Introduction
Intervertebral disc degeneration disease (IDD) is
one of the major causes of low back pain (LBP) (1). Since 2015, overall >540 million
individuals worldwide suffer from varying degrees of LBP, which
causes pain and loss of function. LBP imposes huge burdens on
society, the economy and families; seriously reduces the quality of
life of patients (1). The present
authors have been engaged in orthopaedic trauma-related clinical
work. In clinical practice, we noticed the present of varying
degrees of disc degeneration (most frequent at the level of L4/L5)
in patients with lumbar spine fractures after surgery.
Intervertebral disc degeneration is a very complex process that is
mainly associated with the abnormal apoptosis of intervertebral
disc cells, biomechanical mechanisms and the autoimmune response
(2,3). Regardless of the aetiology, the end
result is that the proliferation capacity of the intervertebral
disc is weakened and the function is reduced, which leads to the
occurrence of disc degeneration (2,3).
As a family of proteins that regulate cell proliferation and cell
matrix biosynthesis, growth factors play important roles in
stimulating cell proliferation and repairing intervertebral disc
injury (3).
The IDD rat model is a good experimental model for
studying the mechanism of intervertebral disc degeneration. Because
the pathological changes in the blood and intervertebral discs in
the model are similar to those of human intervertebral disc
degeneration, the IDD rat model has been widely used to study human
intervertebral disc degeneration (4). Fine needle puncture is currently the
preferred method for establishing models of intervertebral disc
degeneration due to its simplicity, ease of operation and high
repeatability. Masuda et al (4) attempted to puncture rabbit fibre
rings using different sizes of needles (16G, 18G and 21G) and
successfully established an animal model of disc degeneration that
led to decreases in both disc height and magnetic resonance imaging
grading.
Chronic intermittent hypobaric hypoxia (CIHH)
pre-conditioning is the practice of simulating altitude sickness in
humans or animals through intermittent exposure to low pressure and
low oxygen. Moderate low-pressure hypoxia stimulation can
effectively mobilize the body's endogenous protective mechanism to
counter the subsequent stimulation by more serious external injury
(5). CIHH has been widely used in
sports training to enhance the resistance of organs and tissues to
anoxia (6). Previous studies have
demonstrated that CIHH pre-treatment has a protective effect
against collagen-induced arthritis in rats through the
down-regulation of hypoxia-inducible factor 1-α (HIF-1α) and NF-κB,
and through the inhibition of the inflammatory cytokines TNF-α and
IL-17 (5). Therefore, it was
hypothesised that CIHH promotes the expression of basic fibroblast
growth factor (bFGF) and TGFβ1, which play important regulatory
roles in cell proliferation, differentiation and tissue repair,
thereby promoting intervertebral disc tissue repair. The main
objective of the present study was to investigate the reparative
effect of CIHH pre-treatment on degressive intervertebral disc
tissue in rats.
Materials and methods
Chemicals and reagents
The Haematoxylin-Eosin/HE Staining kit, Modified
Safranine O-Fast Green FCF Cartilage Stain kit, and Masson's
Trichrome Stain kit were purchased from Beijing Solarbio Science
& Technology Co., Ltd. Rat bFGF ELISA kit (cat. no.
E-EL-R0091c), Rat HIF-1α ELISA kit (E-EL-R0513c) and TGF-β1 ELISA
kit (E-EL-0162c) were purchased from Elabscience Biotechnology,
Inc. For the western blotting experiments, collagen I(WL0088),
collagen II(WL03082) and TGF-β1 (WL02193) antibodies were purchased
from Wanleibio Co., Ltd., and the bFGF (E-AB-15525) antibody was
purchased from Elabscience Biotechnology, Inc.
Animals and treatments
All experiments were performed in accordance with
the guidelines for the Care and Use of Experimental Animals
(National Research Committee, 1996) and approved by the Ethics
Committee for the Use of Experimental Animals of Hebei Medical
University (approval no. Z2019-012-1; Hebei, China). At total, 48
adult male Sprague-Dawley rats (provided by Hebei Medical
University Experimental Animal Centre; weight, 320±20 g; 8 weeks
old) were randomly divided into three groups: The experimental
group (CIHH-IDD), degenerative group (IDD) and control group (CON).
The IDD model was established in rats in the IDD group (n=16) by
puncturing the tail discs after 28 days of normal feeding. CIHH +
IDD rats (n=16) were treated with CIHH (simulated altitude of 3,000
m, 5 h per day, for 28 consecutive days; PO2=108.8 mmHg)
before undergoing the same treatment as the IDD rats. CON rats
(n=16) were normally bred without IDD induction. At 1, 2, 4 and 8
weeks after IDD surgery, four rats from each group were randomly
selected for X-ray imaging, after which blood from the heart and
tissue from the tail disc were collected after the animals were
anaesthetised.
All animals were kept in a temperature-controlled
room (22±1°C; relative humidity, 40–80%) with a 12 h light/dark
cycle and free access to water and food. The health status and
physical activity of the rats were monitored every day. At the end
of the experiments, rats were fasted overnight and anaesthetised
with pentobarbital sodium (50 mg/kg; intraperitoneal injection).
Left index finger to find the heart apex pulse, right hand holding
blood needle puncture and 3 ml of blood were collected from the
heart. The rats were sacrificed with an overdose of pentobarbital
sodium (100 mg/kg; intraperitoneal injection), and then tail disc
specimens were isolated. In the present study, the preparation of
IDD rats, the collection of samples and the measurement of the
results were performed by the same skilled researchers to reduce
errors caused by subjective and human errors.
IDD establishment
The rats were anaesthetised using pentobarbital
sodium (50 mg/kg, intraperitoneal injection) and fixed in the
supine position. The rat tail discs at C0 6/7, C0 7/8 and C0 8/9
were selected as the research objects. The tail vertebrae were
first disinfected, and then an X-ray instrument was used to locate
the intervertebral disc nucleus centre. A 21G needle was passed
through the disc nucleus, slowly rotated 180° and maintained for 5
sec, after which the needle was removed, pressure was applied to
stop the blood and the area was disinfected again. Because the
endplate is an important structure for maintaining nutrition in the
intervertebral disc, damage to the endplate can exacerbate or
accelerate the degeneration of the intervertebral disc (7–9);
thus, the surgeon avoided damaging the endplate as much as
possible.
CIHH treatment
For CIHH treatment, the animal was placed in a
low-pressure oxygen chamber, and the air was pumped away using a
vacuum, resulting in a pressure of 108.8 mmHg, which represents an
altitude of 3,000 m. At the same time, fresh air flowed into the
chamber through a small ventilation hole to keep enough fresh air
for the animal to breathe. An intermittent oxygen environment
controller was used to maintain a low oxygen environment for 5 min,
and then the pressure was restored to normal. For safety, the
reduced pressure and boost pressure speed were controlled at 2.5
m/s with the vent valve. The time from the low oxygen concentration
to the high oxygen concentration was 30 sec, and the intermittent
low-pressure hypoxia experiment lasted for 5 h.
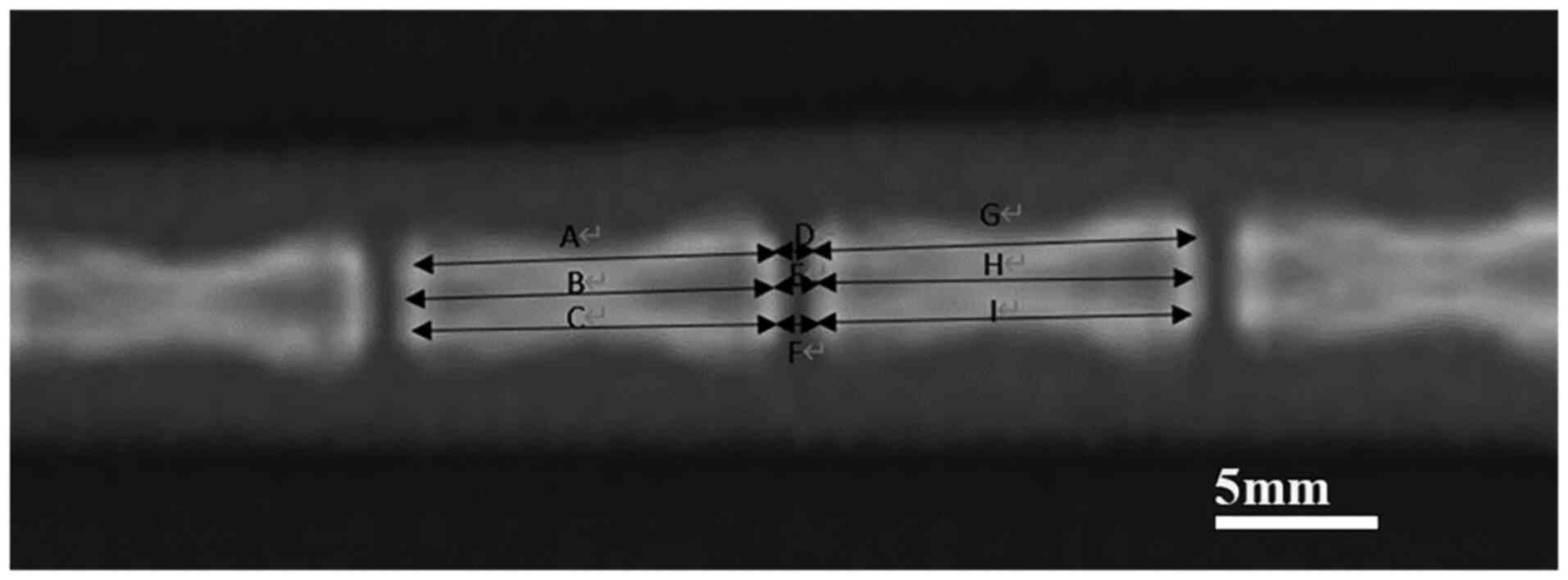
X-ray calculation of the disc height
index (DHI)
After being anaesthetised, the rats were placed in
the prone position for X-ray scans and radiographs before IDD
surgery. The same operation was performed at 1, 2, 4 and 8 weeks
after IDD surgery. According to the method described by Han et
al (10), the width of the
intervertebral disc was divided into four equal points, the image
analysis software ImageJ (V1.8.0.112, National Institutes of
Health) was used to measure the height of the intervertebral disc
and its adjacent vertebral body, and the intervertebral DHI was
calculated, which represented the change in height relative to the
rate of change in the DHI value (10). The specific calculation method was
calculated as follows (Fig. 1):
DHI=2(D+E+F)/(A+B+C+G+H+I), where DHI %=post-operative
DHI/pre-operative DHI ×100%.
