Introduction
Ligamentum flavum (LF) is a connective tissue and
can be affected by hypertrophy, which is the major pathogenic
factor of degenerative lumbar spinal stenosis (LSS) (1). The pathology of LF hypertrophy is a
common fibrosis process of this connective tissue. Upon fibrosis,
LF shows decreased elasticity and increased thickness,
proliferation of effector cells and component and structural change
in the extracellular matrix (2).
Symptoms such as lower back pain, bladder and rectal dysfunctions
are induced by nerves and blood vessels stimulated by hypertrophic
LF in the spinal canal. The mechanism of LF hypertrophy remains to
be elucidated.
Recently, the major pathological elements of LF
hypertrophy have been proved to be mechanical and metabolic
(3,4). Pathologically, the hypertrophy of LF
is associated with scar repair secondary to mechanically induced
micro-injury and metabolic accumulation mediated biochemical
reaction, among which the inflammatory reaction is important
(5,6). Inflammatory factors serve a vital
role in the hypertrophy process of LF (7). TGFβ1 can significantly promote the
proliferation of LF fibroblasts and the overexpression of collagen
fibers, while MMP13 has a distinct function of elastic fiber
degradation in the LF extracellular matrix (8,9). A
connection exists between macrophage migration inhibitory factor
[MIF; novoprotein recombinant human MIF (n-6his) (ch33)] and TGFβ1
and MMP (10). As a multipotent
pro-inflammatory factor, MIF and its mediated inflammatory reaction
are driving factors of LF hypertrophy. MIF can be activated by
several factors, including high glucose, infection and hypoxia
(11–13). Our previous study found that the
concentration of MIF was positively associated with the thickness
of LF but the mechanism remains to be elucidated (14). Thus, the present study aims to
explore the MIF effect on LF fibroblasts to explore the potential
mechanism of LF hypertrophy.
Materials and methods
Ethics and study subjects
This project was approved by Wuhan Municipal Health
Commission Medical Research Ethics Committee (Wuhan, Hubei;
approval no. 672HREC2020N01B). Written informed consents were
acquired from all patients. Random and consecutive patients
underwent surgery for single segment (L4/5) LSS with the LF
thickness >4 mm and lumbar disc herniation (LDH) between
February 2021 and July 2021 were included. Patients with spinal
tumors, tuberculosis, infection, L4 spinal instability or
spondylolisthesis were excluded. LF specimens removed from these
two groups [lumbar spinal stenosis (LSS; n=12) and lumbar disc
herniation (LDH; n=15)] were initially processed to measure the
concentration of MIF, TGFβ1 and MMP13 by ELISA. Basic data of
patients are in Table I.
 | Table I.Basic information of LSS and LDH
groups. |
Table I.
Basic information of LSS and LDH
groups.
| Characteristic | LSS (n=12) | LDH (n=15) | t|χ2 | P-value |
|---|
| Age (year) | 60.40±4.27 | 56.67±8.56 | 1.479 | 0.152 |
| BMI | 23.88±1.96 | 25.27±1.99 | 1.819 | 0.081 |
| Male/Female | 48 | 69 | 0.127 | 0.722 |
ELISA
The ligamentum flavum tissue was milled with PBS and
centrifuged with 10,000 × g at 4°C for 15 min. The supernatant was
collected. Protein concentrations were measured by the BCA kit
(Beyotime Institute of Biotechnology). ELISA kits (Human MIF, cat.
no. JL11770; Shanghai Future Industry Co., Ltd.; Human TGF-β1, cat.
no. JL10706; Shanghai Future Industry Co., Ltd.; Human MMP 13, cat.
no. JL12202; Shanghai Future Industry Co., Ltd.) were used
following the manufacturer's instructions. The sample was added to
the well and covered to incubate 1 h at 37°C. The enzyme-labeled
plate was taken out, the liquid was discarded and the biotinylated
antibody working solution was added before covering and incubating
for 1 h at 37°C. The liquid was discarded, it was wash three times.
The enzyme conjugate working solution was added, and the
plate-sealing film was covered to incubate 30 min at 37°C. The
liquid was discard again and washed 5 times. Substrate was added
and incubated 15 min at 37°C in the dark. The stop solution was
added and the concentrations of MIF, TGF-β1 and MMP-13 were
measured under the wavelength of 450 nm.
Culture of primary LFs
The LF tissue from a 35 year old male patient who
was recruited in February 2021 without other history of disease in
the LDH group was harvested. It was washed three times with sterile
physiologic saline containing penicillin and streptomycin. LF
tissue was cut into 0.5 mm3 and placed into a 15 ml
centrifuge tube. LF tissue was digested with 0.2% type I
collagenase at 37°C for 1 h. Tissue was washed with low-glucose
DMEM and centrifuged at 118 × g for 5 min at room temperature. LF
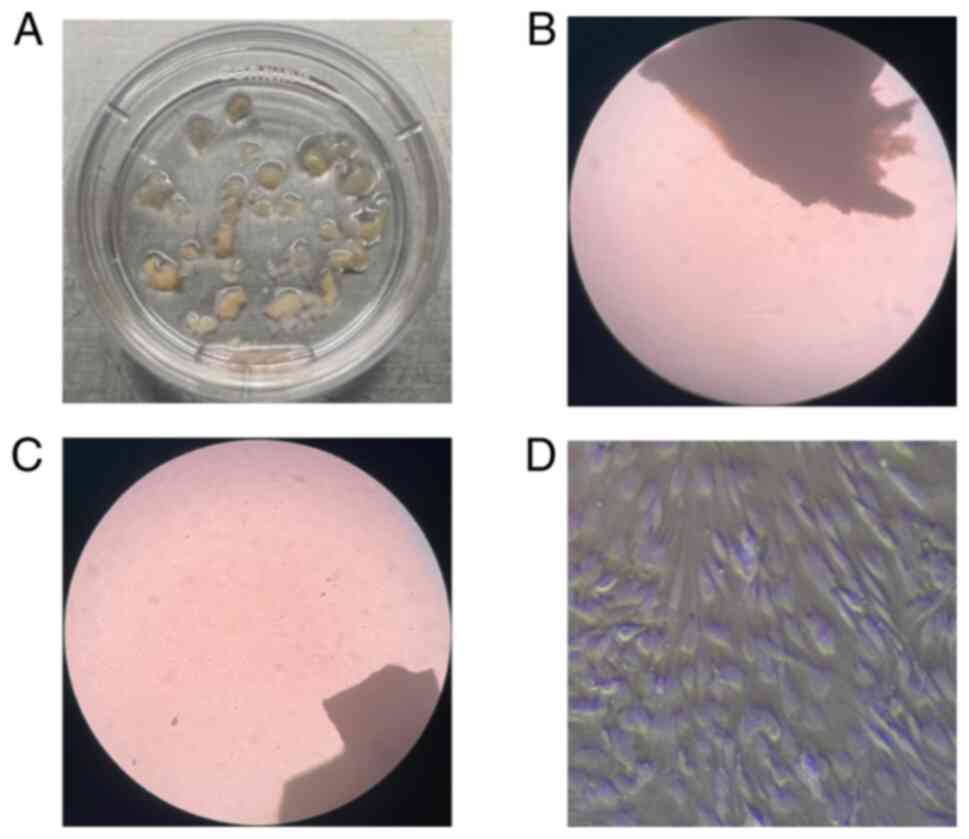
tissue was evenly inoculated in a culture dish (Fig. 1A) with high-glucose DMEM
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.), 1% penicillin-streptomycin (Gibco; Thermo Fisher
Scientific, Inc.) at 37°C, 5% CO2 under controlled
humidity. When the cell growth area reached 80% area, passage was
performed.
