Introduction
Reactive oxygen species (ROS) mainly refer to
superoxide anion (•O2-), hydroxyl radical (•OH),
hydrogen peroxide (H2O2) and singlet oxygen,
which are derived from the metabolism of O2. These
chemically reactive molecules are generated as metabolites of
oxidative reactions in mitochondria, the nicotinamide adenine
dinucleotide phosphate (NADPH) oxidase system and endoplasmic
reticulum (ER) (1). In order to
counteract ROS overproduction, multiple antioxidant enzymes exist
to maintain redox homeostasis, including superoxide dismutase,
catalase, and glutathione peroxidase (2). ROS are capable of reacting with
proteins, lipids and nucleic acids, as well as regulate signaling
pathways that are involved in various cellular processes (3). Thus, a state of equilibrium between
oxidants and reductants is required for physiological processes,
such as cell proliferation, differentiation and survival. However,
the imbalance in ROS generation and removal causes excessive
accumulation of ROS and thus oxidative stress, resulting in
detrimental oxidative DNA damage, which is regarded as a major
factor for carcinogenesis (4).
Controlling ROS metabolism is beneficial for the prevention and
treatment of cancer.
Autophagy is an evolutionarily conserved and
lysosome-dependent catabolic process whereby cytoplasmic
components, such as damaged organelles, protein aggregates and
lipid droplets, are degraded and further recycled in autophagosomes
for the maintenance of cellular homeostasis (5). In response to ROS-mediated oxidative
damage, autophagy can be induced as an adaptive reaction to remove
ROS and oxidative biomolecules and organelles, thus alleviating
oxidative stress (6). The
interaction between ROS and autophagy has been widely investigated
in various cancer types, as it participates in tumorigenesis,
metastasis and chemoresistance (7). As one of the most aggressive forms
of cancer, melanoma is a type of skin cancer with a high metastasis
potential and poor survival rate. In comparison with other solid
tumors, ROS levels are particularly abundant in melanoma, which is
considered a ROS-driven tumor (8). However, the crosstalk between ROS
and autophagy in the development of melanoma remains elusive.
During tumor progression or chemotherapy-induced stress, obsolete
organelles and useless proteins are recycled by autophagy to foster
cancer cell growth and chemoresistance. Understanding the role of
autophagy in the development of melanoma is of great significance,
and autophagy regulation represents a new potential therapeutic
target in this disease. Chemotherapeutic agents both induce
oxidative damage and autophagy via generation of amounts of ROS.
However, cytoprotective autophagy limits the chemotherapy efficacy.
Moreover, ROS-mediated autophagy is induced in diverse melanoma
chemotherapies where it exerts double functions: i) Promoting cell
survival, which is a mechanism of chemoresistance (9); and ii) triggering autophagic cell
death, which improves antitumor efficacy (10). The aim of the present review was
to emphasize the role of ROS-mediated autophagy in melanomagenesis
and melanoma treatments, as well as discuss the therapeutic
potential of combination chemotherapy with autophagy-regulating
agents according to the functional status of ROS-induced autophagy
in patients with melanoma.
In order to summarize the role of ROS-mediated
autophagy in melanoma, a PubMed search was performed in April 2022.
Articles containing the following key words were considered for
inclusion: ‘reactive oxygen species’ (or ‘ROS’ or ‘oxidative
stress’) AND ‘autophagy’ AND ‘melanoma’ (or ‘melanomagenesis’).
Relevant articles were also identified from a manual search of
reference lists within those included. The abstracts of identified
articles were screened and classified for inclusion in the review.
A total of 60 original articles that have been published in a
peer-reviewed journal and written in English were included.
ROS generation in melanoma
Ultraviolet (UV) radiation is a major risk factor
for melanoma development. UV can transduce its electromagnetic
energy into chemical, hormonal, and neural signals upon absorption,
thus regulating homeostatic activity, including activation of the
central nervous system and endocrine glands through neural
transmission or chemical messengers, which exerts systemic effects
on patients with melanoma (11).
The oncogenic effect of UV on skin is induced by ROS-mediated DNA
damage (12). UV penetrates the
epidermis and dermis of the skin and is absorbed by various
biomolecules, which generates high levels of ROS in skin cells
(13). By tracing the
luminescence of singlet oxygen, high ROS levels can be detected
during UV exposure both in vitro and in vivo
(14). Photosensitizer molecules
facilitate ROS production. Vitamins, well-known endogenous
photosensitizers, whose chemical structure is altered upon
absorption of UV radiation, are susceptible to producing ROS via
photosensitized reactions (15).
ROS oxidizes fatty acids in cell membranes, lipoproteins, and other
lipid-containing molecules, and ultimately leads to impairment of
cellular structures and functions. Oxidized fatty acids also
produce ROS under UV irradiation and become strong photosensitizers
under continuous UV irradiation (16). Photosensitizers such as flavins,
urocanic acids, and cholesterols have been shown to produce ROS by
absorbing the energy of UV radiation (17). ROS accumulation in the skin
changes the absorption of those molecules, which in turn increase
ROS production (18). Thus, an
increase of ROS initiates a vicious cycle that amplifies the
UV-mediated damaging effects on skin cells. Furthermore, UV
radiation induces the activation of ROS-producing enzymes. Valencia
and Kochevar revealed that UV activated NADPH oxidase (NOX)1 to
generate ROS in human keratinocytes, which stimulates prostaglandin
E2 synthesis and contributes to skin injury (19). Notably, UV radiation has been
revealed to improve the expression of sestrin2 in melanocytes and
melanoma cells, which inhibits the antioxidant response factor
nuclear factor erythroid 2-related factor 2 (Nrf2) and further
aggravates ROS production (20).
Excessive ROS produced by UV radiation is considered to cause skin
damage and ultimately tumorigenesis, mainly via oxidative
stress-induced DNA damage (21).
Compared with those of keratinocytes and
fibroblasts, both melanocytes and melanoma cells exhibit higher
basal levels of ROS (22).
Suppressing melanin synthesis in melanocytes by N phenylthiourea
alleviates intracellular ROS (23). These findings indicate that the
process of synthesis of melanin is an essential source of ROS.
