Introduction
Intervertebral disc degeneration (IDD) is a
degenerative condition that is primarily associated with age
(1). IDD is a major cause of pain
in the lower back, which reduces the quantity of life and wellbeing
of the patient (2). In addition,
IDD applies pressure on the global healthcare structure and
inflicts substantial financial costs (2). Numerous external and
patient-specific factors have been reported to contribute to the
occurrence and progression of IDD, including mechanical stress, age
and genetic predispositions (3–5).
However, the precise molecular mechanism underlying the
pathogenesis of IDD remains poorly understood. As a result,
effective long-term treatment strategies for this condition remains
elusive.
The intervertebral disc (IVD) is an avascular organ
that is comprised of three main structures: Central nucleus
pulposus (NP); adjacent annulus fibrosus; and the bony endplate.
Collectively, these entities guarantee the precise mechanical
functionality of the disc, relieving any compressive axial forces
that act on the spinal column to confer multiaxial flexibility
(6,7). Various stimuli, such as mechanical
stress and senescence, have been found to trigger the death of
cells in the IVD by activating numerous signal transduction
pathways (including the ER and death receptor pathways) (8). It has been proposed that cell death
can serve an instrumental role in the progression of IDD (8,9).
Indeed, excessive NP cell apoptosis can trigger IDD and has been
previously reported to be a node of therapeutic intervention for
treating IDD (8,10).
Under physiological conditions, in a healthy IVD the
NP is abundantly hydrated and produces copious quantities of
aggrecan and collagen II (11).
In addition, sufficient quantities of extracellular matrix (ECM)
maintains the internal pressure within the IVD, which provide a
stable hydrodynamic structure to facilitate IVD functionality
(12,13). A specialized catabolic enzyme
system in IVD primarily regulates ECM metabolism. The system
includes MMPs, a disintegrin and metalloproteinase with
thrombospondin motifs (ADAMTS) (14). Previous studies reported these
enzymes to serve key roles in the progression of degenerative
cartilaginous diseases (15,16). In particular, MMPs 1, 2, 3 and 9
were found to be expressed at high levels in NP cells from
degenerate discs (17). In
addition, another study found that compared with those in healthy
tissues, the expression of ADAMTS-4 and ADAMTS-5 was considerably
elevated in degenerated disc tissues (18).
Taurine is a free amino acid that is abundant in
mammalian tissues, particularly in myocardial tissues (19). However, the plasma levels of
taurine typically decrease with age in both humans and rats
(20,21). This age-related reduction in
plasma taurine levels may result in adverse effects on the
regulation of blood pressure, cardiovascular function and CNS
function (20). Due to its
numerous reported physiological properties, taurine has been
applied as an anti-stress supplement, with effects including the
regulation of Ca2+ homeostasis, antioxidant and
anti-apoptotic action (22,23). There is accumulating evidence that
taurine can also regulate energy metabolism and endoplasmic
reticulum (ER) stress-induced cell damage (23). Furthermore, taurine deficiency has
been previously reported in various diseases, such as Parkinson's
disease and epilepsy (24,25).
Adequate taurine levels was only noted in healthy disc tissues,
whilst it was only found in small amounts in degenerated discs
(21). This suggests a potential
role of taurine on IDD. However, there is an insufficient number of
studies that aimed to characterize the effects of taurine on IDD
and its associated mechanism. Therefore, the present study
attempted to examine the effects of IL-1β on NP cells in
vitro and investigate the functional characteristics of ER
stress. In addition, the present study explored the possible
mechanism of taurine on NP cells following exposure to thapsigargin
(TG), which is a classical inducer of ER stress.
Materials and methods
Chemicals and materials
TG and taurine were purchased from Sigma-Aldrich;
Merck KGaA. IL-1β was purchased from R&D Systems, Inc.
Caspase-12 antibodies (cat. no. sc-21747) were acquired from Santa
Cruz Biotechnology, Inc. C/EBP homologous protein (CHOP; cat. no.
2895), Bax (cat. no. 14796), cleaved caspase-3 (cat. no. 9664) and
β-actin (cat. no. 3700) antibodies were purchased from Cell
Signaling Technology, Inc. Collagen-II (cat. no. ab34712), Bcl-2
(cat. no. ab194583), caspase-9 (cat. no. ab184786) and glucose
regulatory protein 78 (GRP78; cat. no. ab21685) antibodies were
purchased from Abcam. HRP-conjugated anti-rabbit IgG (cat. no.
ab288151), Alexa Fluor® 488-conjugated goat anti-rabbit
IgG H&L (cat. no. ab150077), Dylight® 488-conjugated
goat anti-mouse IgG H&L (cat. no. ab96879) and goat anti-rat
IgG H&L (Alexa Fluor 647; cat. no. ab150167; Abcam) secondary
antibodies were purchased from Abcam. The In Situ Cell Death
Detection Kit was purchased from Roche Diagnostics (cat. no.
11684817910).
NP cell culture
Each empirical procedure involving animals performed
in the present study adhered to the rules in the Animal Care and
Use Committee of Shanghai Jing'an District Zhabei Central Hospital.
The present study was approved by the Animal Care and Use Committee
of Shanghai Jing'an District Zhabei Central Hospital (Shanghai,
China). Collectively, 15 male Sprague-Dawley rats (mean weight,
201.4±12.4 g; weight range, 180–220 g, 5–7 weeks; Animal Center of
Chinese Academy of Sciences) were housed under specific
pathogen-free laboratory conditions (18–29°C; 40–70% humidity),
with free access to food and water and controlled lighting (12 h
light/dark cycle). All rats were aseptically collected after
euthanasia by CO2 inhalation (40% vol/min for 5 min).
Death was confirmed when the rat lacked a heartbeat or respiratory
activity. The annulus fibrosus was first secluded to separate the
gelatinous NP. The NP cells of the rats were then isolated using
protocols described previously (26). Briefly, the lumbar disc was
exposed and the annulus fibrosus was cut open using a pair of
ophthalmic scissors under aseptic conditions. The gelatinous NP was
then isolated, which was cut into 1–3 mm-thick slices in sterile
D-Hanks solution (MilliporeSigma). This tissue was incubated with
0.1% collagenase type II (2 h at 37°C, MilliporeSigma) and 10 U/ml
hyaluronidase (2 h at 37°C, MilliporeSigma). The cells were
centrifuged (300 × g; 3 min; 37°C) and cultured in DMEM-F12 medium
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 15% FBS
(Gibco; Thermo Fisher Scientific, Inc.) at 37°C and 5%
CO2. In general, the intervertebral discs of three to
four rats were collected and pooled into one culture. Subsequently,
1×105 cells were transferred into each well of six-well
plates before they were pre-treated with various concentrations of
taurine [0 (Control), 5, 10, 20, 40, 80 nmol/l] for 2 h at 37°C
prior to treating the cells with 10 ng/ml IL-1β or 10 µM TG for 24
h at 37°C (27).
Cell viability assay
NP cells (5–6×103 cells per well) were
seeded into 96-well plates. Different dosages of taurine were used
for treating the cells (0, 5, 10, 20, 40, 80 nmol/l) alongside
IL-1β (10 ng/ml) for 24 h at 37°C. However, the control group were
not treated with IL-1β. The NP cells were then incubated for 2 h at
37°C with the Cell Counting Kit-8 reagent (CCK8; 10 µl per well;
Dojindo Molecular Technologies, Inc.). Subsequently, a microplate
reader (Molecular Devices, LLC) was utilized to measure the
absorbance at 450 nm.