Intervertebral disc tissue specimen
collection
At 1, 2, 4 and 8 weeks after the IDD operation, four
rats in each group were sacrificed by an intraperitoneal injection
of excessive levels of anaesthetic drugs. The tail skin was
carefully removed, the tail was detached and the C0 6/7, C0 7/8 and
C0 8/9 discs were separated. One intervertebral disc tissue sample
was soaked in 10% neutral-buffered formalin fixation solution for
48 h (23–26°C). The specimens were routinely dehydrated,
decalcified and paraffin-embedded to prepare wax blocks. The
intervertebral disc tissue was cut into 5-µm thick slices that were
used for haemotoxylin-eosin (HE), modified Safranine O-Fast Green
and Masson's trichrome staining. HE staining can clearly show the
layers of tissue, modified Safranine O-Fast Green staining can
better show the cartilage layers and subchondral bone structure,
and Masson's trichrome staining can clearly show fibrous ring
tissue and nucleus pulposus tissue (11). The other two intervertebral disc
tissues were quickly placed at −80°C and used to measure the
protein expression levels of bFGF, TGFβ1, Collagen I and Collagen
II using western blotting.
ELISA determination of bFGF, TGF β1
and HIF-1α in serum
The rats were anaesthetised with pentobarbital
sodium (50 mg/kg; intraperitoneal injection), and 3 ml of blood
were collected from the heart and centrifuged for 5 min (2716 × g,
23–26°C) to get serum. The serum was collected and stored at −20°C
for later use. The expression levels of bFGF, TGFβ1 and HIF-1α in
the serum were determined using ELISA kits as aforementioned.
According to the manufacturer's procedures indicated in the kits,
the optical density values were measured at 450 nm (ELX-800, BioTek
Instruments, Inc.) 15 min after the cessation of the reaction. The
concentrations of bFGF, TGFβ1 and HIF-1α were determined from a
standard log-log graph.
Western blotting
The expression levels of bFGF, TGFβ1, Collagen I and
Collagen II in degenerative disc tissue were measured using western
blotting. Degenerated intervertebral disc tissue was frozen at
−80°C, homogenised and lysed and then placed on ice for 5 min. The
samples were centrifuged at 23,188 × g. and 4°C for 10 min, and the
protein was extracted using RIPA lysis buffer (WLA019, Wanleibio
Co.). and separated by electrophoresis. The protein concentration
in the supernatant was determined using the BCA method, and each
sample was boiled in water for 5 min at 100°C. The samples (20 µl
per lane, containing 40 µg of protein) were examined using 7.5–15%
SDS-PAGE and transferred to PVDF membranes. The PVDF membrane was
blocked with 5% (M/V) non-fat milk powder for 1 h at 4°C. The blots
were incubated first at 4°C with primary antibodies against bFGF
(1:2,000), TGFβ1 (1:500), Collagen I (1:500) and Collagen II
(1:500) overnight. The samples were then incubated with secondary
antibodies (Goat Anti-rabbit IgG-HRP, 1:5000, WLA023, Wanleibio
Co.) for 45 min at 37°C. The reaction was visualised by
chemiluminescence, and the optical density of the target band was
analysed by a gel image processing system (Gel-Pro-Analyzer 6.0;
Media Cybernetics, Inc.). The protein levels were normalised to
that of β-actin (1:1,000, WL01845, Wanleibio Co.).
Statistical analysis
Statistical analysis was performed using SPSS 26.0
(IBM Corp.). Experiments were repeated three times. The data are
expressed as the mean ± SEM. n represents the number of animals in
functional or western blotting experiments. Statistical analysis
was conducted using one-way ANOVA followed by
Student-Newman-Keuls's post hoc test for comparisons among multiple
groups. The paired Student's t-tests were used for comparisons
between two groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Imaging analysis of the effect of CIHH
on intervertebral disc degeneration in rats
Vertebral imaging of each group of four rats was
performed using digital X-ray instruments at 1, 2, 4 and 8 weeks
after surgery, and the results are shown below. The DR images
indicated that the density of the intervertebral space in the
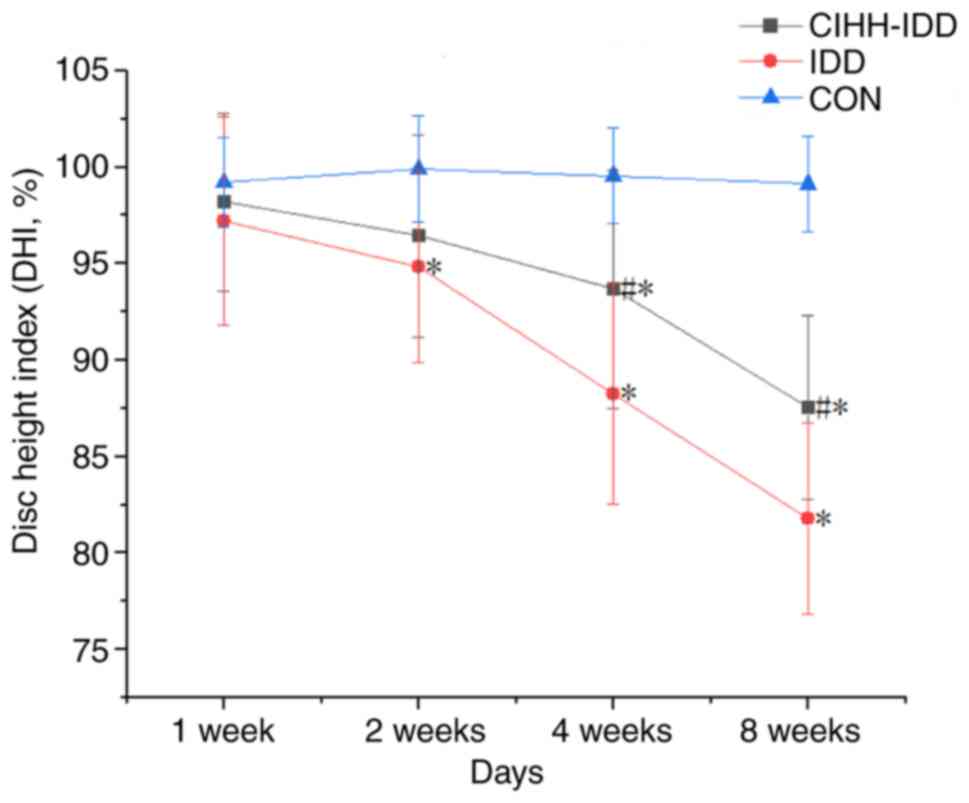
CIHH-IDD and IDD groups increased gradually after surgery (Fig. 2). The intervertebral space height
(DHI %) of each group of rats at different times was measured using
image analysis software and is presented in Table I. In the second week after
surgery, the differences between the IDD group and CON group were
significant (P<0.05), the results of the CIHH-IDD group were not
significant compared with those of CON group (P>0.05), and the
CIHH-IDD group was significantly different compared with the IDD
group (P<0.05). At 4 weeks after surgery, The DHI (%) of the IDD
group was significantly different compared with that in the
CIHH-IDD group (P<0.05), and the difference between the CIHH-IDD
group and the CON group was significant (P<0.05).
