Molecular and genetic studies of the endocrine
system have progressed rapidly over the past few decades. Cyclic
adenosine monophosphate (cAMP) is the most important secondary
messenger involved in endocrine system development and function.
Dysregulation of cAMP expression and signaling perturbs the
endocrine physiology and causes disease. cAMP production and
degradation is mediated by ACs and phosphodiesterases (PDEs)
respectively (1–5). PDEs hydrolyze the phosphate bonds of
cyclic nucleotides; 11 PDE gene families have been identified based
on amino acid sequences, biochemical properties, and inhibitor
profiles (6,7). PDEs may share a catalytic function
but differ in subcellular localization and tissue expression status
(7). PDEs hydrolyze cAMP and
cyclic guanosine monophosphate (cGMP) to AMP and GMP. PDEs may
degrade cAMP (PDE4, 7, 8), cGMP (PDE5, 6, 9), or both (PDE1, 2, 3,
10, 11) (1,8–10).
Thus, PDEs perform various roles depending on their location and
expression status. For example, inhibitors of PDE5 serve as
therapeutic agents for male erectile dysfunction and pulmonary
hypertension (11,12). PDE9A and PDE10A are widely
distributed throughout the central nervous system (CNS); modulation
of their expression usefully treats Alzheimer's disease (13) and schizophrenia (14–16). PDE11A degrades both cAMP and cGMP
(17–21). PDE11A features four splice
variants (1–4) varying in terms of tissue expression
and the N-terminal regulatory regions. The N-terminal domain is
regulatory in nature and the C-terminal domain catalytic. The
longest isoforms of PDE11A in the mouse and human share ~95%
protein sequence homology. The PDE11A level is highest in the
prostate (22) of the various
splice variants, PDE11A1 and PDE11A3 are found in the spleen
(23,24) and PDE11A4 in the hippocampus
(25). PDE11A is also expressed
in the liver, skeletal muscle, pituitary gland, pancreas, and
kidneys (18,19,22). Thus, PDE11A expression and
structural characteristics vary by tissue location. This
mini-review summarizes the locations of PDE11A expression, the
effects of structural differences, and disease involvement.
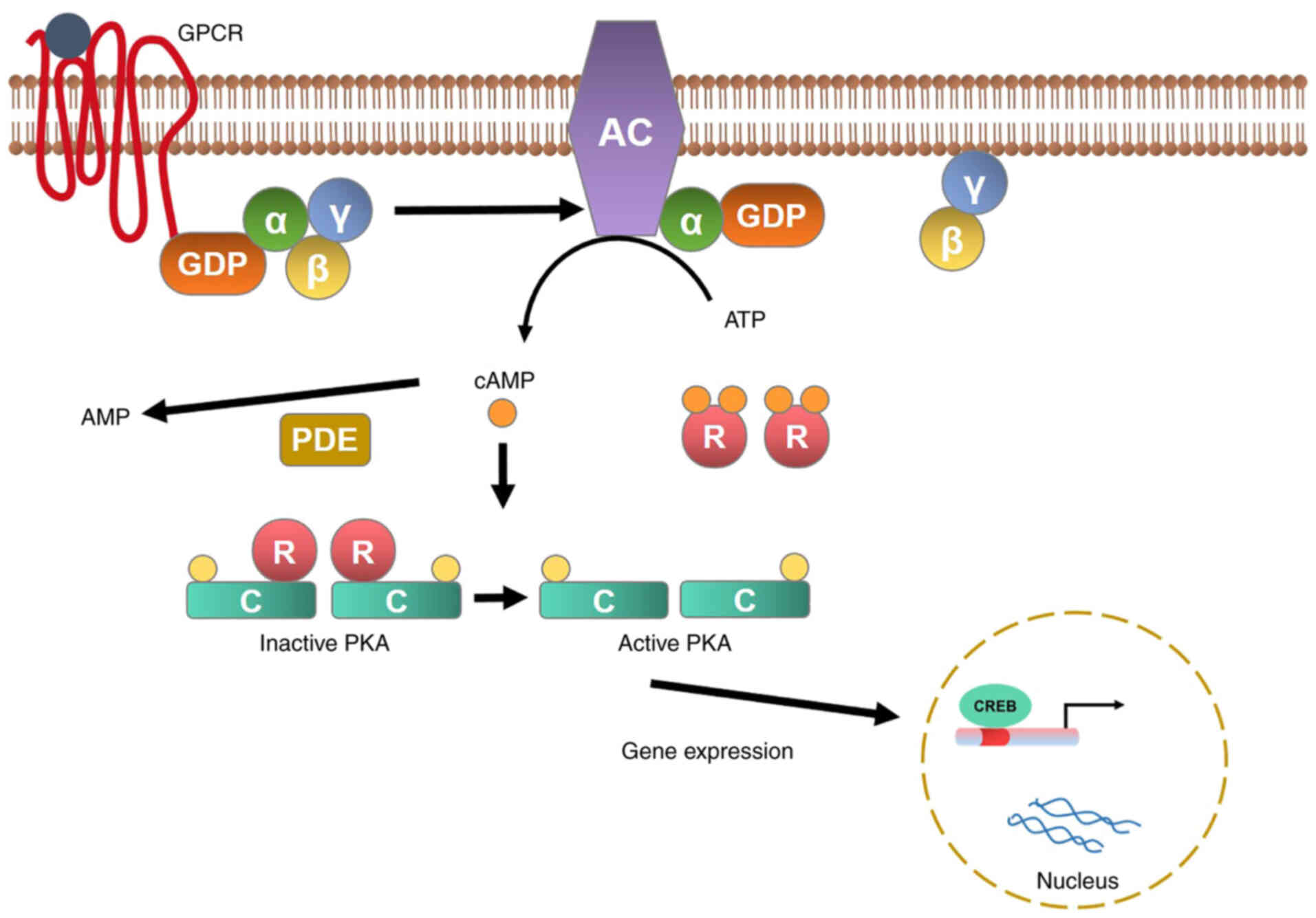
cAMP and cGMP are important secondary messengers
involved in cell regulation and metabolism (6) driven by the GPCR. AC catalyzes
conversion of ATP (Adenosine triphosphate) to cAMP and inorganic
pyrophosphate; cAMP activates protein kinase A (PKA), which in turn
phosphorylates intracellular proteins that mediate specific
responses (26). cAMP activation
is triggered by adrenocorticotropic hormone bound to the
adrenocorticotropic hormone receptor; this in turn induces
dissociation of the Gsα subunit (encoded by the GNAS gene) from
G-protein, AC activation, cAMP generation, and PKA activation. PKA
is a tetrameric complex of two regulatory subunits (PRKACA and
PRKACB). The latter is responsible for phosphorylation of various
enzymes and transcription factors, including the cAMP response
element-binding protein (CREB) (Fig.
