Introduction
Lung cancer is one of the most commonly diagnosed
malignancies and one of the main causes of cancer-related deaths
worldwide (1). In 2018, 2.09
million new cases and 1.76 million deaths were estimated to be
attributed to lung cancer, as determined using the Global Cancer
Incidence, Mortality and Prevalence database (1). The morbidity and mortality rates of
lung cancer have also shown an annual increase (1). Non-small cell lung cancer (NSCLC),
the dominant type of lung cancer, represents ~85% of total lung
cancer cases (2). The main
subtypes of NSCLC are lung adenocarcinoma and lung squamous cell
carcinoma (2). At present,
advancements in the treatment of NSCLC, including small-molecule
tyrosine kinase inhibitors (TKIs) and immunotherapy, have increased
patient survival rates in certain patients (2). However, the overall survival rate of
patients with NSCLC remains low, especially in patients with
metastatic NSCLC (2). Traditional
Chinese medicine (TCM) in the adjuvant therapy in patients with
NSCLC is considered to have potential therapeutic value in
improving prognosis (3).
In TCM, Salvia miltiorrhiza Bunge (SM) is
used to treat numerous diseases, including various cancer types,
based on its efficacy in promoting circulation and removing stasis
(4). The activity of SM is a
result of lipophilic compounds, including tanshinones and
cryptotanshinones, and hydrophilic phenolic acids, including
salvianolic acid A (Sal A) and salvianolic acid B (Sal B) (5). Sal B has the highest content of the
aforementioned compounds in SM. It has been reported to be a
potential cytotoxic polyphenol and is therefore a potential
therapeutic in a number of different cancers, including,
hepatocellular carcinoma, breast cancer, head and neck squamous
cell carcinoma, gastric cancer and colorectal cancer, based on
laboratory data (6–11). Furthermore, Sal B was observed to
inhibit NSCLC A549 cell growth; its half maximal inhibitory
concentration (IC50) value was determined to be a
concentration of 279.6 µM (12).
A previous study suggested that Sal B is also likely to be a
potential therapeutic candidate for NSCLC (12). However, the use of Sal B for NSCLC
treatment has not yet been adequately elucidated, and the molecular
mechanisms of the anti-NSCLC activities of Sal B remain
unclear.
Epithelial-mesenchymal transition (EMT) is a cell
phenotype transformation process, which is a crucial
patho-mechanism for enhanced tumorigenesis and metastasis,
contributing to the malignant progression of cancer (13). Transforming growth factor
β1 (TGF-β1), a common pluripotential
cytokine, induces EMT and therefore contributes to tumor invasion
and metastasis, inhibiting apoptotic stimuli in various cancer
cells including NSCLC (14).
Inhibition of TGF-β signaling is a novel and effective strategy for
NSCLC therapy (15). Sal B has
been reported to serve an important role in anti-pulmonary fibrosis
via inhibition of the TGF-β signaling pathway, which suggests that
suppression of TGF-β signaling could be a crucial mechanism in the
biological activity of Sal B (16). It can therefore be hypothesized
that Sal B has a therapeutic effect in NSCLC via inhibition of the
TGF-β signaling pathway. In the present study,
TGF-β1-stimulated NSCLC A549 cells were employed and
co-cultured with different concentrations of Sal B. Changes in cell
function and the intracellular mechanism of the TGF-β signaling
pathway were detected.
Materials and methods
Drugs and reagents
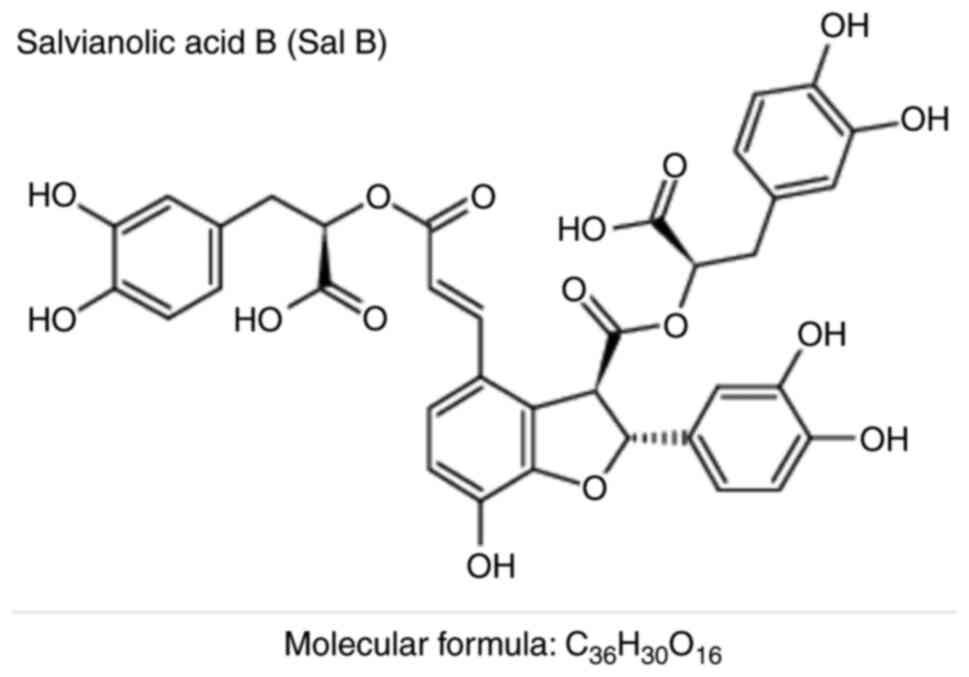
Sal B (chemical abstracts service no. 115939-25-8;
molecular formula, C36H30O16;
molecular weight, 718.61 Da; the chemical structure is displayed in
Fig. 1) was purchased from
Nantong FeiYu Biotechnology Co., Ltd. with a purity of >98%
(cat. no. FY1167B013). Recombinant human TGF-β1 (cat.
no. 100-21) was purchased from PeproTech, Inc. The Cell Cycle
Detection kit (cat. no. BB-4104-2) and BBcellProbe™
Annexin V-FITC Double-staining Cell Apoptosis Detection kit (cat.
no. BB-4101-2) were obtained from BestBio Co. Cell lysis buffer for
western blotting (cat. no. P0013) and phenylmethylsulfonyl fluoride
(PMSF) (cat. no. ST506) were purchased from the Beyotime Institute
of Biotechnology. The following primary antibodies were used in the
present study: rabbit polyclonal anti-E-cadherin (cat. no.
WL01482), anti-N-cadherin (cat. no. WL01047), anti-vimentin (cat.
no. WL01960), anti-Snail (cat. no. WL01960), anti-cyclin B1 (cat.
no. WL01760), anti-cyclin-dependent kinase inhibitor 1 (p21)/WAF1
(cat. no. WL0362), anti-LC3α/β (cat. no. WL01506), anti-p62 (cat.
no. WL02385), anti-Beclin1 (cat. no. WL02508), anti-Bax (cat. no.
WL01637), anti-Bcl-2 (cat. no. WL01556),
anti-caspase-3/cleaved-caspase-3 (cat. no. WL02117),
anti-phosphorylated (p)-ERK1/2
(Thr202/Thr204; cat. no. WLP1512),
anti-ERK1/2 (cat. no. WL01864), anti-p-JNK1/2
(Thr183/Tyr185; cat. no. WL01813),
anti-JNK1/2 (cat.no. WL01295), anti-p-p38
(Thr180/Thr182; cat. no. WLP1576), anti-p38
(cat. no. WL00764), anti-p-Smad2
(Ser465/Ser467)/p-Smad3
(Ser423/Ser425; cat. no. WL02305),
anti-Smad2/3 (cat. no. WL01520), anti-plasminogen activator
inhibitor-1 (PAI-1; cat. no. WL01486) and anti-GAPDH (cat. no.
WL03412) antibodies were acquired from Wanleibio Co. Ltd.
Anti-p-Smad3 linker region (Ser208/Ser213,
p-Smad3L; cat. no. 28029) was purchased from Immuno-Biological
Laboratories Co., Ltd. The secondary antibody, HRP-conjugated goat
anti-rabbit IgG (heavy chain + light chain; cat. no. ZB-2301) was
obtained from OriGene Technologies, Inc. ECL Plus Western Blotting
Substrate (cat. no. C05-07004) was purchased from BIOSS.
Cell culture
The human NSCLC A549 cell line was purchased from
the American Type Culture Collection (ATCC). A549 cells were
cultured as a sub-confluent monolayer in RPMI-1640 medium (cat. no.