Immunofluorescence staining
Following treatments, the NP cells were first seeded
into six-well plates (5×105 on cover glass). They were
then fixed with 4% (v/v) paraformaldehyde for 1 h at room
temperature, then incubated with 0.5% (v/v), Triton X-100 for 10
min and incubation with 5% (w/v) BSA (MilliporeSigma) for 1 h at
37°C. Primary antibodies against collagen II (1:50) and caspase-12
(1:50) were then added to the cells followed by incubation for 12 h
at 4°C. Next, [Alexa Fluor® 488-conjugated goat
anti-rabbit IgG H&L (cat. no. ab150077; 1:500) and goat
anti-rat IgG H&L (Alexa Fluor 647; ab150167; 1:500)] were
utilized to treat all cells for a 60 min at room temperature. DAPI
(Abcam; cat. no. ab285390) was then added to incubate the cell
nuclei for 5 min at room temperature (28), which allowed for the visualization
of the NP cells using a Nikon Eclipse Ti confocal microscope (Nikon
Corporation; magnification, ×40).
Western blotting
Following treatment, protein samples were extracted
from the NP cells using RIPA buffer (MilliporeSigma). Bicinchoninic
acid method was used to measure the protein concentrations in each
sample. SDS-PAGE (8–12%) was used to separate the proteins (40 µg)
before transferring onto PVDF membranes. The membranes were then
incubated in 5% non-fat milk for 1 h at room temperature before
incubation with primary antibodies. Specifically, anti-Bax
(1:1,000), anti-Bcl-2 (1:1,000), cleaved-caspase-3 (1:1,000),
caspase-9 (1:1,000), anti-CHOP (1:1,000), anti-GRP78 (1:1,000) and
anti-collagen II (1:1,000) antibodies were used, with incubations
for 12 h at 4°C. Subsequently, the membranes were incubated with
secondary antibodies [HRP-conjugated anti-rabbit IgG (1:5,000) and
Dylight 488-conjugated goat anti-mouse IgG H&L (1:5,000)] for a
further 1 h. A ChemiDoc™ XRS+ imaging system (v4.0,
Bio-Rad Laboratories, Inc.) and visualization reagent (Thermo
Fisher Scientific, Inc.; cat. no. 32106) was used to quantify the
intensities of the blots.
TUNEL assay
Following treatment, NP cells (5×105 on
cover glass) were incubated in 4% paraformaldehyde for 30 min at
room temperature, 3% (v/v) H2O2 for 10 min
and 0.1% Triton X-100 for 7 min. The TUNEL reagent was then applied
to the NP cells for 15 min at room temperature before the nuclei
were stained with DAPI for 7 min at room temperature. After washing
with PBS, the slides were sealed with sealing agent (Thermo Fisher
Scientific, Inc.; cat. no. P36970). Images in five fields were
captured using a Nikon Eclipse Ti confocal microscope (Nikon
Corporation, magnification, ×40) to evaluate the apoptotic activity
(v5.0, GraphPad Prism; GraphPad Software, Inc.).
Reverse transcription-quantitative
PCR
Following treatment, TRIzol® reagent
(Thermo Fisher Scientific, Inc.) was used to isolate total RNA from
the extracted NP cells, before being reverse transcribed using a
cDNA Synthesis kit (cat. no. 6130, Takara Biotechnology Co., Ltd.)
using the following conditions: 15 min 37°C and 5 sec 85°C.
Subsequently, SYBR Premix Ex Taq mixture (cat. no. B110032; Sangon
Biotech Co., Ltd.) was used for qPCR using the following
thermocycling conditions: 10 min at 95°C, followed by 40 cycles of
15 sec at 95°C and 1 min at 60°C. The 2−ΔΔCq method
(29) was adopted to quantify the
expression levels of mRNA associated with the targeted gene in NP
cells, and the primer sequences of targeted genes are listed in
Table I. All process were
performed according to the protocols outlined by the manufacturers
(30).
 | Table I.Primer sequences of the targeted
genes. |
Table I.
Primer sequences of the targeted
genes.
| Genes | Primer
sequences |
|---|
| MMP-1 | Forward:
5′-GCTTAGCCTTCCTTTGCTGTTGC-3′ |
|
| Reverse:
5′-GACGTCTTCACCCAAGTTGTAGTAG-3′ |
| MMP-3 | Forward:
5′-CTGGGCTATCCGAGGTCATG-3′ |
|
| Reverse:
5′-TGGACGGTTTCAGGGAGGC-3′ |
| MMP-9 | Forward:
5′-CCACCGAGCTATCCACTCAT-3′ |
|
| Reverse:
5′-GTCCGGTTTCAGCATGTTTT-3′ |
| ADAMTS-4 | Forward:
5′-AAGCATCCGAAACCCTGTCAACG-3′ |
|
| Reverse:
5′-AGCCATACCCAGAGCGTCAC-3′ |
| ADAMTS-5 | Forward:
5′-AGAGTCCGAACGAGTTTACG-3′ |
|
| Reverse:
5′-GTGCCAGTTCTGTGCGTC-3′ |
| SRY-box
transcription factor 9 | Forward:
5′-GCGACGTCATCTCCAACATC-3′ |
|
| Reverse:
5′-ATGCCGTAGCTGCCAGTGTA-3′ |
| Aggrecan | Forward:
5′-GACCCAAACAGCAGAAACAGC-3′ |
|
| Reverse:
5′-CGGGAAAGTGGCGATAACAG-3′ |
| Collagen II | Forward:
5′-CACCCAGAGTGGAAGAGCG-3′ |
|
| Reverse:
5′-TCAGTGGACAGTAGACGGAGGA-3′ |
| β-actin | Forward:
5′-TCAGGTCATCACTATCGGCAAT-3′ |
|
| Reverse:
5′-AAAGAAAGGGTGTAAAACGCA-3′ |
Statistical analysis
All data were analyzed using GraphPad Prism 7
software (GraphPad Software, Inc.). Data were presented as the mean
± standard deviation. Each assay was repeated at least three times.
One-way ANOVA followed by Tukey's test was used to compare the data
in the present study. P<0.05 was considered to indicate a
statistically significant difference.
Results
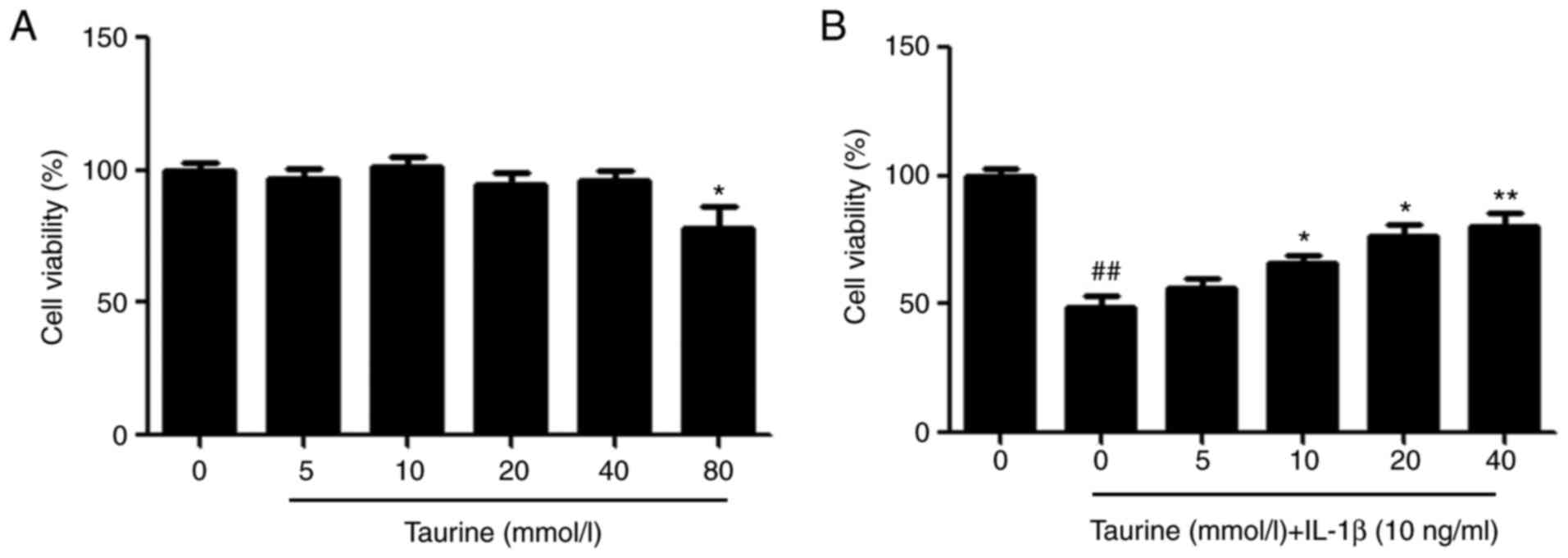
Taurine elevates the viability of NP
cells induced by IL-1β
CCK-8 assay was used for assessing the effects of
taurine on NP cells. Ascending doses of taurine were used to treat
the NP cells. No cytotoxic effects could be observed at
concentrations ≤40 µM (Fig. 1A).