1). cAMP signaling plays roles during several steps of
tumorigenesis. Inactivation of germline mutations in the alpha
regulatory subunit gene of PKA induces the Carney complex (27–32) (an autosomal-dominant disease
characterized by cardiac myxoma, schwannoma, and endocrine tumors;
Carney complex is one of the most common types of primary
pigmentary crystalline adrenocortical disease associated
hyperplasia) (33). Such
mutations are also implicated in cancer cell formation via
activation of the stimulatory G protein of AC, increasing the cAMP
level (34). Similar to cAMP,
cGMP is degraded by class I phosphodiesterase in metazoans, some of
which are activated by cGMP binding to the GAF domain (35). Cyclic GMP signaling is not
observed in eubacteria, plants, and yeast but is found in
vertebrates. Besides that, It was also found in Drosophila and
Caenorhabditis elegans with cGMP signaling, which is mediated by
cGMP regulatory protein kinase G, possibly Ras guanine nucleotide
exchange factor, and ion channels, is similar to that of
vertebrates. Furthermore, these regulators contain the cyclic
nucleotide-binding domain instead of the GAF domain (36,37). In metazoans, cGMP is synthesized
by two guanylyl cyclases, one membrane-bound and the other soluble,
and has a common phylogenetic precursor. Although no close homologs
of this protein have been found in Dictyostelium, Dictyostelium
guanylyl cyclases (38), guanylyl
cyclases A and soluble guanylyl cyclases are similar to AC.
guanylyl cyclases A has a dozen ubiquitous topologies on metazoan
AC, whereas soluble guanylyl cyclases is just homologous to a small
family of soluble AC present in vertebrates and bacteria (39,40). Upregulation of cGMP levels by PDEs
induces activation of PKG, which promotes vasodilation and
increases blood flow, particularly in the brain (41). cAMP also contributes to
tumorigenesis via PDE (6), which
increases cAMP and cGMP levels (1,8–10),
resulting in sustained activation of the cAMP/PKA cascade. PDE is
expressed in many different cancer cells, which may also host PDE
mutations (examples PDE11A R804H, and R867G (6,42,43). An association between PDE genetic
changes and tumorigenesis has been noted, particularly in the
prostate, testis, and adrenal cortex (44,45). Hence, mutations in PDE and
circulation of cAMP and cGMP are essential not only for human
development but also for cancer and many diseases.
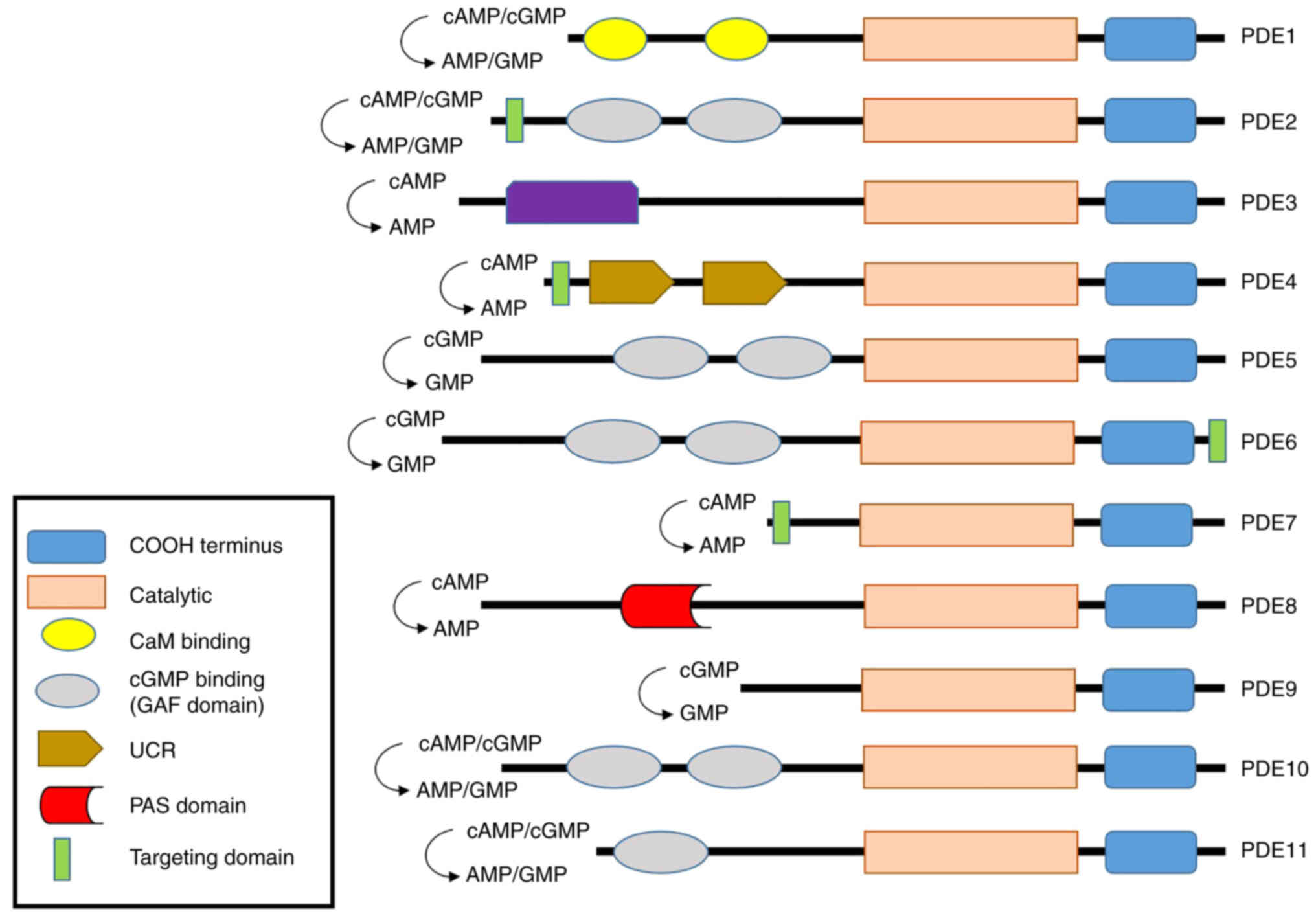
PDEs regulate cAMP and cGMP production and are
essential enzymes. PDEs are found in various tissues where they
perform different roles. PDE features 11 different isoforms
(Fig. 2). The four PDE1 isoforms
(PDE1A, PDE1B, PDE1B1-2, and PDE1C1-2) are found in the brain,
sperm, kidney, liver, pancreas, and thyroid gland (46–48); the heart (49); immune cells (50); and the olfactory epithelium
(51) respectively, and regulate
both cAMP and cGMP action. The PDEs play roles in vascular smooth
muscle contraction and proliferation, sperm function, dopamine
signaling, and immune cell activation. The common PDE1 subtype
inhibitors include Vinpocetine, IC224 (PDE1A), SCH51866,
8-MeoM-IBMX (PDE1B), Zaprinast (PDE1B1-2), and Sildenafil
(PDE1C1-2). PDE2A1-3 is expressed in the adrenal glomerulosa
(52). The PDE2 proteins regulate
both cAMP and cGMP actions and control aldosterone and ACTH
secretion and long-term memory. Common PDE2A inhibitors include
EHNA, BAY60-7550, PDP, and IC933. PDE3 includes PDE3A1-3 and PDE3B.