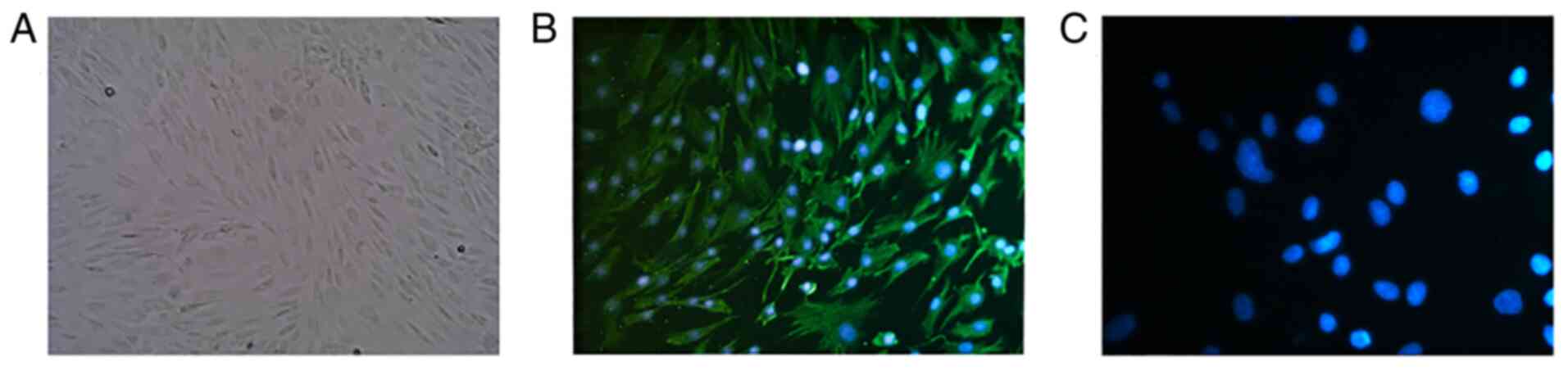
Human primary LFs identification
The third generation cells were first observed under
the light microscope (three fields of vision were randomly selected
and the typical images were shown in the figure; magnification,
×200). The cells were fully digested by 0.25% trypsin and 0.02%
EDTA (Dalian Meilun Biotech Co., Ltd.) The cells were transferred
to a culture dish and the culture medium was removed when the cells
grew to 90%. The cells were washed with PBS 3 times for 5 min each
and then fixed with 4% paraformaldehyde solution for 10 min. The
cells were washed with PBS for 3 times, 5 min per time. Then, the
cells were permeabilized with 0.5% Triton (Beijing Biosynthesis
Biotechnology Co., Ltd.) for 2 min and washed twice with PBS for 5
min per time. Cells were blocked with 1% BSA (Beijing Biosynthesis
Biotechnology Co., Ltd.) for 30 mins at room temperature.
Subsequently, the cells were incubated at 4°C overnight with mouse
anti vimentin monoclonal antibody (1:100; cat. no. bsm-33170M) and
rabbit anti type I collagen (COL-1) polyclonal antibody (1:100;
cat. no. bs-10423R), both from Beijing Biosynthesis Biotechnology
Co., Ltd. Then the cells were incubated with FITC labeled Goat
Anti-Mouse IgG and Goat Anti-rabbit IgG (1:50; cat. no. bs-0296G
and bs-0295G respectively, Beijing Biosynthesis Biotechnology Co.,
Ltd.) for 1 h in the dark at room temperature. Nuclei were stained
with 4′, 6-diamino-2-phenylindole. Images were acquired using a
laser confocal fluorescence microscope (magnification, ×400).
Cell treatments
There was a two part cell treatment in the present
study. In the first, LFs (50–60% confluence) were treated with 20
and 40 nM MIF at 37°C for 24 or 48 h to observe the difference in
the proliferation of the cells and record the MIF concentration and
treatment time. For the second, LFs PP1 (5 µM; Dalian Meilun
Biology Technology Co., Ltd.) was added to pretreat the cells for 1
h in a blank group (PP1 group) and MIF treatment group (MIF+PP1
group; treated by MIF at the concentration and time as the
aforementioned). A control group was treated with the same amount
of DMSO under basic medium.
Cell proliferation assay
Treated LFs were plated into a 96-well plate at a
density of 4,500 per well and proliferation was measured by CCK-8
kit. (Beyotime Institute of Biotechnology) according to the
manufacturer's instructions. The absorbance at 450 nm was
determined by a model 680 microplate reader (Bio-Rad Laboratories,
Inc.). The experiment was repeated three times.
Western blot analysis
The cells were lysed in RIPA buffer with 1%
phenylmethylsulfonyl fluoride. Total protein concentrations were
measured by a BCA kit (Beyotime Institute of Biotechnology). Equal
amounts of protein (25 µg) were separated on 8 or 10% SDS-PAGE gels
(Dalian Meilun Biology Technology Co., Ltd.) and transferred onto
polyvinylidene difluoride membranes (MilliporeSigma). The membrane
was sealed with 5% skimmed milk powder for 1 h at room temperature.
Then, the membranes were incubated with the corresponding primary
antibodies (rabbit anti-human Src; cat. no. bs-1135R; rabbit
anti-human TGFβ1; cat. no. bs-0086R; rabbit anti-humanMMP13; cat.
no. bs-0575R; rabbit anti-human COL-1; cat. no. bs-7158R; rabbit
anti-human COL-3; cat. no. bs-0549R; rabbit anti-human all from
Beijing Biosynthesis Biotechnology Co., Ltd. Co., Ltd.)
respectively, overnight at 4°C, washed three times in Western Blot
Wash Buffer (Beyotime Institute of Biotechnology) and incubated
with the corresponding secondary antibody (anti-rabbit IgG-HRP
linked antibody; 1:10,000; cat. no. 111-035-003 Jackson
ImmunoResearch) for 1.5 h at room temperature. The membranes were
washed three times in Wash Buffer and subjected to western blotting
using ECL chemiluminescence kit (Beyotime Institute of
Biotechnology). The immunoreactive protein was determined by
chemiluminescent center detection, and software (image
lab,6.1.0.07, Bio-Rad Laboratories) was used for densitometry.