Compared with that in the control group, the viability of
IL-1β-induced NP cells was significantly lower (Fig. 1B), which was reversed by taurine
treatment (10–40 µM, Fig. 1B).
Collectively, these findings suggest that taurine can mediate
protective effects on the NP cells.
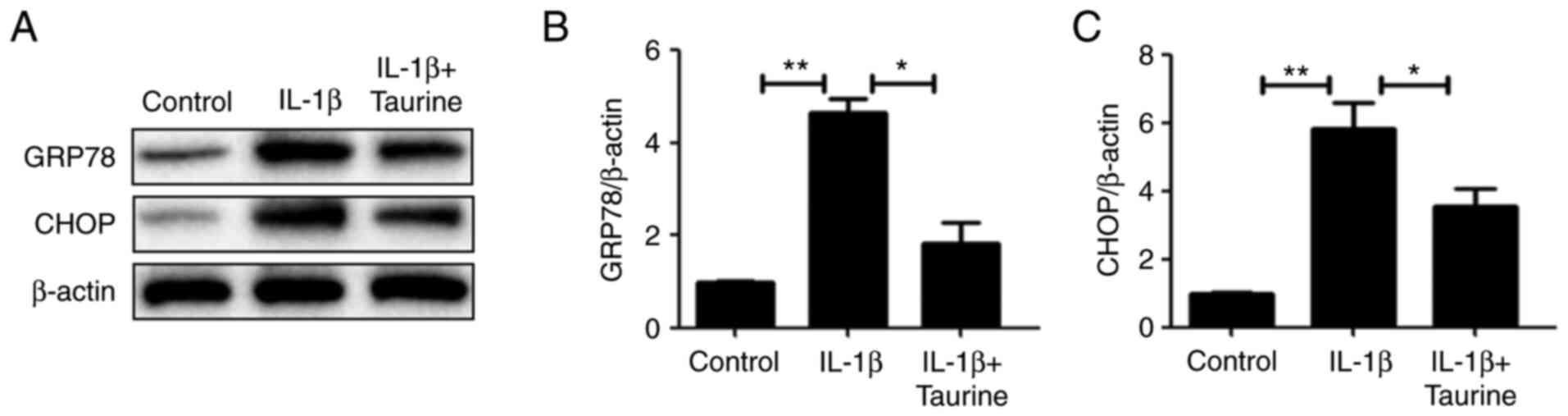
Taurine reverses ER stress and
apoptosis in NP cells treated with IL-1β
CCK-8 results and a previous report (28) revealed that IL-1β (10 ng/ml)
reduced cell viability by 50%, which is a quantifiable level of
cell injury. Therefore, IL-1β (10 ng/ml) was selected for treating
the NP cells. In the present study, the expression of ER
stress-associated protein CHOP and GRP78 was measured to determine
the extent of ER stress in the IL-1β-treated cohort in response to
taurine treatment. The expression levels of all two proteins
exhibited significantly elevated expression levels in response to
IL-1β compared with those in the control group. However, taurine
treatment significantly reversed this increment (Fig. 2).
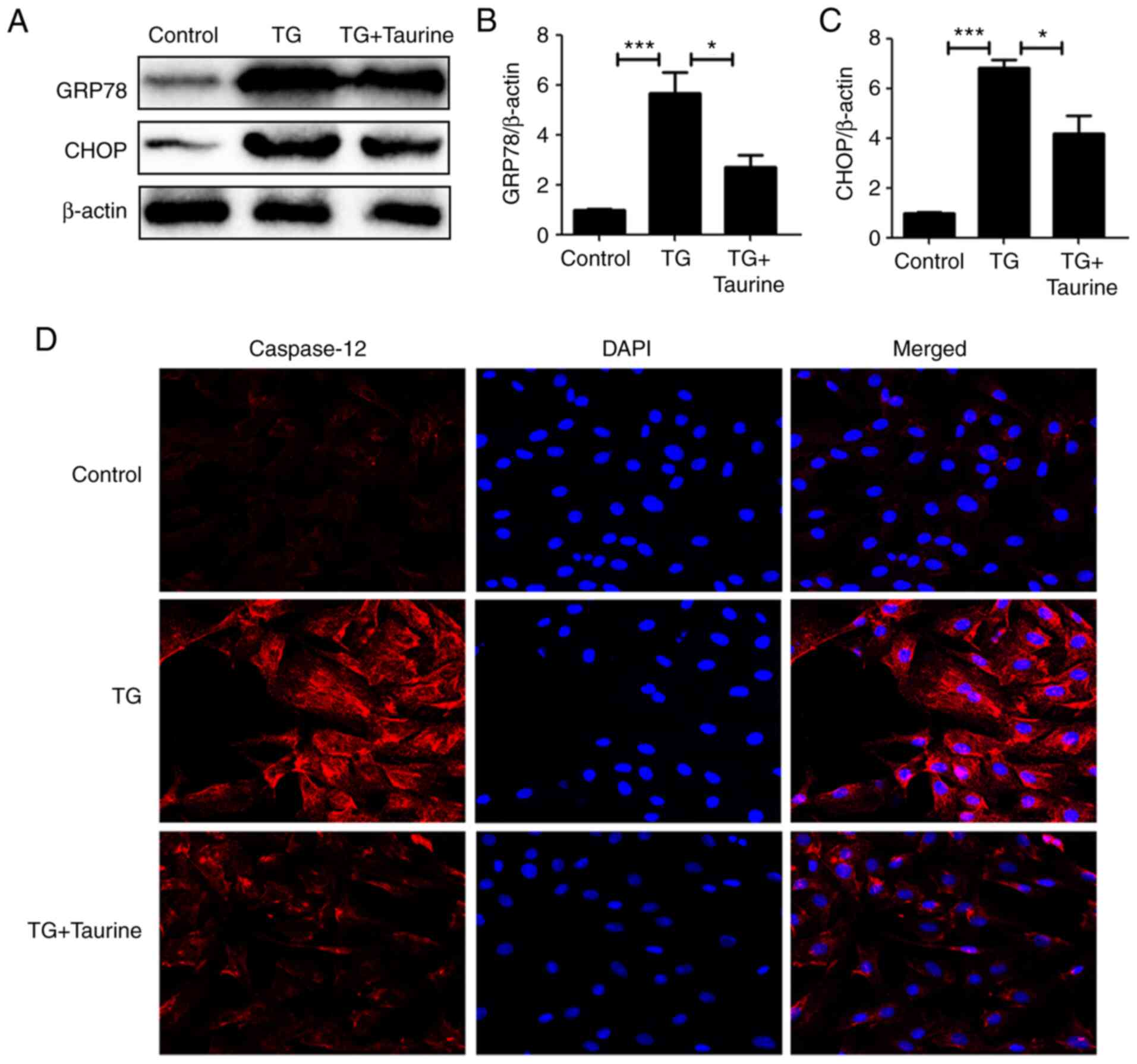
Taurine reverses TG-triggered ER
stress and apoptosis in NP cells
TG, suppressor of the sarcoplasmic reticulum/ER
Ca2+ ATPase, can increase cytoplasmic Ca2+ by
inhibiting the ability of the cell to pump Ca2+ back
into the ER. A previous study (27) showed that TG at 10 µM could reduce
cell viability of PC12 cells by 50%, which is a quantifiable level
of cell injury. In addition, other concentrations of TG either
failed to induce cell injury or induced ~100% cell death.