SH30809.01; Hyclone; Cytiva) containing 10% FBS (cat. no.
11011-8611; Hangzhou Sijiqing Biological Engineering Materials Co.,
Ltd.), penicillin (100 U/ml)/streptomycin (0.1 mg/ml; cat. no.
C0222; Beyotime Institute of Biotechnology) in a humidified
incubator with sterile air containing 5% CO2 at 37°C.
Cells in the logarithmic growth phase were routinely cultured for
24 h after being plated and were subsequently used in the following
experiments. All experiments were performed in triplicate.
Cell migration assay
The effect of Sal B on A549 cell migration was
visualized using the wound healing assay. Cells in the logarithmic
growth phase were digested using trypsin-EDTA solution (cat. no.
C0201; Beyotime Institute of Biotechnology) and were subsequently
collected, counted and reseeded into a sterile 6-well plate
(1×106 cells/well). These cells were cultured in
RPMI-1640 medium containing 10% FBS. When these cells reached
almost 100% confluency, serum-free RPMI-1640 medium was used for 12
h to synchronize cell growth. Scratches were made on the inner
surface of the 6-well plates using a sterile 200-µl pipette tip.
Subsequently, these cells were washed using PBS and cultured in
serum-free RPMI-1640 medium with or without Sal B (25, 50 and 100
µM) and TGF-β1 (9 pM) for 24 h (37°C, 5%
CO2). Representative images were captured using an
inverted microscope (×100 magnification; CKX53; Olympus
Corporation) at 0, 12 and 24 h. The width of the scratch area was
also measured, and the healing rate of the scratch (%) was
quantified using the following formula: Healing rate of scratch (%)
= (the width of scratch area at 0 h-the width of scratch area at
12/24 h)/the width of scratch area at 0 h ×100% (17).
Cell cycle detection
The effect of Sal B on the cell cycle in A549 cells
was detected using flow cytometry (FCM). Briefly, logarithmic
growth phase A549 cells were collected, counted and reseeded in a
sterile 6-well plate to a density of 5×105 cells/well.
Cells were cultured in RPMI-1640 medium containing 10% FBS. Once
the cells reached 90% confluency they were synchronized by
culturing in serum-free RPMI-1640 medium for 12 h (37°C, 5%
CO2). Subsequently, concentration-graded Sal B (25, 50
and 100 µM) and/or TGF-β1 (9 pM) were added to
serum-free medium and cells were incubated for 24 h (37°C, 5%
CO2). After culturing, the cells were collected and
washed using cooled PBS twice. Cells were fixed using cooled 70%
ethanol at 4°C overnight. On the following day, the cells were
resuspended in 300 µl cooled PBS following washing. RNase A
solution (20 µl/sample) was added and these cells were incubated at
37°C for 30 min. Subsequently, the cells were stained with
propidium iodide (PI) solution (400 µl/sample) at 4°C for 1 h in
the dark. The cell cycle distribution was determined using a BD
FACSCelesta™ flow cytometer (BD Biosciences). These
results were analyzed using FlowJo 7.6.1 software (BD
Biosciences).
Cell apoptosis detection
The apoptotic rate was evaluated using the
BBcellProbe™ Annexin V-FITC Double-staining Cell
Apoptosis Detection kit according to the manufacturer's protocol.
Briefly, A549 cells in the logarithmic phase were collected,
counted and seeded into a sterile 6-well plate (5×105
cells/well). Subsequently, these cells were treated with Sal B (25,
50 and 100 µM) and TGF-β1 (9 pM) for 24 h (37°C, 5%
CO2). Following treatment, these cells were collected
and washed with pre-cooled PBS twice and then suspended in 1X
Annexin V binding buffer (400 µl/sample). Annexin V-FITC (5
µl/sample) was added and the cells were incubated at 4°C for 15 min
in the dark. PI staining-solution (5 µl/sample) was added and these
cells were re-incubated at 4°C for 5 min in the dark. Cell
apoptosis in each sample was analyzed using the BD
FACSCelesta™ flow cytometer. These data were quantified
using FlowJo 7.6.1 software.
Extraction of total cell protein and
western blotting
A549 cells were treated with Sal B (25, 50 and 100
µM) and TGF-β1 (9 pM) for 24 h (37°C, 5%
CO2). Following treatment, total protein was extracted
from the cells using cell lysis buffer for western blotting
containing a proteinase inhibitor, PMSF (1 mM), according to the
manufacturer's protocol. Protein expression levels were detected
via western blotting as previously described (18). In brief, proteins (50 µg/sample)
were separated using 10% SDS-PAGE and the separated proteins were
transferred to a PVDF membrane (MilliporeSigma). Membranes were
blocked using 5% skimmed milk powder (room temperature, 2 h).
Subsequently, the membranes were incubated with the following
primary antibodies (4°C overnight): rabbit anti-N-cadherin
(1:1,000), anti-vimentin (1:500), anti-Snail (1:1,000),
anti-E-cadherin (1:1,000), anti-cyclin B1 (1:1,000), anti-p21/WAF1
(1:1,000), anti-LC3α/β (1:1,000), anti-p62 (1:500), anti-Beclin1
(1:1,000), anti-Bax (1:1,000), anti-Bcl-2 (1:500),
anti-caspase-3/cleaved-caspase-3 (1:500), anti-p-ERK1/2 (1:300),
anti-ERK1/2 (1:500), anti-p-JNK1/2 (1:1,000), anti-JNK1/2
(1:1,000), anti-p-p38 (1:1,000), anti-p38 (1:1,000), anti-p-Smad2/3
(1:1,000), anti-p-Smad3L (1:1,000), anti-Smad2/3 (1:1,000),
anti-PAI-1 (1:1,000) and anti-GAPDH (1:1,000). Following primary
incubation, the membranes were incubated with goat anti-rabbit
IgG-HRP antibody (1:10,000). Protein bands were visualized using
ECL Plus Western Blotting Substrate under the UVP ChemiStudio
Imaging System (Analytik Jena GmbH). Densitometric analysis of the
protein bands was performed using ImageJ 2.x software (National
Institutes of Health). GAPDH was used as the internal reference
gene. The ratio of semi-quantified protein to GAPDH in the control
group was assigned a value of 1.
Statistical analysis
Data are presented as the mean ± SD. Statistical
analyses were performed using SPSS 16.0 software for Windows (SPSS,
Inc.). Pairwise comparison of multiple group means were determined
using a one-way ANOVA followed by Tukey's multiple comparison test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Sal B inhibits the
TGF-β1-induced EMT and cell migration in A549 cells
EMT-inducing factors, including the TGF-β family,
contribute to tumor cell malignant transformation, which results in
enhanced metastasis (19).