Further mechanistic investigations revealed that the melanosome and
its melanin contents were associated with oxidative reactions,
where superoxide anion and H2O2 were produced
(24). Melanin has both
photoprotective and phototoxic properties based on diverse
conditions. The biosynthesis of melanin requires a series of
oxidoreduction reactions and consumes oxygen, which generates
cytotoxic intermediates, such as free radicals. Under physiological
conditions, the process of melanin synthesis is limited within the
boundaries of melanosomes, and plays a protective role against
UV-induced carcinogenesis; however, this process can be
dysregulated with cytotoxic intermediates leaking outside
melanosomes under pathological conditions, thus contributing to the
malignant transformation of melanocytes (25). Melanin exerts a double role in
determining ROS levels: It absorbs UV radiation and thus mitigates
UV-induced ROS production in melanocytes and keratinocytes;
however, melanocytes are in a state of pro-oxidation during melanin
synthesis, and become more predisposed to intracellular ROS
accumulation and carcinogenesis (23). One explanation could be that the
pro-oxidant activity is different between the two forms of melanin
in the skin, namely the reddish-yellow pheomelanin and the
brown-black eumelanin. L-tyrosine and L-dihydroxyphenylalanine
serve as substrates for melanin pigmentation, which is regulated by
transcriptional factors including tyrosinase and tyrosinase-related
proteins, and signaling pathways involving cAMP and protein kinase
C (26). In addition, metal
cations such as Mn+2 and Cu+2 stimulate
L-dihydroxyphenylalanine auto-oxidation to melanin (27). Melanocytes with high pheomelanin
content become pro-oxidant and thus generate ROS upon exposure to
UV radiation and metal ions (28,29), while this effect can be prevented
by eumelanin, if present in sufficient quantity (30). Thus, the enhanced ratio of
pheomelanin to eumelanin in isolated melanosomes leads to
mutagenesis through enhanced ROS production (30). Furthermore, eumelanin expression
is regulated by the melanocortin 1 receptor gene (MC1R) signaling,
which is responsible for ROS scavenging and DNA repair (31). MC1R inactivation results in
elevated ROS production and compromised DNA repair, thereby leading
to increased risk of carcinogenesis (32). However, increased melanin
pigmentation is associated with shorter overall survival and
disease-free survival time in patients with melanoma, and
inhibition of melanin synthesis improves the radiotherapeutic
response (33). The forms of
melanin that are responsible for the negative effect of melanin on
melanoma therapy remain to be investigated.
The cellular ROS pool in melanocytes and melanoma
cells can be also derived from NOX family enzymes. Among them,
NOX1, NOX4, and NOX5 have been demonstrated to be expressed in the
melanocytic lineage (34). ROS
generation induced by these NOX isoforms is required for the
proliferation and malignant transformation of melanoma cells. NOX1
was shown to be upregulated in melanoma cell lines and is activated
to produce ROS by the mutation of N-RAS, an oncogene, which is
involved in melanoma progression (35). NOX4 expression is significantly
higher in melanoma tumors compared with that in primary tumors,
indicating its oncogenic role in melanoma. Govindaraja et al
(36) revealed that
NOX4-generated ROS activated by AKT, a serine-threonine kinase
highly expressed in melanoma, facilitated the transformation of
radial growth to vertical growth that was required for the invasive
and metastatic phenotype. In addition, ROS produced by NOX4 promote
cell survival through activating the focal adhesion kinase pathway,
maintaining cell adhesion and viability (37). Of note, in cultured melanoma cells
under hypoxic conditions, NOX4 is involved in the generation of ROS
and cell apoptosis mediated by α-melanocyte-stimulating hormone
(α-MSH), an inducer of melanin (38). A possible explanation for this
proapoptotic role of NOX4 is that NOX4-dependent ROS production
sensitizes melanoma cells to TNF-related apoptosis-inducing ligand
TRAIL-induced apoptosis via Bax phosphorylation (39). In addition, α-MSH activates its
downstream signal transducer melanocyte-inducing transcription
factor to stimulate NOX4 gene expression and further drive ROS
generation, ultimately suppressing melanin synthesis (40). Silencing of NOX4 expression in
melanoma cells attenuates ROS production and thereby suppresses
cell growth and tumorigenicity in vivo by regulating G2-M
cell cycle progression, suggesting that NOX4-generated ROS elicit a
transformation in the phenotype of melanoma cells (41). Furthermore, NOX5 is overexpressed
in melanoma, and affects cell proliferation through the
ROS-mediated hypoxia-inducible factor (HIF)-1α and p27Kip1
signaling pathways (42).
Accordingly, these results indicate that NOX-induced ROS production
is associated with melanomagenesis and melanoma progression.
Differences in signals and resultant effects involving these
ROS-producing NOX enzymes merit further investigation.
In summary, the main contributors to ROS generation
in melanoma cells include UV radiation, melanin synthesis and NOX
family enzymes (Fig. 1).
Excessive ROS leads to oxidative stress, which enhances the
melanomagenesis and progression of melanoma.
Oxidative stress and melanomagenesis
Excessive ROS causes oxidative stress, which is
recognized as the initiator and promoter of melanoma. The main
mechanism by which ROS overproduction promotes melanomagenesis has
been well established, and involves the induction of oxidative DNA
damage and mutagenesis. The by-products of DNA damage include
8-hydroxydeoxyguanosine (8-OHdG), cyclobutane pyrimidine dimers,
pyrimidone adducts, DNA strand breaks, and DNA crosslinks, which
result in genomic instability and transcriptional silencing
(43). 8-OHdG, a major form of
oxidative DNA damage, is regarded as a premutagenic DNA lesion and
is highly expressed in melanocytes compared with its levels in
keratinocytes (44). Similarly,
the expression level of 8-OHdG is lower in patients with melanoma,
who have a significantly longer survival time (45). These findings indicate that
ROS-mediated DNA oxidation exacerbates a malignant phenotype, and
has been implicated in the poor prognosis of patients with
melanoma.
Oxidative stress also promotes the occurrence and
development of melanoma via genotoxicity. On one hand, it causes
oncogene activation. For example, mutations of the BRAF oncogene,
occurring in ~50% of melanoma cases, are induced by oxidative
stress (46). In addition, ROS
stabilize the expression of HIF-1α, a transcriptional regulator of
the hypoxic response, to activate the Met protooncogene, which
drives the proliferation and metastasis of melanoma cells, and
induces angiogenesis (47).