Therefore, 10 µM TG was chosen for treating the NP cells. The NP
cells were treated with TG in the present study to assess if ER
stress had an association with the cytoprotective effects of
taurine. Compared with the control groups, TG significantly
elevated GRP78 and CHOP expression, a downstream apoptotic protein.
Taurine partially but significantly reversed this increase in GRP78
and CHOP expression (Fig. 3A-C).
In addition, immunofluorescence staining of cleaved caspase-12 also
supported the notion that taurine inhibited TG-activated apoptosis
(Fig. 3D).
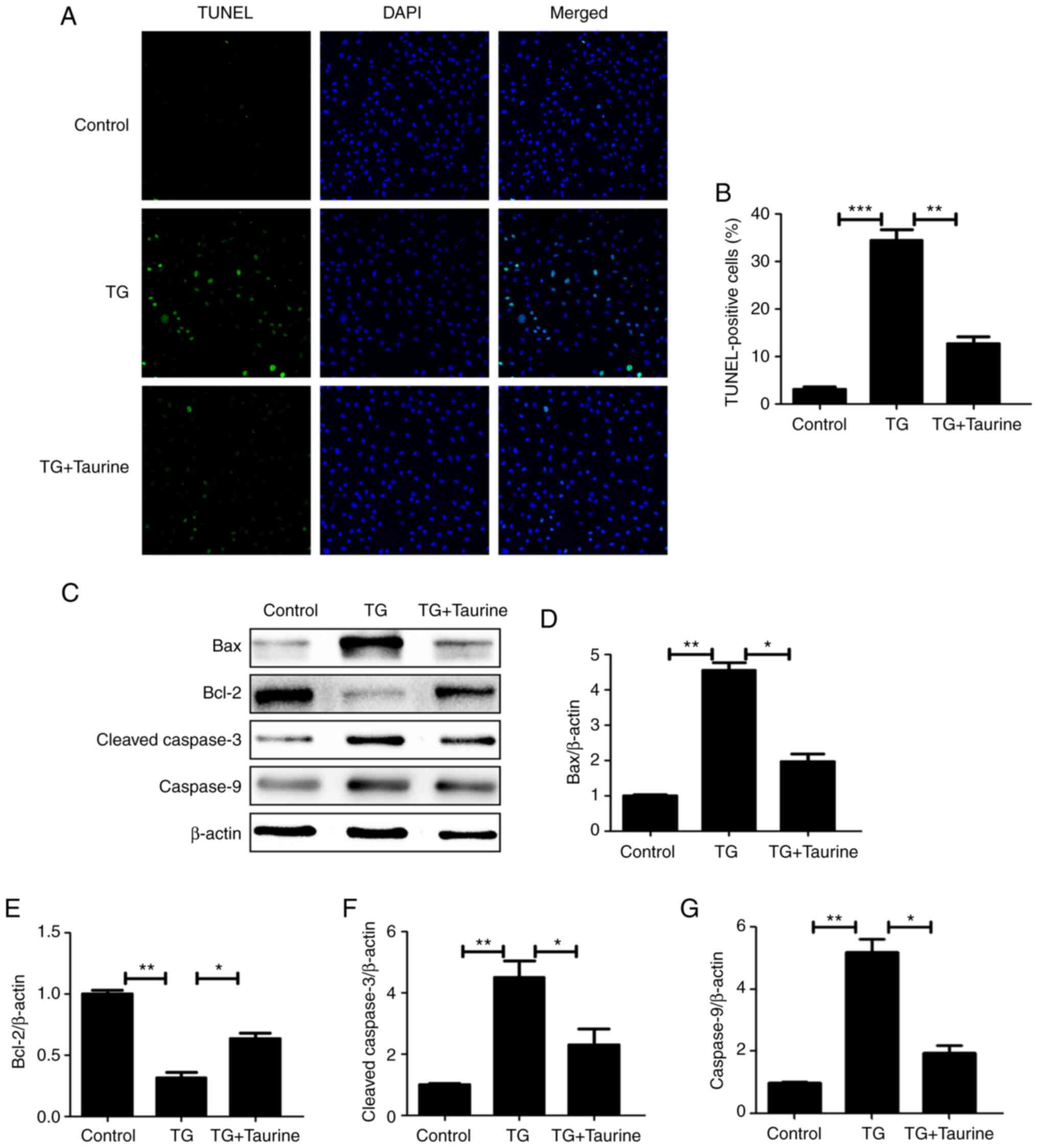
Taurine suppresses TG-induced
apoptosis in NP cells
To further explore the effects of taurine on the
apoptosis of TG-treated NP cells, TUNEL and western blotting were
performed to measure the apoptotic activity of NP cells. TUNEL
results demonstrated that taurine significantly decreased the
number of apoptotic TG-induced NP cells (Fig. 4A and B), suggesting that taurine
exerted anti-apoptotic effects on TG-induced NP cells.
Administration of TG also increased the protein expression of
cleaved caspase-3 and caspase-9, whilst decreasing that of Bcl-2
(Fig. 4C-G). By contrast, these
TG-induced effects on the expression of proteins associated with
apoptosis were significantly reversed by treatment with taurine
(Fig. 4C-G). These findings
suggest that taurine suppressed TG-mediated apoptosis in NP
cells.