Therefore, the effect of Sal B on TGF-β1-induced EMT and
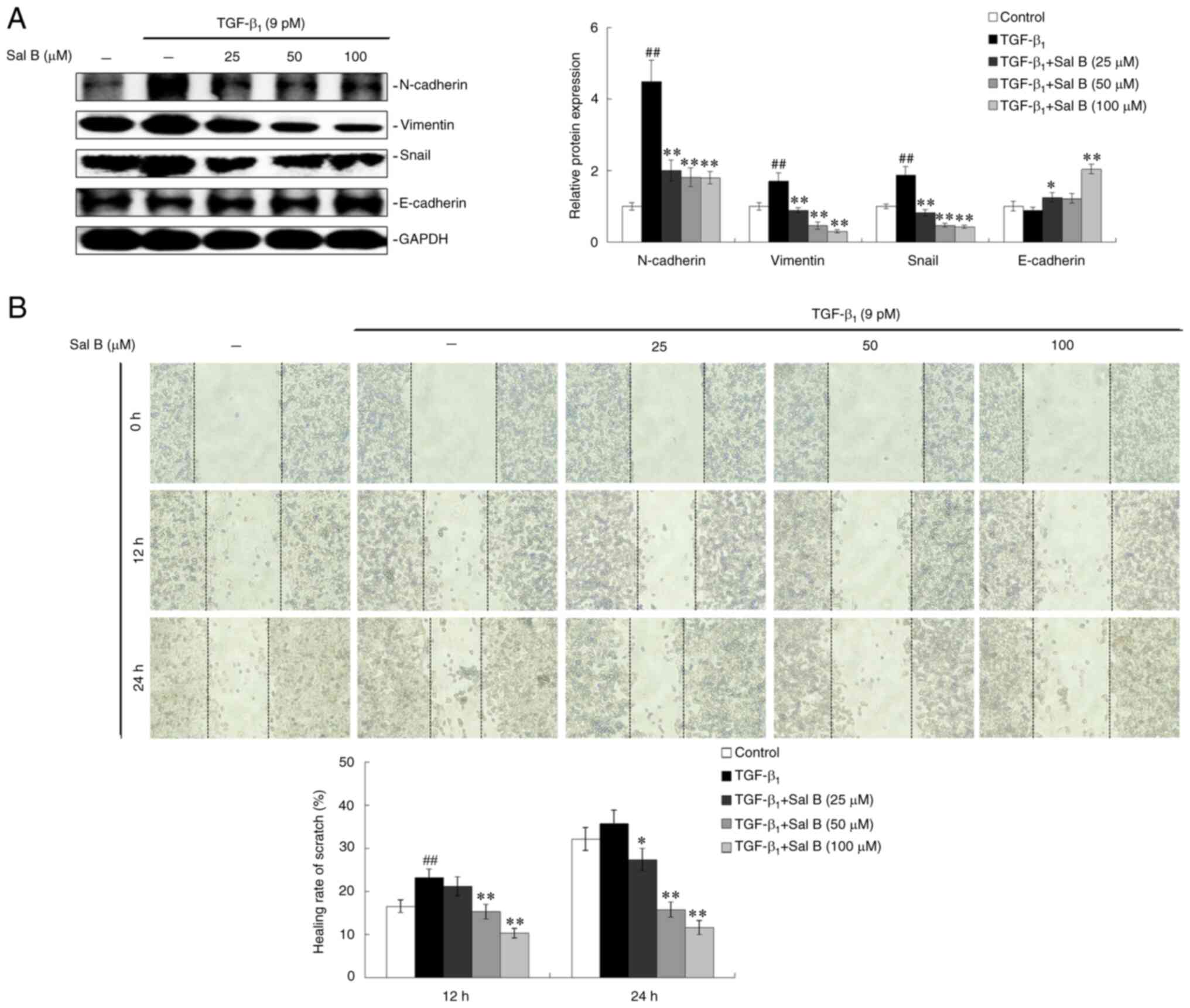
migration of human NSCLC A549 cells was investigated. The results
demonstrated that important marker proteins in EMT, including
N-cadherin, vimentin and Snail, presented with increased protein
expression levels in A549 cells following TGF-β1
stimulation, whereas co-treatment with three different
concentrations of Sal B with TGF-β1 resulted in a
significant dose-dependent decrease in these aforementioned protein
expression levels (Fig. 2A).
E-cadherin, a protective protein that inhibits the EMT, was
observed to increase in a dose-dependent manner in Sal B-treated
A549 cells compared with those cells treated with TGF-β1
only (Fig. 2A). Moreover, the
migration of A549 cells was enhanced by TGF-β1
treatment, whereas Sal B co-treatment resulted in a dose-dependent
inhibitory effect on TGF-β1-induced cell migration at 12
and 24 h (Fig. 2B).
Sal B inhibits the cell cycle
progression of TGF-β1-stimulated A549 cells
Vigorous cell proliferation reflected by rapid cell
division is a typical characteristic of cancer cells (20). Whether Sal B induces NSCLC cell
cycle arrest, resulting in the inhibition of the proliferation of
TGF-β1-stimulated A549 cells, was therefore
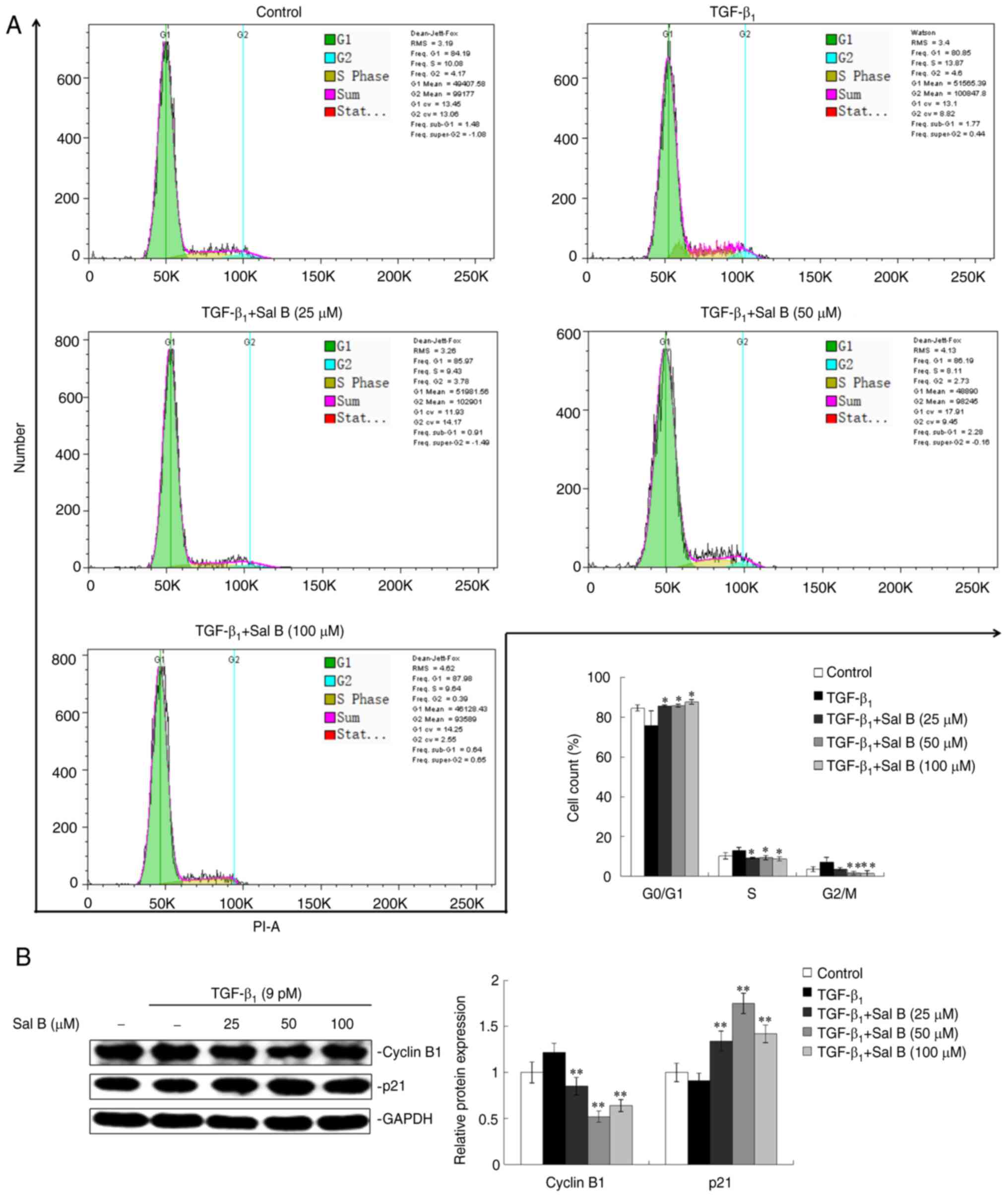
investigated. A decreased percentage of A549 cells in the
G0/G1 phase and an increased percentage of
cells in the S and G2/M phase under TGF-β1
stimulation were observed, compared with the control group.
Co-treatment with Sal B and TGF-β1 induced
G0/G1 phase arrest in A549 cells (Fig. 3A). Cyclin B1 and p21/WAF1 (p21)
are crucial for cell cycle progression; p21 promotes cyclin B1
proteasomal degradation to contribute to arrest of the cell cycle
(21). The results demonstrated
increased protein expression levels of cyclin B1 and decreased p21
protein expression levels in A549 cells induced by
TGF-β1. However, co-treatment with Sal B resulted in
inhibition of the TGF-β1-induced elevation of cyclin B1
and reduction in p21 (Fig.
3B).