Increased expression of HIF-1α can also originate from induction of
melanogenesis in melanoma cells, and further regulates cellular
metabolism and the behavior of cancer cells (48). Another oncogene, RAC1, which is
associated with an increased risk of melanoma, can be activated by
high levels of ROS to accelerate the migration and invasion of B16
melanoma cells; however, these effects are weakened by the
suppression of ROS-mediated RAC1 activation (49). Notably, tumor suppressor genes
compromised in melanoma aggravate oxidative stress. Jenkins et
al (50) reported that
depletion of p16 expression contributed to marked increases in ROS
levels in cultured human melanocytes, which triggers oxidative DNA
damage. Additionally, silencing large tumor suppressor kinase 1, a
tumor suppressor in melanoma, was demonstrated to lead to enhanced
oxidative stress, which is highly engaged in melanoma growth
(51). Thus, the loss of tumor
suppressor genes increases the susceptibility of melanocytes to
oxidative stress and expedites carcinogenesis. On the other hand,
oxidative stress mediates epigenetic modifications to induce
melanomagenesis. Molognoni et al (52) revealed that ROS increased DNA
methyltrasferase 1 and DNA hypermethylation, which led to Ras
activation and malignant transformation in melanoma via activation
of the ERK signaling pathway.
Furthermore, oxidative stress favors the development
of melanoma by the activation of specific signaling pathways. For
instance, ROS-mediated oxidation of light chain 8 (LC8), a
multifunctional protein of the dynein motor complex, activates the
NF-κΒ signaling pathway due to the impaired ability of LC8 to bind
to the NF-κB component I-κBα, suppressing its phosphorylation by
IKK and ultimately leading to NF-κB activation (53). This effect facilitates melanoma
progression by its antiapoptotic effects and creates an
inflammatory microenvironment (54). Other signaling pathways,
particularly the PI3K/AKT, MAPK/ERK and Nrf2 signaling pathways,
are implicated in the initiation and progression of melanoma
(35,43).
Melanogenesis is an essential process in melanoma
cells. By interacting with hormones, neuropeptides and vitamin D,
these cells participate in steroidogenesis and sex hormone
conversion, which is required for the production of melanin
(55). Local metabolites, such as
HIF-1α, are upregulated during this process, and their accumulation
can activate HIF-1-related pathways to affect the progression of
melanoma (48). Melanosomes act
as vital boundaries of the process of melanin synthesis, and
restrict cytotoxic intermediates to leak into the surrounding
environment, which influences the behavior of melanoma cells,
triggering tumorigenesis (56).
The induction of melanogenesis is associated with changes in
glucose metabolism in melanoma cells (57). UV irradiation and UV-induced ROS
also cause a persistent increase in glucose consumption, which is
accompanied by increased glycolysis in these cells, and promotes
the invasion of melanoma (58).
In addition, intermediates of melanogenesis including quinones,
semiquinones, quinonimines, and ROS produced during this process
exert immunosuppressive functions in melanoma (59). The activity of T lymphocytes and
cytokines, such as IL-1, IL-6, TNF-α, and IL-10, is suppressed
during this process, and inhibition of melanogenesis increases the
lymphocyte-mediated killing effect on melanoma cells (60). Mitochondrial ROS also promote
inflammatory cytokines, including TGF-β and IL-13, which induce the
activation and M2 polarization of macrophages, thus leading to an
immunosuppressive microenvironment and metastatic behaviors in
melanoma (61). Therefore,
ROS-induced oxidative stress can facilitate melanoma progression
through the promotion of glucose metabolism and
immunosuppression.
ROS-mediated oxidative stress possibly leads to
melanomagenesis through two potentially important effects:
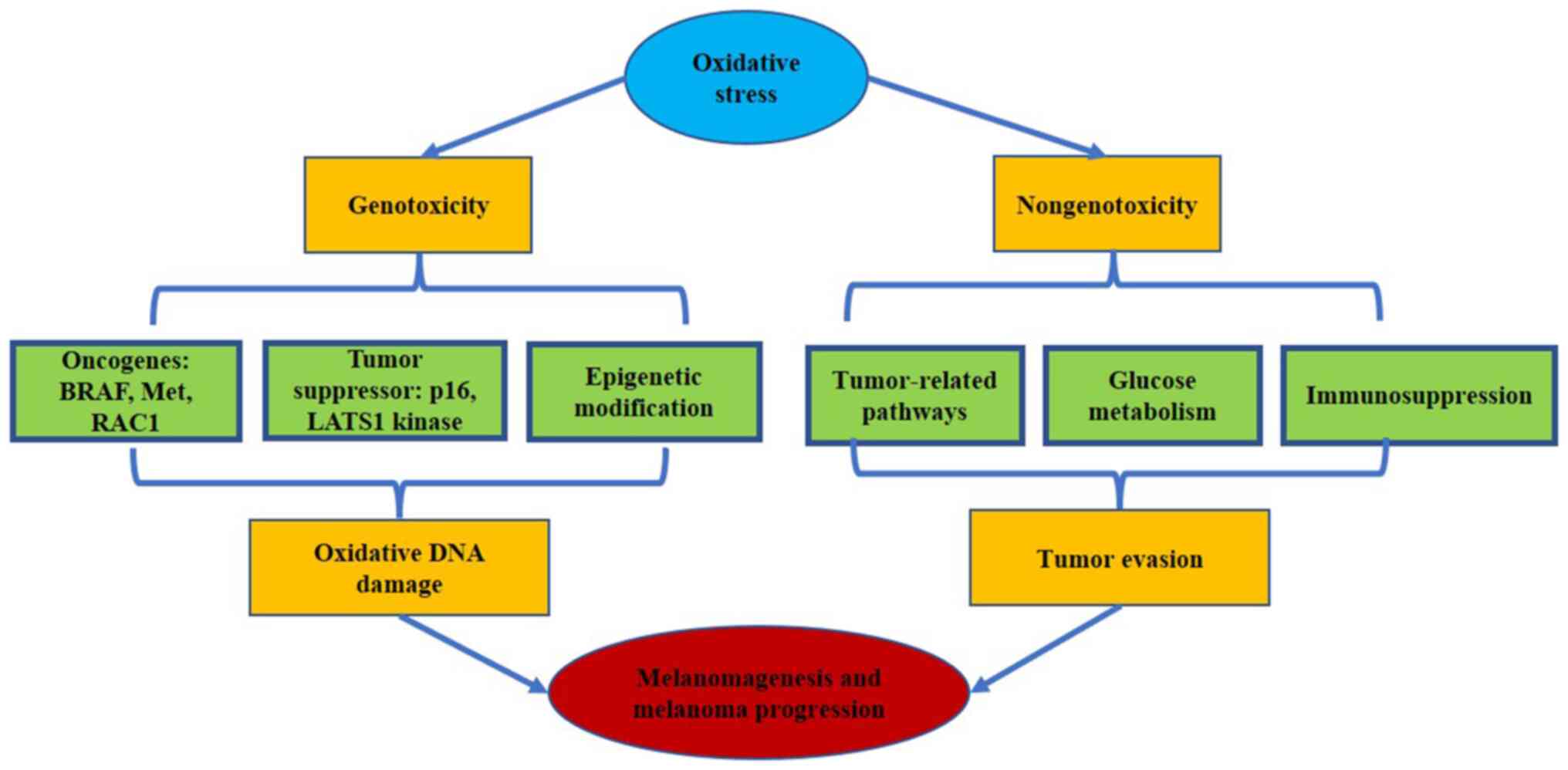
Genotoxicity and nongenotoxicity (Fig. 2). The genotoxic effect is induced
by oxidative stress-mediated damage to DNA, which causes genetic
and epigenetic changes in melanoma-related genes. The nongenotoxic
effect is enhanced by the activation of specific signaling pathways
that influence numerous cellular processes linked to carcinogenesis
and melanoma progression. Moreover, oxidative stress triggers
glucose metabolism and immunosuppression and further accelerates
melanoma evasion.