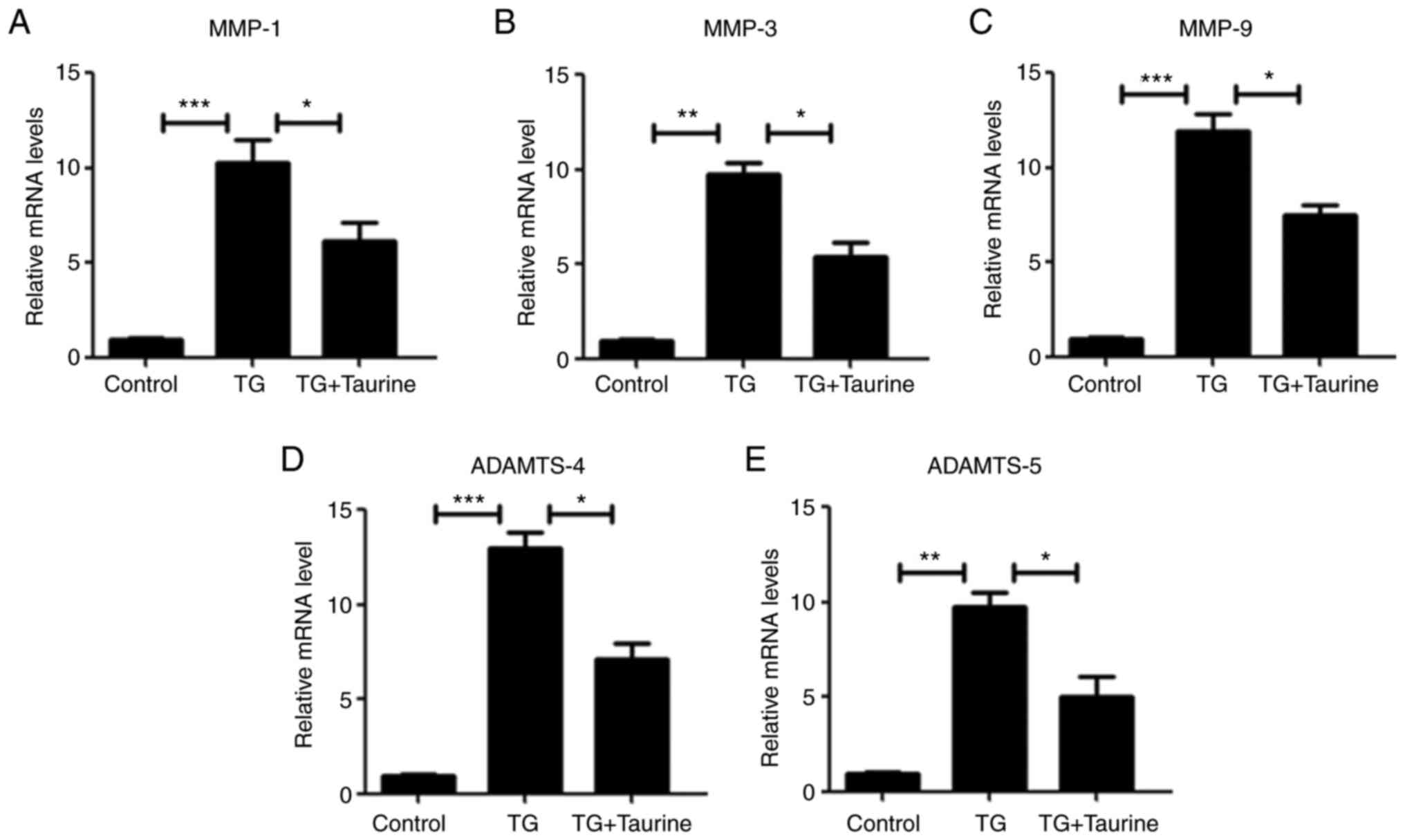
Taurine attenuates the ER
stress-induced expression of catabolic enzymes of the ECM in NP
cells
Previous studies have reported catabolic changes
(such as MMP-1, MMP-3, MMP-9, ADAMTS-4 and ADAMTS-5) in the
degenerative disc (31,32). Therefore, the expression
ECM-degrading enzymes MMP-1, MMP-3, MMP-9, ADAMTS-4 and ADAMTS-5
was measured by RT-qPCR. Accordingly, TG treatment triggered
significant elevations in the mRNA and protein expression of MMP-1,
MMP-3, MMP-9, ADAMTS-4 and ADAMTS-5 enzymes (Fig. 5). However, taurine significantly
reversed this TG-induced increase (Fig. 5).
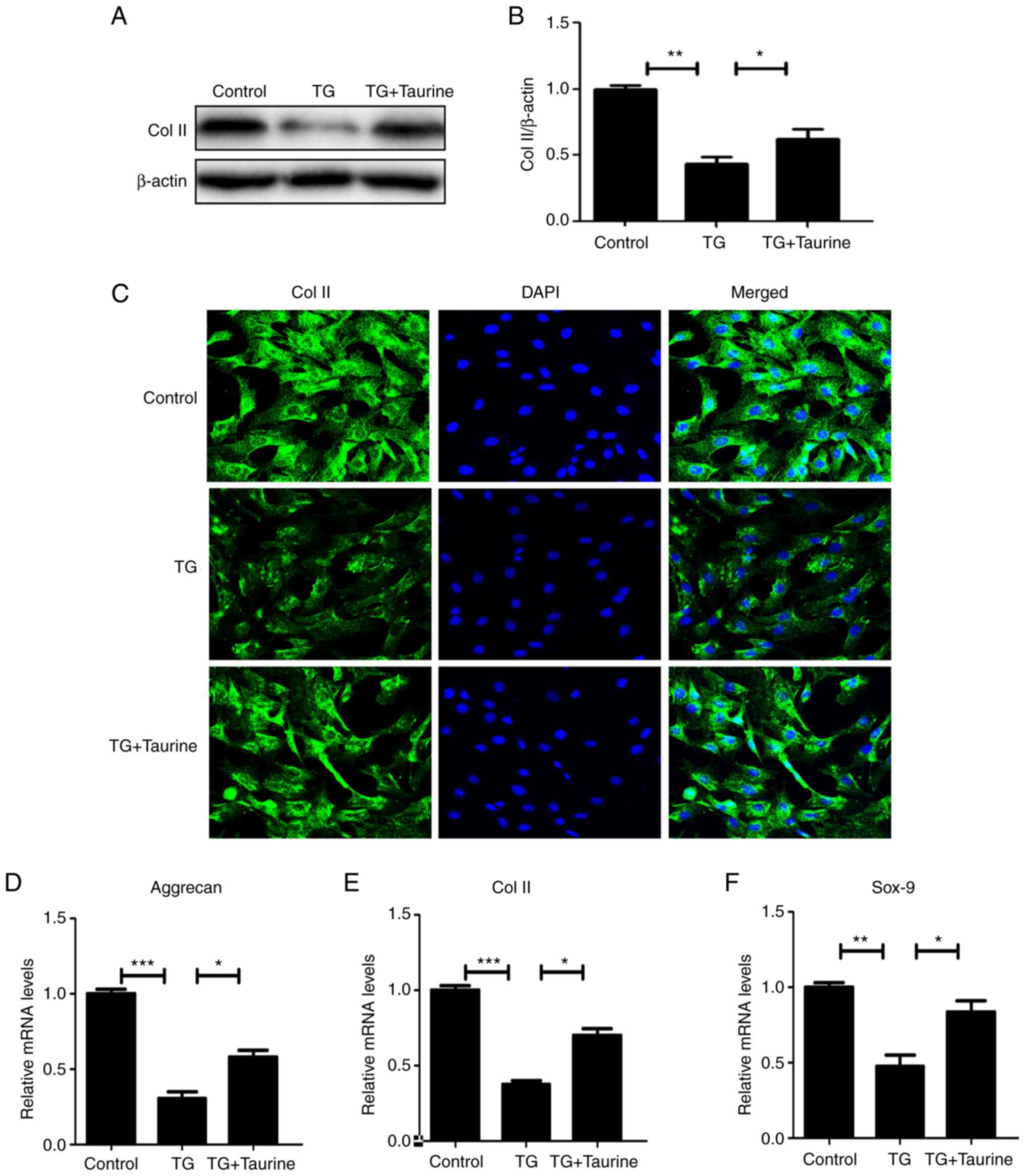
Taurine restores the ECM in TG-treated
in NP cells
Western blotting and immunofluorescence analysis
were utilized to evaluate the extent of ECM metabolism in NP cells,
which enabled the measurement of collagen-II and aggrecan. As shown
in Fig. 6A and B, in the TG
group, collagen-II expression were markedly suppressed by TG,
indicating increased ECM catabolism in response to ER stress.