Sal B induces the autophagy of
TGF-β1-stimulated A549 cells
Autophagy inhibition is a common phenomenon in
numerous types of cancer cells including NSCLC (22). The effect of Sal B on NSCLC cell
autophagy in TGF-β1-stimulated A549 cells was therefore
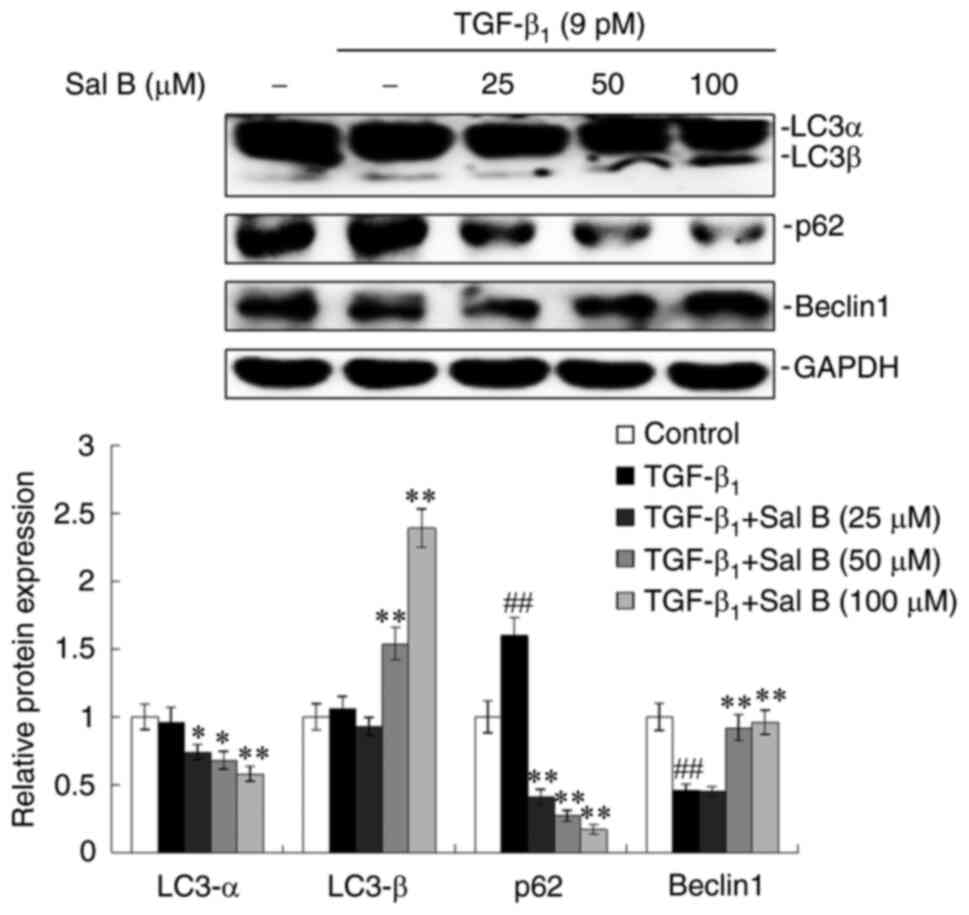
investigated. A few important marker proteins of autophagy were
assessed. The results demonstrated that LC3α exhibited reduced
protein expression levels, whereas increased protein expression
levels were displayed by LC3β in the TGF-β1-stimulated
A549 cells when treated with concentration-graded Sal B, especially
in the 100 µM Sal B-treatment group. TGF-β1 increased
the protein expression levels of p62 whereas Beclin1 protein
expression levels were decreased, which were reversed by combined
treatment with concentration-graded Sal B. The most obvious change
was observed for p62 (Fig.
4).
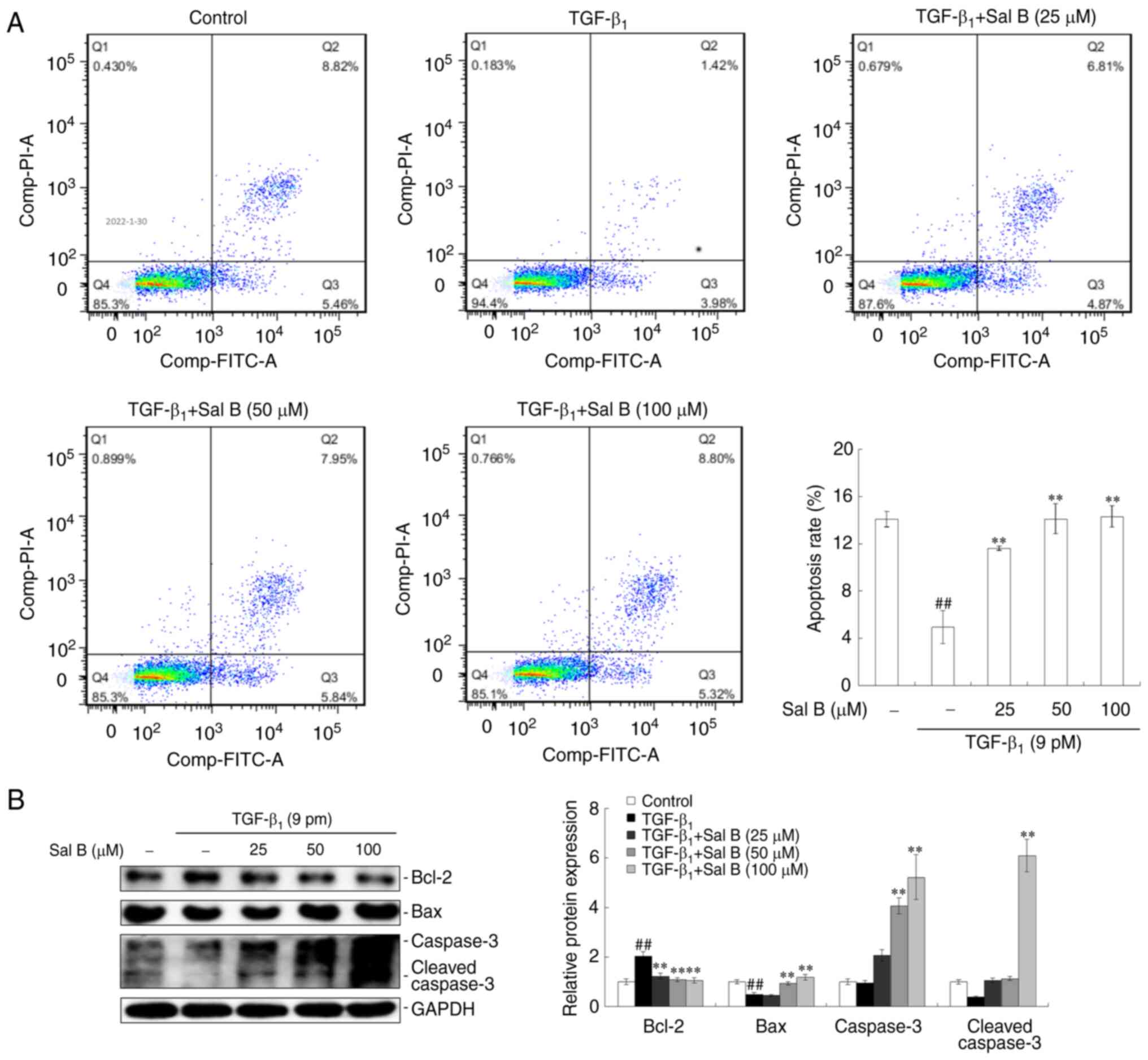
Sal B induces apoptosis in
TGF-β1-stimulated A549 cells
Inducing cell apoptosis is a major strategy in
cancer treatment (23). Therefore
the effect of Sal B on NSCLC cell apoptosis was examined via FCM,
which was used to quantify crucial marker proteins. The results
demonstrated that a repressed apoptotic rate was observed in A549
cells under TGF-β1-stimulation alone, whereas
co-treatment with Sal B resulted in the induction of apoptosis in
A549 cells. Increased apoptotic rates were demonstrated at all
three Sal B concentrations compared with the
TGF-β1-stimulated only group (Fig. 5A). Investigation of crucial
apoptotic marker protein expression levels demonstrated that
TGF-β1 had an inhibitory effects on Bax, caspase-3 and
cleaved-caspase-3, but had a stimulative effect on Bcl-2 protein
expression levels. Co-treatment using three different
concentrations of Sal B reversed these effects caused by
TGF-β1 treatment (Fig.
5B).