Autophagy as a response to alleviate
oxidative stress
A complex interaction between ROS and autophagy
exists. In response to various stimuli, ROS and autophagy can
regulate each other through a variety of signaling pathways, thus
determining cell fate, which is largely dependent on the quantity
of ROS produced and the antioxidant ability of cells (62). Under hypoxic conditions, large
amounts of ROS are accumulated in cancer cells, which subsequently
stimulate autophagy (63). As the
major species of ROS, H2O2 is generated in
these starved tumor cells as a result of PI3K activation, which
induces the formation of autophagosomes through oxidation of
autophagy-related gene (ATG)4 (64). In addition, both O2 and
H2O2 trigger autophagy by AMP-activated
protein kinase activation and subsequent mTOR inhibition, as well
as through transcriptional regulation of ATGs such as p62 and
Beclin 1 (BECN1) (65,66). Consequently, autophagy functions
as an antioxidant defense mechanism to mitigate ROS damage to cells
and maintains cellular homeostasis by engulfing oxidized substances
(67). However, autophagy may
provide nutrients and a favorable environment for tumor progression
via the degradation of ROS-damaged organelles and proteins
(62). In this context,
ROS-mediated autophagy may play double roles in melanomagenesis and
melanoma progression.
As aforementioned, UV radiation is a major resource
for ROS generation in melanoma. Exposure to UV light increases the
expression of p62 in an ROS-dependent manner, which involves Nrf2
activity to counteract oxidative stress (68). Deletion of ATG7 in a
BRAFV600E/PTEN null model of melanoma was revealed to be associated
with enhanced oxidative stress and senescence, which prevent
melanoma tumorigenesis (69).
These findings indicate that oxidative stress-induced autophagy
exerts a tumor-promoting role. Autophagy is also essential for the
survival of cancer cells under starvation, which further promotes
melanoma metastasis (70).
Moreover, in melanoma cells in response to hypoxia and
reoxygenation treatment, the persisted accumulation of
intracellular ROS is accompanied by increased autophagy (71). Suppression of autophagy markedly
accelerates cell death induced by cycling hypoxia via increased ROS
generation (72). Therefore,
excessive ROS could induce autophagy to protect the survival of
melanoma cell under stress conditions. In addition, BRAFV600E has
been demonstrated to increase the ER stress response, which
subsequently activates cytoprotective autophagy (73). Alterations in the oxidative
environment of the ER cause the generation of ER stress-induced ROS
(74). For example, NOX4, an
essential ROS-producing NOX enzyme in melanoma, can be activated to
generate ROS during the ER stress response (75). This process is responsible for
high basal autophagy during melanomagenesis. Mechanistically, the
JNK-mediated phosphorylation of Bcl-2 and Bcl-xl releases BECN1;
meanwhile, tribbles homolog 3 inactivates mTOR signaling,
triggering the autophagy process (76). Thus, melanoma cells employ
autophagy as an adaptive mechanism to moderate oxidative
stress.
In conclusion, autophagy plays a prominent
regulatory role in tumor cell proliferation by counteracting
ROS-mediated oxidative stress and maintaining cellular homeostasis.
However, ROS overproduction prolongs the activation of autophagy
and its excessive induction may culminate in autophagic cell death.
In this regard, ROS could switch autophagic cell survival to death.
Thus, clarification of the role of ROS-induced autophagy in
melanoma progression may provide a promising therapeutic strategy
for this disease.
Role of ROS-induced autophagy in melanoma
treatment
Autophagy as a protective role to
ameliorate ROS-induced apoptosis
Several studies have reported that various
anticancer agents are employed to treat melanoma through ROS
production and ROS-mediated apoptosis (77,78). Kalantuboside B, a natural
bufadienolide derivative, has been demonstrated to enhance
intracellular ROS levels, which induce apoptosis and autophagy;
moreover, apoptosis was revealed to be potentiated by the autophagy
inhibitors chloroquine and 3-methyladenine in A2058 melanoma cells
and xenografts, suggesting that autophagy plays a protective role
in melanoma (9). Further
mechanistic evaluation revealed that this natural agent triggered
ROS-induced autophagy via activating the ERK signaling pathway and
downregulating the calcium-dependent p53 signaling (9). Dihydromyricetin, another natural
compound, was also found to promote autophagy to alleviate cell
apoptosis through the ROS-NF-κB signaling pathway in human melanoma
cells (79). In fact, autophagy
serves as a modulator of ROS generation and contributes to cell
survival under stress. Esomeprazole, a proton pump inhibitor, was
revealed to induce ROS accumulation and ROS-mediated cell death by
mitochondrial dysfunction and involvement of NADPH oxidase, while
it also elicited early autophagy to reduce its cytotoxicity against
melanoma (80). Similarly,
shikonin, a botanical anticancer drug, was demonstrated to
facilitate ER stress-mediated apoptosis by increasing the
generation of ROS, which was accompanied by prosurvival autophagy
through activation of the p38 signaling pathway in A375 melanoma
cells (81). These findings
indicate that autophagy represents an adaptive survival response to
overcome drug-induced cellular stress and cytotoxicity. In
addition, Zn(II) phthalocyanine photodynamic therapy, an emerging
therapy for melanoma, causes ROS-mediated oxidative stress that
further activates both apoptosis and autophagy; however,
suppression of autophagy strengthens phototoxicity and prevents
apoptotic cell death (82).