Reverse transcription-quantitative
(RT-q) PCR
Total cell with density of 2×106/well RNA
was extracted using TRIzol reagent (cat. no. 15596026; Thermo
Fisher Scientific, Inc.). According to the manufacturer's
protocols, total RNA was reverse transcribed with mRNA reverse
transcription kit (Bestar; cat. no. DBI-2220; DBI Bioscience) The
resulting cDNA was used as template for RT-qPCR using SYBR-Green
(cat. nos. QPK-201 and QPK-201T; Toyobo Life Science).
Amplification with 95°C 5 sec (denaturation), 55°C 10 sec
(annealing) and 72°C 15 sec (extension) was followed by a melting
curve analysis with continual fluorescence data acquisition during
the 55–95°C melt. The threshold cycle (CT) values were normalized
to GAPDH and the relative expression was calculated by the ΔΔCq
method (15). The RT-qPCR primer
sequences are shown in Table II.
The experiment was repeated three times.
 | Table II.Primers used for reverse
transcription-quantitative PCR. |
Table II.
Primers used for reverse
transcription-quantitative PCR.
| Name |
| Primer Sequences
(5′-3′) | Melting
Temperature, ° | CG % |
|---|
| COL-1 | Sense |
AAGACAGTGATTGAATACAAAACCAC | 58.6 | 34.6 |
|
| Antisense |
GGGAGTTTACAGGAAGCAGACAG | 60.9 | 52.1 |
| COL-3 | Sense |
CAAGGCTGAAGGAAATAGCAAA | 59.4 | 40.9 |
|
| Antisense |
TCTCACAGCCTTGCGTGTTC | 59.3 | 55 |
| MMP13 | Sense |
CAGAACTTCCCAACCGTATTGAT | 60.1 | 43.5 |
|
| Antisense |
TGTATTCAAACTGTATGGGTCCG | 59.5 | 43.5 |
| TGFβ1 | Sense |
CAGCAACAATTCCTGGCGATA | 61.2 | 47.6 |
|
| Antisense |
GCTAAGGCGAAAGCCCTCAAT | 62.7 | 52.4 |
| Src | Sense |
CCTCAACGTGAAGCACTACAAG | 59.5 | 50 |
|
| Antisense |
GGCGTGTTTGGAGTAGTAGGC | 61 | 57 |
| GAPDH | Sense |
CATCATCCCTGCCTCTACTGG | 59.4 | 57.1 |
|
| Antisense |
GTGGGTGTCGCTGTTGAAGTC | 60.1 | 57.1 |
Drugs and treatments
MIF was dissolved in DMEM incomplete culture medium.
PP1 (cat. no. MB3961; Dalian Meilun Biotech Co., Ltd.), the Src
family inhibitor, was dissolved in DMSO with the concentration of
working solution <1.0%.
Statistical analysis
Statistical data was analyzed by GraphPad 6
(GraphPad Software, Inc.) and SPSS 16.0 (SPSS, Inc.) software. Mean
± standard deviation were applied to describe all quantitative
data. Quantitative data of normal distribution was compared using
independent t-tests. Chi-square test was applied for comparison of
male/female rate between two groups. Comparison among the three and
four groups was assessed using one-way ANOVA and LSD and Bonferroni
post hoc tests were used following ANOVA. P<0.05 was considered
to indicate a statistically significant difference.
Results
Concentration of fibrotic factors in
the LF specimens
The concentration of MIF, TGFβ1 and MMP13 were
higher in LSS group compared with the LDH group. (Table III).
 | Table III.Fibrotic factors in the LF between
LSS and LDH groups. (pg/mg protein). |
Table III.
Fibrotic factors in the LF between
LSS and LDH groups. (pg/mg protein).
| Factors | LSS (n=12) | LDH (n=15) | t | P-value |
|---|
| MIF | 340.56±42.86 | 189.20±51.17 | 8.20 | 0.000 |
| TGFβ1 |
3,045.60±595.79 |
2,185.50±734.57 | 3.28 | 0.003 |
| MMP13 | 649.08±135.31 | 366.13±75.92 | 6.88 | 0.000 |
Primary cell culture and
identification
The LF tissues (~0.5 mm3) were placed
onto culture dish (Fig. 1A). LF
cells (LFs) migrated out of the tissues a week later (Fig. 1B). LF tissues was removed when the
LFs growth occupied ~50% of the area of culture dish (Fig. 1C) and passage was performed. The
LFs were identified on the third passage (Fig. 1D). LFs identification were
performed by microscopic observation (Fig. 2A) and immunofluorescence (Fig. 2B and C) analysis.
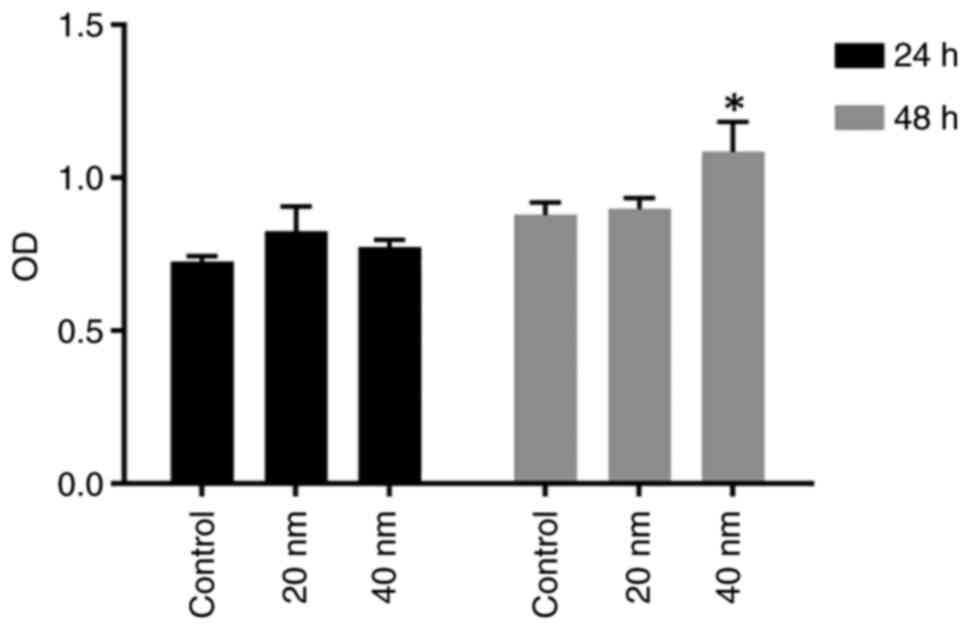
Time and concentration of MIF
treatment for LFs proliferation
Fibroblast proliferation is the vital pathological
process in the hypertrophy of LF. The time of proliferation of LF
cells under MIF treatment was unclear; two different MIF
concentrations (20 and 40 nM) were used for 24 and 48 h. The
proliferation condition of LFs was tested by CCK8 following MIF
treatment. No significant difference in proliferation was observed
in cells treated by MIF at the concentration of 20 and 40 nM for 24
h, while significant difference was observed with the concentration
of 40 nM for 48 h (Fig. 3).