Taurine appeared to have reversed this effect, consistent with
results aforementioned in the present study. Immunofluorescence
staining results of collagen-II were consistent with the western
blot analysis results (Fig. 6C),
whereby taurine treatment attenuated the TG-associated catabolism
of the ECM in NP cells. In addition, RT-qPCR analysis showed that
downregulation of aggrecan, Col II and Sox-9 by IL-1β are
attenuated by the treatment of Taurine (Fig. 6D-F).
Discussion
Recent reports have suggested that IDD is a major
trigger of lower back pain (33,34). Conventional treatment methods for
IDD treatment include physical therapy and pain management
medication (35). Surgical
interventions are necessary if conventional methods cannot
alleviate the symptoms, which can lead to a succession of sequelae
(36). In addition, in the
majority of cases, patients with IDD remain largely asymptomatic,
such that early-stage IDD can only be revealed by MRI (37). Therefore, by exploiting all the
available information on the pathophysiology of IDD, it is vital to
explore novel treatment strategies to promote endogenous repair
whilst halting IDD progression, particularly during early stages of
the disease (38).
Accumulating evidence suggests that excessive ECM
degradation and elevated NP cell apoptosis serve key roles in the
pathology underlying IDD (11).
Numerous biomechanical and biochemical activities can promote the
deterioration of NP cells, which can in turn induce cell apoptosis
to disrupt the equilibrium between ECM catabolism and anabolism
(39). Since NP cells have
limited capacities for resisting stress and self-repair (40), the molecular pathways underlying
NP cell apoptosis require characterization for ameliorating NP
degeneration in patients with IDD.
Aging and excessive mechanical loading are primary
stressors that can cause NP cell apoptosis (41). Both can activate numerous
signaling pathways, including those such as the NLRX1 and NF-κB
pathways associated with inflammation (42). IL-1β is a potent proinflammatory
mediator that can trigger the apoptosis of NP cells by inducing
mitochondrial dysfunction and activating caspase-3 (43). According to CCK-8 assay results,
the viability of IL-1β-induced NP cells was found to be lower
compared with that in the control group. By contrast, taurine
treatment reversed this IL-1β-induced effect, suggesting that
taurine can exert cytoprotective effects on NP cells.
ER stress and the unfolded protein response (UPR)
downstream have been previously associated with the pathology of a
number of diseases, including cancer, skeletal disorders and
neurodegeneration (44). In terms
of musculoskeletal disorders, a previous study revealed that in
human osteoarthritis, chondrocytes were chronically exposed to ER
stress, where CHOP served a key role in mediating apoptosis and
cartilage degeneration downstream of ER stress (45). By contrast, only a small number of
studies have studied the potential contribution of ER stress to IDD
(7). GRP78 is a 78-kDa
glucose-regulated protein that is also known as immunoglobulin
heavy chain binding protein (46). Eukaryotic translation initiation
factor 2A (eIF2α) phosphorylation is instrumental for regulating
the global rate of protein synthesis (47). In addition, protein kinase R-like
ER kinase (PERK) has been reported to induce cell apoptosis by
promoting the accumulation of CHOP under severe ER stress (48). ER stress can induce apoptosis by
activating the UPR, upregulating the expression of GRP78 and
activating PERK/eIF2α signaling (47). After dissociation from PERK, eIF2α
is then phosphorylated. This eIF2α phosphorylation then activate
the regulatory factors of ER stress, such as activating
transcription factor (ATF) 4 and CHOP (48). Specifically, caspase-12 also has a
role in cell apoptosis resulting from ER stress (49). Taurine has been documented to
protect cells by either inhibiting mitochondrial dysfunction or ER
stress (50,51). Nonaka et al (52) previously found that taurine can
alleviate homocysteine-triggered ER stress in vascular smooth
muscle cells. According to the present study, the ER stress
signaling pathway (GRP78 and CHOP) appeared to be more active in
the IL-1β groups compared with that in the control groups,
suggesting that IL-1β can trigger ER stress. Consequently, the
administration of taurine can attenuate this ER stress, indicating
the potential role of taurine in suppressing ER stress. In present
study, TG was chosen to assess the extent to which ER stress
associates with the cytoprotective effects of taurine in NP cells.
TG can be extracted from the plant Thapsia garganica and is
a classical inhibitor of ER Ca2+ ATPase (53). ER Ca2+ATPase
dysfunction has been demonstrated to serve a role in various
diseases, including IDD (54). By
suppressing ER Ca2+ ATPase, TG interferes with
Ca2+ flux in the ER lumen, which contributes to
accumulation of cytoplasmic Ca2+ (55). This Ca2+ overload
causes mitochondrial dysfunction and disrupts a number of metabolic
pathways, including lipid turnover, which is reliant on lipase
maturation factor 1, an ER chaperone (56). Furthermore, depletion of this
Ca2+ store results in the rapid accumulation of unfolded
proteins in the ER, promoting the dissociation of GRP78 from
inositol-requiring enzyme 1 (IRE1), PERK and ATF6 pathways, thereby
activating UPR signaling (47).
Regulation of store-operated Ca2+ entry has also been
associated with the expression of specific microRNAs (57) and may regulate the
Ca2+-associated modulation of the UPR (IRE1 activity) in
the ER (58). According to the
present study, compared with that in the control groups, TG
treatment elevated GRP78 expression in addition to increasing the
expression of its downstream apoptotic mediator CHOP. Taurine
partially reversed the upregulation of both GRP78 and CHOP. In
addition, immunofluorescence staining results of cleaved caspase-12
were also consistent with the GRP78 and CHOP western blotting data.
TUNEL and western blot analysis of apoptotic activity also revealed
that taurine suppressed the TG-mediated apoptosis of NP cells.
According to these results, in TG-treated NP cells, taurine
partially reversed the activation of ER stress and ER
stress-associated apoptosis.
Increasing the expression of ECM-degrading enzymes
and proinflammatory mediators are conducive for IDD progression
(59). Production of inflammatory
factors, such as IL-1β, in turn elevates the expression of
catabolic proteins, such as MMP and ADAMTS, to promote continuous
collagen and proteoglycan degradation (59). Numerous reports have presented
evidence that ER stress is a key process in mediating inflammation
and matrix degradation (54,60). A previous NP cell secretome
analysis demonstrated an influence of ER stress induction on the
secretion of the ECM, which is accompanied with the reduction in
the expression of collagen and cell adhesion-related proteins
(60). Pharmacologically blocking
ER Ca2+ release using Ca2+ antagonists was
found to ameliorate ER stress and Ca2+ overload to
prevent the apoptosis of NP cells and partially halt the
development of IDD (54). The
present study revealed that NP cells treated with TG exhibited
characteristics indicative of ECM degradation, including the
upregulation of MMP-1, MMP-3, MMP-9 and ADAMTS-4, ADAMTS-5
expression and the downregulation of collagen-II expression.
Taurine reversed these TG-induced effects on increasing the
expression of these catabolic enzymes. Collectively, these results
suggest that taurine attenuated the ER stress-associated metabolic
processes of the NP cells.
In conclusion, the present study revealed that
taurine can promote NP cell viability whilst inhibiting apoptosis.
These protective effects were associated with the prevention of ER
stress in NP cells in vitro. In addition, taurine was able
to attenuate ER stress-associated ECM catabolic activity in NP
cells. Therefore, taurine should be investigated for its
potentially feasibility as a therapeutic agent for preventing NP
degeneration patients diagnosed with IDD.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LY and YO designed the study. LY and ZL contributed
to experiments and statistical analysis. LY and YO confirm the
authenticity of all the raw data. All authors read and approved the
version of the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Care
and Use Committee of Shanghai Jing'an District Zhabei Central
Hospital (Shanghai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang F, Cai F, Shi R, Wang XH and Wu XT:
Aging and age related stresses: A senescence mechanism of
intervertebral disc degeneration. Osteoarthritis Cartilage.