The former is expressed in the heart (53), adipocytes, oocytes, cardiac and
vascular smooth muscle, myocardium, and platelets (54). PDE3B is expressed in heart muscle
(55), the immune system
(56), endothelial cells
(mediating permeability and cell proliferation) (57), the brain (58) and the liver (59). PDE3A and PDE3B regulate both cAMP
and cGMP production; PDE3A controls cardiac contraction, platelet
aggregation, vascular smooth muscle contraction, cell maturation,
and renin release. PDE3B modulates lipolysis, glycogenolysis,
insulin secretion, and heart function. Common PDE3 inhibitors
include amrinone, cilostazol, milrinone, and enoximone. In
addition, many inhibitors have been reported to modulate PDEs
(Table I). PDE4 includes PDE4A,
PDE4B, PDE4C, and PDE4D. PDE4 is expressed in the heart and small
intestine (60), immune cells
(61), and the brain (62). Unlike PDEs 1, 2, and 3, PDE4
exhibits a higher affinity for cAMP than cGMP and controls brain
function, monocyte and macrophage activation, neutrophil
infiltration, vascular smooth muscle proliferation, fertility, and
heart β-adrenergic signaling and excitatory/contract coupling. PDE5
includes PDE5A1-3 expressed in the lung, penis, smooth muscle
(28), platelets (63), brain (64) and cardiac muscle (65). Both PDE5 enzymes regulate cGMP;
the nitrous oxide (NO)/cGMP effects in vascular smooth muscle,
platelets, and the lower urinary tract; and the cardiac stress
response. PDE6 includes PDE6A, PDE6B, and PDE6C expressed in
photoreceptors (66) and the
pineal gland (67). PDE6
regulates cGMP action, controls the cGMP concentrations of rod and
cone photoreceptors, and is the primary effector enzyme of the
phototransduction cascade. PDE7 features PDE7A1-2 and PDE7B1-3
found in immune cells (68),
skeletal and cardiac muscles (69) and the brain (70). PDE7 modulates the cAMP activity
and plays an important role in the regulation of human T cell
function. PDE8 includes PDE8A1-5 and PDE8B1-3 found in immune cells
(71), the heart (72), the ovary and testes (73), the thyroid gland (74), placenta, brain (75) and the adrenal gland (76). Both PDE8s regulate cAMP activation
and TSH levels, adrenal steroid production, luteinizing hormone
signaling, and steroidogenesis in Leydig cells, and activate T
cells. PDE9 includes PDE9A1-6 expressed in the kidney, spleen, gut,
and prostate (77). PDE9
regulates cGMP activation to play a role in energy balance. PDE10
includes PDE10A1-2 expressed in the brain, testis, and thyroid
(78). PDE10 regulates both cAMP
and cGMP actions and plays roles in striatal activation and
behavioral activity. PDE11 includes PDE11A1-4 of the testis,
pituitary gland, heart, kidney, liver (18,19), prostate, adrenal gland, and colon
(22), but only the A4 splice
mutant is expressed in adrenal tissue. PDE11A regulates both cAMP
and cGMP actions and is involved in spermatogenesis. PDE11A4 was
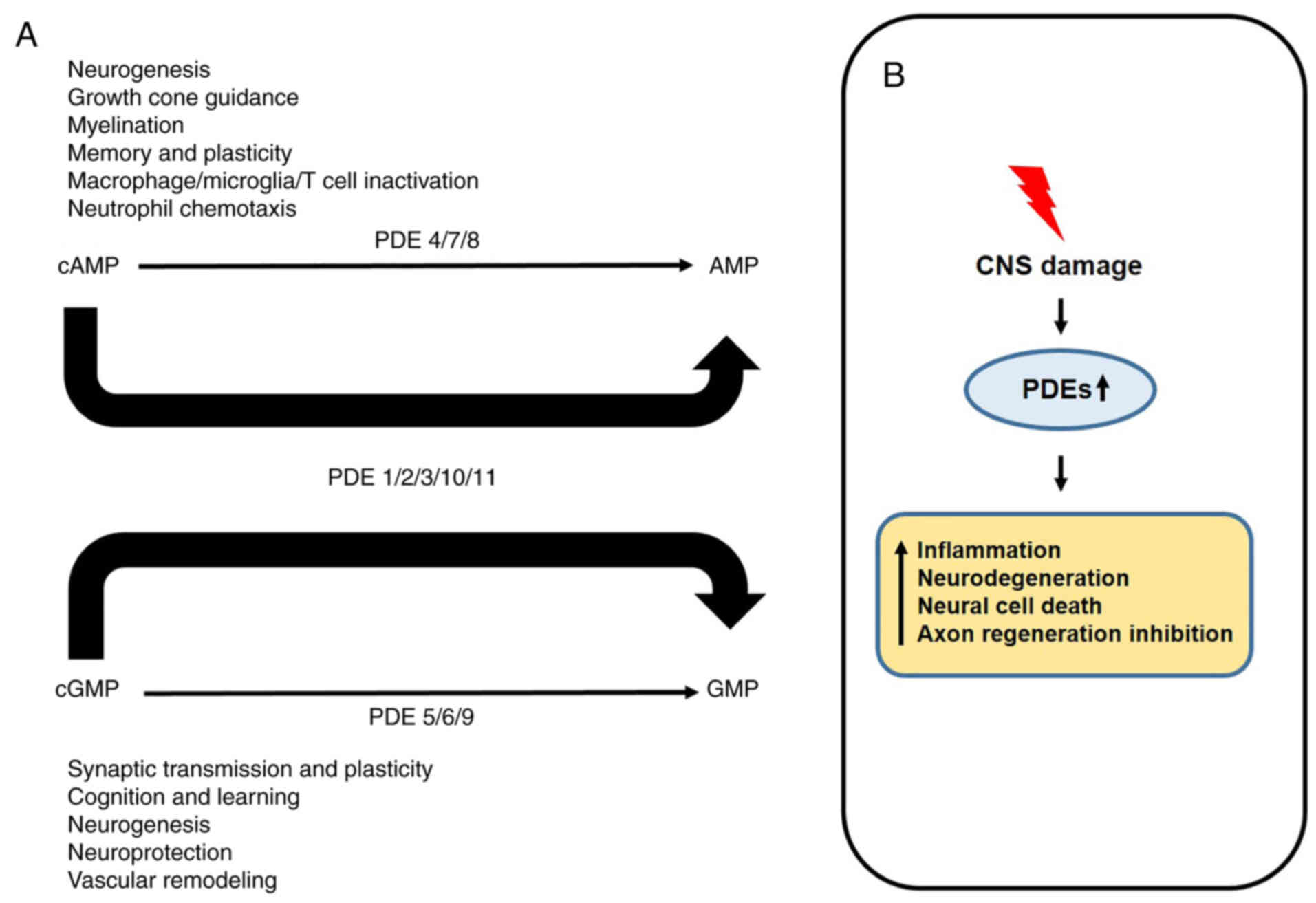
recently found in the hippocampus (23,79). Besides that, all PDEs are
expressed somewhere in the CNS and hydrolyze cAMP and cGMP to
perform their respective roles (Fig.
3A) (80). However, when the
central nervous system is damaged, the increase of PDEs expression
activates immune cells and decreases the regeneration of neuronal
cells, resulting in the death of neural cells (Fig. 3B) (81). Therefore, all PDE families can
perform their respective roles depending on the expression site,
and all PDEs also can contribute to the growth and development of
nerve cells and cancer cells.
cAMP and cGMP are important GPCR-driven secondary
messengers controlling cellular regulation and metabolism. cAMP is
formed by the actions of AC and PDE and mediates cellular responses
by activating PKA to phosphorylate intracellular proteins (4,5).
In addition, the cAMP has been implicated in various tumorigenesis
due to either increasing expression levels by activating the
stimulatory G protein of AC or degraded by PDE11A. PDE11A encoded
on chromosome 2q31.2 is highly polymorphic and was also the first
PDE associated with an adrenocortical tumor-associated genetic
condition. Furthermore, PDE11A degrades not only cAMP but also cGMP
(82). Besides, several previous
studies found that PDE11A mutations were mainly expressed in
abnormal adrenal glands (19,83). Three PDE11A mutations have been
reported in Cushing syndrome patients with a primary pigmented
nodular adrenocortical disease or isolated micronodular
adrenocortical disease without other genetic defects. An
association between the GWA single-nucleotide polymorphism (SNP) of
PDE11 and adrenocortical tumors has been also confirmed (43). Mutations and relationships of
PDE11A have been reported in numerous types of cancer, as well as
the most studied adrenal cortical tumors (https://www.cbioportal.org/). The heterozygous
inactivation strains of PDE11A in patients were identified in
non-secreting adrenal cortical adenoma, and heterozygous missense
strains were more common in Primary bilateral macronodular adrenal
hyperplasia (24%) and adrenocortical carcinomas (19%) compared to
control group (5.7%) (84).