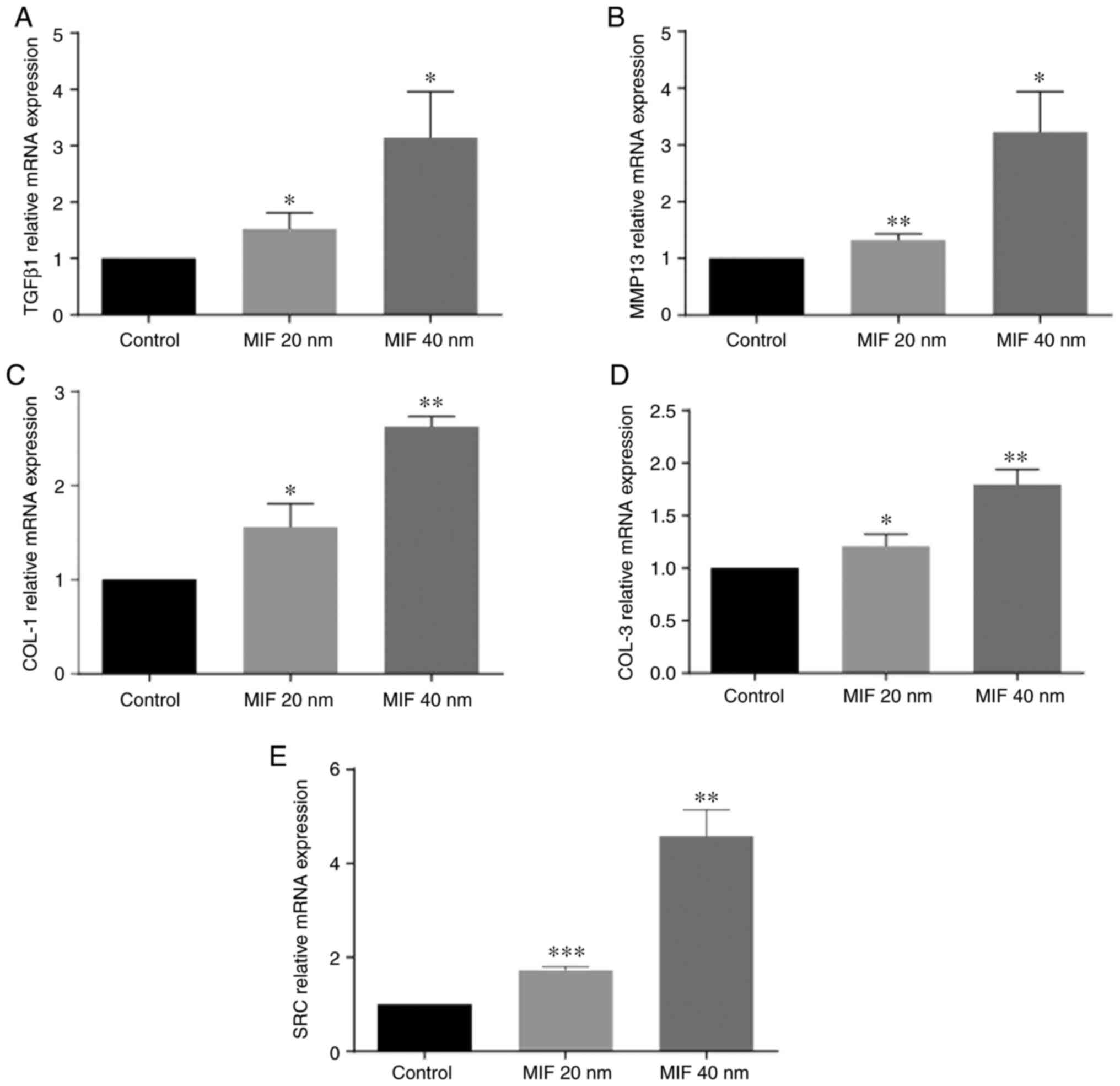
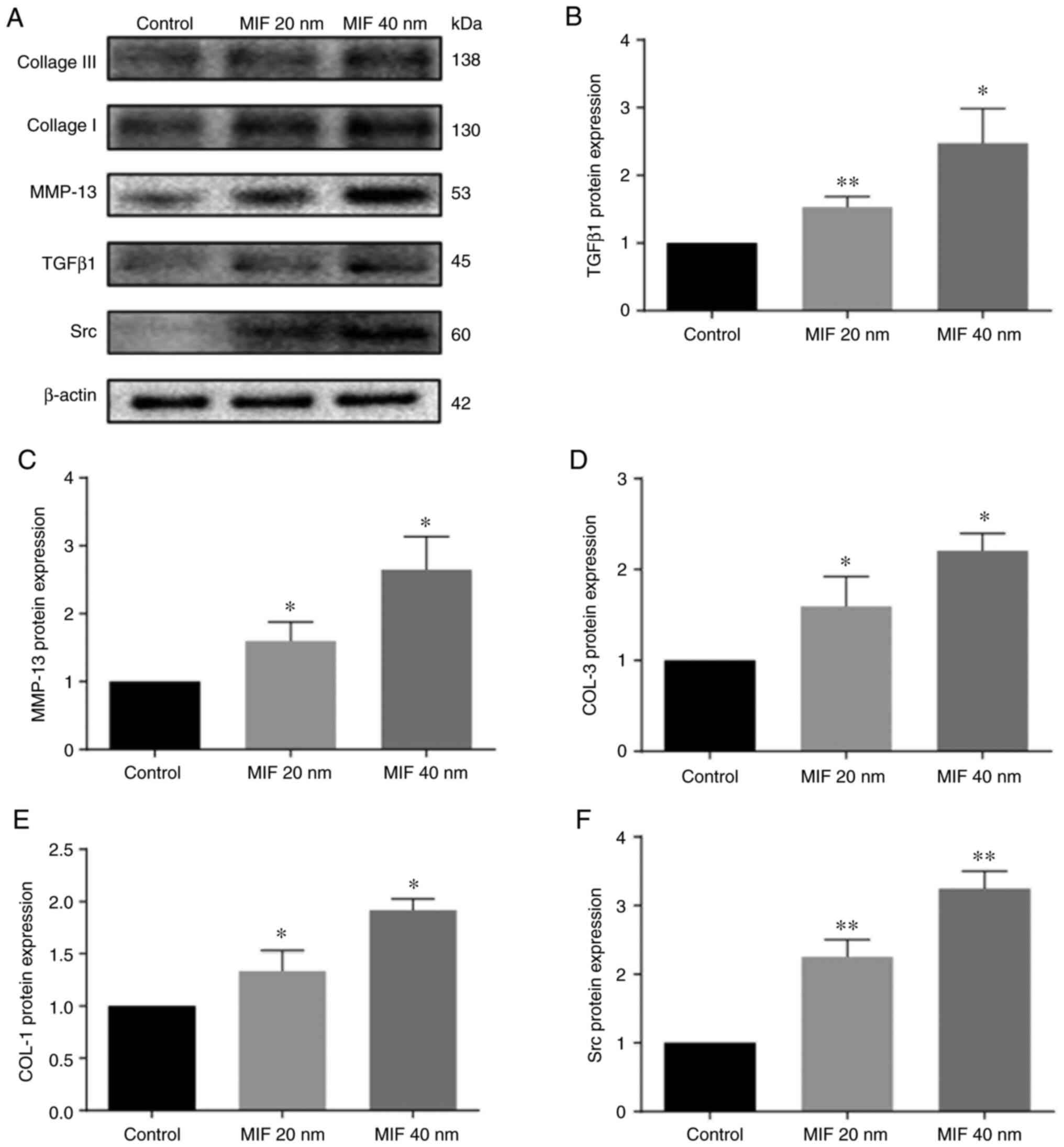
MIF promotes expression of TGFβ1,
MMP13, COL-1 and COL-3 and Src
To investigate the effect of MIF on protein
expression of LFs, LFs were stimulated with different
concentrations of human recombinant MIF to observe the expression
of important downstream LF fibrosis factors and the condition of
collagen deposition. LFs were treated with MIF at 20 and 40 nM for
48 h and, compared with the control groups, the expression of
TGFβ1, MMP13, COL-1, COL-3 and Src in the MIF stimulated groups
were significantly increased and those in 40 nM MIF treatment were
more evident (Figs. 4 and
5).