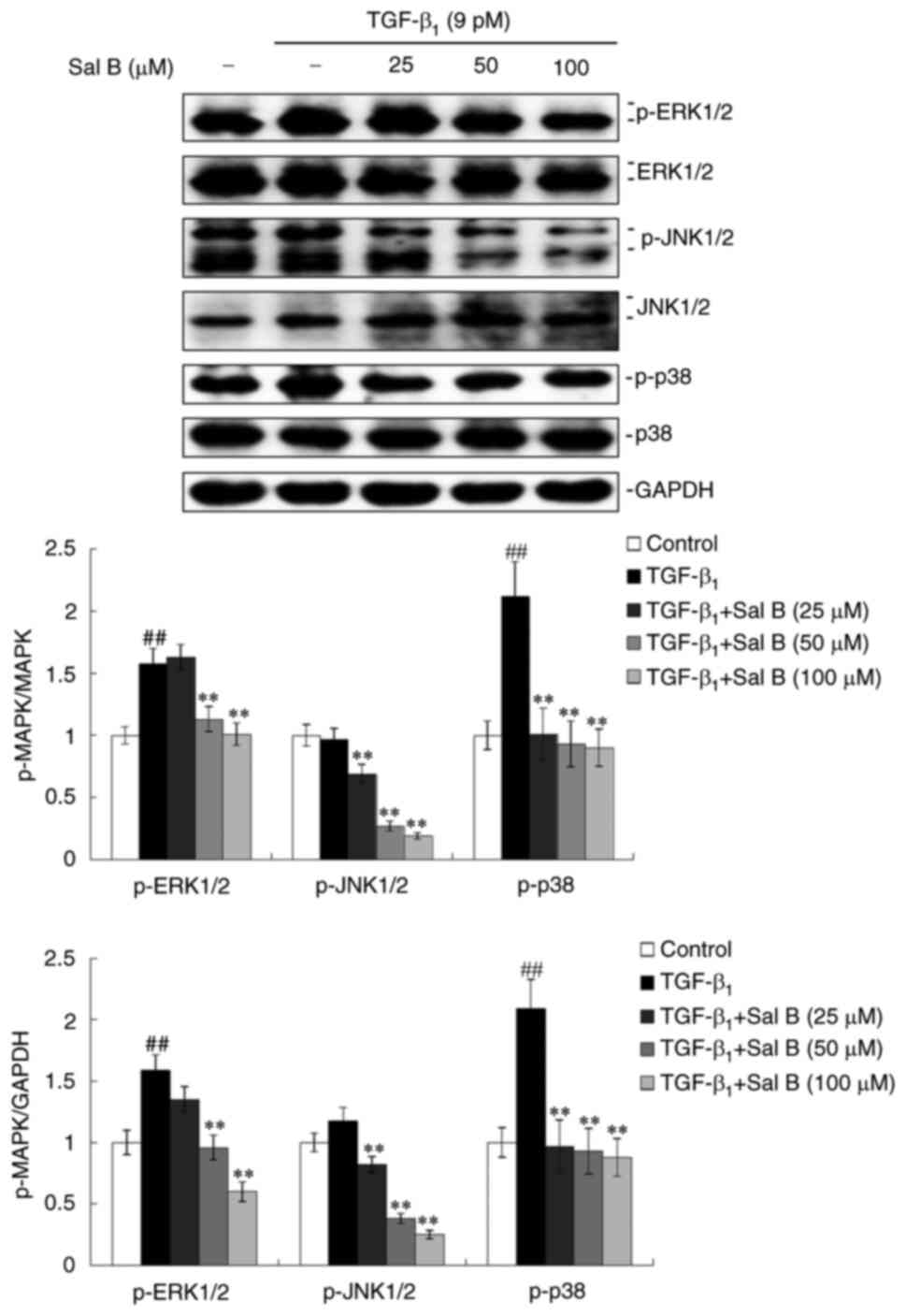
Sal B inactivates the MAPK signaling
pathway in TGF-β1-stimulated A549 cells
MAPK signaling pathways are regarded as noncanonical
TGF-β signaling pathways and serve an important role in the
cytological effects mediated by TGF-β1 (24). Therefore, the activation of MAPK
signaling pathways in TGF-β1-stimulated A549 cells under
Sal B treatment was investigated. The activation of MAPK signaling
pathways can be assessed using the phosphorylation levels of the
following three crucial MAPKs: ERK1/2, JNK1/2 and p38. It was
observed that TGF-β1 induced increased protein
expression levels of p-ERK1/2, p-JNK1/2 and p-p38, whereas
co-treatment with Sal B resulted in the dose-dependent inhibition
of ERK1/2, JNK1/2 and p38 phosphorylation. Furthermore,
significantly inhibited protein expression levels of ERK1/2 were
observed under Sal B co-treatment in TGF-β1-stimulated
A549 cells at 24 h (Fig. 6).
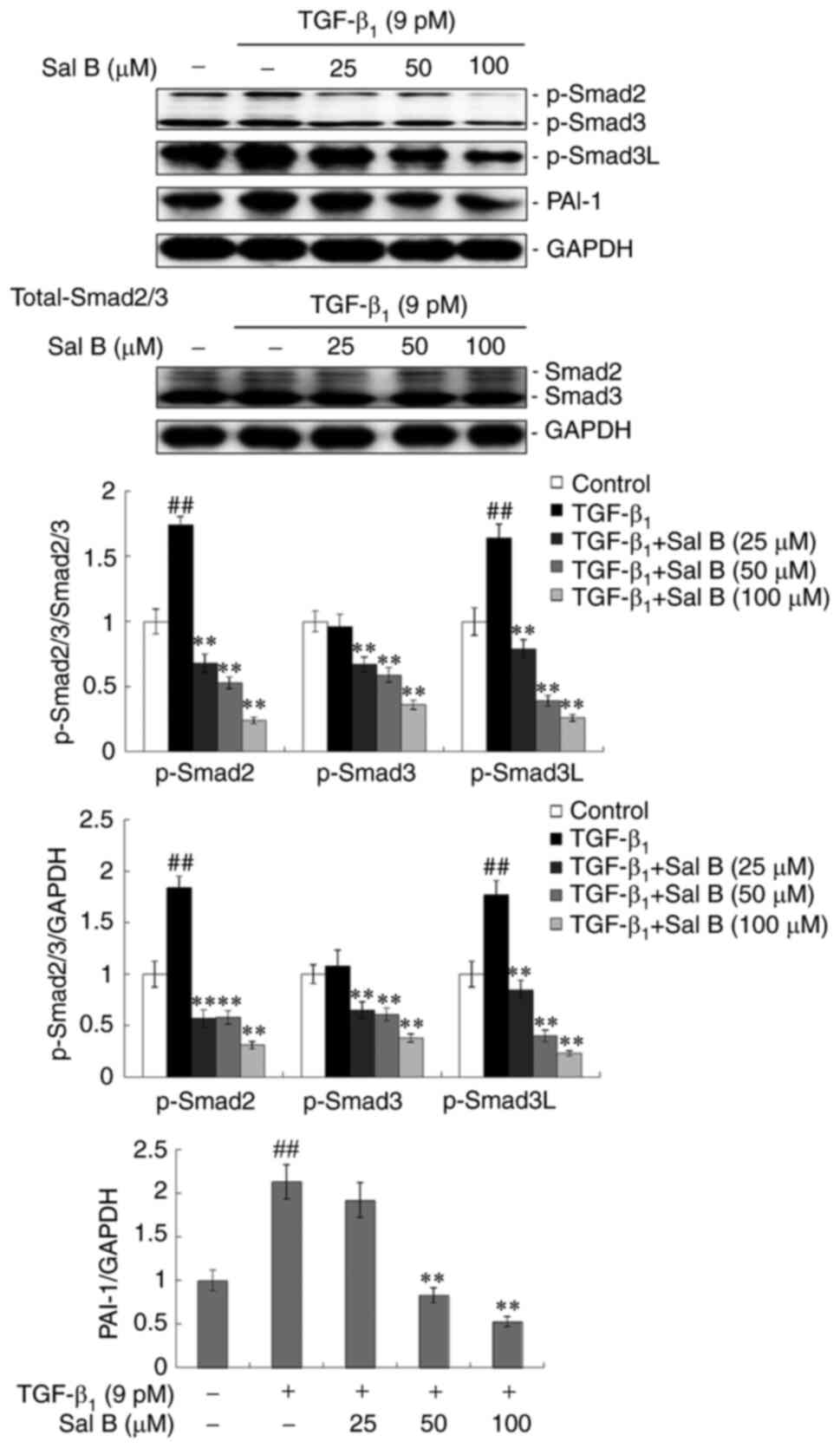
Sal B inhibits the phosphorylation of
Smad2/3 and PAI-1 expression in TGF-β1-stimulated A549
cells
Smad2/3 is regarded as the main intracellular
effector of canonical TGF-β signal transduction, which is
phosphorylated and nuclear-translocated to regulate target genes,
such as PAI-1, as a transcription factor (25). The results demonstrated that
p-Smad2 protein expression levels were significantly increased in
A549 cells following TGF-β1 stimulation for 24 h.