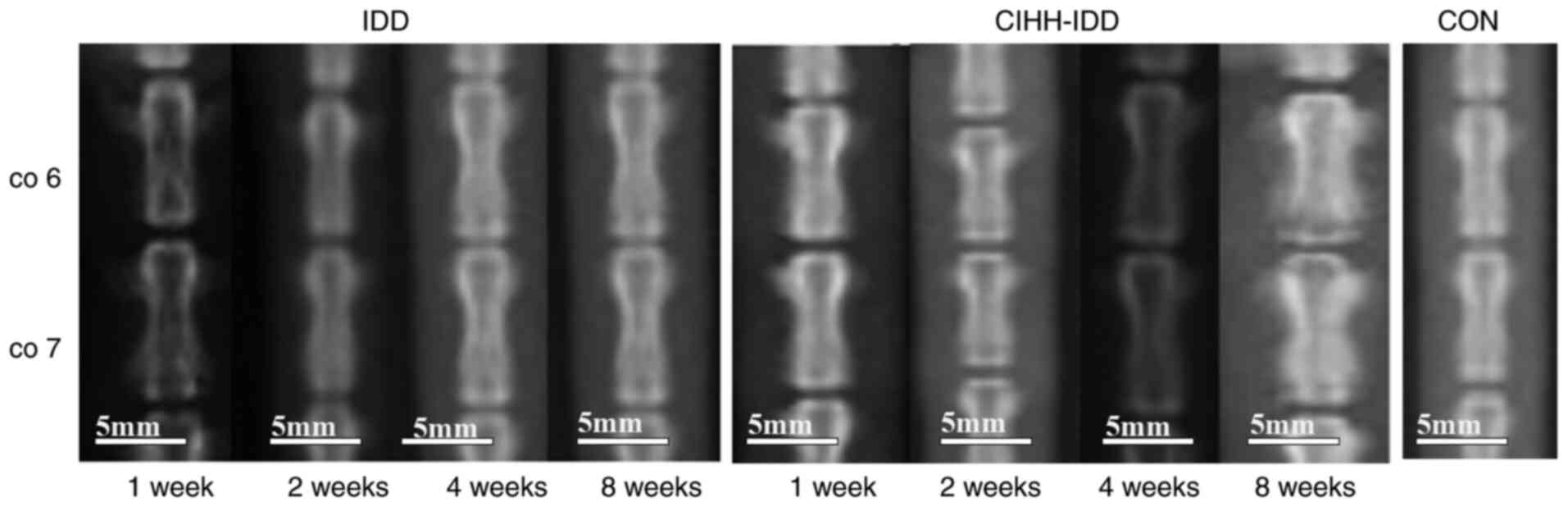 | Figure 2.Images of intervertebral discs in
rats. Images of CO6, CO7 and intervertebral space in rats of IDD
group and CIHH-IDD group, at 1, 2, 4 and 8 W after surgery. Scale
bar, 50 nm. W, weeks; IDD, CIHH-IDD; CON, control; Scale bar, 50
nm. W, weeks; IDD, CIHH-IDD; CON, control. |
 | Table I.Intervertebral disc height index (DHI
%). |
Table I.
Intervertebral disc height index (DHI
%).
| Post-operation | CIHH-IDD | IDD | CON |
|---|
| 1 week | 98.20±4.61 | 97.20±5.37 | 99.20±2.31 |
| 2 weeks |
96.45±5.24b |
94.83±4.97a | 98.90±2.78 |
| 4 weeks |
93.67±6.17a,b |
88.26±5.71c | 99.56±2.51 |
| 8 weeks |
87.54±4.76b,c |
81.79±4.96c | 99.12±2.47 |
The results indicated that the intervertebral disc
height of rats in the IDD group decreased at 2 weeks after surgery
compared with the control, and the decreasing trend was gradually
but significantly worsened over time (Fig. 3). By contrast, the intervertebral
disc height of rats in the CIHH-IDD group demonstrated a
significant decrease at 4 weeks after surgery compared with the
control, with a slower downward trend compared with that in the IDD
group. Overall, CIHH treatment significantly inhibited the degree
of disc degeneration in the CIHH-IDD group compared with the IDD
group.
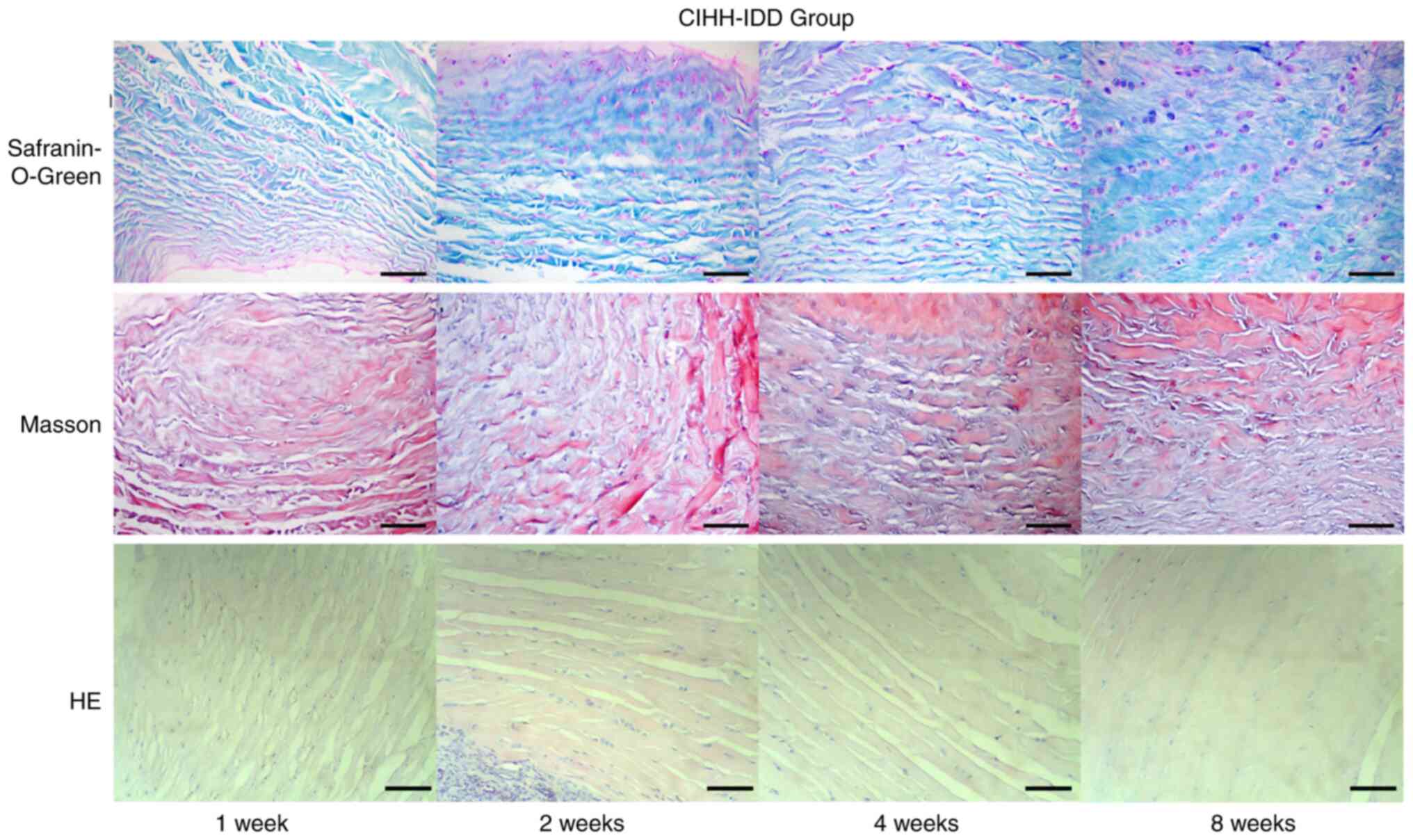
Effect of CIHH on disc pathology in
rats
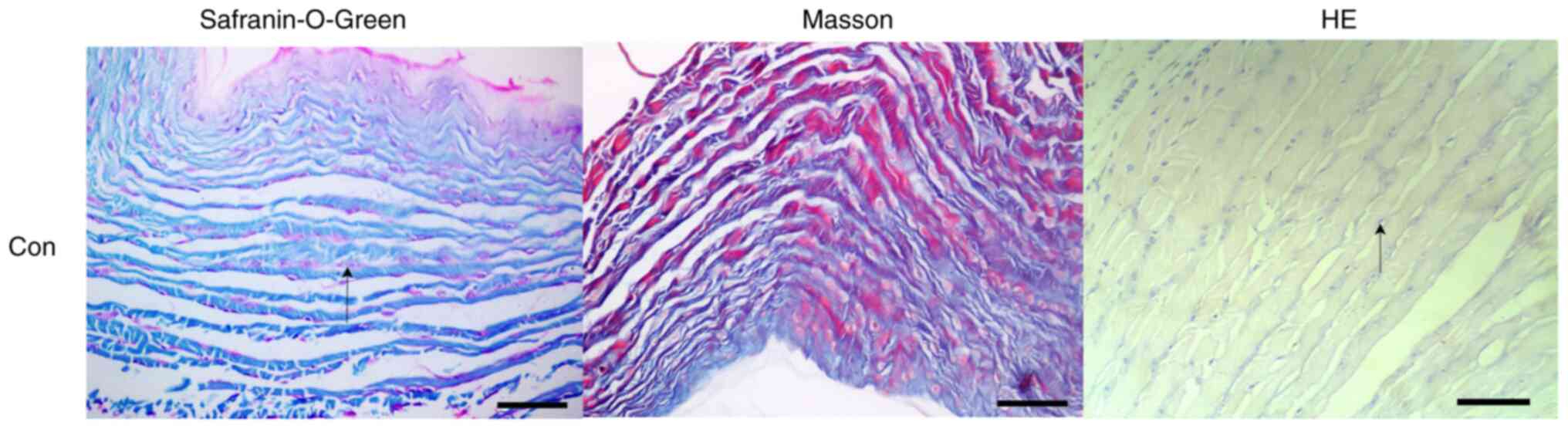
Disc tissue staining in the CON group indicated that
the intervertebral discs of rats presented a similar oval
appearance and that the nucleus pulposus was increased, accounting
for more than half of the total intervertebral disc volume. The
proteoglycans in the nucleus pulposus were stained red and have
small round cells spaced at intervals. The boundary between nucleus
pulposus tissue and the surrounding fibre ring was clear. The fibre
ring was arranged in an orderly and concentric circular lamella,
and there was no obvious fracture or crack between each lamella.