Furthermore, combination of photodynamic therapy with the natural
agent curcumin was shown to aggravate oxidative stress-mediated
cell apoptosis and facilitate the formation of autophagosomes,
which favor ROS-damaged melanoma cells to escape apoptosis
(83). Therefore, these studies
suggest that these anti-melanoma therapies induce a protective
autophagy to eliminate ROS, which compromises ROS-mediated
apoptosis.
Autophagic cell death exacerbates
ROS-induced death
A variety of anticancer drugs mediate autophagic
melanoma cell death by increasing the production of ROS. Liang
et al (84) revealed that
squalene synthase (SQS) III, a derivative of Schima crenata
Korth. with antitumor activities, induced apoptosis and autophagic
cell death in the human melanoma cell line A375. These effects were
reversed by the ROS scavenger N-acetylcysteine, indicating
that SQS III-induced autophagic cell death resulted from ROS
generation, which was further demonstrated to be a messenger to
inhibit the AKT/mTOR signaling pathway, which is a negative
regulator of the autophagy process (84). These findings demonstrated that
drug-mediated ROS caused melanoma cell death by regulating
autophagy-related signaling pathways. A previous study has reported
that dimethylacrylshikonin, isolated from the roots of
Boraginaceae, triggered the loss of mitochondrial membrane
potential and thus ROS accumulation in melanoma cells, leading to
autophagic cell death by mitochondrial dysfunction (85). A similar study revealed that
inhibition of dihydrolipoyl dehydrogenase, a mitochondrial
oxidoreductase enzyme, contributed to ROS overproduction and
alteration of mitochondrial energy metabolism, and thus triggered
autophagic melanoma cell death (86). Therefore, ROS generation
attributed to mitochondrial dysfunction appears to be a major
contributor of autophagic cell death. Additionally,
berberine-photodynamic therapy activated ER stress, which
contributed to a marked increase in ROS, and thus enhanced
autophagy to facilitate apoptosis in human melanoma cells (87). Photodynamic therapy-induced ROS
accumulation was sufficient to elicit oxidative stress, which
further mediated autophagy and consequently inhibited cell
proliferation in B16F10 melanoma cells (10). Furthermore, the
bis(phenylidenebenzeneamine)-1-disulfide (88), graveoline (89) and terfenadine (90) have been found to induce autophagic
cell death in melanoma cells through increasing ROS production.
Collectively, ROS-induced autophagy elicited by
anticancer agents exerts double functions in the treatment of
melanoma, either via autophagic cell survival or death (Fig. 1). It has been reported that low
doses of nitrogen-doped titanium dioxide, a photodynamic therapy
for melanoma, stimulate a protective autophagy flux response,
whereas therapeutic doses impair autophagy and induce necroptosis
via ROS production (91). A
possible explanation is that the functional effects of ROS-induced
autophagy on cancer cell death are dependent on the type of
oxidative stress, as well as the quantity and location of the ROS
produced (92). It should be
noted that anticancer drug-induced ROS also inhibit cytoprotective
autophagy to promote apoptosis, and that blocking autophagy fails
to affect ROS generation (78,89), suggesting that ROS serve as
messengers to modulate autophagy and thus determine the cell fate.
In this context, further clarifying the role of anticancer
drug-induced ROS and the resultant autophagy is essential for
melanoma treatment.
Future directions
As a ROS-driven tumor, melanoma is susceptible to
ROS production and thus to oxidative stress. The main resources of
ROS in melanocytes comprises UV radiation, melanin synthesis and
activation of NOX. Excessive ROS generation causes oxidative damage
to melanocytes, including oxidative DNA damage, which is
responsible for gene mutations and epigenetic alterations, as well
as aberrant activation of signaling pathways that are involved in
cell proliferation and differentiation, ultimately leading to
melanomagenesis and melanoma progression. Autophagy is elicited as
an adaptive response to remove ROS and oxidative components, which
maintains genetic stability and inhibits carcinogenesis; however,
once a tumor is formed, autophagy is induced to provide metabolic
demands and nutrients for tumor progression. Furthermore, the
sustained activation of autophagy may contribute to cell death,
which is also known as autophagic cell death (Fig. 1).
The crosstalk between ROS and autophagy in
melanomagenesis remains unclear. Understanding the mechanism of
ROS-regulated autophagy in melanoma will provide new therapeutic
strategies for this disease. In addition, it is essential to
investigate whether anticancer drug-induced autophagy promotes cell
survival or facilitates cell death. Agents that are cytoprotective
autophagy-inducing drugs combined with autophagy inhibitors and
autophagic cell death-mediating agents combined with autophagy
activators are both effective therapeutic strategies for melanoma.
Therefore, clarification of the functional status of ROS-induced
autophagy is crucial in melanoma treatment. Of note, prolonged
autophagy causes the removal of excessive ROS and the degradation
of dysfunctional mitochondria, which leads to reductive stress.
This novel reductive stress mechanism of cell death offers a novel
approach for cancer therapy, including transfer-hydrogenation
catalysts (93–95). Further studies on the role of
reductive stress in melanoma may provide insights into the
treatment of this disease.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Traditional Chinese Medicine
research projects of Heilongjiang Province (grant no. ZHY2020-041)
and the Doctoral Program of Heilongjiang Province (grant no.
LBH-Z21218).
Availability of data and materials
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the current
study.
Authors' contributions
XZ performed the literature search. XZ, HL and CL
wrote the manuscript. XY planned and supervised the writing of the
present review article. Data authentication is not applicable. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Saikolappan S, Kumar B, Shishodia G, Koul
S and Koul HK: Reactive oxygen species and cancer: A complex
interaction. Cancer Lett. 452:132–143. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sarmiento-Salinas FL, Perez-Gonzalez A,
Acosta-Casique A, Ix-Ballote A, Diaz A, Treviño S, Rosas-Murrieta
NH, Millán-Perez-Peña L and Maycotte P: Reactive oxygen species:
Role in carcinogenesis, cancer cell signaling and tumor
progression. Life Sci. 284:1199422021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Venza I, Venza M, Visalli M, Lentini G,
Teti D and d'Alcontres FS: ROS as regulators of cellular processes
in melanoma. Oxid Med Cell Longev. 2021:12086902021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pizzimenti S, Ribero S, Cucci MA,
Grattarola M, Monge C, Dianzani C, Barrera G and Muzio G: Oxidative
stress-related mechanisms in melanoma and in the acquired
resistance to targeted therapies. Antioxidants (Basel).