24:398–408. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Simon J, McAuliffe M, Shamim F, Vuong N
and Tahaei A: Discogenic low back pain. Phys Med Rehabil Clin N Am.
25:305–317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Petersen T, Laslett M and Juhl C: Clinical
classification in low back pain: Best-evidence diagnostic rules
based on systematic reviews. BMC Musculoskelet Disord. 18:1882017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barrey CY and Le Huec JC; French Society
for Spine Surgery, : Chronic low back pain: Relevance of a new
classification based on the injury pattern. Orthop Traumatol Surg
Res. 105:339–346. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liang D, Hong D, Tang F, Wang Y, Li J, Li
L and Chen H: Upregulated lnc-HRK-2:1 prompts nucleus pulposus cell
senescence in intervertebral disc degeneration. Mol Med Rep.
22:5251–5261. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pattappa G, Li Z, Peroglio M, Wismer N,
Alini M and Grad S: Diversity of intervertebral disc cells:
Phenotype and function. J Anat. 221:480–496. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sakai D and Grad S: Advancing the cellular
and molecular therapy for intervertebral disc disease. Adv Drug
Deliv Rev. 84:159–171. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ding F, Shao ZW and Xiong LM: Cell death
in intervertebral disc degeneration. Apoptosis. 18:777–785. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu S, Liang T and Li S: Correlation
between polymorphism of TRAIL gene and condition of intervertebral
disc degeneration. Med Sci Monit. 21:2282–2287. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao Z, Zheng J, Ye Y, Zhao K and Wang R
and Wang R: MicroRNA-25-3p regulates human nucleus pulposus cell
proliferation and apoptosis in intervertebral disc degeneration by
targeting Bim. Mol Med Rep. 22:3621–3628. 2020.PubMed/NCBI
|
|
11
|
Tsingas M, Ottone OK, Haseeb A, Barve RA,
Shapiro IM, Lefebvre V and Risbud MV: Sox9 deletion causes severe
intervertebral disc degeneration characterized by apoptosis, matrix
remodeling, and compartment-specific transcriptomic changes. Matrix
Biol. 94:110–133. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Borrelli C and Buckley CT: Injectable
disc-derived ECM hydrogel functionalised with chondroitin sulfate
for intervertebral disc regeneration. Acta Biomater. 117:142–155.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ohnishi T, Novais EJ and Risbud MV:
Alterations in ECM signature underscore multiple sub-phenotypes of
intervertebral disc degeneration. Matrix Biol Plus. 6–7, 100036.
2020.PubMed/NCBI
|
|
14
|
Le Maitre CL, Pockert A, Buttle DJ,
Freemont AJ and Hoyland JA: Matrix synthesis and degradation in
human intervertebral disc degeneration. Biochem Soc Trans.
35:652–655. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang WJ, Yu XH, Wang C, Yang W, He WS,
Zhang SJ, Yan YG and Zhang J: MMPs and ADAMTSs in intervertebral
disc degeneration. Clin Chim Acta. 448:238–246. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ding F and Li X: Apigenin mitigates
intervertebral disc degeneration through the amelioration of tumor
necrosis factor α (TNF-α) signaling pathway. Med Sci Monit.
26:e9245872020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roberts S, Caterson B, Menage J, Evans EH,
Jaffray DC and Eisenstein SM: Matrix metalloproteinases and
aggrecanase: Their role in disorders of the human intervertebral
disc. Spine (Phila Pa 1976). 25:3005–3013. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pockert AJ, Richardson SM, Le Maitre CL,
Lyon M, Deakin JA, Buttle DJ, Freemont AJ and Hoyland JA: Modified
expression of the ADAMTS enzymes and tissue inhibitor of
metalloproteinases 3 during human intervertebral disc degeneration.
Arthritis Rheum. 60:482–491. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamori Y, Nara Y, Ikeda K and Mizushima S:
Is taurine a preventive nutritional factor of cardiovascular
diseases or just a biological marker of nutrition? Adv Exp Med
Biol. 403:623–629. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wallace DR and Dawson R Jr: Decreased
plasma taurine in aged rats. Gerontology. 36:19–27. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pacholczyk-Sienicka B, Radek M, Radek A
and Jankowski S: Characterization of metabolites determined by
means of 1H HR MAS NMR in intervertebral disc degeneration. MAGMA.
28:173–183. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Goodman CA, Horvath D, Stathis C, Mori T,
Croft K, Murphy RM and Hayes A: Taurine supplementation increases
skeletal muscle force production and protects muscle function
during and after high-frequency in vitro stimulation. J Appl
Physiol (1985). 107:144–154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ghosh S, Chowdhury S, Das AK and Sil PC:
Taurine ameliorates oxidative stress induced inflammation and ER
stress mediated testicular damage in STZ-induced diabetic Wistar
rats. Food Chem Toxicol. 124:64–80. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rainesalo S, Keränen T, Palmio J, Peltola
J, Oja SS and Saransaari P: Plasma and cerebrospinal fluid amino
acids in epileptic patients. Neurochem Res. 29:319–324. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Engelborghs S, Marescau B and De Deyn PP:
Amino acids and biogenic amines in cerebrospinal fluid of patients
with Parkinson's disease. Neurochem Res. 28:1145–1150. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao Z, Lin Y, Zhang P, Cheng Q, Ye L, Wu
F, Chen Y, Fu M, Cheng C and Gao Y: Sinomenine ameliorates
intervertebral disc degeneration via inhibition of apoptosis and
autophagy in vitro and in vivo. Am J Transl Res. 11:5956–5966.
2019.PubMed/NCBI
|
|
27
|
Liu D, Gu Y, Wang W and Chen W: Astragalin
alleviates ischemia/reperfusion-induced brain injury via
suppression of endoplasmic reticulum stress. Mol Med Rep.
22:4070–4078. 2020.PubMed/NCBI
|
|
28
|
Chen J, Xuan J, Gu YT, Shi KS, Xie JJ,
Chen JX, Zheng ZM, Chen Y, Chen XB, Wu YS, et al: Celastrol reduces
IL-1β induced matrix catabolism, oxidative stress and inflammation
in human nucleus pulposus cells and attenuates rat intervertebral
disc degeneration in vivo. Biomed Pharmacother. 91:208–219. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Martirosyan NL, Patel AA, Carotenuto A,
Kalani MY, Belykh E, Walker CT, Preul MC and Theodore N: Genetic
alterations in intervertebral disc disease. Front Surg. 3:592016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yurube T, Takada T, Suzuki T, Kakutani K,
Maeno K, Doita M, Kurosaka M and Nishida K: Rat tail static
compression model mimics extracellular matrix metabolic imbalances
of matrix metalloproteinases, aggrecanases, and tissue inhibitors
of metalloproteinases in intervertebral disc degeneration.