However, respective co-treatment with three different
concentrations of Sal B led to reduced p-Smad2 and p-Smad3 protein
expression levels, compared with those with TGF-β1
treatment only. MAPK-mediated Smad3 phosphorylation at the linker
region serves an important role in TGF-β signaling and exerts a
carcinogenic effect (26). The
results demonstrated that the protein expression levels of p-Smad3L
were significantly reduced under Sal B treatment in
TGF-β1-stimulated A549 cells (Fig. 7). An important target, PAI-1, of
the TGF-β/Smad signaling pathway displayed increased protein
expression levels in A549 cells under TGF-β1 stimulation
for 24 h, which were subsequently inhibited with Sal B co-treatment
in a dose-dependent manner (Fig.
7).
Discussion
Natural polyphenols are potential active ingredients
for the prevention and treatment of cancer (27). Phenolic acids [including
salvianolic acid A (Sal A) and Sal B] from Salvia
miltiorrhiza Bunge (SM), one of the major polyphenol classes,
have been reported for their therapeutic properties against cancer
in various solid tumors (11).
Sal A has been experimentally verified to inhibit cell growth and
induce partial apoptosis (28),
and reverse cisplatin resistance in human non-small cell lung
cancer (NSCLC) A549 cells (29).
Sal B and Sal A have both structural and functional similarities
(11). Related research has shown
that the percentage of Sal B concentration in SM is approximately
5.0% of the root dry weight, which occupies approximately 70% of
water-soluble phenolic acids extracted from SM, which is far higher
than the concentration of Sal A in SM (30). Moreover, Sal B has been observed
to be converted into Sal A under conditions of high temperature,
high pressure and high humidity (31), which provides the possibility for
united and continuous pharmacological activities resulting from Sal
B-converted Sal A in vivo. The above findings hint that Sal
B may have more potential and applied value than those of Sal A.
However, Sal B has been only reported to inhibit A549 cell growth
(12), while the pharmacological
activity and the molecular mechanism of Sal B in regards to human
NSCLC remain unsubstantiated. Therefore, Sal B was investigated to
establish whether it possesses therapeutic properties against the
human NSCLC A549 cell line via regulating TGF-β signaling. The
results from the present study demonstrated that Sal B exhibited
in vitro anticancer activity against NSCLC, which was
reflected in the inhibition of A549 cell epithelial-mesenchymal
transition (EMT), migration and cell cycle progression, the
induction of cell autophagy and apoptosis-related inhibition of
TGF-β signaling.
The occurrence and progression of NSCLC are closely
involved in aberrant serial gene expression and numerous signaling
pathways (32). Among these,
TGF-β signaling serves a vitally important role in the
patho-mechanism of NSCLC, as a result of its crosstalk with
numerous molecules and signaling pathways, which results in the
mediation of various cell biological behaviors (33,34). Therefore, TGF-β1, a
leading initiator of TGF-β signaling in mammals, was used in the
present study to induce a human NSCLC progression model in
vitro in A549 cells prior to treatment with Sal B. The present
study was therefore designed to investigate the effects of Sal B on
human NSCLC progression via TGF-β signaling. The results
demonstrated that TGF-β1 was able to induce EMT, which
was indicated by upregulated mesenchymal markers including
N-cadherin, Vimentin and Snail, and downregulated epithelial marker
E-cadherin. These changes simultaneously led to increased migration
in A549 cells, whereas Sal B reversed these effects induced by
TGF-β1 and inhibited the EMT and migration of A549
cells. However, how Sal B intervenes in the process of EMT will be
investigated in-depth in further research by using more
experimental techniques such as observing cellular morphology under
high-power microscope by immunofluorescent staining marked
N-cadherin/E-cadherin in the near future. Furthermore, cell
proliferation was inhibited by Sal B treatment, which was reflected
by suppressed cell cycle progression and by the regulation of
protein expression levels of critical markers, including cyclin B1
and p21 in TGF-β1-stimulated A549 cells. Notably, the
changes in Sal B-regulated cell cycle progression and its markers
were found to be relatively weak in the present study, which may
have resulted from the Sal B concentrations used which were less
than its IC50 value in A549 cells (12). Unexpectedly, the protein level of
cyclin B1 was slightly higher at 100 µM Sal B than that at 50 µM,
which may have resulted from different regulatory mechanisms
regarding the Sal B's concentration difference on cyclin B1
expression, considering that cyclin B1 has a dual face for
regulating progression of the cell cycle (35). Subsequently, activation of cell
autophagy and apoptosis by Sal B treatment were observed, which
were indicated by Sal B-induced increased protein expression levels
of autophagy marker proteins, LC3β and Beclin1 and decreased
protein expression levels of p62. Yet, the mechanism regarding the
influence of the entire process of A549 cell autophagy by Sal B
requires further investigation. Further studies, including cell
submicroscopic structure by using transmission electron microscopy,
will be performed in the near future. Sal B induced increased
apoptotic rates and protein expression levels of apoptotic marker
proteins, including caspase-3/cleaved-caspase-3 and Bax, whereas
protein expression levels of Bcl-2 were decreased. Numerous types
of cancer cells, including NSCLC cells, have increased survival
rates as a result of EMT, which is characterized by vigorous cell
proliferation and migration and attenuated cell autophagy and
apoptosis (36). The present
study demonstrated that the in vitro anti-NSCLC efficacy of
Sal B was reflected in the reduced cell EMT, migration and cell
cycle progression, as well as activated autophagy and apoptosis via
the TGF-β1-induced human NSCLC progression model in A549
cells. Although the presented anticancer effects of Sal B on
TGF-β1-induced human NSCLC A549 progression were overall
relatively weak in the study, this may have resulted from
overactive TGF-β signaling in NSCLC itself and the used
concentrations of Sal B much less than its IC50 value.
Therefore, further research to investigate the effect of Sal B on
NSCLC utilizing a more aggressive cell model to examine the
intrinsic cause of the disease is required. For example, NSCLC
cells with KRAS-mutations from lung cancer patients used for
assessing the pharmacological activity of Sal B (37), which will be performed in the near
future.
Moreover, the molecular mechanism of the anti-NSCLC
effect of Sal B was investigated in the present study. The
important role of TGF-β signaling in NSCLC and the inhibitory
effects of Sal B on TGF-β1-induced human NSCLC
progression, resulted in the canonical and noncanonical TGF-β
signaling pathways being analyzed to determine the association
between Sal B and NSCLC. The canonical TGF-β signaling pathway is a
Smad-dependent signaling pathway, which includes the following five
stages. i) TGF-β1, the dominating ligand of TGF-β
signaling, attaches to the TGF-β receptor type II, located on the
target cell membrane, and subsequently recruits and
trans-phosphorylates TGF-β receptor type I (TβRI), located in the
cytoplasm; ii) p-TβRI induces receptor-regulated Smad2/3 activation
to produce p-Smad2/3; iii) p-Smad2/3 binds to the common mediator,
Smad4, to produce heterotrimer/heterodimer complexes, p-Smad2/3/4
or p-Smad3/4; iv) p-Smad2/3/4 and/or p-Smad3/4 are transported into
the cell nucleus to regulate the expression of downstream target
genes, including PAI-1, as a transcription factor; and v)
inhibitory type Smad7 is transported into the nucleus to
depolymerize p-Smad2/3/4 or p-Smad3/4, which results in the
termination of TGF-β/Smad signal transduction (25). Smad2/3 phosphorylation is
therefore an indispensable and central step for TGF-β/Smad signal
transduction. The present study demonstrated that Sal B markedly
inhibited TGF-β1-induced Smad2/3 phosphorylation, which
contributed to the inhibition of PAI-1 protein expression
levels.