10:19422021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Morishita H and Mizushima N: Diverse
cellular roles of autophagy. Annu Rev Cell Dev Biol. 35:453–475.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Catalani E, Giovarelli M, Zecchini S,
Perrotta C and Cervia D: Oxidative stress and autophagy as key
targets in melanoma cell fate. Cancers (Basel). 13:57912021.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao L, Loveless J, Shay C and Teng Y:
Targeting ROS-Mediated crosstalk between autophagy and apoptosis in
cancer. Adv Exp Med Biol. 1260:1–12. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fried L and Arbiser JL: The reactive
oxygen-driven tumor: Relevance to melanoma. Pigment Cell Melanoma
Res. 21:117–122. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hseu YC, Cho HJ, Gowrisankar YV,
Thiyagarajan V, Chen XZ, Lin KY, Huang HC and Yang HL:
Kalantuboside B induced apoptosis and cytoprotective autophagy in
human melanoma A2058cells: An in vitro and in vivo study. Free
Radic Biol Med. 143:397–411. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Santos GMP, Oliveira SCPS, Monteiro JCS,
Fagnani SR, Sampaio FP, Correia NA, Crugeira PJL and Pinheiro ALB:
ROS-induced autophagy reduces B16F10 melanoma cell proliferative
activity. Lasers Med Sci. 33:1335–1340. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Slominski AT, Zmijewski MA, Plonka PM,
Szaflarski JP and Paus R: How UV Light Touches the Brain and
Endocrine System Through Skin, and Why. Endocrinology.
159:1992–2007. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
von Thaler AK, Kamenisch Y and Berneburg
M: The role of ultraviolet radiation in melanomagenesis. Exp
Dermatol. 19:81–88. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Terra VA, Souza-Neto FP, Pereira RC, Silva
TN, Costa AC, Luiz RC, Cecchini R and Cecchini AL: Time-dependent
reactive species formation and oxidative stress damage in the skin
after UVB irradiation. J Photochem Photobiol B. 109:34–41. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baumler W, Regensburger J, Knak A,
Felgentrager A and Maisch T: UVA and endogenous
photosensitizers-the detection of singlet oxygen by its
luminescence. Photochem Photobiol Sci. 11:107–117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Knak A, Regensburger J, Maisch T and
Baumler W: Exposure of vitamins to UVB and UVA radiation generates
singlet oxygen. Photochem Photobiol Sci. 13:820–829. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Regensburger J, Maisch T, Knak A, Gollmer
A, Felgentraeger A, Lehner K and Baeumler W: UVA irradiation of
fatty acids and their oxidized products substantially increases
their ability to generate singlet oxygen. Phys Chem Chem Phys.
15:17672–17680. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baier J, Maisch T, Maier M, Engel E,
Landthaler M and Baumler W: Singlet oxygen generation by UVA light
exposure of endogenous photosensitizers. Biophys J. 91:1452–1459.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Regensburger J, Knak A, Maisch T,
Landthaler M and Baumler W: Fatty acids and vitamins generate
singlet oxygen under UVB irradiation. Exp Dermatol. 21:135–139.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Valencia A and Kochevar IE: Nox1-based
NADPH oxidase is the major source of UVA-induced reactive oxygen
species in human keratinocytes. J Invest Dermatol. 128:214–222.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao B, Shah P, Qiang L, He TC, Budanov A
and He YY: Distinct Role of Sesn2 in Response to UVB-Induced DNA
Damage and UVA-Induced Oxidative Stress in Melanocytes. Photochem
Photobiol. 93:375–381. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cadet J, Douki T and Ravanat JL:
Oxidatively generated damage to cellular DNA by UVB and UVA
radiation. Photochem Photobiol. 91:140–155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meyskens FL Jr, McNulty SE, Buckmeier JA,
Tohidian NB, Spillane TJ, Kahlon RS and Gonzalez RI: Aberrant redox
regulation in human metastatic melanoma cells compared to normal
melanocytes. Free Radic Biol Med. 31:799–808. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jenkins NC and Grossman D: Role of melanin
in melanocyte dysregulation of reactive oxygen species. Biomed Res
Int. 2013:9087972013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arslanbaeva LR and Santoro MM: Adaptive
redox homeostasis in cutaneous melanoma. Redox Biol. 37:1017532020.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Slominski RM, Sarna T, Plonka PM, Raman C,
Brozyna AA and Slominski AT: Melanoma, melanin, and melanogenesis:
The Yin and Yang relationship. Front Oncol. 12:8424962022.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Slominski A, Tobin DJ, Shibahara S and
Wortsman J: Melanin pigmentation in mammalian skin and its hormonal
regulation. Physiol Rev. 84:1155–1228. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Slominski A, Zmijewski MA and Pawelek J:
L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators
of melanocyte functions. Pigment Cell Melanoma Res. 25:14–27. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Panzella L, Leone L, Greco G, Vitiello G,
D'Errico G, Napolitano A and d'Ischia M: Red human hair pheomelanin
is a potent pro-oxidant mediating UV-independent contributory
mechanisms of melanomagenesis. Pigment Cell Melanoma Res.
27:244–252. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Panzella L, Szewczyk G, d'Ischia M,
Napolitano A and Sarna T: Zinc-induced structural effects enhance
oxygen consumption and superoxide generation in synthetic
pheomelanins on UVA/visible light irradiation. Photochem Photobiol.
86:757–764. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shain AH and Bastian BC: From melanocytes
to melanomas. Nat Rev Cancer. 16:345–358. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Swope VB and Abdel-Malek ZA: MC1R: Front
and center in the bright side of dark eumelanin and DNA repair. Int
J Mol Sci. 19:26672018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nasti TH and Timares L: MC1R, eumelanin
and pheomelanin: Their role in determining the susceptibility to
skin cancer. Photochem Photobiol. 91:188–200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brozyna AA, Jozwicki W, Roszkowski K,
Filipiak J and Slominski AT: Melanin content in melanoma metastases
affects the outcome of radiotherapy. Oncotarget. 7:17844–17853.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu-Smith F, Dellinger R and Meyskens FL
Jr: Updates of reactive oxygen species in melanoma etiology and
progression. Arch Biochem Biophys. 563:51–55. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Meierjohann S: Oxidative stress in
melanocyte senescence and melanoma transformation. Eur J Cell Biol.