Furthermore, the p.R867G PDE11A mutation was found in one patient
with familial Primary bilateral macronodular adrenal hyperplasia
(85). In a Primary bilateral
macronodular adrenal hyperplasia cohort, the frequency of all
PDE11A variants was significantly higher in Primary bilateral
macronodular adrenal hyperplasia patients (28%) than in controls
(7.2%). The inactivating PDE11A mutation (p.R307) was also found in
adrenocortical cancer-associated genetic condition patients
(19). Not only that, in the New
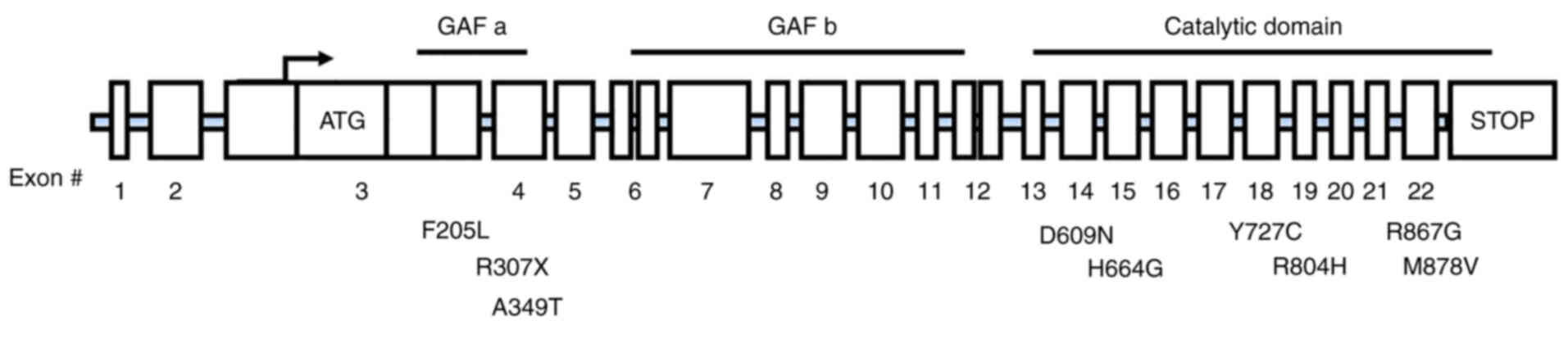
York Cancer Project, Horvath et al (43,86) studied 745 patients with
adrenocortical tumors and found PDE11A sequence changes including
three truncation mutations (c.171Tdel/fs41X, c.919C>T/p.R307X
and c.1655_1657TCTdelCCins/fs15X) and two missense substitutions
[c.2411G>A(R804H) and c.2599C>(R867G)] (Fig. 4). Mutations in PDE11A have also
been reported in testicular germ cell tumors. In 259 patients with
testicular German cell tumors, 55 PDE11A strains (20 missense, 4
splice sites, 2 non-sense sites, 7 synonyms, 22 introductions, 10
missense strains, 9 transcriptions) were identified. Among them,
rare mutations (p.F258Y, p.G291R, p.V820M, p.R545X, p.K568R) were
found. Mutations in PDE11A testicular germ cell tumors degrade
PDE11A function, ultimately increasing the cAMP/cGMP level
(87). Mutation of PDE11A was
also observed in Carney complex, somatic dystrophy and various
endocrine tumors (kidney, prostate, colon, lung and breast)
(22,44,82,88,89). In addition, mutations in Y727C and
E840K of PDE11A have been reported to be extremely high expressed
in prostate cancer (90).
Moreover, the role of PDE11A in brain tumors has recently been
studied and reported due to the brain belongs to an essential part
of the human body and is responsible for a critical part of the CNS
(91). All brain cancers are
graded from 1 to 4 based on how the cancer cells look under the
microscope and how well they reproduce. The most aggressive and
fast-growing malignant Grade 4 tumor is called glioblastoma
(92). Currently open surgery,
radiation therapy (93), and
chemotherapy (94) are all using
for the treatment of glioblastoma. However, they are ineffective or
have a very high probability of side effects. Therefore, to develop
an effective treatment strategy for glioblastoma, the studies of
analytical methods using molecular targeting are necessary.
Besides, with the increase of studies on the association between
PDE11A and the brain, it was found in the brain's hippocampus
(62), and the deletion of PDE11A
in the brain has been shown to increase microglial activation
(25). More specially, recently,
PDE11A was also found to be highly expressed in glioblastoma. Lee
et al (95) found that the
PDE11A expression level in glioblastomas was higher than in normal
brains and PDE11A knockdown reduced cancer cell proliferation. This
suggests that the expression of PDE11A can regulate the development
of glioblastoma in patients. Other PDEs (PDE5, PDE8, and PDE10) are
also involved in cancer cell proliferation; various mutations have
been described (96–98). Therefore, PDE11A and other PDEs
fail to act as regulators of cAMP and cGMP, affecting the growth
and development of cancer cells. Furthermore, it is thought that
alternatives to PDEs related to such mutation can play a clear role
in CNS and testicular cancer, where PDEs are highly expressed.
PDE11A is expressed in the human testis, pituitary
gland, heart, kidney, liver, prostate, adrenal gland, colon, and
hippocampus (18,19,22,23,79), and is associated with tumors and
other diseases. Adrenocorticotropin independent macronodular
adrenocortical hyperplasia (a bilateral tumor) is a rare cause of
Cushing's syndrome (less than 1% of all cases). Several types of
adrenocortical tumors that cause the Cushing's syndrome were found
to be caused by abnormal cAMP and could be caused by mutations in
PDE11A (86). The bilaterality of
such benign tumors suggests that a genetic factor is in play;
Adrenocorticotropin independent macronodular adrenocortical
hyperplasia has been associated with PDE11A mutations (99,100). This mutation was also observed
in patients with acromegaly. Acromegaly is a condition in which the
body produces excessive growth hormones, causing body tissues and
bones to grow faster. Mutations of PDE11A (Y727C, R804H, R867G,
M878V, FS41X) were reported in patients with acromegaly (101). PDE11A also affects brain
expression and development. The previous paper reported that it
might be related to a bipolar disorder associated with lithium
reactivity (102). Moreover, two
rare PDE11A pentasensory mutations were found in patients with
Alzheimer's disease, and PDE11A levels were reduced in brain
samples (79,103). Expression of PDE11A4 is 3–10
fold higher in the ventral hippocampus than in the eastern
hippocampus. This means that in brain development, it has the
potential to modulate behavior by regulating cytokine and
hippocampal glutamate signaling and protein translation. Therefore,
it is speculated that the expression level of PDE11A4 in the brain
may affect schizophrenia and neurodevelopment (23,104). In reality, PDE11A knockout can
impair protein translation required in abdominal hippocampus
formation in the brian hippocampus, inhibiting memory integration,
and demonstrating reduced expression of RSK2 and lower
phosphorylation of S6 compared to WT mice. Based on these results,
it is suggested that PDE11A can affect perception and association
(105). Besides, PDE11A is also
implicated in sperm physiology and is primarily described in the
prostate. More specifically, PDE11A3 localizes to the testis
(106), and PDE11A4 is highly
expressed in the prostate and developing sperm. In addition,
fertilization is also related to sperm concentration and motility
and the percentage of live sperm (107). It is regulated in part by cAMP
and cGMP (7). Ejaculated sperm
from PDE11A knockout mice that could regulate both cAMP and cGMP
showed reduced sperm concentration and rate of progression. This
suggests that the expression of PDE11A may have physiological
effects on tissues (107).
The authors would like to thank Prof. Guang-Ho Cha
(Chungnam National University, South Korea) and Dr Sung-Jin Park
(National Institute of Health, USA) for fruitful discussions.