Fusiform fibroblasts were observed between each lamella (Fig. 4).
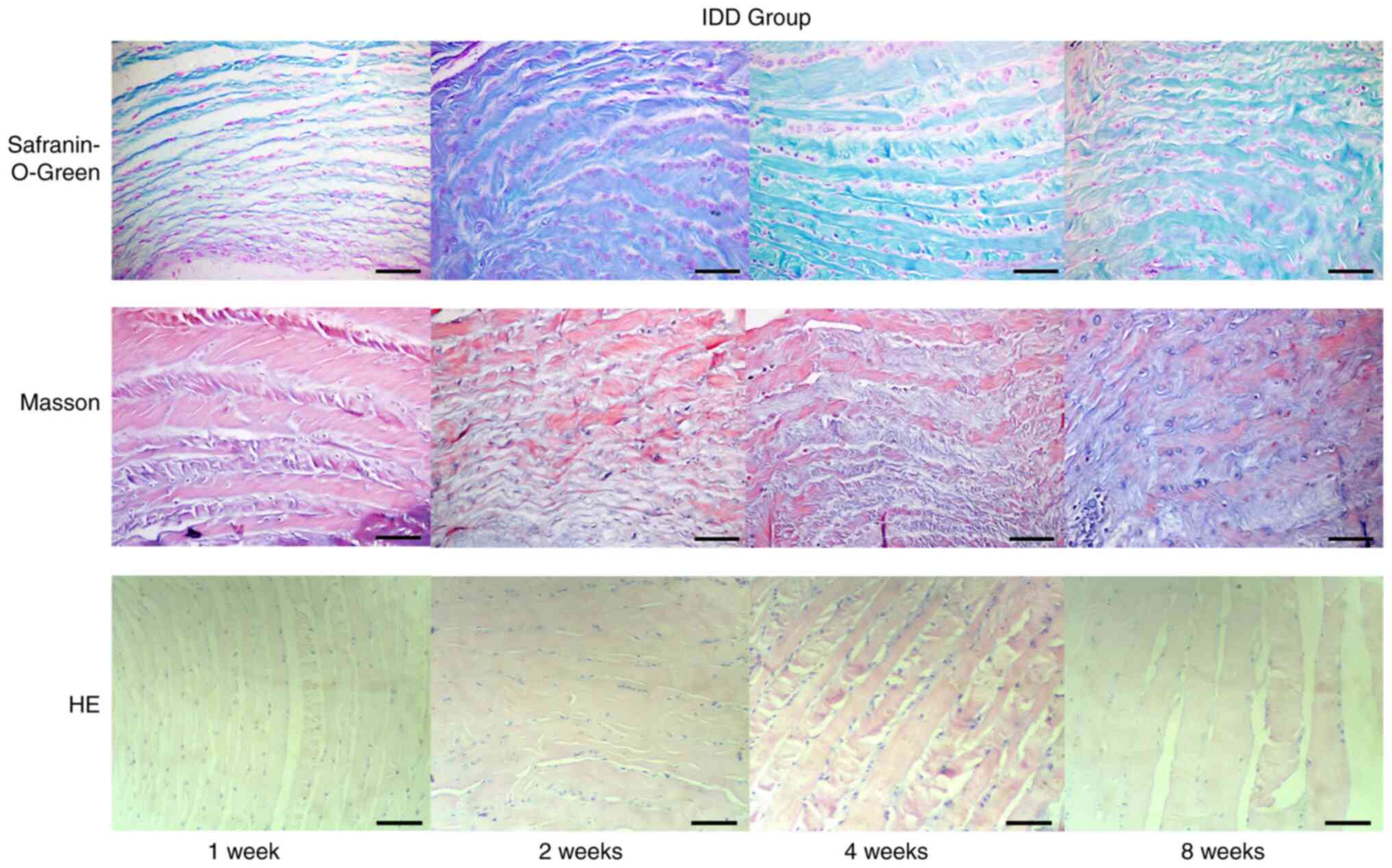
Pathologically stained disc slices from the IDD
group showed that intervertebral disc histology was mainly changed
in the following manners (Fig.
5): Reduced volume of the nucleus pulposus, fractures or
disordered arrangement between the annulus fibrosus, decreased
numbers of nucleus pulposus cells and extracellular matrix (ECM)
and metaplasia of cells. After the operation, the nucleus pulposus
became irregular and smaller, and some tissue of the nucleus
pulposus was lost, resulting in space. Over time, the degree of
shrinkage gradually increased, and the space in part of the nucleus
pulposus increased. Furthermore, the number of cells in the nucleus
pulposus was markedly reduced. Although some small round cells
could be seen in the nucleus pulposus, these cells were
significantly different compared with the uniformly distributed
normal cells in the nucleus pulposus, which were separated by ECM
and distributed in clusters. The original small circular spinal
cord cells became large round cartilage-like cells, some cells
appeared to have large empty bubbles and the cells and their
surroundings were dyed red by safranin-O-green staining or blue by
Masson staining. The boundary between the nucleus pulposus and the
annulus fibrosus became unclear. The annulus was disorganised, with
radiation-like or edge tearing, which sometimes extended from the
inside out to the perimeter of the fibre ring. Metaplasia of cells
was also observed in the annulus fibrosus; in particular, the
number of chondroid cells between the lamellar layers of the inner
annulus fibrosus increased, and the amount of red-stained
proteoglycan increased. Masson staining showed blue collagen
fibres, which were markedly increased. Chondroid cell proliferation
in the annulus fibrosus was present. The structure of cartilage,
subchondral bone and bone tissue can be indicated by
safranin-O-green staining. The cartilage matrix appears red, and
the subchondral bone and bone tissue appear green, which can
robustly the cartilage tissue.
By observing the pathological sections of CIHH rats
with intervertebral disc disease, the arrangement of the annulus
fibrosus was observed to be more similar to that of the CON group
compared with the IDD group, and the arrangement disorder and
tearing degree were reduced compared with those in the IDD group
(Fig. 6). Both nucleus pulposus
volume reductions and extracellular matrix reductions occurred but
did not further worsen. Cavitation of the nucleus pulposus was
rare. CIHH treatment resulted in some repair of the degenerative
discs in rats.
Effect of CIHH on the expression
levels of bFGF, TGF 1 and HIF-1 in rat blood
The serum level of HIF-1α at 1 week after surgery
was significantly higher in the CIHH-IDD group compared with the
CON and IDD groups (P<0.01). There was no significant difference
in the level of HIF-1α between the three groups beginning at 2 week
(P>0.05). The expression level of HIF-1α in CIHH-pre-treated
rats returned to normal after normal feeding for 2 weeks (Table II).
 | Table II.Effect of chronic intermittent
hypobaric hypoxia on the expression of bFGF, TGF 1 and HIF-1 in rat
blood. |
Table II.
Effect of chronic intermittent
hypobaric hypoxia on the expression of bFGF, TGF 1 and HIF-1 in rat
blood.
| Group | bFGF | TGFβ1 | HIF-1α |
|---|
| CIHH-IDD |
|
|
|
| 1
week |
11.12±3.41a,b |
48.53±7.97a,b |
7.76±2.23a,b |
| 2
week |
9.78±3.12a,c |
42.19±6.76a,b | 4.88±1.56 |
| 4
week |
9.85±3.31a |
36.85±6.45a,b | 4.01±1.22 |
| 8
week |
8.52±2.76a |
32.88±5.87a,b | 4.39±1.32 |
| IDD |
|
|
|
| 1
week | 7.53±2.74 |
30.91±5.45a | 4.13±1.01 |
| 2
week |
8.31±3.25d |
34.47±5.94a | 4.12±1.13 |
| 4
week |
9.83±3.49a |
26.32±2.67d | 4.22±1.22 |
| 8
week |
8.73±2.78a |
26.98±3.83d | 4.32±1.32 |
| CON |
|
|
|
| 1
week | 7.75±2.46 | 23.59±2.97 | 4.01±1.21 |
| 2
week | 7.57±2.66 | 24.37±3.42 | 4.02±1.01 |
| 4
week | 7.49±2.58 | 23.26±3.17 | 4.22±0.99 |
| 8
week | 7.53±2.62 | 23.63±3.29 | 4.21±1.02 |
The serum level of TGFβ1 in the IDD group was higher
compared with that in the CON group beginning in the first week
after surgery (P<0.05), indicating that the expression of TGFβ1
was increased in rats after disc puncture (Table II). After CIHH pre-treatment, the
serum level of TGFβ1 in the CIHH-IDD group was significantly higher
compared with that in the IDD group beginning in the first week
after surgery (P<0.01), indicating that CIHH pre-treatment
significantly increased the serum level of TGFβ1 in rats.
The expression of bFGF was measured, and the serum
levels of bFGF in rats that were pre-treated with CIHH were
observed to significantly increase compared with that in the IDD
groups and CON groups at week 1 (P<0.01). Furthermore, the serum
level of bFGF in rats in the IDD group showed a tendency to
increase over time (Table
II).
Effect of CIHH on bFGF, TGFβ1,
Collagen I and Collagen II expression in degenerative disc tissue
in rats
At 1 week after surgery, the expression of bFGF in
the three groups was not statistically significant. At 2 weeks
after the operation, the expression of bFGF in the CIHH-IDD group
was significantly increased compared with that in the CON group
(P<0.01). Compared with that in the IDD group, the expression of
bFGF in the CIHH-IDD group was significantly up-regulated at 2 and
8 weeks (P<0.05), but there was no significant difference
between the two groups in the fourth weeks (Fig. 7).