MAPK signaling pathways, noncanonical TGF-β
signaling pathways, which include the ERK, JNK and p38 signaling
pathways, are an attractive therapeutic target. Certain inhibitors
of MEK have been used in an attempt to treat NSCLC by correcting
aberrant MAPK signaling (38). In
the present study, three MAPKs were assessed. The results
demonstrated that Sal B markedly inhibited
TGF-β1-induced ERK1/2, JNK1/2 and p38 phosphorylation to
reduce the activation of the ERK, JNK and p38 MAPK signaling
pathways in A549 cells. For nearly 20 years, a series of studies
from our research group and others have strongly suggested that
Smad3 phosphorylation of Smad3L may be a crucial mechanism in TGF-β
signaling, enabling a carcinogenic effect (26,39,40). Consequently, protein expression
levels of p-Smad3L were assessed and the results demonstrated that
Sal B decreased p-Smad3L protein expression levels in
TGF-β1-treated A549 cells. These results are supported
by previous studies in which MAPK-regulated Smad2/3 phosphorylation
has been demonstrated to enhance Smad2/3L phosphorylation via
activated MAPK, which results in the occurrence and development of
tumors and has been widely confirmed in keloid, HCC and colorectal
cancer (18,41,42). PAI-1, an endogenous inhibitor of
the urokinase-type plasminogen activator system, is induced by
being directly bound to Smad3/Smad4, TGF-β-induced elements, at the
PAI-1 promoter (43). Previous
studies have reported that secreted PAI-1 increases EMT marker
expression and enhances cell migration in in vitro and in
vivo models of NSCLC (44,45). Extracellular PAI-1 activates the
ERK1/2 and AKT signaling pathways and inhibits caspase-3 activity,
which results in cell apoptosis inhibition in NSCLC (45). The present study observed that Sal
B inhibited the ERK1/2 signaling pathway and Smad2/3
phosphorylation, which resulted in the inhibition of the downstream
target gene PAI-1 and inhibited EMT and cell migration, whereas
apoptosis was induced, in NSCLC A549 cells. However, whereas the
characteristics of natural products have multiple target effects,
Sal B maybe have multiple targets not only like-inhibitor of TGF-β
signaling indicated in the study and/or disrupting of COX-2
activity reported previously in NSCLC (12). Therefore, further research using
NSCLC models in vivo and in vitro are required to
investigate the underlying mechanisms of Sal B.
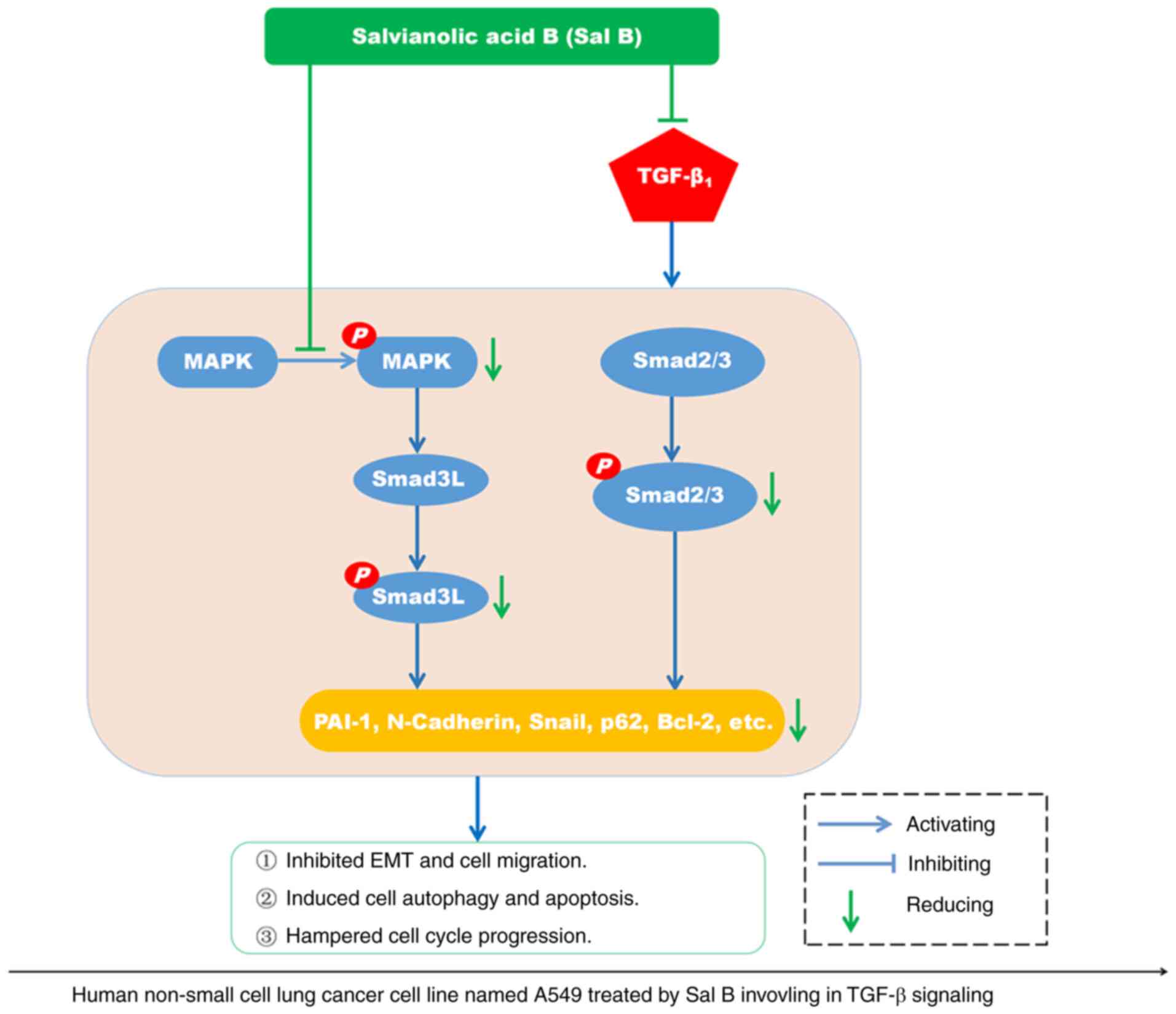
In summary, Sal B inhibited
TGF-β1-induced human NSCLC progression by inactivating
the phosphorylation of MAPK and Smad2/3, which led to impeded TGF-β
signaling transduction (Fig. 8).
However, further research needs to be performed to determine the
pharmacological efficacy of Sal B in vivo in NSCLC animal
models or humans. Therefore, continued research into Sal B and
other new therapeutics is urgently required. Animal models of NSCLC
should be established and treated with suitable doses of Sal B to
further support the potential clinical benefits of Sal B in
patients with NSCLC, which may improve the survival rates and
prognosis of patients with NSCLC.
Acknowledgements
Not applicable.
Funding
The present study obtained funding from the National Talent
Project of Traditional Chinese Medicine Characteristic Technology
Inheritance Supported National Administration of Traditional
Chinese Medicine in China [no. 168 of Chinese Traditional Chinese
Medicine Education Letter (2015)] and the National Natural Science
Foundation of China (grant no. 81573652).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CW and YY conceptualized and designed the study. GH,
YW, TL, FD and MC performed all experiments included in the study.
GH, JG and CW contributed to the data collection, analysis,
confirmed the authenticity of all the raw data and wrote the
manuscript. CW and YY reviewed and edited the manuscript. GH and YY
acquired funding. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bade BC and Dela Cruz CS: Lung cancer
2020: Epidemiology, etiology, and prevention. Clin Chest Med.
41:1–24. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Herbst RS, Morgensztern D and Boshoff C:
The biology and management of non-small cell lung cancer. Nature.
553:446–454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang LC, Chang YY, Lee IC, Kuo HC and Tsai
MY: Systematic review and meta-analysis of Chinese herbal medicine
as adjuvant treatment in advanced non-small cell lung cancer
patients. Complement Ther Med. 52:1024722020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen X, Guo J, Bao J, Lu J and Wang Y: The
anticancer properties of Salvia miltiorrhiza Bunge
(Danshen): A systematic review. Med Res Rev. 34:768–794. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hung YC, Pan TL and Hu WL: Roles of
reactive oxygen species in anticancer therapy with Salvia
miltiorrhiza Bunge. Oxid Med Cell Longev. 2016:52932842016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gong L, Di C, Xia X, Wang J, Chen G, Shi
J, Chen P, Xu H and Zhang W: AKT/mTOR signaling pathway is involved
in salvianolic acid B-induced autophagy and apoptosis in
hepatocellular carcinoma cells. Int J Oncol. 49:2538–2548. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Katary MA, Abdelsayed R, Alhashim A,
Abdelhasib M and Elmarakby AA: Salvianolic acid B slows the
progression of breast cancer cell growth via enhancement of
apoptosis and reduction of oxidative stress, inflammation, and
angiogenesis. Int J Mol Sci. 20:56532019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hao Y, Xie T, Korotcov A, Zhou Y, Pang X,
Shan L, Ji H, Sridhar R, Wang P, Califano J and Gu X: Salvianolic
acid B inhibits growth of head and neck squamous cell carcinoma in
vitro and in vivo via cyclooxygenase-2 and apoptotic pathways. Int
J Cancer. 124:2200–2209. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen B, Huang C, Zhang Y, Tang X, Li S,
Wang Q and Lin Y: Salvia bowleyana Dunn root is a novel
source of salvianolic acid B and displays antitumor effects against
gastric cancer cells. Oncol Lett. 20:817–827. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jing Z, Fei W, Zhou J, Zhang L, Chen L,
Zhang X, Liang X, Xie J, Fang Y, Sui X, et al: Salvianolic acid B,
a novel autophagy inducer, exerts antitumor activity as a single
agent in colorectal cancer cells. Oncotarget. 7:61509–61519. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qin T, Rasul A, Sarfraz A, Sarfraz I,
Hussain G, Anwar H, Riaz A, Liu S, Wei W, Li J and Li X:
Salvianolic acid A & B: Potential cytotoxic polyphenols in
battle against cancer via targeting multiple signaling pathways.