This work was financially supported by a research fund of
Chungnam National University (2020) (grant no. CNU-2020SHK) and by
the National Research Foundation of Korea (NRF) grant funded by the
Korea Government (MEST) (grant nos. NRF-2021R1A2C1008492,
NRF-2020R1F1A1049801 and NRF-2021R1C1C2008456).
Not applicable.
GK and JoP conceived the present study. GK and HL
analyzed the PDE11A literature and made substantial contributions
to finalization of this manuscript. TTV, UJ, JiP and SHK assessed
and analyzed the oncogene database (https://www.cbioportal.org/). SHK, JiP, JoP and SK
commented on previous versions of the manuscript. JoP and SK were
involved in data interpretation and writing the discussion. Data
authentication is not applicable. All authors read and approved the
final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Hannah-Shmouni F, Faucz FR and Stratakis
CA: Alterations of phosphodiesterases in adrenocortical tumors.
Front Endocrinol (Lausanne). 7:1112016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rall TW and Sutherland EW: Formation of a
cyclic adenine ribonucleotide by tissue particles. J Biol Chem.
232:1065–1076. 1958. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Butcher RW and Sutherland EW: Adenosine
3′,5′-phosphate in biological materials. I. purification and
properties of cyclic 3′,5′-Nucleotide phosphodiesterase and use of
this enzyme to characterize adenosine 3′,5′-phosphate in human
urine. J Biol Chem. 237:1244–1250. 1962. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu Y, Chen J, Fontes SK, Bautista EN and
Cheng Z: Physiological and pathological roles of protein kinase a
in the heart. Cardiovasc Res. 118:386–398. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Calamera G, Moltzau LR, Levy FO and
Andressen KW: Phosphodiesterases and compartmentation of camp and
cgmp signaling in regulation of cardiac contractility in normal and
failing hearts. Int J Mol Sci. 23:21452022. View Article : Google Scholar
|
|
6
|
Levy I, Horvath A, Azevedo M, de Alexandre
RB and Stratakis CA: Phosphodiesterase function and endocrine
cells: Links to human disease and roles in tumor development and
treatment. Curr Opin Pharmacol. 11:689–697. 2011. View Article : Google Scholar
|
|
7
|
Makhlouf A, Kshirsagar A and Niederberger
C: Phosphodiesterase 11: A brief review of structure, expression
and function. Int J Impot Res. 18:501–519. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang J, Hu B, Xu Z, Ye Y, Wang H, Wang S,
Liu Z and Wang J: Selectivity mechanism of phosphodiesterase
isoform inhibitor through in silico investigations. J Mol Model.
28:92021. View Article : Google Scholar
|
|
9
|
Omori K and Kotera J: Overview of PDEs and
their regulation. Circ Res. 100:309–327. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ke H and Wang H: Crystal structures of
phosphodiesterases and implications on substrate specificity and
inhibitor selectivity. Curr Top Med Chem. 7:391–403. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rotella DP: Phosphodiesterase 5
inhibitors: Current status and potential applications. Nat Rev Drug
Discov. 1:674–682. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Galie N, Ghofrani HA, Torbicki A, Barst
RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A,
et al: Sildenafil citrate therapy for pulmonary arterial
hypertension. N Engl J Med. 353:2148–2157. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kleiman RJ, Chapin DS, Christoffersen C,
Freeman J, Fonseca KR, Geoghegan KF, Grimwood S, Guanowsky V, Hajós
M, Harms JF, et al: Phosphodiesterase 9a regulates central cgmp and
modulates responses to cholinergic and monoaminergic perturbation
in vivo. J Pharmacol Exp Ther. 341:396–409. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schmidt CJ: Phosphodiesterase inhibitors
as potential cognition enhancing agents. Curr Top Med Chem.
10:222–230. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Blokland A, Schreiber R and Prickaerts J:
Improving memory: A role for phosphodiesterases. Curr Pharm Des.
12:2511–2523. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Menniti FS, Faraci WS and Schmidt CJ:
Phosphodiesterases in the Cns: Targets for drug development. Nat
Rev Drug Discov. 5:660–670. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hetman JM, Robas N, Baxendale R, Fidock M,
Phillips SC, Soderling SH and Beavo JA: Cloning and
characterization of two splice variants of human phosphodiesterase
11A. Proc Natl Acad Sci USA. 97:12891–12895. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fawcett L, Baxendale R, Stacey P,
McGrouther C, Harrow I, Soderling S, Hetman J, Beavo JA and
Phillips SC: Molecular Cloning and characterization of a distinct
human phosphodiesterase gene family: PDE11A. Proc Natl Acad Sci
USA. 97:3702–3707. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yuasa K, Kotera J, Fujishige K, Michibata
H, Sasaki T and Omori K: Isolation and characterization of two
novel phosphodiesterase PDE11A variants showing unique structure
and tissue-specific expression. J Biol Chem. 275:31469–31479. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yuasa K, Ohgaru T, Asahina M and Omori K:
Identification of rat cyclic nucleotide phosphodiesterase 11A
(PDE11A): Comparison of rat and human PDE11A Splicing variants. Eur
J Biochem. 268:4440–4448. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Weeks JL II, Zoraghi R, Francis SH and
Corbin JD: N-Terminal domain of phosphodiesterase-11A4 (PDE11A4)
decreases affinity of the catalytic site for substrates and
tadalafil, and is involved in oligomerization. Biochemistry.
46:10353–10364. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
D'Andrea MR, Qiu Y, Haynes-Johnson D,
Bhattacharjee S, Kraft P and Lundeen S: Expression of PDE11A in
normal and malignant human tissues. J Histochem Cytochem.
53:895–903. 2005. View Article : Google Scholar
|
|
23
|
Kelly MP: A role for phosphodiesterase 11A
(PDE11A) in the formation of social memories and the stabilization
of mood. Adv Neurobiol. 17:201–230. 2017. View Article : Google Scholar
|
|
24
|
Kelly MP: Does phosphodiesterase 11A
(PDE11A) hold promise as a future therapeutic target? Curr Pharm
Des. 21:389–416. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pilarzyk K, Farmer R, Porcher L and Kelly
MP: The role of PDE11A4 in social isolation-induced changes in
intracellular signaling and neuroinflammation. Front Pharmacol.
12:7496282021. View Article : Google Scholar
|
|
26
|
Wettschureck N and Offermanns S: Mammalian
G proteins and their cell type specific functions. Physiol Rev.
85:1159–1204. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stratakis CA: Mutations of the gene
encoding the protein kinase a type I-Alpha regulatory subunit
(PRKAR1A) in patients with the ‘complex of spotty skin
pigmentation, myxomas, endocrine overactivity, and schwannomas’
(Carney Complex). Ann N Y Acad Sci. 968:3–21. 2002. View Article : Google Scholar
|
|
28
|
Bertherat J, Horvath A, Groussin L, Grabar
S, Boikos S, Cazabat L, Libe R, René-Corail F, Stergiopoulos S,
Bourdeau I, et al: Mutations in regulatory subunit type 1A of
cyclic adenosine 5′-Monophosphate-dependent protein kinase
(PRKAR1A): Phenotype analysis in 353 patients and 80 different
genotypes. J Clin Endocrinol Metab. 94:2085–2091. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Greene EL, Horvath AD, Nesterova M,
Giatzakis C, Bossis I and Stratakis CA: In vitro functional studies
of naturally occurring pathogenic PRKAR1A mutations that are not
subject to nonsense mRNA decay. Hum Mutat. 29:633–639. 2008.
