayashi Y, Taniwaki M, Takamatsu Y, Naoe T, Tobinai K, Munakata
W, et al: Phase I study of OPB-51602, an oral inhibitor of signal
transducer and activator of transcription 3, in patients with
relapsed/refractory hematological malignancies. Cancer Sci.
106:896–901. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Bharadwaj U, Eckols TK, Xu X, Kasembeli
MM, Chen Y, Adachi M, Song Y, Mo Q, Lai SY and Tweardy DJ:
Small-molecule inhibition of STAT3 in radioresistant head and neck
squamous cell carcinoma. Oncotarget. 7:26307–26330. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Xi S, Gooding WE and Grandis JR: In vivo
antitumor efficacy of STAT3 blockade using a transcription factor
decoy approach: Implications for cancer therapy. Oncogene.
24:970–979. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Shen J, Li R and Li G: Inhibitory effects
of decoy-ODN targeting activated STAT3 on human glioma growth in
vivo. In Vivo. 23:237–243. 2009.PubMed/NCBI
|
|
79
|
Sun Z, Yao Z, Liu S, Tang H and Yan X: An
oligonucleotide decoy for Stat3 activates the immune response of
macrophages to breast cancer. Immunobiology. 211:199–209. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhang X, Zhang J, Wang L, Wei H and Tian
Z: Therapeutic effects of STAT3 decoy oligodeoxynucleotide on human
lung cancer in xenograft mice. BMC Cancer. 7:1492007. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhang X, Liu P, Zhang B, Mao H, Shen L and
Ma Y: Inhibitory effects of STAT3 decoy oligodeoxynucleotides on
human epithelial ovarian cancer cell growth in vivo. Int J Mol Med.
32:623–628. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Chan KS, Sano S, Kiguchi K, Anders J,
Komazawa N, Takeda J and DiGiovanni J: Disruption of Stat3 reveals
a critical role in both the initiation and the promotion stages of
epithelial carcinogenesis. J Clin Invest. 114:720–728. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zhang Q, Hossain DM, Duttagupta P, Moreira
D, Zhao X, Won H, Buettner R, Nechaev S, Majka M, Zhang B, et al:
Serum-resistant CpG-STAT3 decoy for targeting survival and immune
checkpoint signaling in acute myeloid leukemia. Blood.
127:1687–1700. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Sun XJ, Jiang TH, Zhang XP and Mao AW:
Role of the tumor microenvironment in pancreatic adenocarcinoma.
Front Biosci (Landmark Ed). 21:31–41. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Eggert T and Greten TF: Tumor regulation
of the tissue environment in the liver. Pharmacol Ther. 173:47–57.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Peng NF, Li LQ, Qin X, Guo Y, Peng T, Xiao
KY, Chen XG, Yang YF, Su ZX, Chen B, et al: Evaluation of risk
factors and clinicopathologic features for intrahepatic
cholangiocarcinoma in Southern China: A possible role of hepatitis
B virus. Ann Surg Oncol. 18:1258–1266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Jarnicki A, Putoczki T and Ernst M: Stat3:
Linking inflammation to epithelial cancer-more than a ‘gut’
feeling? Cell Div. 5:142010. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Liu Y, Liao S, Bennett S, Tang H, Song D,
Wood D, Zhan X and Xu J: STAT3 and its targeting inhibitors in
osteosarcoma. Cell Prolif. 54:e129742021. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Bharti AC, Shishodia S, Reuben JM, Weber
D, Alexanian R, Raj-Vadhan S, Estrov Z, Talpaz M and Aggarwal BB:
Nuclear factor-kappaB and STAT3 are constitutively active in CD138+
cells derived from multiple myeloma patients, and suppression of
these transcription factors leads to apoptosis. Blood.
103:3175–3184. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Kanda N, Seno H, Konda Y, Marusawa H,
Kanai M, Nakajima T, Kawashima T, Nanakin A, Sawabu T, Uenoyama Y,
et al: STAT3 is constitutively activated and supports cell survival
in association with survivin expression in gastric cancer cells.
Oncogene. 23:4921–4929. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Haura EB, Zheng Z, Song L, Cantor A and
Bepler G: Activated epidermal growth factor receptor-Stat-3
signaling promotes tumor survival in vivo in non-small cell lung
cancer. Clin Cancer Res. 11:8288–8294. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Liu B, Ren Z, Shi Y, Guan C, Pan Z and
Zong Z: Activation of signal transducers and activators of
transcription 3 and overexpression of its target gene CyclinD1 in
laryngeal carcinomas. Laryngoscope. 118:1976–1980. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Bournazou E and Bromberg J: Targeting the
tumor microenvironment: JAK-STAT3 signaling. JAKSTAT.
2:e238282013.PubMed/NCBI
|
|
94
|
Yu H and Jove R: The STATs of cancer-new
molecular targets come of age. Nat Rev Cancer. 4:97–105. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Schmidt-Arras D and Rose-John S: IL-6
pathway in the liver: From physiopathology to therapy. J Hepatol.
64:1403–1415. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Liu Z, Zhang M, Li Y, Zhang Y and She Z:
Effect of small interfering RNA targeting p63 on the proliferation
and invasiveness of human cholangiocarcinoma cells in vitro. Nan
Fang Yi Ke Da Xue Xue Bao. 32:207–210. 2012.(In Chinese).
PubMed/NCBI
|
|
97
|
Sia D, Tovar V, Moeini A and Llovet JM:
Intrahepatic cholangiocarcinoma: Pathogenesis and rationale for
molecular therapies. Oncogene. 32:4861–4870. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Montal R, Sia D, Montironi C, Leow WQ,
Esteban-Fabró R, Pinyol R, Torres-Martin M, Bassaganyas L, Moeini
A, Peix J, et al: Molecular classification and therapeutic targets
in extrahepatic cholangiocarcinoma. J Hepatol. 73:315–327. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Liu S, Xiu P, Liu N, et al: Effects of
STAT3 on proliferation and apoptosis of human intrahepatic bile
duct carcinoma cells. Shandong Med J. 55:5–7. 2015.(In
Chinese).
|
|
100
|
Dong J, Cheng XD, Zhang WD and Qin JJ:
Recent update on development of Small-Molecule STAT3 inhibitors for
cancer therapy: From phosphorylation inhibition to protein
degradation. J Med Chem. 64:8884–8915. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Tang Y, Tang Z, Yang J, Liu T and Tang Y:
MicroRNA-7-5p Inhibits Migration, Invasion and Metastasis of
Intrahepatic Cholangiocarcinoma by Inhibiting MyD88. J Clin Transl
Hepatol. 9:809–817. 2021.PubMed/NCBI
|