Arthritis Res Ther. 14:R512012. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ekşi MŞ, Özcan-Ekşi EE, Özmen BB, Turgut
VU, Huet SE, Dinç T, Kara M, Özgen S, Özek MM and Pamir MN: Lumbar
intervertebral disc degeneration, end-plates and paraspinal muscle
changes in children and adolescents with low-back pain. J Pediatr
Orthop B. 31:93–102. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Khan AN, Jacobsen HE, Khan J, Filippi CG,
Levine M, Lehman RA Jr, Riew KD, Lenke LG and Chahine NO:
Inflammatory biomarkers of low back pain and disc degeneration: A
review. Ann N Y Acad Sci. 1410:68–84. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang B, Xu H, Wang J, Liu B and Sun G: A
narrative review of non-operative treatment, especially traditional
Chinese medicine therapy, for lumbar intervertebral disc
herniation. Biosci Trends. 11:406–417. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jacobs WC, van der Gaag NA, Kruyt MC,
Tuschel A, de Kleuver M, Peul WC, Verbout AJ and Oner FC: Total
disc replacement for chronic discogenic low back pain: A Cochrane
review. Spine (Phila Pa 1976). 38:24–36. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ogon I, Takashima H, Morita T, Oshigiri T,
Terashima Y, Yoshimoto M, Fukushi R, Fujimoto S, Emori M, Teramoto
A, et al: Relevance between schmorl's node and lumbar
intervertebral disc degeneration quantified with magnetic resonance
imaging T2 mapping in chronic low back pain. Asian Spine J.
14:621–628. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cherif H, Bisson DG, Mannarino M, Rabau O,
Ouellet JA and Haglund L: Senotherapeutic drugs for human
intervertebral disc degeneration and low back pain. Elife.
9:e546932020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang D, Chen Y, Cao S, Ren P, Shi H, Li H,
Xie L, Huang W, Shi B and Han J: Cyclic mechanical stretch
ameliorates the degeneration of nucleus pulposus cells through
promoting the ITGA2/PI3K/AKT signaling pathway. Oxid Med Cell
Longev. 2021:66993262021.PubMed/NCBI
|
|
40
|
Mobasheri A, Csaki C, Clutterbuck AL,
Rahmanzadeh M and Shakibaei M: Mesenchymal stem cells in connective
tissue engineering and regenerative medicine: Applications in
cartilage repair and osteoarthritis therapy. Histol Histopathol.
24:347–366. 2009.PubMed/NCBI
|
|
41
|
Zhao CQ, Wang LM, Jiang LS and Dai LY: The
cell biology of intervertebral disc aging and degeneration. Ageing
Res Rev. 6:247–261. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xu H, Ji L, Yu C, Chen Q, Ge Q and Lu Y:
MiR-423-5p regulates cells apoptosis and extracellular matrix
degradation via nucleotide-binding, leucine-rich repeat containing
X1 (NLRX1) in interleukin 1 beta (IL-1β)-induced human nucleus
pulposus cells. Med Sci Monit. 26:e9224972020.PubMed/NCBI
|
|
43
|
Guo HT, Yang SD, Zhang F, Liu S, Yang DL,
Ma L, Wang H and Ding WY: 17β-Estradiol protects against
interleukin-1β-induced apoptosis in rat nucleus pulposus cells via
the mTOR/caspase-3 pathway. Mol Med Rep. 20:1523–1530.
2019.PubMed/NCBI
|
|
44
|
Horiuchi K, Tohmonda T and Morioka H: The
unfolded protein response in skeletal development and homeostasis.
Cell Mol Life Sci. 73:2851–2869. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Uehara Y, Hirose J, Yamabe S, Okamoto N,
Okada T, Oyadomari S and Mizuta H: Endoplasmic reticulum
stress-induced apoptosis contributes to articular cartilage
degeneration via C/EBP homologous protein. Osteoarthritis
Cartilage. 22:1007–1017. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shimizu A, Kaira K, Yasuda M, Asao T and
Ishikawa O: Clinical and pathological significance of ER stress
marker (BiP/GRP78 and PERK) expression in malignant melanoma.
Pathol Oncol Res. 23:111–116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chern YJ, Wong JCT, Cheng GSW, Yu A, Yin
Y, Schaeffer DF, Kennecke HF, Morin G and Tai IT: The interaction
between SPARC and GRP78 interferes with ER stress signaling and
potentiates apoptosis via PERK/eIF2α and IRE1α/XBP-1 in colorectal
cancer. Cell Death Dis. 10:5042019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fu YF, Liu X, Gao M, Zhang YN and Liu J:
Endoplasmic reticulum stress induces autophagy and apoptosis while
inhibiting proliferation and drug resistance in multiple myeloma
through the PI3K/Akt/mTOR signaling pathway. Oncotarget.
8:61093–61106. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lee W, Kim DH, Boo JH, Kim YH, Park IS and
Mook-Jung I: ER stress-induced caspase-12 activation is inhibited
by PKC in neuronal cells. Apoptosis. 10:407–415. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yang Y, Zhang Y, Liu X, Zuo J, Wang K, Liu
W and Ge J: Exogenous taurine attenuates mitochondrial oxidative
stress and endoplasmic reticulum stress in rat cardiomyocytes. Acta
Biochim Biophys Sin (Shanghai). 45:359–367. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yang YJ, Han YY, Chen K, Zhang Y, Liu X,
Li S, Wang KQ, Ge JB, Liu W and Zuo J: TonEBP modulates the
protective effect of taurine in ischemia-induced cytotoxicity in
cardiomyocytes. Cell Death Dis. 6:e20252015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Nonaka H, Tsujino T, Watari Y, Emoto N and
Yokoyama M: Taurine prevents the decrease in expression and
secretion of extracellular superoxide dismutase induced by
homocysteine: Amelioration of homocysteine-induced endoplasmic
reticulum stress by taurine. Circulation. 104:1165–1170. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li Z, Guo J, Bian Y and Zhang M:
Intermedin protects thapsigargin-induced endoplasmic reticulum
stress in cardiomyocytes by modulating protein kinase A and
sarco/endoplasmic reticulum Ca2+-ATPase. Mol Med Rep.
23:1072021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Luo R, Song Y, Liao Z, Yin H, Zhan S, Wang
K, Li S, Li G, Ma L, Lu S, et al: Impaired calcium homeostasis via
advanced glycation end products promotes apoptosis through
endoplasmic reticulum stress in human nucleus pulposus cells and
exacerbates intervertebral disc degeneration in rats. FEBS J.
286:4356–4373. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Canová NK, Kmonícková E, Martínek J, Zídek
Z and Farghali H: Thapsigargin, a selective inhibitor of
sarco-endoplasmic reticulum Ca2+-ATPases, modulates
nitric oxide production and cell death of primary rat hepatocytes
in culture. Cell Biol Toxicol. 23:337–354. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Mao HZ, Ehrhardt N, Bedoya C, Gomez JA,
DeZwaan-McCabe D, Mungrue IN, Kaufman RJ, Rutkowski DT and Péterfy
M: Lipase maturation factor 1 (lmf1) is induced by endoplasmic
reticulum stress through activating transcription factor 6α (Atf6α)
signaling. J Biol Chem. 289:24417–24427. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Finger F and Hoppe T: MicroRNAs meet
calcium: Joint venture in ER proteostasis. Sci Signal. 7:re112014.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Groenendyk J, Peng Z, Dudek E, Fan X,
Mizianty MJ, Dufey E, Urra H, Sepulveda D, Rojas-Rivera D, Lim Y,
et al: Interplay between the oxidoreductase PDIA6 and microRNA-322
controls the response to disrupted endoplasmic reticulum calcium
homeostasis. Sci Signal. 7:ra542014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lama P, Zehra U, Balkovec C, Claireaux HA,
Flower L, Harding IJ, Dolan P and Adams MA: Significance of
cartilage endplate within herniated disc tissue. Eur Spine J.
23:1869–1877. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Novais EJ, Choi H, Madhu V, Suyama K, Anjo
SI, Manadas B, Shapiro IM, Salgado AJ and Risbud MV: Hypoxia and
hypoxia-inducible factor-1α regulate endoplasmic reticulum stress
in nucleus pulposus cells: Implications of endoplasmic reticulum
stress for extracellular matrix secretion. Am J Pathol.
191:487–502. 2021. View Article : Google Scholar : PubMed/NCBI
|