93:36–41. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Govindarajan B, Sligh JE, Vincent BJ, Li
M, Canter JA, Nickoloff BJ, Rodenburg RJ, Smeitink JA, Oberley L,
Zhang Y, et al: Overexpression of Akt converts radial growth
melanoma to vertical growth melanoma. J Clin Invest. 117:719–729.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ribeiro-Pereira C, Moraes JA, Souza Mde J,
Laurindo FR, Arruda MA and Barja-Fidalgo C: Redox modulation of FAK
controls melanoma survival-role of NOX4. PLoS One. 9:e994812014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu GS, Wu JC, Tsai HE, Dusting GJ, Chan
EC, Wu CS and Tai MH: Proopiomelanocortin gene delivery induces
apoptosis in melanoma through NADPH oxidase 4-mediated ROS
generation. Free Radic Biol Med. 70:14–22. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Quast SA, Berger A and Eberle J:
ROS-dependent phosphorylation of Bax by wortmannin sensitizes
melanoma cells for TRAIL-induced apoptosis. Cell Death Dis.
4:e8392013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu GS, Peshavariya H, Higuchi M, Brewer
AC, Chang CW, Chan EC and Dusting GJ: Microphthalmia-associated
transcription factor modulates expression of NADPH oxidase type 4:
A negative regulator of melanogenesis. Free Radic Biol Med.
52:1835–1843. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yamaura M, Mitsushita J, Furuta S, Kiniwa
Y, Ashida A, Goto Y, Shang WH, Kubodera M, Kato M, Takata M, et al:
NADPH oxidase 4 contributes to transformation phenotype of melanoma
cells by regulating G2-M cell cycle progression. Cancer Res.
69:2647–2654. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Antony S, Jiang G, Wu Y, Meitzler JL,
Makhlouf HR, Haines DC, Butcher D, Hoon DS, Ji J, Zhang Y, et al:
NADPH oxidase 5 (NOX5)-induced reactive oxygen signaling modulates
normoxic HIF-1α and p27Kip1 expression in malignant
melanoma and other human tumors. Mol Carcinog. 56:2643–2662. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xian D, Lai R, Song J, Xiong X and Zhong
J: Emerging Perspective: Role of Increased ROS and Redox Imbalance
in Skin Carcinogenesis. Oxid Med Cell Longev. 2019:81273622019.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mouret S, Forestier A and Douki T: The
specificity of UVA-induced DNA damage in human melanocytes.
Photochem Photobiol Sci. 11:155–162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Murtas D, Piras F, Minerba L, Ugalde J,
Floris C, Maxia C, Demurtas P, Perra MT and Sirigu P: Nuclear
8-hydroxy-2′-deoxyguanosine as survival biomarker in patients with
cutaneous melanoma. Oncol Rep. 23:329–335. 2010.PubMed/NCBI
|
|
46
|
Landi MT, Bauer J, Pfeiffer RM, Elder DE,
Hulley B, Minghetti P, Calista D, Kanetsky PA, Pinkel D and Bastian
BC: MC1R germline variants confer risk for BRAF-mutant melanoma.
Science. 313:521–522. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Comito G, Calvani M, Giannoni E, Bianchini
F, Calorini L, Torre E, Migliore C, Giordano S and Chiarugi P:
HIF-1α stabilization by mitochondrial ROS promotes Met-dependent
invasive growth and vasculogenic mimicry in melanoma cells. Free
Radic Biol Med. 51:893–904. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Slominski A, Kim TK, Brozyna AA,
Janjetovic Z, Brooks DL, Schwab LP, Skobowiat C, Jóźwicki W and
Seagroves TN: The role of melanogenesis in regulation of melanoma
behavior: Melanogenesis leads to stimulation of HIF-1α expression
and HIF-dependent attendant pathways. Arch Biochem Biophys.
563:79–93. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Park SJ, Kim YT and Jeon YJ: Antioxidant
dieckol downregulates the Rac1/ROS signaling pathway and inhibits
Wiskott-Aldrich syndrome protein (WASP)-family verprolin-homologous
protein 2 (WAVE2)-mediated invasive migration of B16 mouse melanoma
cells. Mol Cells. 33:363–369. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jenkins NC, Liu T, Cassidy P, Leachman SA,
Boucher KM, Goodson AG, Samadashwily G and Grossman D: The
p16(INK4A) tumor suppressor regulates cellular oxidative stress.
Oncogene. 30:265–274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kazimierczak U, Dondajewska E,
Zajaczkowska M, Karwacka M, Kolenda T and Mackiewicz A: LATS1 Is a
Mediator of Melanogenesis in Response to Oxidative Stress and
Regulator of Melanoma Growth. Int J Mol Sci. 22:31082021.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Molognoni F, de Melo FH, da Silva CT and
Jasiulionis MG: Ras and Rac1, frequently mutated in melanomas, are
activated by superoxide anion, modulate Dnmt1 level and are
causally related to melanocyte malignant transformation. PLoS One.
8:e819372013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lingappan K: NF-κB in Oxidative Stress.
Curr Opin Toxicol. 7:81–86. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Dolcet X, Llobet D, Pallares J and
Matias-Guiu X: NF-kB in development and progression of human
cancer. Virchows Arch. 446:475–482. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Slominski A and Wortsman J:
Neuroendocrinology of the skin. Endocr Rev. 21:457–487. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Slominski RM, Zmijewski MA and Slominski
AT: The role of melanin pigment in melanoma. Exp Dermatol.
24:258–259. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Li W, Slominski R and Slominski AT:
High-resolution magic angle spinning nuclear magnetic resonance
analysis of metabolic changes in melanoma cells after induction of
melanogenesis. Anal Biochem. 386:282–284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kamenisch Y, Ivanova I, Drexler K and
Berneburg M: UVA, metabolism and melanoma: UVA makes melanoma
hungry for metastasis. Exp Dermatol. 27:941–949. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Slominski A, Paus R and Mihm MC:
Inhibition of melanogenesis as an adjuvant strategy in the
treatment of melanotic melanomas: Selective review and hypothesis.
Anticancer Res. 18((5B)): 3709–3715. 1998.PubMed/NCBI
|
|
60
|
Slominski A, Zbytek B and Slominski R:
Inhibitors of melanogenesis increase toxicity of cyclophosphamide
and lymphocytes against melanoma cells. Int J Cancer.
124:1470–1477. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kuo CL, Chou HY, Chiu YC, Cheng AN, Fan
CC, Chang YN, Chen CH, Jiang SS, Chen NJ and Lee AY: Mitochondrial
oxidative stress by Lon-PYCR1 maintains an immunosuppressive tumor
microenvironment that promotes cancer progression and metastasis.