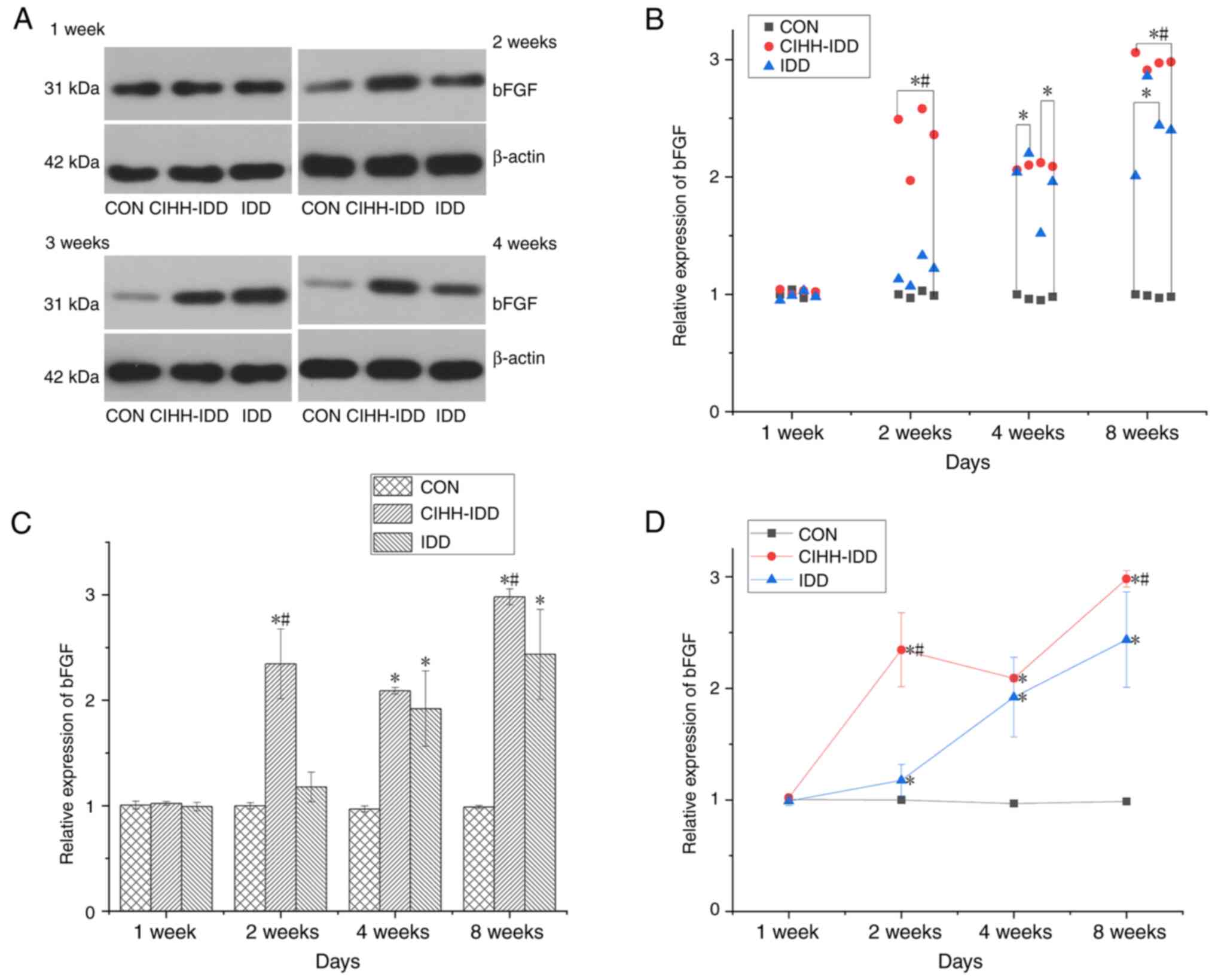 | Figure 7.Effect of CIHH on the expression of
bFGF protein in degeneration disc tissue. (A) Western blotting of
bFGF protein at 1, 2, 4 and 8 weeks. (B) Western blotting data
distribution of bFGF proteins at 1, 2, 4, and 8 weeks. (C) bFGF
protein expression levels at 1, 2, 4, and 8 weeks in three groups.
(D) Expression trend of bFGF protein. n=16 for each group; n=4 for
each time point. *P<0.05 vs. CON group; #P<0.05
vs. IDD group. bFGF, basic fibroblast growth factor; CIHH, chronic
intermittent hypobaric hypoxia; IDD, intervertebral disc
degeneration disease group; CON, control. |
Compared with that in the CON group, the expression
of TGFβ1 in rat degenerative discs (IDD group) demonstrated a
significant trend of increased expression beginning at 1 week
(P<0.01). By contrast, the expression of TGFβ1 in the CIHH-IDD
group was not significantly different at 1 week compared with that
in the IDD group. Starting at 2 weeks, the protein expression of
TGFβ1 in the CIHH-IDD group increased significantly compared with
both the CON and IDD groups (P<0.05) (Fig. 8).
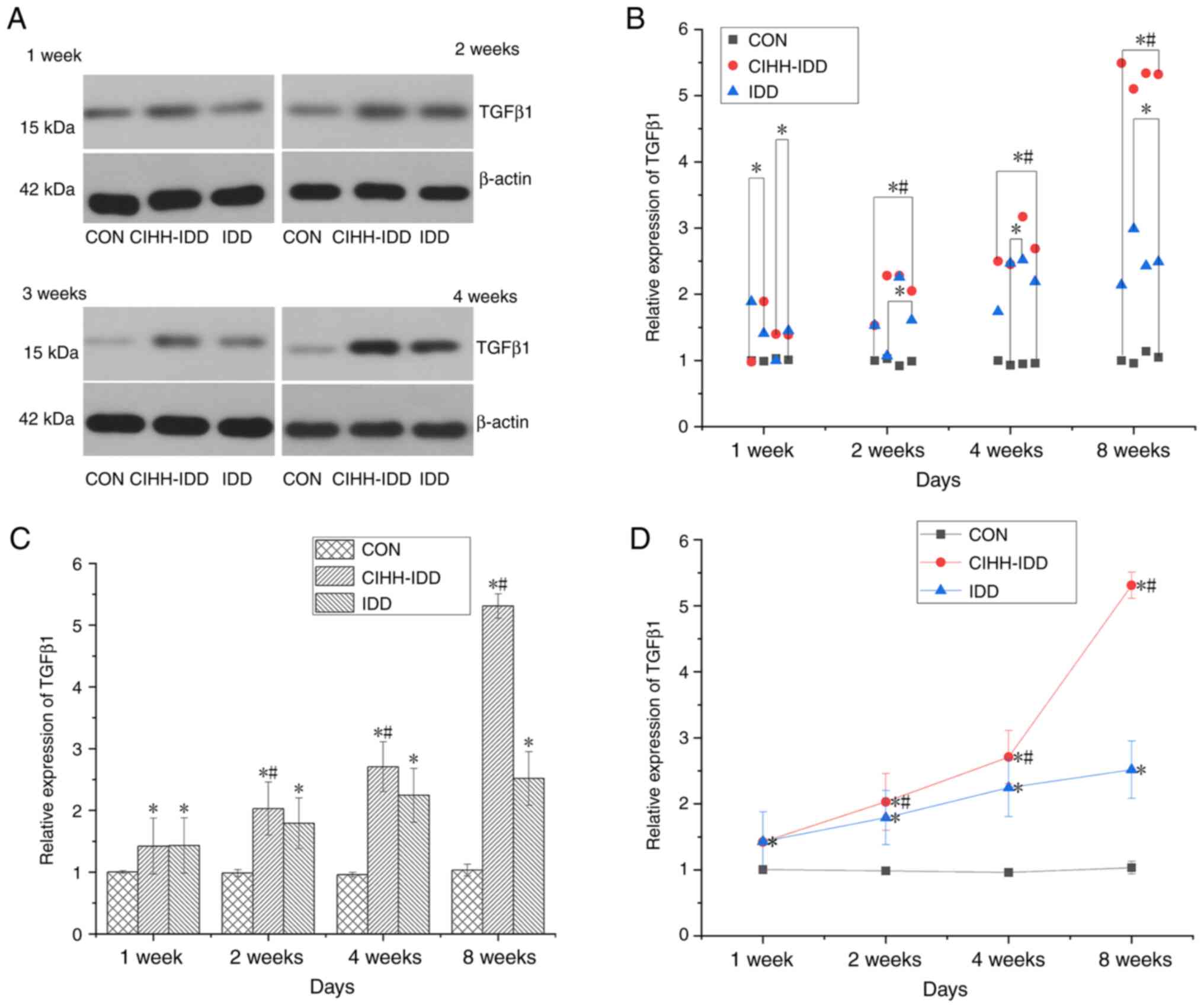 | Figure 8.Effect of CIHH on the expression of
TGFβ1 protein in degeneration disc tissue. (A) Western blotting of
TGFβ1 protein at 1, 2, 4 and 8 weeks in the three groups. (B)
Western blotting data distribution of TGFβ1 proteins at 1, 2, 4,
and 8 weeks. (C) TGFβ1 protein expression levels at 1, 2, 4, and 8
weeks in three groups. (D) The expression trend of TGFβ1 protein.
n=16 for each group, n=4 for each time point. *P<0.05 vs. CON
group; #P<0.05 vs. IDD group. CIHH, chronic
intermittent hypobaric hypoxia; IDD, intervertebral disc
degeneration disease group; CON, control |
After establishing the animal models of IDD, the
expression of Collagen I in the degenerative intervertebral discs
of rats in the CIHH-IDD group and IDD group was significantly
increased compared with that in the CON group at week 1
(P<0.05). The data demonstrated that at 2 weeks after surgery,
the protein expression of Collagen I was not significantly
different among the three groups, and it was considered that this
effect might be associated with deviations during surgery and the
experiments. At 1, 4 and 8 weeks after surgery, the protein
expression of collagen I in the CIHH-IDD group was inhibited
compared to that in the IDD group (P<0.05) (Fig. 9).