View Article : Google Scholar
|
|
30
|
Groussin L, Kirschner LS, Vincent-Dejean
C, Perlemoine K, Jullian E, Delemer B, Zacharieva S, Pignatelli D,
Carney JA, Luton JP, et al: Molecular analysis of the cyclic
AMP-Dependent Protein Kinase A (PKA) regulatory subunit 1A
(PRKAR1A) gene in patients with carney complex and primary
pigmented nodular adrenocortical disease (PPNAD) reveals novel
mutations and clues for pathophysiology: Augmented PKA signaling is
associated with adrenal tumorigenesis in PPNAD. Am J Hum Genet.
71:1433–1442. 2002. View
Article : Google Scholar
|
|
31
|
Horvath A, Bertherat J, Groussin L,
Guillaud-Bataille M, Tsang K, Cazabat L, Libé R, Remmers E,
René-Corail F, Faucz FR, et al: Mutations and polymorphisms in the
gene encoding regulatory subunit type 1-alpha of protein kinase A
(PRKAR1A): An update. Hum Mutat. 31:369–379. 2010. View Article : Google Scholar
|
|
32
|
Sandrini F and Stratakis C: Clinical and
molecular genetics of carney complex. Mol Genet Metab. 78:83–92.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kirschner LS, Carney JA, Pack SD, Taymans
SE, Giatzakis C, Cho YS, Cho-Chung YS and Stratakis CA: Mutations
of the gene encoding the protein kinase a type I-alpha regulatory
subunit in patients with the carney complex. Nat Genet. 26:89–92.
2000. View Article : Google Scholar
|
|
34
|
Weinstein LS, Shenker A, Gejman PV, Merino
MJ, Friedman E and Spiegel AM: Activating mutations of the
stimulatory G protein in the McCune-albright syndrome. N Engl J
Med. 325:1688–1695. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stewart V and Yanofsky C: Role of leader
peptide synthesis in tryptophanase operon expression in Escherichia
Coli K-12. J Bacteriol. 167:383–386. 1986. View Article : Google Scholar
|
|
36
|
Velterop JS, Sellink E, Meulenberg JJ,
David S, Bulder I and Postma PW: Synthesis of pyrroloquinoline
quinone in vivo and in vitro and detection of an intermediate in
the biosynthetic pathway. J Bacteriol. 177:5088–5098. 1995.
View Article : Google Scholar
|
|
37
|
Meulenberg JJ, Sellink E, Riegman NH and
Postma PW: Nucleotide sequence and structure of the klebsiella
pneumoniae Pqq operon. Mol Gen Genet. 232:284–294. 1992. View Article : Google Scholar
|
|
38
|
Roelofs J, Smith JL and Van Haastert PJ:
Cgmp signalling: Different ways to create a pathway. Trends Genet.
19:132–134. 2003. View Article : Google Scholar
|
|
39
|
Ochman H: Distinguishing the ORFs from the
ELFs: Short bacterial genes and the annotation of genomes. Trends
Genet. 18:335–337. 2002. View Article : Google Scholar
|
|
40
|
Yanofsky C: Transcription Attenuation. J
Biol Chem. 263:609–612. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
You JY, Liu XW, Bao YX, Shen ZN, Wang Q,
He GY, Lu J, Zhang JG, Chen JW and Liu PQ: A novel
phosphodiesterase 9A inhibitor LW33 protects against ischemic
stroke through the cGMP/PKG/CREB Pathway. Eur J Pharmacol.
925:1749872022. View Article : Google Scholar
|
|
42
|
Libe R, Fratticci A, Coste J, Tissier F,
Horvath A, Ragazzon B, Rene-Corail F, Groussin L, Bertagna X,
Raffin-Sanson ML, et al: Phosphodiesterase 11A (PDE11A) and genetic
predisposition to adrenocortical tumors. Clin Cancer Res.
14:4016–4024. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Horvath A, Boikos S, Giatzakis C,
Robinson-White A, Groussin L, Griffin KJ, Stein E, Levine E,
Delimpasi G, Hsiao HP, et al: A genome-wide scan identifies
mutations in the gene encoding phosphodiesterase 11A4 (PDE11A) in
individuals with adrenocortical hyperplasia. Nat Genet. 38:794–800.
2006. View
Article : Google Scholar
|
|
44
|
Horvath A, Korde L, Greene MH, Libe R,
Osorio P, Faucz FR, Raffin-Sanson ML, Tsang KM, Drori-Herishanu L,
Patronas Y, et al: Functional phosphodiesterase 11A mutations may
modify the risk of familial and bilateral testicular germ cell
tumors. Cancer Res. 69:5301–5306. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
de Alexandre RB, Horvath AD, Szarek E,
Manning AD, Leal LF, Kardauke F, Epstein JA, Carraro DM, Soares FA,
Apanasovich TV, et al: Phosphodiesterase Sequence variants may
predispose to prostate cancer. Endocr Relat Cancer. 22:519–530.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lefievre L, de Lamirande E and Gagnon C:
Presence of Cyclic nucleotide phosphodiesterases PDE1A, existing as
a stable complex with calmodulin, and PDE3A in human spermatozoa.
Biol Reprod. 67:423–430. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fidock M, Miller M and Lanfear J:
Isolation and differential tissue distribution of two human cDNAs
encoding PDE1 splice variants. Cell Signal. 14:53–60. 2002.
View Article : Google Scholar
|
|
48
|
Michibata H, Yanaka N, Kanoh Y, Okumura K
and Omori K: Human Ca2+/Calmodulin-dependent phosphodiesterase
PDE1A: Novel splice variants, their specific expression, genomic
organization, and chromosomal localization. Biochim Biophys Acta.
1517:278–287. 2001. View Article : Google Scholar
|
|
49
|
Loughney K, Martins TJ, Harris EA, Sadhu
K, Hicks JB, Sonnenburg WK, Beavo JA and Ferguson K: Isolation and
Characterization of CDNAs corresponding to two human calcium,
calmodulin-regulated, 3′,5′-cyclic nucleotide phosphodiesterases. J
Biol Chem. 271:796–806. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kanda N and Watanabe S: Regulatory roles
of adenylate cyclase and cyclic nucleotide phosphodiesterases 1 and
4 in interleukin-13 production by activated human T cells. Biochem
Pharmacol. 62:495–507. 2001. View Article : Google Scholar
|
|
51
|
Yan C, Zhao AZ, Bentley JK, Loughney K,
Ferguson K and Beavo JA: Molecular cloning and characterization of
a calmodulin-dependent phosphodiesterase enriched in olfactory
sensory neurons. Proc Natl Acad Sci USA. 92:9677–9681. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Nikolaev VO, Gambaryan S, Engelhardt S,
Walter U and Lohse MJ: Real-Time Monitoring of the PDE2 activity of
live cells: Hormone-stimulated camp hydrolysis is faster than
hormone-stimulated camp synthesis. J Biol Chem. 280:1716–1719.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Maurice DH, Palmer D, Tilley DG, Dunkerley
HA, Netherton SJ, Raymond DR, Elbatarny HS and Jimmo SL: Cyclic
nucleotide phosphodiesterase activity, expression, and targeting in
cells of the cardiovascular system. Mol Pharmacol. 64:533–546.
2003. View Article : Google Scholar
|
|
54
|
Degerman E, Belfrage P and Manganiello VC:
Structure, localization, and regulation of cgmp-inhibited
phosphodiesterase (PDE3). J Biol Chem. 272:6823–6826. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mongillo M, Tocchetti CG, Terrin A,
Lissandron V, Cheung YF, Dostmann WR, Pozzan T, Kass DA, Paolocci
N, Houslay MD and Zaccolo M: Compartmentalized phosphodiesterase-2
activity blunts beta-adrenergic cardiac inotropy via an
No/cGMP-dependent pathway. Circ Res. 98:226–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bender AT, Ostenson CL, Giordano D and
Beavo JA: Differentiation of human monocytes in vitro with
granulocyte-macrophage colony-stimulating factor and macrophage
colony-stimulating factor produces distinct changes in cGMP
phosphodiesterase expression. Cell Signal. 16:365–374. 2004.