Int J Biol Sci. 15:2256–2264. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tao L, Wang S, Zhao Y, Sheng X, Wang A,
Zheng S and Lu Y: Phenolcarboxylic acids from medicinal herbs exert
anticancer effects through disruption of COX-2 activity.
Phytomedicine. 21:1473–1482. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dongre A and Weinberg RA: New insights
into the mechanisms of epithelial-mesenchymal transition and
implications for cancer. Nat Rev Mol Cell Biol. 20:69–84. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jeong JH, Jang HJ, Kwak S, Sung GJ, Park
SH, Song JH, Kim H, Na Y and Choi KC: Novel TGF-β1 inhibitor
antagonizes TGF-β1-induced epithelial-mesenchymal transition in
human A549 lung cancer cells. J Cell Biochem. 120:977–987. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eser PÖ and Jänne PA: TGFβ pathway
inhibition in the treatment of non-small cell lung cancer.
Pharmacol Ther. 184:112–130. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Q, Chu H, Ma Y, Wu T, Qian F, Ren X,
Tu W, Zhou X, Jin L, Wu W and Wang J: Salvianolic acid B attenuates
experimental pulmonary fibrosis through inhibition of the TGF-β
signaling pathway. Sci Rep. 6:276102016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu C, Chen W, Ding H, Li D, Wen G, Zhang
C, Lu W, Chen M and Yang Y: Salvianolic acid B exerts anti-liver
fibrosis effects via inhibition of MAPK-mediated phospho-Smad2/3 at
linker regions in vivo and in vitro. Life Sci. 239:1168812019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Boye A, Kan H, Wu C, Jiang Y, Yang X, He S
and Yang Y: MAPK inhibitors differently modulate TGF-β/Smad
signaling in HepG2 cells. Tumour Biol. 36:3643–3651. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hao Y, Baker D and Ten Dijke P:
TGF-β-mediated epithelial-mesenchymal transition and cancer
metastasis. Int J Mol Sci. 20:27672019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu X, Chen Y, Li Y, Petersen RB and Huang
K: Targeting mitosis exit: A brake for cancer cell proliferation.
Biochim Biophys Acta Rev Cancer. 1871:179–191. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mateen S, Raina K, Jain AK, Agarwal C,
Chan D and Agarwal R: Epigenetic modifications and p21-cyclin B1
nexus in anticancer effect of histone deacetylase inhibitors in
combination with silibinin on non-small cell lung cancer cells.
Epigenetics. 7:1161–1172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu G, Pei F, Yang F, Li L, Amin AD, Liu
S, Buchan JR and Cho WC: Role of autophagy and apoptosis in
non-small-cell lung cancer. Int J Mol Sci. 18:3672017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim R, Emi M and Tanabe K: The role of
apoptosis in cancer cell survival and therapeutic outcome. Cancer
Biol Ther. 5:1429–1442. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mulder KM: Role of Ras and Mapks in
TGFbeta signaling. Cytokine Growth Factor Rev. 11:23–35. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Derynck R and Zhang YE: Smad-dependent and
Smad-independent pathways in TGF-beta family signalling. Nature.
425:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ooshima A, Park J and Kim SJ:
Phosphorylation status at Smad3 linker region modulates
transforming growth factor-β-induced epithelial-mesenchymal
transition and cancer progression. Cancer Sci. 110:481–488. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou Y, Zheng J, Li Y, Xu DP, Li S, Chen
YM and Li HB: Natural polyphenols for prevention and treatment of
cancer. Nutrients. 8:5152016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bi L, Chen J, Yuan X, Jiang Z and Chen W:
Salvianolic acid A positively regulates PTEN protein level and
inhibits growth of A549 lung cancer cells. Biomed Rep. 1:213–217.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tang XL, Yan L, Zhu L, Jiao DM, Chen J and
Chen QY: Salvianolic acid A reverses cisplatin resistance in lung
cancer A549 cells by targeting c-met and attenuating Akt/mTOR
pathway. J Pharmacol Sci. 135:1–7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun Y, Zhu H, Wang J, Liu Z and Bi J:
Isolation and purification of salvianolic acid A and salvianolic
acid B from Salvia miltiorrhiza by high-speed
counter-current chromatography and comparison of their antioxidant
activity. J Chromatogr B Analyt Technol Biomed Life Sci.
877:733–737. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xia H, Sun L, Lou H and Rahman MM:
Conversion of salvianolic acid B into salvianolic acid A in tissues
of radix salviae miltiorrhizae using high temperature, high
pressure and high humidity. Phytomedicine. 21:906–911. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Petty RD, Nicolson MC, Kerr KM,
Collie-Duguid E and Murray GI: Gene expression profiling in
non-small cell lung cancer: From molecular mechanisms to clinical
application. Clin Cancer Res. 10:3237–3248. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jeon HS and Jen J: TGF-beta signaling and
the role of inhibitory Smads in non-small cell lung cancer. J
Thorac Oncol. 5:417–419. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang J, Shao N, Ding X, Tan B, Song Q,
Wang N, Jia Y, Ling H and Cheng Y: Crosstalk between transforming
growth factor-β signaling pathway and long non-coding RNAs in
cancer. Cancer Lett. 370:296–301. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schnittger A and De Veylder L: The dual
face of cyclin B1. Trends Plant Sci. 23:475–478. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mittal V: Epithelial mesenchymal
transition in aggressive lung cancers. Adv Exp Med Biol. 890:37–56.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Calles A, Sholl LM, Rodig SJ, Pelton AK,
Hornick JL, Butaney M, Lydon C, Dahlberg SE, Oxnard GR, Jackman DM
and Jänne PA: Immunohistochemical loss of LKB1 is a biomarker for
more aggressive biology in KRAS-mutant lung adenocarcinoma. Clin
Cancer Res. 21:2851–2860. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim C and Giaccone G: MEK inhibitors under
development for treatment of non-small-cell lung cancer. Expert
Opin Investig Drugs. 27:17–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Murata M, Yoshida K, Yamaguchi T and
Matsuzaki K: Linker phosphorylation of Smad3 promotes
fibro-carcinogenesis in chronic viral hepatitis of hepatocellular
carcinoma. World J Gastroenterol. 20:15018–15027. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gong Y, Li D, Li L, Yang J, Ding H, Zhang
C, Wen G, Wu C, Fang Z, Hou S and Yang Y: Smad3 C-terminal
phosphorylation site mutation attenuates the hepatoprotective
effect of salvianolic acid B against hepatocarcinogenesis. Food
Chem Toxicol. 147:1119122021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
He S, Liu X, Yang Y, Huang W, Xu S, Yang
S, Zhang X and Roberts MS: Mechanisms of transforming growth factor
beta(1)/Smad signalling mediated by mitogen-activated protein
kinase pathways in keloid fibroblasts. Br J Dermatol. 162:538–546.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Matsuzaki K, Kitano C, Murata M, Sekimoto
G, Yoshida K, Uemura Y, Seki T, Taketani S, Fujisawa J and Okazaki
K: Smad2 and Smad3 phosphorylated at both linker and COOH-terminal
regions transmit malignant TGF-beta signal in later stages of human
colorectal cancer. Cancer Res. 69:5321–5330. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dennler S, Itoh S, Vivien D, ten Dijke P,
Huet S and Gauthier JM: Direct binding of Smad3 and Smad4 to
critical TGF beta-inducible elements in the promoter of human
plasminogen activator inhibitor-type 1 gene. EMBO J. 17:3091–3100.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lin X, Lin BW, Chen XL, Zhang BL, Xiao XJ,
Shi JS, Lin JD and Chen X: PAI-1/PIAS3/Stat3/miR-34a forms a
positive feedback loop to promote EMT-mediated metastasis through
Stat3 signaling in non-small cell lung cancer. Biochem Biophys Res
Commun. 493:1464–1470. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kang J, Kim W, Kwon T, Youn H, Kim JS and
Youn B: Plasminogen activator inhibitor-1 enhances radioresistance
and aggressiveness of non-small cell lung cancer cells. Oncotarget.
7:23961–23974. 2016. View Article : Google Scholar : PubMed/NCBI
|