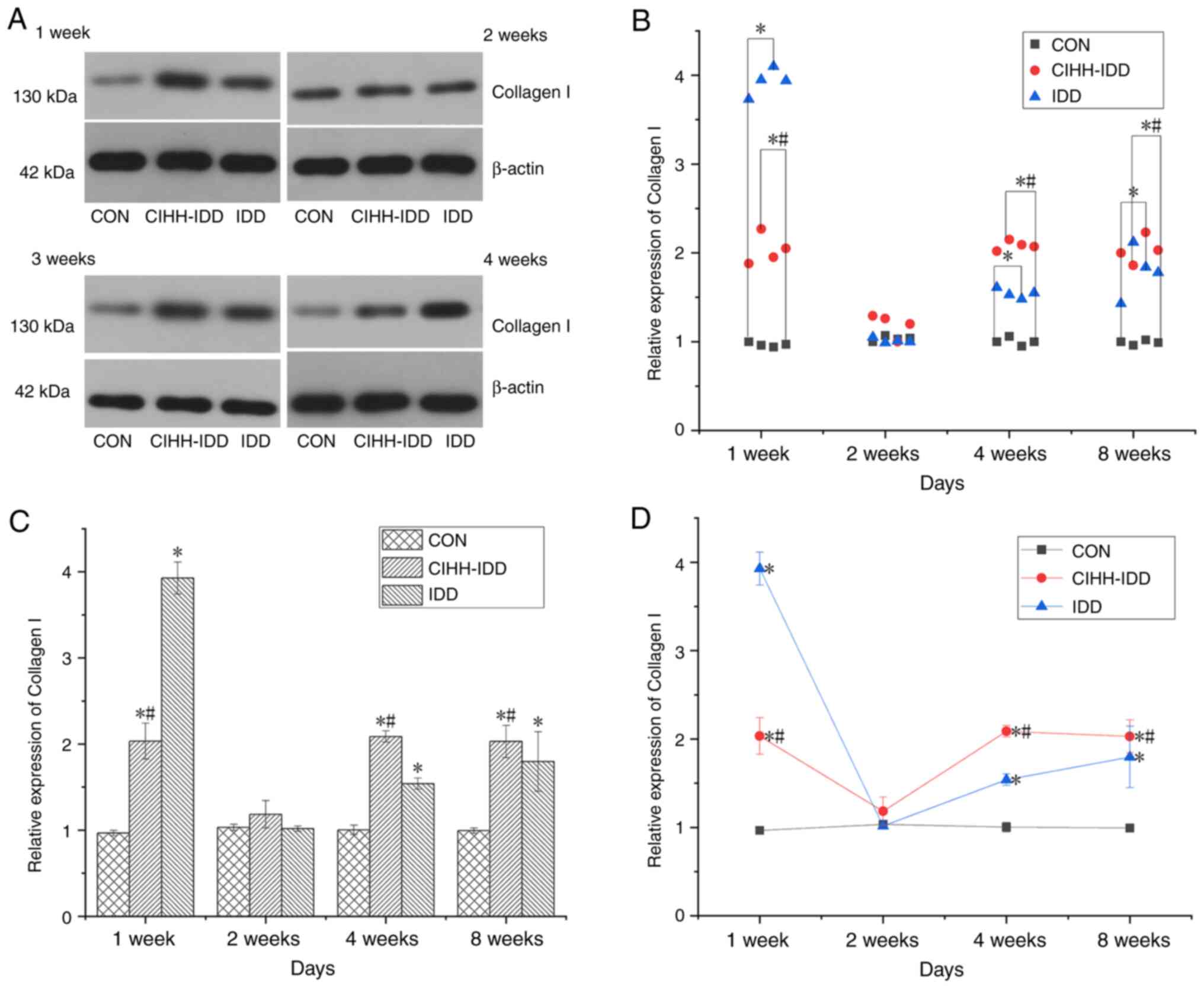 | Figure 9.Effect of CIHH on the expression of
Collagen I protein in degeneration disc tissue. (A) Western
blotting of Collagen I protein at 1, 2, 4 and 8 weeks in the three
groups. (B) Western blotting data distribution of Collagen I
proteins at 1, 2, 4, and 8 weeks. (C) Collagen I protein expression
levels at 1, 2, 4, and 8 weeks in three groups. (D) The expression
trend of Collagen I protein. n=16 for each group, n=4 for each time
point. *P<0.05 vs. CON group; #P<0.05 vs. IDD
group. CIHH, chronic intermittent hypobaric hypoxia; IDD,
intervertebral disc degeneration disease group; CON, control. |
At 1 week after surgery, the protein expression of
collagen II in the CIHH-IDD group and IDD group was significantly
increased compared with that in the CON group (P<0.05). Compared
with that in the IDD group, the protein expression of collagen II
in the CIHH-IDD was significantly increased from 2 weeks after
surgery (P<0.05) (Fig.
10).
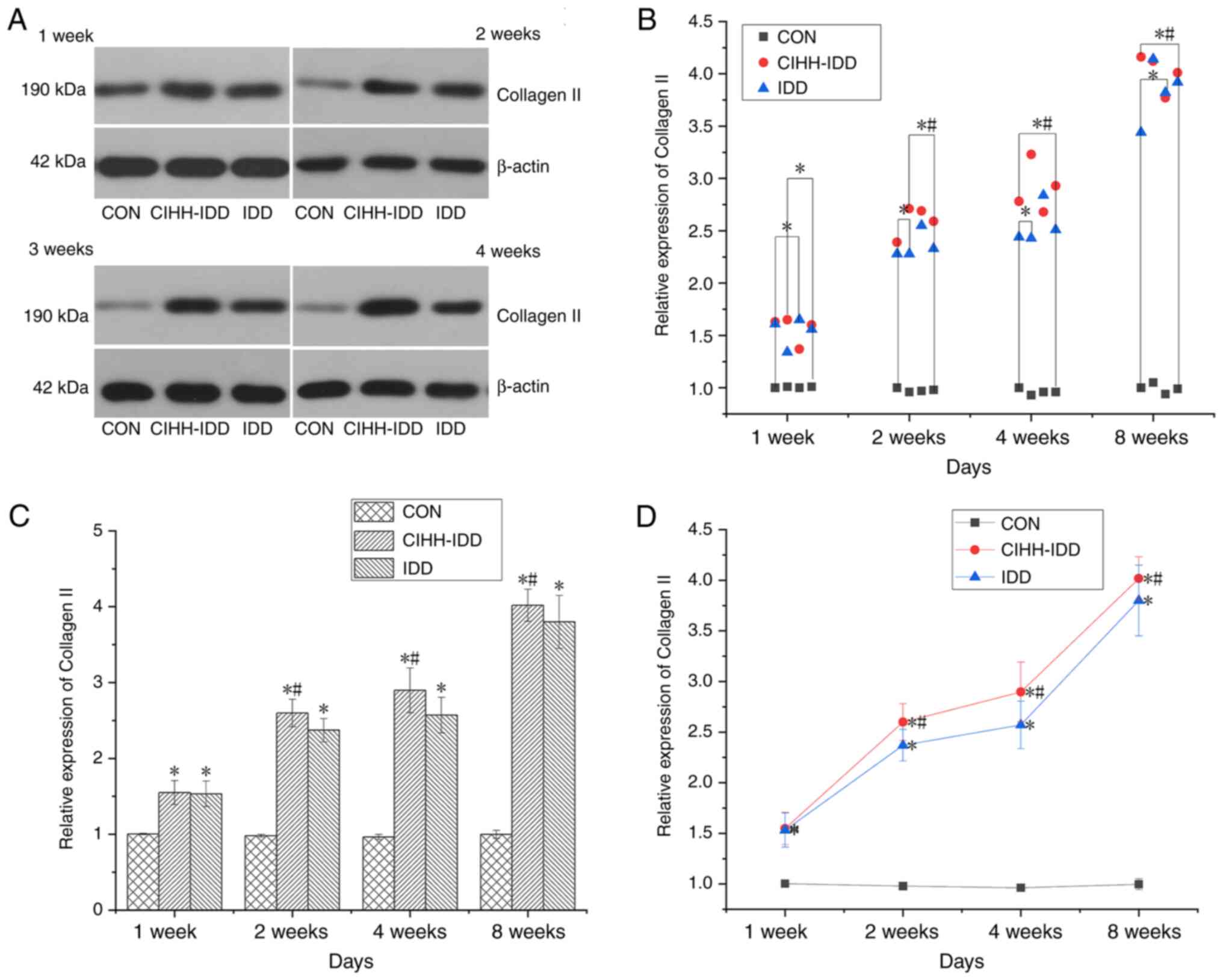 | Figure 10.Effect of CIHH on the expression of
Collagen II protein in degeneration disc tissue. (A) Western
blotting of Collagen II protein at 1, 2, 4 and 8 weeks in the three
groups. (B) Western blotting data distribution of Collagen II
proteins at 1, 2, 4, and 8 weeks. (C) Collagen II protein
expression levels at 1, 2, 4, and 8 weeks in three groups. (D)
Expression trend of Collagen II protein. n=16 for each group; n=4
for each time point. *P<0.05 vs. CON group;
#P<0.05 vs. IDD group. CIHH, chronic intermittent
hypobaric hypoxia; IDD, intervertebral disc degeneration disease
group; CON, control. |
Discussion
Intervertebral disc degeneration is a complex
process that gradually occurs under the combined effects of various
factors, including natural and environmental factors (12). In recent years, due to the
increasing burden of lumbago on individuals, families and society,
research on the aetiology and pathogenesis of disc degeneration has
increased (13). A number of
clinical studies have demonstrated that the mechanism of
intervertebral disc degeneration may be a complex pathological
phenomenon caused by reduced nutrient supply in intervertebral disc
tissue, changes in extracellular matrix components in
intervertebral discs, excessive apoptosis, biomechanical changes
and autoimmunity, but the exact mechanism has not been clarified
(14–16).