View Article : Google Scholar
|
|
57
|
Seybold J, Thomas D, Witzenrath M, Boral
S, Hocke AC, Burger A, Hatzelmann A, Tenor H, Schudt C, Krüll M, et
al: Tumor necrosis factor-alpha-dependent expression of
phosphodiesterase 2: Role in endothelial hyperpermeability. Blood.
105:3569–3576. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Domek-Lopacinska K and Strosznajder JB:
The effect of selective inhibition of cyclic GMP hydrolyzing
phosphodiesterases 2 and 5 on learning and memory processes and
nitric oxide synthase activity in brain during aging. Brain Res.
1216:68–77. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
de Oliveira SK and Smolenski A:
Phosphodiesterases link the aryl hydrocarbon receptor complex to
cyclic nucleotide signaling. Biochem Pharmacol. 77:723–733. 2009.
View Article : Google Scholar
|
|
60
|
Rena G, Begg F, Ross A, MacKenzie C,
McPhee I, Campbell L, Huston E, Sullivan M and Houslay MD:
Molecular cloning, genomic positioning, promoter identification,
and characterization of the novel cyclic amp-specific
phosphodiesterase PDE4A10. Mol Pharmacol. 59:996–1011. 2001.
View Article : Google Scholar
|
|
61
|
Wang P, Wu P, Ohleth KM, Egan RW and
Billah MM: Phosphodiesterase 4B2 is the predominant
phosphodiesterase species and undergoes differential regulation of
gene expression in human monocytes and neutrophils. Mol Pharmacol.
56:170–174. 1999. View Article : Google Scholar
|
|
62
|
Bolger G, Michaeli T, Martins T, St John
T, Steiner B, Rodgers L, Riggs M, Wigler M and Ferguson K: A family
of human phosphodiesterases homologous to the dunce learning and
memory gene product of drosophila melanogaster are potential
targets for antidepressant drugs. Mol Cell Biol. 13:6558–6571.
1993. View Article : Google Scholar
|
|
63
|
Dunkern TR and Hatzelmann A: The effect of
sildenafil on human platelet secretory function is controlled by a
complex interplay between phosphodiesterases 2, 3 and 5. Cell
Signal. 17:331–339. 2005. View Article : Google Scholar
|
|
64
|
Prickaerts J, Sik A, van Staveren WC,
Koopmans G, Steinbusch HW, van der Staay FJ, de Vente J and
Blokland A: Phosphodiesterase type 5 inhibition improves early
memory consolidation of object information. Neurochem Int.
45:915–928. 2004. View Article : Google Scholar
|
|
65
|
Miller CL and Yan C: Targeting cyclic
nucleotide phosphodiesterase in the heart: Therapeutic
implications. J Cardiovasc Transl Res. 3:507–515. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ridge KD, Abdulaev NG, Sousa M and
Palczewski K: Phototransduction: Crystal clear. Trends Biochem Sci.
28:479–487. 2003. View Article : Google Scholar
|
|
67
|
Morin F, Lugnier C, Kameni J and Voisin P:
Expression and role of phosphodiesterase 6 in the chicken pineal
gland. J Neurochem. 78:88–99. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Bloom TJ and Beavo JA: Identification and
tissue-specific expression of PDE7 phosphodiesterase splice
variants. Proc Natl Acad Sci USA. 93:14188–14192. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Han P, Zhu X and Michaeli T: Alternative
splicing of the high affinity cAMP-specific phosphodiesterase
(PDE7A) mRNA in human skeletal muscle and heart. J Biol Chem.
272:16152–16157. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Sasaki T, Kotera J and Omori K:
Transcriptional activation of phosphodiesterase 7B1 by dopamine d1
receptor stimulation through the cyclic AMP/Cyclic AMP-dependent
protein kinase/cyclic AMP-response element binding protein pathway
in primary striatal neurons. J Neurochem. 89:474–483. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Glavas NA, Ostenson C, Schaefer JB, Vasta
V and Beavo JA: T cell activation up-regulates cyclic nucleotide
phosphodiesterases 8A1 and 7A3. Proc Natl Acad Sci USA.
98:6319–6324. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Patrucco E, Albergine MS, Santana LF and
Beavo JA: Phosphodiesterase 8A (PDE8A) regulates
excitation-contraction coupling in ventricular myocytes. J Mol Cell
Cardiol. 49:330–333. 2010. View Article : Google Scholar
|
|
73
|
Mehats C, Andersen CB, Filopanti M, Jin SL
and Conti M: Cyclic nucleotide phosphodiesterases and their role in
endocrine cell signaling. Trends Endocrinol Metab. 13:29–35. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hayashi M, Matsushima K, Ohashi H, Tsunoda
H, Murase S, Kawarada Y and Tanaka T: Molecular cloning and
characterization of human PDE8B, a novel thyroid-specific isozyme
of 3′,5′-cyclic nucleotide phosphodiesterase. Biochem Biophys Res
Commun. 250:751–756. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Hayashi M, Shimada Y, Nishimura Y, Hama T
and Tanaka T: Genomic organization, chromosomal localization, and
alternative splicing of the human phosphodiesterase 8B gene.
Biochem Biophys Res Commun. 297:1253–1258. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Horvath A, Giatzakis C, Tsang K, Greene E,
Osorio P, Boikos S, Libè R, Patronas Y, Robinson-White A, Remmers
E, et al: A cAMP-specific phosphodiesterase (PDE8B) that is mutated
in adrenal hyperplasia is expressed widely in human and mouse
tissues: A novel PDE8B isoform in human adrenal cortex. Eur J Hum
Genet. 16:1245–1253. 2008. View Article : Google Scholar
|
|
77
|
Rentero C, Monfort A and Puigdomenech P:
Identification and distribution of different mRNA variants produced
by differential splicing in the human phosphodiesterase 9A gene.
Biochem Biophys Res Commun. 301:686–692. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Furukawa T, Youssef EM, Yatsuoka T,
Yokoyama T, Makino N, Inoue H, Fukushige S, Hoshi M, Hayashi Y,
Sunamura M and Horii A: Cloning and characterization of the human
Udp-N-Acetylglucosamine: Alpha-1,3-D-mannoside
beta-1,4-N-acetylglucosaminyltransferase IV-Homologue (hGnT-IV-H)
gene. J Hum Genet. 44:397–401. 1999. View Article : Google Scholar
|
|
79
|
Kelly MP, Logue SF, Brennan J, Day JP,
Lakkaraju S, Jiang L, Zhong X, Tam M, Sukoff Rizzo SJ, Platt BJ, et
al: Phosphodiesterase 11A in brain is enriched in ventral
hippocampus and deletion causes psychiatric disease-related
phenotypes. Proc Natl Acad Sci USA. 107:8457–8462. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Kleppisch T: Phosphodiesterases in the
central nervous system. Handb Exp Pharmacol. 71–92. 2009.
View Article : Google Scholar
|
|
81
|
Knott EP, Assi M, Rao SN, Ghosh M and
Pearse DD: Phosphodiesterase inhibitors as a therapeutic approach
to neuroprotection and repair. Int J Mol Sci. 18:6962017.
View Article : Google Scholar
|
|
82
|
Libe R, Horvath A, Vezzosi D, Fratticci A,
Coste J, Perlemoine K, Ragazzon B, Guillaud-Bataille M, Groussin L,
Clauser E, et al: Frequent phosphodiesterase 11A gene (PDE11A)
defects in patients with carney complex (CNC) Caused by PRKAR1A
Mutations: PDE11A may contribute to adrenal and testicular tumors
in CNC as a modifier of the phenotype. J Clin Endocrinol Metab.