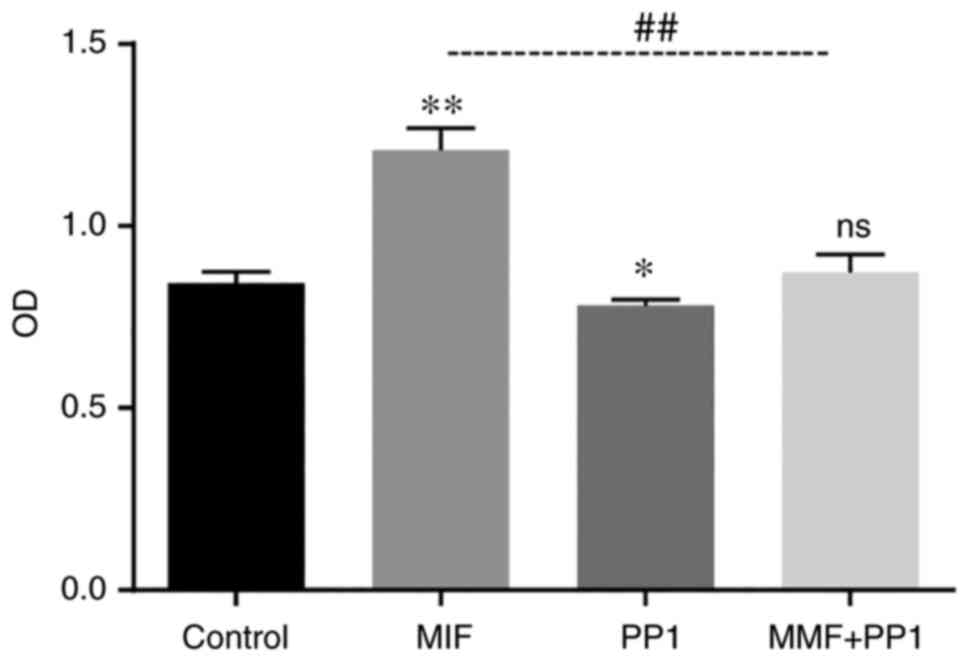
Src kinase is involved in LFs
proliferation induced by MIF
The experimental LFs were divided into control
group, MIF group (40 nM recombinant MIF treatment), PP1 group (Src
kinase specific antagonist 5 µM PP1 pretreatment for 1 h) and
MIF+PP1 group (MIF combined with PP1) and were treated for 48 h.
The purpose of the experiment was to explore whether Src kinase was
involved in MIF-induced fibroblasts proliferation. Accordingly, the
experiment demonstrated that the efficacy of MIF in promoting cell
proliferation decreased following PP1 intervention (Fig. 6).
Discussion
MIF level in hypertrophic LF was higher compared
with normal LF and the concentration of TGF β 1 and MMP13 also
showed this trend in hypertrophic LF. MIF can promote LFs to
secrete LF hypertrophy factors such as TGFβ1 and MMP13. The former
can directly promote the expression of the main components of
matrix collagen fibers, which were composed of type I collagen
(COL-1) and type III collagen (COL-3) and the latter can degrade
the elastic fibers in LF extracellular matrix. MIF can promote LFs
proliferation with concentration-dependent. The effect of MIF in
promoting LFs proliferation was decreased by PTK inhibitor, so MIF
may promote the proliferation of LFs through the Src kinase
signaling pathway. MIF and related inflammatory reactions take part
in the procedure of LF hypertrophy and MIF may occupy the dominant
role.
The lumbar LF is a connective tissue with the
thickness <4 mm. Anatomically, it connects the upper and lower
vertebral lamina and covers the central spinal canal, the nerve
root canal and the dorsal part of the intervertebral foramen
(16). The LF mainly consists of
fibroblast and extracellular matrix secreted by it (and most of the
original secretory type fibroblasts) transform into stable
fibroblast when LF tissue becomes mature (2). The extracellular matrix is mainly
constituted of elastic fibers, collagen fibers, proteoglycans and
glycoproteins. Elastic fibers and collagen fibers are mixed with a
ratio of 4:1 (16). The collagen
fibers are mainly comprised of type I collagen (COL-1) and type III
collagen (COL-3) (16,17). The pathological manifestations of
LF hypertrophy are stable fibroblast transforming into secretory
type fibroblast to proliferate in the LF (16). Inflammatory reactions mediated by
inflammatory cell infiltration cause overexpression of collagen
fibers in the matrix, degradation of elastic fibers, aggregation of
endothelial cells and microvascular neogenesis accelerated scar
formation in the LF tissues (5).
Specifically, elastic fibers are degraded, collagen fibers are
increased and proliferous collagen fibers are arranged in disorder.
These microstructural changes affect matrix remodeling, which
result in an increase in the thickness of the LF tissue, decrease
in elasticity and increase in brittleness (18). Hence, Lumbar, lower extremities
pain and numbness and bladder and bowel disorder induced by
intraspinal neurovascular stimulation secondary to the stenosis of
the central spinal canal, nerve root canal (lateral recess) and
intervertebral foramen, which have close association with the
hypertrophy of lumbar LF (19).