The present study investigated the internal
mechanism by which CIHH promoted disc repair in IDD rats. It was
revealed that that CIHH pre-treatment could significantly promote
the expression of bFGF and TGFβ1 in blood and intervertebral disc
tissue, thus effectively reducing the degree of intervertebral disc
degeneration and playing a role in the repair of intervertebral
disc degeneration. Of course, the effect of CIHH on degenerative
intervertebral discs in rats not only involved repair but was also
associated with the suppression of inflammatory factors, Such as
IL-4, TNF-α, and IL-17.
Through CIHH pre-treatment, an increase in HIF-1α
was observed in the serum of rats. Increased HIF expression is a
marker of the hypoxia response and a signal of hypoxia in tissues.
The body's adaptation to a low-pressure, low-oxygen environment is
achieved by enhancing HIF-1 levels (17). Hypoxia has both positive and
negative effects on the body. Severe hypoxia is involved in the
occurrence and prognosis of acute and chronic diseases such as
diabetes, cardiovascular disease and pulmonary oedema (18). On the other hand, controlled
hypoxia (intensity and time) plays a beneficial role through the
adaptation mechanism of body's response (19). For example, hypoxia can resist
heart ischaemia/reperfusion injury (20), inhibit arrhythmia (21) and protect the liver (22). Ambalavanan et al have
demonstrated the presence of low levels of bFGF in serum under
normal conditions, while in the case of hypoxia, the pulmonary
vascular endothelium is damaged, which destroys cellular integrity
and then bFGF is released (23).
The present study observed that the expression of
bFGF in blood peaked at 4 weeks after CIHH pre-treatment and then
declined steadily to the same level as that in IDD rats (Table II), which was consistent with the
conclusion reported in the literature that bFGF is released after
vascular endothelial injury in a hypoxic environment (24). TGF-β1 is a multifunctional growth
factor with fibrotic and immunoregulatory properties. TGF-β1 is
considered to be an important regulatory factor in COPD and other
inflammatory lung diseases (25–27). Notably, hypoxia can induce the
up-regulation of TGF-β1, platelet-derived growth factor (PDGF) and
HIF-1 protein expression in pulmonary artery smooth muscle cells,
thereby promoting the occurrence of pulmonary arterial hypertension
(28–30). In addition, TGF-β1 and PDGF play
important roles in hypoxia-induced lysyl oxidase expression and the
promotion of vascular smooth muscle cell growth (31). In the present study, the serum
level of TGF-β1 was significantly up-regulated in rats that were
pre-treated with CIHH compared with those in the CON and IDD
groups. Hypoxia caused an increase in TGF-β1 expression, which
played a role in inducing tissue differentiation.
Degeneration of the intervertebral disc first
affects the nucleus pulposus through necrosis and apoptosis of
nucleus pulposus cells. Then, the synthesis and secretion of
extracellular matrix proteins such as proteoglycan is decreased,
Collagen II is transformed to Collagen I and the decrease in
extracellular matrix changes the microenvironment of the nucleus
pulposus, further reducing the number of nucleus pulposus cells and
forming a vicious cycle (32).
The role of bFGF in intervertebral disc degeneration
and repair is relatively complex, and some studies have suggested
that bFGF has dual effects on intervertebral disc degeneration,
such as preventing and accelerating disc degeneration (33–35). bFGF, as a powerful mitogen,
stimulates the proliferation of capillary endothelial cells and
chondrocytes via the ERK and AKT signalling pathways (36). Since there are no blood vessels in
mature intervertebral disc tissue, oxygen and nutrients are
obtained by the intervertebral disc through penetration of the
endplate (37). Therefore, in
intervertebral disc development, bFGF is highly expressed in the
capillary inner skin, suggesting that it promotes the growth and
development of intervertebral discs through blood vessel formation.
The present study demonstrated that there was no significant change
in the expression of bFGF in the degenerated disc tissues of
CIHH-IDD rats compared with those of IDD rats and CON rats at 1
week after surgery.
TGF-β1 induces the differentiation and proliferation
of intervertebral disc mesenchymal cells and participates in the
repair process in damaged tissue (38). TGF-β1 is present in normal human
disc tissue, and the level of TGF-β1 can increase when disc
degeneration occurs. TGF-β1 repairs degenerative discs in the early
stages by promoting ECM synthesis (39). The present study observed
increased levels of TGF-β1 in rats in the IDD and CIHH-IDD groups
at the early stage of postoperative disc degeneration. The
expression of TGF-β1 in the IDD group decreased with time, while
that in the CIHH-IDD group remained increased. Light microscopy was
used to observe that the number of nucleus pulposus cells and
amount of ECM in the degenerated intervertebral discs of IDD rats
were reduced compared with those of CIHH-IDD rats. In addition, it
was observed that with increasing degeneration degrees, the nucleus
pulposus decreased, the boundary between the nucleus pulposus and
annulus fibrosus became increasingly blurred and the annulus
arrangement was disordered and broken. The differentiation and
proliferation of degenerated mesenchymal cells were observed in
CIHH-IDD rats, and the degree of intervertebral disc degeneration
was significantly inhibited.
Walsh et al (40) used static pressure to induce disc
degeneration, and then injected TGF-β1 and bFGF into intervertebral
discs. This study demonstrated that the number of fibre cells, the
expression of proteoglycan and collagen II and the height of the
intervertebral disc increases in the exogenous growth factor
injection group compared with those of the control group (injected
with normal saline) (40). Walsh
et al (40) demonstrated
that bFGF can repair the degenerative disc. The present study
observed that CIHH-IDD rats still had degeneration after disc
injury compared with those in the CON group. However, the present
study showed that the degree of intervertebral disc degeneration
was significantly reduced compared with that in the IDD group
(Fig. 3). These results indicated
that CIHH treatment could relieve and repair intervertebral disc
degeneration in rats.
The present study observed the effect of CIHH
pre-treatment on degenerative discs and obtained positive results,
which suggested that CIHH pre-treatment had a preventive effect on
IDD in the clinic. However, the therapeutic effect of CIHH
pre-treatment on degenerated discs could not be determined because
no recovery of disc height was observed. This is a shortcoming of
the present study, and further studies need to be performed.
In summary, the present study experimentally
demonstrated that CIHH preconditioning had a protective effect on
the degeneration of intervertebral discs in rats. CIHH played a
role in repairing and preventing disc degeneration by increasing
the expression of related inflammatory factors, such as TGFβ1 and
bFGF, in blood and disc tissues.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural Science
Foundation of Hebei Province (Shijiazhuang, China).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PCW conceptualised the study and investigated and
analysed the data. SRL, DR, HTW, SQY, ZHS and LDG collected and
analysed the data and developed the methodology. PCW and SRL
investigation and developed methodology. SRL wrote the manuscript.
All authors have read and approved the final manuscript. PCW and
SRL confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
All experiments are in accordance with the
guidelines for the Care and Use of Experimental Animals (National
Research Committee, 1996), and approved by the Ethics Committee for
the use of experimental animals of Hebei Medical University
(approval no. Z2019-012-1).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
GBD 2015 DALYs and HALE Collaborators, .
Global, regional, and national disability-adjusted life-years
(DALYs) for 315 diseases and injuries and healthy life expectancy
(HALE), 1990–2015: A systematic analysis for the global burden of
disease study 2015. Lancet. 388:1603–1658. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kepler CK, Ponnappan RK, Tannoury CA,
Risbud MV and Anderson DG: The molecular basis of intervertebral
disc degeneration. Spine J. 13:318–330. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Risbud MV and Shapiro IM: Role of
cytokines in intervertebral disc degeneration: Pain and disc
content. Nat Rev Rheumatol. 10:44–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Masuda K, Aota Y, Muehleman C, Imai Y,
Okuma M, Thonar EJ, Andersson GB and An HS: A novel rabbit model of
mild, reproducible disc degeneration by an anulus needle puncture:
Correlation between the degree of disc injury and radiological and
histological appearances of disc degeneration. Spine (Phila Pa
1976). 30:5–14. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shi M, Cui F, Liu AJ, Ma HJ, Cheng M, Song
SX, Yuan F, Li DP and Zhang Y: The protective effects of chronic
intermittent hypobaric hypoxia pretreatment against
collagen-induced arthritis in rats. J Inflamm (Lond). 12:232015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roels B, Bentley DJ, Coste O, Mercier J
and Millet GP: Effects of intermittent hypoxic training on cycling
performance in well-trained athletes. Eur J Appl Physiol.