96:E208–E214. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Jager R, Russwurm C, Schwede F, Genieser
HG, Koesling D and Russwurm M: Activation of PDE10 and PDE11
phosphodiesterases. J Biol Chem. 287:1210–1219. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Pitsava G and Stratakis CA: Genetic
alterations in benign adrenal tumors. Biomedicines. 10:10412022.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Hsiao HP, Kirschner LS, Bourdeau I, Keil
MF, Boikos SA, Verma S, Robinson-White AJ, Nesterova M, Lacroix A
and Stratakis CA: Clinical and genetic heterogeneity, overlap with
other tumor syndromes, and atypical glucocorticoid hormone
secretion in adrenocorticotropin-independent macronodular adrenal
hyperplasia compared with other adrenocortical tumors. J Clin
Endocrinol Metab. 94:2930–2937. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Horvath A, Giatzakis C, Robinson-White A,
Boikos S, Levine E, Griffin K, Stein E, Kamvissi V, Soni P, Bossis
I, et al: Adrenal hyperplasia and adenomas are associated with
inhibition of phosphodiesterase 11A in carriers of PDE11A sequence
variants that are frequent in the population. Cancer Res.
66:11571–11575. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Pathak A, Stewart DR, Faucz FR, Xekouki P,
Bass S, Vogt A, Zhang X, Boland J, Yeager M, Loud JT, et al: Rare
inactivating PDE11A variants associated with testicular germ cell
tumors. Endocr Relat Cancer. 22:909–917. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Dal J, Nielsen EH, Klose M,
Feldt-Rasmussen U, Andersen M, Vang S, Korbonits M and Jørgensen
JOL: Phenotypic and genotypic features of a large kindred with a
germline AIP variant. Clin Endocrinol (Oxf). 93:146–153. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Pinto EM, Faucz FR, Paza LZ, Wu G,
Fernandes ES, Bertherat J, Stratakis CA, Lalli E, Ribeiro RC,
Rodriguez-Galindo C, et al: Germline variants in phosphodiesterase
genes and genetic predisposition to pediatric adrenocortical
tumors. Cancers (Basel). 12:5062020. View Article : Google Scholar
|
|
90
|
Faucz FR, Horvath A, Rothenbuhler A,
Almeida MQ, Libe R, Raffin-Sanson ML, Bertherat J, Carraro DM,
Soares FA, Molina Gde C, et al: Phosphodiesterase 11A (PDE11A)
genetic variants may increase susceptibility to prostatic cancer. J
Clin Endocrinol Metab. 96:E135–E140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Dono A, Nickles J, Rodriguez-Armendariz
AG, McFarland BC, Ajami NJ, Ballester LY, Wargo JA and Esquenazi Y:
Glioma and the gut-brain axis: Opportunities and future
perspectives. Neurooncol Adv. 4:vdac0542022.PubMed/NCBI
|
|
92
|
Schwartz KA, Noel M, Nikolai M, Olson LK,
Hord NG, Zakem M, Clark J, Elnabtity M, Figueroa B and Chang HT:
Long term survivals in aggressive primary brain malignancies
treated with an adjuvant ketogenic diet. Front Nutr. 9:7707962022.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Burns TC, Awad AJ, Li MD and Grant GA:
Radiation-induced brain injury: Low-hanging fruit for
neuroregeneration. Neurosurg Focus. 40:E32016. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Lee H, Park S, Kong G, Kwon SH, Park J,
Park J and Kim SH: Phosphodiesterase 11A (PDE11A), a potential
biomarker for glioblastoma. Toxicol Res. 2022. View Article : Google Scholar
|
|
96
|
Rothenbuhler A, Horvath A, Libe R, Faucz
FR, Fratticci A, Raffin Sanson ML, Vezzosi D, Azevedo M, Levy I,
Almeida MQ, et al: Identification of novel genetic variants in
phosphodiesterase 8B (PDE8B), a cAMP-specific phosphodiesterase
highly expressed in the adrenal cortex, in a cohort of patients
with adrenal tumours. Clin Endocrinol (Oxf). 77:195–199. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Hou Y, Wren A, Mylarapu N, Browning K,
Islam BN, Wang R, Vega KJ and Browning DD: Inhibition of colon
cancer cell growth by phosphodiesterase inhibitors is independent
of cGMP Signaling. J Pharmacol Exp Ther. 381:42–53. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Di Iorio P, Ronci M, Giuliani P, Caciagli
F, Ciccarelli R, Caruso V, Beggiato S and Zuccarini M: Pros and
cons of pharmacological manipulation of cGMP-PDEs in the prevention
and treatment of breast cancer. Int J Mol Sci. 23:2622021.
View Article : Google Scholar
|
|
99
|
Vezzosi D, Cartier D, Regnier C, Otal P,
Bennet A, Parmentier F, Plantavid M, Lacroix A, Lefebvre H and
Caron P: Familial adrenocorticotropin-independent macronodular
adrenal hyperplasia with aberrant serotonin and vasopressin adrenal
receptors. Eur J Endocrinol. 156:21–31. 2007. View Article : Google Scholar
|
|
100
|
Vezzosi D, Libe R, Baudry C, Rizk-Rabin M,
Horvath A, Levy I, René-Corail F, Ragazzon B, Stratakis CA,
Vandecasteele G and Bertherat J: Phosphodiesterase 11A (PDE11A)
gene defects in patients with acth-independent macronodular adrenal
hyperplasia (AIMAH): Functional variants may contribute to genetic
susceptibility of bilateral adrenal tumors. J Clin Endocrinol
Metab. 97:E2063–E2069. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Peverelli E, Ermetici F, Filopanti M, Elli
FM, Ronchi CL, Mantovani G, Ferrero S, Bosari S, Beck-Peccoz P,
Lania A and Spada A: Analysis of genetic variants of
phosphodiesterase 11A in acromegalic patients. Eur J Endocrinol.
161:687–694. 2009. View Article : Google Scholar
|
|
102
|
Pathak G, Agostino MJ, Bishara K, Capell
WR, Fisher JL, Hegde S, Ibrahim BA, Pilarzyk K, Sabin C, Tuczkewycz
T, et al: PDE11A negatively regulates lithium responsivity. Mol
Psychiatry. 22:1714–1724. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Qin W, Zhou A, Zuo X, Jia L, Li F, Wang Q,
Li Y, Wei Y, Jin H, Cruchaga C, et al: Exome Sequencing Revealed
PDE11A as a novel candidate gene for early-onset Alzheimer's
disease. Hum Mol Genet. 30:811–822. 2021. View Article : Google Scholar
|
|
104
|
Pilarzyk K, Klett J, Pena EA, Porcher L,
Smith AJ and Kelly MP: Loss of function of phosphodiesterase 11A4
shows that recent and remote long-term memories can be uncoupled.
Curr Biol. 29:2307–2321. e52019. View Article : Google Scholar
|
|
105
|
Hegde S, Capell WR, Ibrahim BA, Klett J,
Patel NS, Sougiannis AT and Kelly MP: Phosphodiesterase 11A
(PDE11A), enriched in ventral hippocampus neurons, is required for
consolidation of social but not nonsocial memories in mice.
Neuropsychopharmacology. 41:2920–2931. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Loughney K, Taylor J and Florio VA:
3′,5′-cyclic nucleotide phosphodiesterase 11A: Localization in
human tissues. Int J Impot Res. 17:320–325. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Wayman C, Phillips S, Lunny C, Webb T,
Fawcett L, Baxendale R and Burgess G: Phosphodiesterase 11 (PDE11)
regulation of spermatozoa physiology. Int J Impot Res. 17:216–223.
2005. View Article : Google Scholar : PubMed/NCBI
|