Although sex, obesity, spinal mechanics and
metabolic substances factors are influential elements in LF
hypertrophy, studies on the effect of MIF on hypertrophy are rare
(20–22). Other factors need to be first
controlled as much as possible during the experimental design of
MIF effects. Mechanics stress is a vital element during the LF
hypertrophy procedure on lumbar region. LDH mostly occurs in L5/S1
and L4/5 and LSS mostly occurs in L4/5 segment (23). Therefore, in order to control the
mechanics stress influence in present study, all LF samples were
taken from the same segment (L4/5), excluding patients with
instability or spondylolisthesis of L4. Despite the ventral side of
LF being furnished with little blood supply, the upper, lower and
dorsal part can directly contact with the blood supplied periosteum
and muscles. Therefore, patients with tumors, tuberculosis,
infection or metabolic disease were excluded to reduce the
hematogenous influence in this study. Coincidentally, participants
revealed no statistically significant difference in sex and BMI
between two groups in this study.
Inflammatory mediators serve major roles in the
fibrosis of LF hypertrophy (7).
The content of MIF measured by ELISA in the study tended to be
higher in the hypertrophic LF samples. It has been confirmed that
IL1, IL-6 and TNF-α are the downstream factors of MIF (24). Among them, IL1 and IL-6 can
promote the expression of COL-1 and TNF-α promotes the expression
of type III collagen of LFs (7).
Theoretically, MIF also can affect the fibroblasts of LF.
Furthermore, TGFβ1, one of the most closely related factors to LF
hypertrophy, can not only promote the proliferation of fibroblasts
but also contribute to the COL-1 and COL-3 collagen fibers in LF
(8). MMP has the function of
dissociating the extracellular matrix (9). Sugimoto et al (25) suggested that MMP is the major
degradation factor for elastic fiber of LF, mainly MMP13, MMP2 and
MMP9. Studies related to rheumatoid and cardiovascular explain that
MIF can promote the expression of MMP and TGFβ1 (10,26). In the present study, higher
concentration of TGFβ1 (3,045.60±595.79 vs. 2,185.50±734.57 pg/mg
protein) and MMP13 (649.08±135.31 vs. 366.13±75.92 pg/mg protein)
were found in the hypertrophic LF of patients. MIF concentration
differences (340.56±42.86 vs. 189.20±51.17 pg/mg protein) were also
discovered in LF specimens between the LSS and LDH groups. MIF
concentration in hypertrophic LF of LSS patients was higher
compared with that in LDH groups. TGFβ1and MMP13 also shared this
characteristic. The effect of higher level MIF on the LF and its
influence on the expression of TGFβ1 and MMP13 in LFs remains to be
elucidated.
Classified as a multipotent cytokine with enzymatic,
chemokine and hormonal properties, MIF can promote cell
proliferation, directly mediate inflammation and participate in
multiple organ fibrosis processes (27). A number of types of cell
proliferation can be promoted by MIF. The MIF promoter region is a
type of multipotent proinflammatory cytokine which contains DNA
binding sites binding to a variety of transcription factors, so MIF
is able to promote the release of a number of inflammatory factors
such as TNF-α, IL-1β, IL-6, IL-8 and IL-12 and the synthesis of
several matrix metalloproteinases, MMP-1, MMP-3, MMP-9 and MMP-13
(22,28). The effect of MIF on the
proliferation and fibrosis factor expression of ligamentum flavum
fibroblasts has not been reported to the best of the authors'
knowledge. Given the reported biological abilities of MIF and the
similar enhancement trend of TGFβ1 and MMP13 in LF samples of LSS
group, it was hypothesized that MIF could promote LFs proliferation
and the expression of the two typical fibrotic factors associated
with the hypertrophy of LF tissues. Primary LFs were obtained from
one patient of the LDH group (the aforementioned 35 year old). In
order to explore the time of the reaction of LFs under the
intervention of different concentrations of MIF, two different MIF
concentrations (20 and 40 nM) were used for 24 and 48 h. The
results showed that there was a significant difference in cell
proliferation at the concentration of 40 nM for 48 h. Therefore,
the intervention time for subsequent experiments was set at 48 h.
In in vitro LFs experiments TGFβ1 and MMP13 showed
concentration dependent increases at genetic and protein levels
with subsequent increase of COL-I and COL-III following stimulation
with MIF at 20 and 40 nM for 48 h (Figs. 5 and 6). In addition to the increase of
fibrosis factor, it was also found that the Src kinase gene and
protein level were closely associated with proliferation also
increased significantly.
As a core family member of the Src family kinases
(SFKs), Src kinase is a group of intracellular non-receptor
tyrosine kinases involved in the regulation of cell proliferation,
differentiation, angiogenesis and other biological behaviors
(29). In normal tissues, Src
kinase is the signal transduction center that coordinates cellular
responses to extracellular stimulation (30). As a ligand for several growth
factor receptors, including fibroblast growth factor receptors,
epidermal growth factor receptor and platelet-derived growth factor
receptor, Src can promote cell proliferation and differentiation
(30,31). Furthermore, Src can be activated
by MIF and high blood glucose (32,33). A study on cardiovascular diseases
indicates that tyrosine kinases in SFKs are recruited to activate
ERK1/2 by phosphorylation after MIF combines with its receptors
(34). In the present study, LFs
stimulated by MIF (40 nM) for 48 h in vitro showed the same
phenomenon of LFs proliferation compared with the control group.
From qPCR and western blotting results, MIF could promote the
expression of Src kinase in LFs. In order to explore the
relationship between Src kinase stimulated by MIF and the
proliferation of LFS, the specific SRC antagonist PP1 was used. The
results showed that the proliferation of LFS induced by MIF was
also inhibited by PP1, which suggested that MIF promoted the
proliferation of CFs through Src kinase signal transduction
pathway.