101:359–368. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wei F, Zhong R, Pan X, Khaleel M, Hammoud
A, Zhou Z, Liu S, Sun H, Zhao Y, Zou X, et al: Computed
tomography-guided sub-end plate injection of pingyangmycin for a
novel rabbit model of slowly progressive disc degeneration. Spine
J. 19:e6–e18. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yuan W, Che W, Jiang YQ, Yuan FL, Wang HR,
Zheng GL, Li XL and Dong J: Establishment of intervertebral disc
degeneration model induced by ischemic sub-endplate in rat tail.
Spine J. 15:1050–1059. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kang R, Li H, Ringgaard S, Rickers K, Sun
H, Chen M, Xie L and Bünger C: Interference in the endplate
nutritional pathway causes intervertebral disc degeneration in an
immature porcine model. Int Orthop. 38:1011–1017. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Han B, Zhu K, Li FC, Xiao YX, Feng J, Shi
ZL, Lin M, Wang J and Chen QX: A simple disc degeneration model
induced by percutaneous needle puncture in the rat tail. Spine
(Phila Pa 1976). 33:1925–1934. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schwan S, Ludtka C, Friedmann A, Heilmann
A, Baerthel A, Brehm W, Wiesner I, Meisel HJ and Goehre F:
Long-term pathology of ovine lumbar spine degeneration following
injury via percutaneous minimally invasive partial nucleotomy. J
Orthop Res. 37:2376–2388. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de Campos MF, de Oliveira CP, Neff CB, de
Toledo Correa OM, Pinhal MA and Rodrigues LM: Studies of molecular
changes in intervertebral disc degeneration in animal model. Acta
Ortop Bras. 24:16–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ruiz-Fernández C, Francisco V, Pino J,
Mera A, González-Gay M, Gómez R, Lago F and Gualillo O: Molecular
relationships among obesity, inflammation and intervertebral disc
degeneration: Are adipokines the common link? Int J Mol Sci.
20:20302019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jin L, Balian G and Li XJ: Animal models
for disc degeneration-an update. Histol Histopathol. 33:543–554.
2018.PubMed/NCBI
|
|
15
|
Xu TT, Liao F, Jin HT, Tong PJ, Xiao LW
and Wu CL: Research advance on intervertebral disc degeneration and
cell death. Zhongguo Gu Shang. 28:673–678. 2015.(In Chinese).
PubMed/NCBI
|
|
16
|
Vergroesen PP, Kingma I, Emanuel KS,
Hoogendoorn RJ, Welting TJ, van Royen BJ, van Dieën JH and Smit TH:
Mechanics and biology in intervertebral disc degeneration: A
vicious circle. Osteoarthritis Cartilage. 23:1057–1070. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fujita N, Markova D, Anderson DG, Chiba K,
Toyama Y, Shapiro IM and Risbud MV: Expression of prolyl
hydroxylases (PHDs) is selectively controlled by HIF-1 and HIF-2
proteins in nucleus pulposus cells of the intervertebral disc:
distinct roles of PHD2 and PHD3 proteins in controlling HIF-1α
activity in hypoxia. J Biol Chem. 287:16975–16986. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tissot van Patot MC, Serkova NJ, Haschke
M, Kominsky DJ, Roach RC, Christians U, Henthorn TK and Honigman B:
Enhanced leukocyte HIF-1alpha and HIF-1 DNA binding in humans after
rapid ascent to 4300 m. Free Radic Biol Med. 46:1551–1557. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meerson F, Pozharov V and Minyailenko T:
Superresistance against hypoxia after preliminary adaptation to
repeated stress. J Appl Physiol (1985). 76:1856–1861. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou JJ, Ma HJ, Liu Y, Guan Y, Maslov LN,
Li DP and Zhang Y: The anti-arrhythmic effect of chronic
intermittent hypobaric hypoxia in rats with metabolic syndrome
induced with fructose. Can J Physiol Pharmacol. 93:227–232. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kushwah N, Jain V, Deep S, Prasad D, Singh
SB and Khan N: Neuroprotective role of intermittent hypobaric
hypoxia in unpredictable chronic mild stress induced depression in
rats. PLoS One. 11:e01493092016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu XH, Yan HC, Zhang J, Qu HD, Qiu XS,
Chen L, Li SJ, Cao X, Bean JC, Chen LH, et al: Intermittent hypoxia
promotes hippocampal neurogenesis and produces antidepressant-like
effects in adult rats. J Neurosci. 30:12653–12663. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ambalavanan N, Bulger A and Philips JB
III: Hypoxia-induced release of peptide growth factors from
neonatal porcine pulmonary artery smooth muscle cells. Biol
Neonate. 76:311–319. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ruszkowska-Ciastek B, Sokup A, Socha M,
Ruprecht Z, Hałas L, Góralczyk B, Góralczyk K, Gadomska G and Rość
D: A preliminary evaluation of VEGF-A, VEGFR1 and VEGFR2 in
patients with well-controlled type 2 diabetes mellitus. J Zhejiang
Univ Sci B. 15:575–581. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mahmood MQ, Reid D, Ward C, Muller HK,
Knight DA, Sohal SS and Walters EH: Transforming growth factor
(TGF) β1 and Smad signalling pathways: A likely key to
EMT-associated COPD pathogenesis. Respirology. 22:133–140. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang JC, Chen G, Chen L, Meng ZJ, Xiong
XZ, Liu HJ, Jin Y, Tao XN, Wu JH and Sun SW: TGF-β/BAMBI pathway
dysfunction contributes to peripheral Th17/Treg imbalance in
chronic obstructive pulmonary disease. Sci Rep. 6:319112016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Verhamme FM, Bracke KR, Joos GF and
Brusselle GG: Transforming growth factor-β superfamily in
obstructive lung diseases. more suspects than TGF-β alone. Am J
Respir Cell Mol Biol. 52:653–662. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schietke R, Warnecke C, Wacker I, Schödel
J, Mole DR, Campean V, Amann K, Goppelt-Struebe M, Behrens J,
Eckardt KU and Wiesener MS: The lysyl oxidases LOX and LOXL2 are
necessary and sufficient to repress E-cadherin in hypoxia: Insights
into cellular transformation processes mediated by HIF-1. J Biol
Chem. 285:6658–6669. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Green RS, Lieb ME, Weintraub AS, Gacheru
SN, Rosenfield CL, Shah S, Kagan HM and Taubman MB: Identification
of lysyl oxidase and other platelet-derived growth factor-inducible
genes in vascular smooth muscle cells by differential screening.
Lab Invest. 73:476–482. 1995.PubMed/NCBI
|
|
30
|
Atsawasuwan P, Mochida Y, Katafuchi M,
Kaku M, Fong KS, Csiszar K and Yamauchi M: Lysyl oxidase binds
transforming growth factor-beta and regulates its signaling via
amine oxidase activity. J Biol Chem. 283:34229–34240. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rodríguez C, Alcudia JF, Martínez-González
J, Raposo B, Navarro MA and Badimon L: Lysyl oxidase (LOX)
down-regulation by TNFalpha: A new mechanism underlying
TNFalpha-induced endothelial dysfunction. Atherosclerosis.
196:558–564. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xia W, Zhang LL, Mo J, Zhang W, Li HT, Luo
ZP and Yang HL: Effect of static compression loads on
intervertebral disc: An in vivo bent rat tail model. Orthop Surg.
10:134–143. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Thompson JP, Oegema TR Jr and Bradford DS:
Stimulation of mature canine intervertebral disc by growth factors.
Spine (Phila Pa 1976). 16:253–260. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nagano T, Yonenobu K, Miyamoto S, Tohyama
M and Ono K: Distribution of the basic fibroblast growth factor and
its receptor gene expression in normal and degenerated rat
intervertebral discs. Spine (Phila Pa 1976). 20:1972–1978. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tolonen J, Grönblad M, Vanharanta H, Virri
J, Guyer RD, Rytömaa T and Karaharju EO: Growth factor expression
in degenerated intervertebral disc tissue. An immunohistochemical
analysis of transforming growth factor beta, fibroblast growth
factor and platelet-derived growth factor. Eur Spine J. 15:588–596.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pratsinis H and Kletsas D: PDGF, bFGF and
IGF-I stimulate the proliferation of intervertebral disc cells in
vitro via the activation of the ERK and Akt signaling pathways. Eur
Spine J. 16:1858–1866. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bibby SR and Urban JP: Effect of nutrient
deprivation on the viability of intervertebral disc cells. Eur
Spine J. 13:695–701. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Singh K, Masuda K, Thonar EJ, An HS and
Cs-Szabo G: Age-related changes in the extracellular matrix of
nucleus pulposus and anulus fibrosus of human intervertebral disc.
Spine (Phila Pa 1976). 34:10–16. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xie J, Li B, Yao B, Zhang P, Wang L, Lu H
and Song X: Transforming growth factor-β1-regulated Fas/FasL
pathway activation suppresses nucleus pulposus cell apoptosis in an
inflammatory environment. Biosci Rep. 40:BSR201917262020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Walsh AJ, Bradford DS and Lotz JC: In vivo
growth factor treatment of degenerated intervertebral discs. Spine
(Phila Pa 1976). 29:156–163. 2004. View Article : Google Scholar : PubMed/NCBI
|