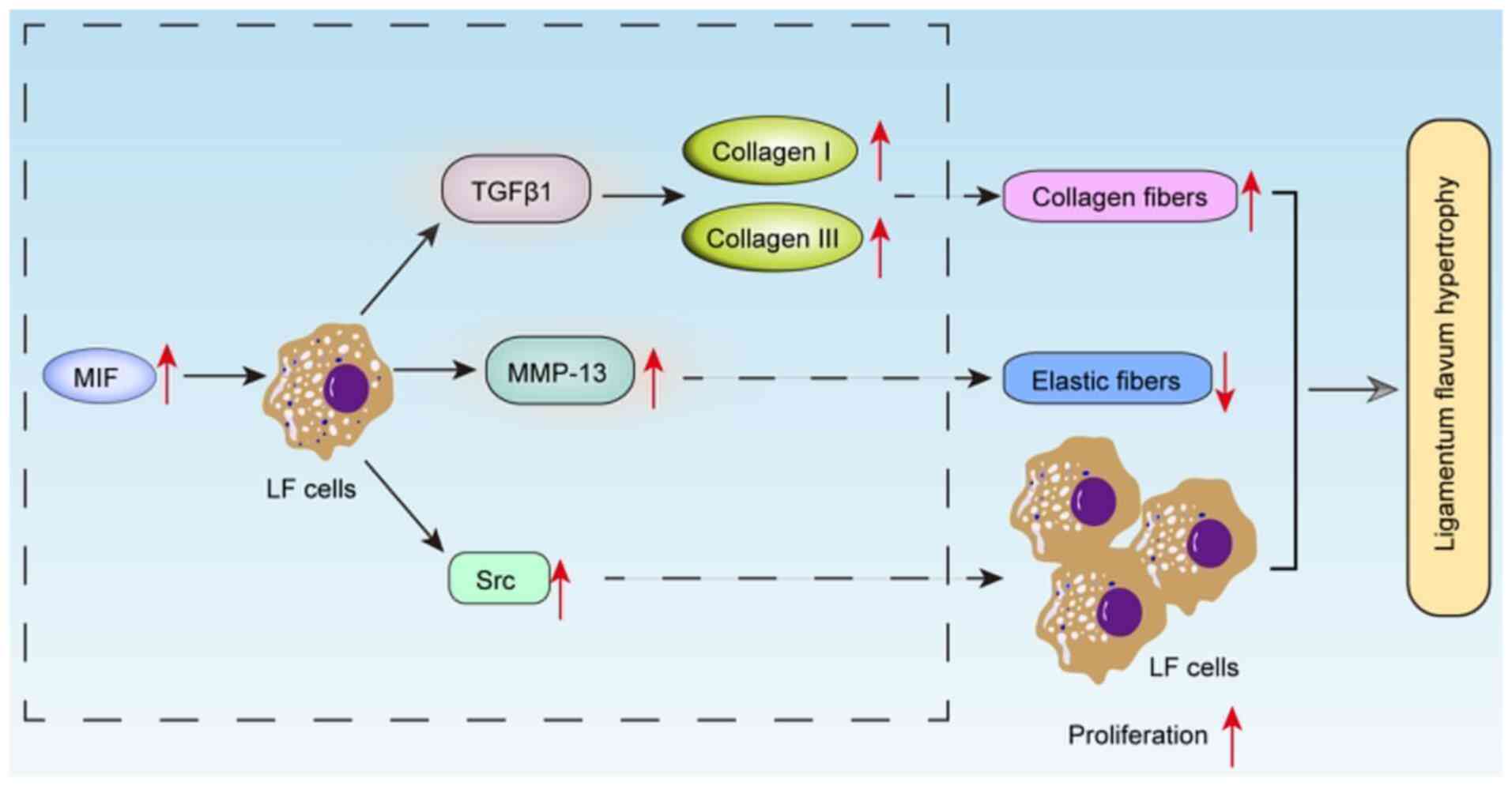
It was concluded that MIF promoted LFs to express
the vital fibrosis related factors TGFβ1 and MMP13 (Fig. 7). TGFβ1 could increase COL-I and
COL-III of collagen fiber and MMP13 decreased the elastic fiber in
LF. MIF also stimulated the expression of Src kinase to promote the
LFs proliferation. These processes contributed to the LF
hypertrophy. There were some experimental limitations in the
present study. The sample size was small for lumbar spinal stenosis
(LSS) and lumbar disc herniation (LDH) patients. The in
vitro LFs experiments cannot exactly simulate MIF concentration
and accurate time treatment for human LF fibroblasts. The mechanism
of MIF promoting the expression of fibrosis TGFβ1 and MMP13 require
further study. CD74, CXCR2, CXCR4 and CXCR7 are the receptors for
MIF and CD74 is more common (35,36). The receptors blocked or knocked
down will be a useful direction of further research. LFs can be
directly affected by peripheral blood, so whether the higher level
of MIF in hypertrophic LFs is exogenous or of local origination is
also a direction worthy of exploration.
Acknowledgements
The authors would like to thank Dr Chaochao Yu
(Zhongnan Hospital of Wuhan University) for preparing part of the
figures.
Funding
The present study was supported by a grant of the General
Hospital of Central Theater Command Postdoctoral Research Fund
grant no. 48610.
Availability of data and materials
The raw data and materials generated and used during
this study are available from the corresponding author upon
reasonable request.
Authors' contributions
QLL, ZXZ and YHY designed the present study from
literature search and manuscript preparation. JYL, YQZ and CS
performed the clinical and experimental studies. CJX, FX and GXY
designed the present study, performed data acquisition, data
analysis and statistical analysis. FX and GXY confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by Wuhan Municipal
Health Commission Medical Research Ethics Committee (Wuhan, Hubei;
approval no. 672HREC2020N01B). Written informed consents were
acquired from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sakai Y, Ito S, Hida T, Ito K, Harada A
and Watanabe K: Clinical outcome of lumbar spinal stenosis based on
new classification according to hypertrophied ligamentum flavum. J
Orthop Sci. 22:27–33. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shafaq N, Suzuki A, Terai H, Wakitani S
and Nakamura H: Cellularity and cartilage matrix increased in
hypertrophied ligamentum flavum: Histopathological analysis
focusing on the mechanical stress and bone morphogenetic protein
signaling. J Spinal Disord Tech. 25:107–115. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Salimi H, Suzuki A, Habibi H, Orita K,
Hori Y, Yabu A, Terai H, Tamai K and Nakamura H: Biglycan
expression and its function in human ligamentum flavum. Sci Rep.
11:48672021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang B, Gao C, Zhang P, Sun W, Zhang J and
Gao J: The increased motion of lumbar induces ligamentum flavum
hypertrophy in a rat model. BMC Musculoskelet Disord. 22:3342021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sairyo K, Biyani A, Goel VK, Leaman DW,
Booth R Jr, Thomas J, Ebraheim NA, Cowgill IA and Mohan SE: Lumbar
ligamentum flavum hypertrophy is due to accumulation of
inflammation-related scar tissue. Spine (Phila Pa 1976).
32:E340–E347. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saito T, Hara M, Kumamaru H, Kobayakawa K,
Yokota K, Kijima K, Yoshizaki S, Harimaya K, Matsumoto Y, Kawaguchi
K, et al: Macrophage infiltration is a causative factor for
ligamentum flavum hypertrophy through the activation of collagen
production in fibroblasts. Am J Pathol. 187:2831–2840. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park JO, Lee BH, Kang YM, Kim TH, Yoon JY,
Kim H, Kwon UH, Lee KI, Lee HM and Moon SH: Inflammatory cytokines
induce fibrosis and ossification of human ligamentum flavum cells.
J Spinal Disord Tech. 26:E6–E12. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang L, Chang M, Tian Y, Yan J, Xu W, Yuan
S, Zhang K and Liu X: The role of Smad2 in transforming growth
factor β-Induced hypertrophy of ligamentum flavum. World Neurosurg.
151:e128–e136. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cui G, Watanabe K, Miyauchi Y, Hosogane N,
Tsuji T, Ishii K, Nakamura M, Toyama Y, Chiba K, Miyamoto T and
Matsumoto M: Matrix metalloproteinase 13 in the ligamentum flavum
from lumbar spinal canal stenosis patients with and without
diabetes mellitus. J Orthop Sci. 16:785–790. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xue YM, Deng CY, Wei W, Liu FZ, Yang H,
Liu Y, Li X, Wang Z, Kuang SJ, Wu SL and Rao F: Macrophage
migration inhibitory factor promotes cardiac fibroblast
proliferation through the Src kinase signaling pathway. Mol Med
Rep. 17:3425–3431. 2018.PubMed/NCBI
|
|
11
|
Zheng Y, Li X, Qian X, Wang Y, Lee JH, Xia
Y, Hawke DH, Zhang G, Lyu J and Lu Z: Secreted and O-GlcNAcylated
MIF binds to the human EGF receptor and inhibits its activation.
Nat Cell Biol. 17:1348–1355. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yao Y, Deng Q, Song W, Zhang H, Li Y, Yang
Y, Fan X, Liu M, Shang J, Sun C, et al: MIF plays a key role in
regulating tissue-specific chondro-osteogenic differentiation fate
of human cartilage endplate stem cells under hypoxia. Stem Cell
Reports. 7:249–262. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hertelendy J, Reumuth G, Simons D, Stoppe
C, Kim BS, Stromps JP, Fuchs PC, Bernhagen J, Pallua N and Grieb G:
Macrophage migration inhibitory factor-a favorable marker in
inflammatory diseases? Curr Med Chem. 25:601–605. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu QL, Wang XZ, Xie W, Chen XW, Zhu YL and
Li XG: Macrophage migration inhibitory factor may contribute to
hypertrophy of lumbar ligamentum flavum in type 2 diabetes
mellitus. Chin Med J (Engl). 133:623–625. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arocho A, Chen B, Ladanyi M and Pan Q:
Validation of the 2-DeltaDeltaCt calculation as an alternate method
of data analysis for quantitative PCR of BCR-ABL P210 transcripts.
Diagn Mol Pathol. 15:56–61. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun C, Zhang H, Wang X and Liu X:
Ligamentum flavum fibrosis and hypertrophy: Molecular pathways,
cellular mechanisms and future directions. FASEB J. 34:9854–9868.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takeda H, Nagai S, Ikeda D, Kaneko S,
Tsuji T and Fujita N: Collagen profiling of ligamentum flavum in
patients with lumbar spinal canal stenosis. J Orthop Sci.
26:560–565. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kosaka H, Sairyo K, Biyani A, Leaman D,
Yeasting R, Higashino K, Sakai T, Katoh S, Sano T, Goel VK and
Yasui N: Pathomechanism of loss of elasticity and hypertrophy of
lumbar ligamentum flavum in elderly patients with lumbar spinal
canal stenosis. Spine (Phila Pa 1976). 32:2805–2811. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Melancia JL, Francisco AF and Antunes JL:
Spinal stenosis. Handb Clin Neurol. 119:541–549. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yan B, Zeng C, Chen Y, Huang M, Yao N,
Zhang J, Yan B, Tang J, Wang L and Zhang Z: Mechanical
Stress-Induced IGF-1 Facilitates col-I and col-III Synthesis via
the IGF-1R/AKT/mTORC1 Signaling Pathway. Stem Cells Int.
2021:55536762021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun C, Wang Z, Tian JW and Wang YH:
Leptin-induced inflammation by activating IL-6 expression
contributes to the fibrosis and hypertrophy of ligamentum flavum in
lumbar spinal canal stenosis. Biosci Rep. 38:BSR201712142018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen MH, Hu CK, Chen PR, Chen YS, Sun JS
and Chen MH: Dose-dependent regulation of cell proliferation and
collagen degradation by estradiol on ligamentum flavum. BMC
Musculoskelet Disord. 15:2382014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sudhir G, Vignesh Jayabalan S, Gadde S,
Venkatesh Kumar G and Karthik Kailash K: Analysis of factors
influencing ligamentum flavum thickness in lumbar spine-A
radiological study of 1070 disc levels in 214 patients. Clin Neurol
Neurosurg. 182:19–24. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kasama T, Ohtsuka K, Sato M, Takahashi R,
Wakabayashi K and Kobayashi K: Macrophage migration inhibitory
factor: A multifunctional cytokine in rheumatic diseases.
Arthritis. 2010:1062022010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sugimoto K, Nakamura T, Tokunaga T, Uehara
Y, Okada T, Taniwaki T, Fujimoto T and Mizuta H: Matrix
metalloproteinase promotes elastic fiber degradation in ligamentum
flavum degeneration. PLoS One. 13:e02008722018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Onodera S, Nishihira J, Koyama Y, Majima
T, Aoki Y, Ichiyama H, Ishibashi T and Minami A: Macrophage
migration inhibitory factor up-regulates the expression of
interleukin-8 messenger RNA in synovial fibroblasts of rheumatoid
arthritis patients: Common transcriptional regulatory mechanism
between interleukin-8 and interleukin-1beta. Arthritis Rheum.
50:1437–1447. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leng L and Bucala R: Macrophage migration
inhibitory factor. Crit Care Med. 33 (12 Suppl):S475–S477. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Su Y and Wang Y, Zhou Y, Zhu Z, Zhang Q,
Zhang X, Wang W, Gu X, Guo A and Wang Y: Macrophage migration
inhibitory factor activates inflammatory responses of astrocytes
through interaction with CD74 receptor. Oncotarget. 8:2719–2730.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li H, Zhao C, Tian Y, Lu J, Zhang G, Liang
S, Chen D, Liu X, Kuang W and Zhu M: Src family kinases and
pulmonary fibrosis: A review. Biomed Pharmacother. 127:1101832020.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Koudelková L, Brábek J and Rosel D: Src
kinase: Key effector in mechanosignalling. Int J Biochem Cell Biol.
131:1059082021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ntanasis-Stathopoulos I, Fotopoulos G,
Tzanninis IG and Kotteas EA: The emerging role of tyrosine kinase
inhibitors in ovarian cancer treatment: A systematic review. Cancer
Invest. 34:313–339. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rao F, Deng CY, Wu SL, Xiao DZ, Yu XY,
Kuang SJ, Lin QX and Shan ZX: Involvement of Src in L-type Ca2+
channel depression induced by macrophage migration inhibitory
factor in atrial myocytes. J Mol Cell Cardiol. 47:586–594. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cai T, Kuang Y, Zhang C, Zhang Z, Chen L,
Li B, Li Y, Wang Y, Yang H, Han Q and Zhu Y: Glucose-6-phosphate
dehydrogenase and NADPH oxidase 4 control STAT3 activity in
melanoma cells through a pathway involving reactive oxygen species,
c-SRC and SHP2. Am J Cancer Res. 5:1610–1620. 2015.PubMed/NCBI
|
|
34
|
Zernecke A, Bernhagen J and Weber C:
Macrophage migration inhibitory factor in cardiovascular disease.
Circulation. 117:1594–1602. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jankauskas SS, Wong DWL, Bucala R, Djudjaj
S and Boor P: Evolving complexity of MIF signaling. Cell Signal.
57:76–88. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Presti M, Mazzon E, Basile MS and Maria
CP: Overexpression of macrophage migration inhibitory factor and
functionally-related genes, D-DT, CD74, CD44, CXCR2 and CXCR4, in
glioblastoma. Oncol Lett. 16:2881–2886. 2018.PubMed/NCBI
|