Cancer Lett. 474:138–150. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Li D, Ding Z, Du K, Ye X and Cheng S:
Reactive oxygen species as a link between antioxidant pathways and
autophagy. Oxid Med Cell Longev. 2021:55832152021.PubMed/NCBI
|
|
63
|
Poillet-Perez L, Despouy G,
Delage-Mourroux R and Boyer-Guittaut M: Interplay between ROS and
autophagy in cancer cells, from tumor initiation to cancer therapy.
Redox Biol. 4:184–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Scherz-Shouval R, Shvets E, Fass E, Shorer
H, Gil L and Elazar Z: Reactive oxygen species are essential for
autophagy and specifically regulate the activity of Atg4. EMBO J.
38:e1018122019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Mathew R, Karp CM, Beaudoin B, Vuong N,
Chen G, Chen HY, Bray K, Reddy A, Bhanot G, Gelinas C, et al:
Autophagy suppresses tumorigenesis through elimination of p62.
Cell. 137:1062–1075. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Jain A, Lamark T, Sjøttem E, Larsen KB,
Awuh JA, Øvervatn A, McMahon M, Hayes JD and Johansen T: p62/SQSTM1
is a target gene for transcription factor NRF2 and creates a
positive feedback loop by inducing antioxidant response
element-driven gene transcription. J Biol Chem. 285:22576–22591.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Redza-Dutordoir M and Averill-Bates DA:
Interactions between reactive oxygen species and autophagy: Special
issue: Death mechanisms in cellular homeostasis. Biochim Biophys
Acta Mol Cell Res. 1868:1190412021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Sample A, Zhao B, Wu C, Qian S, Shi X,
Aplin A and He YY: The autophagy receptor adaptor p62 is
Up-regulated by UVA radiation in melanocytes and in melanoma cells.
Photochem Photobiol. 94:432–437. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Xie X, Koh JY, Price S, White E and
Mehnert JM: Atg7 overcomes senescence and promotes growth of
BrafV600E-Driven melanoma. Cancer Discov. 5:410–423. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zhao Y, Wang W, Min I, Wyrwas B, Moore M,
Zarnegar R and Fahey TJ III: BRAF V600E-dependent role of autophagy
in uveal melanoma. J Cancer Res Clin Oncol. 143:447–455. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wang Y, Wang Y, Wu J, Wang W and Zhang Y:
Oxygen partial pressure plays a crucial role in B16 melanoma cell
survival by regulating autophagy and mitochondrial functions.
Biochem Biophys Res Commun. 510:643–648. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Rouschop KM, Ramaekers CH, Schaaf MB,
Keulers TG, Savelkouls KG, Lambin P, Koritzinsky M and Wouters BG:
Autophagy is required during cycling hypoxia to lower production of
reactive oxygen species. Radiother Oncol. 92:411–416. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ma XH, Piao SF, Dey S, McAfee Q,
Karakousis G, Villanueva J, Hart LS, Levi S, Hu J, Zhang G, et al:
Targeting ER stress-induced autophagy overcomes BRAF inhibitor
resistance in melanoma. J Clin Invest. 124:1406–1417. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Victor P, Sarada D and Ramkumar KM:
Crosstalk between endoplasmic reticulum stress and oxidative
stress: Focus on protein disulfide isomerase and endoplasmic
reticulum oxidase 1. Eur J Pharmacol. 892:1737492021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Santos CX, Tanaka LY, Wosniak J and
Laurindo FR: Mechanisms and implications of reactive oxygen species
generation during the unfolded protein response: Roles of
endoplasmic reticulum oxidoreductases, mitochondrial electron
transport, and NADPH oxidase. Antioxid Redox Signal. 11:2409–2427.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Corazzari M, Rapino F, Ciccosanti F,
Giglio P, Antonioli M, Conti B, Fimia GM, Lovat PE and Piacentini
M: Oncogenic BRAF induces chronic ER stress condition resulting in
increased basal autophagy and apoptotic resistance of cutaneous
melanoma. Cell Death Differ. 22:946–958. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ghaemi B, Moshiri A, Herrmann IK, Hajipour
MJ, Wick P, Amani A and Kharrazi S: Supramolecular insights into
domino effects of simpleAg@ZnO-Induced oxidative
stress in melanoma cancer cells. ACS Appl Mater Interfaces.
11:46408–46418. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Chang SN, Khan I, Kim CG, Park SM, Choi
DK, Lee H, Hwang BS, Kang SC and Park JG: Decursinol angelate
arrest melanoma cell proliferation by initiating cell death and
tumor shrinkage via induction of apoptosis. Int J Mol Sci.
22:40962021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhou DZ, Sun HY, Yue JQ, Peng Y, Chen YM
and Zhong ZJ: Dihydromyricetin induces apoptosis and cytoprotective
autophagy through ROS-NF-κB signalling in human melanoma cells.
Free Radic Res. 51:517–528. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Marino ML, Fais S, Djavaheri-Mergny M,
Villa A, Meschini S, Lozupone F, Venturi G, Della Mina P, Pattingre
S, Rivoltini L, et al: Proton pump inhibition induces autophagy as
a survival mechanism following oxidative stress in human melanoma
cells. Cell Death Dis. 1:e872010. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Liu Y, Kang X, Niu G, He S, Zhang T, Bai
Y, Li Y, Hao H, Chen C, Shou Z and Li B: Shikonin induces apoptosis
and prosurvival autophagy in human melanoma A375 cells via
ROS-mediated ER stress and p38 pathways. Artif Cells Nanomed
Biotechnol. 47:626–635. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Valli F, García Vior MC, Roguin LP and
Marino J: Crosstalk between oxidative stress-induced apoptotic and
autophagic signaling pathways in Zn(II) phthalocyanine photodynamic
therapy of melanoma. Free Radic Biol Med. 152:743–754. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Niu T, Tian Y, Mei Z and Guo G: Inhibition
of autophagy enhances curcumin united light irradiation-induced
oxidative stress and tumor growth suppression in human melanoma
cells. Sci Rep. 6:313832016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Liang QP, Xu TQ, Liu BL, Lei XP, Hambrook
JR, Zhang DM and Zhou GX: Sasanquasaponin III from Schima crenata
Korth induces autophagy through Akt/mTOR/p70S6K pathway and
promotes apoptosis in human melanoma A375 cells. Phytomedicine.
58:1527692019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Kretschmer N, Deutsch A, Durchschein C,
Rinner B, Stallinger A, Higareda-Almaraz JC, Scheideler M,
Lohberger B and Bauer R: Comparative gene expression analysis in
WM164 melanoma cells revealed that β-β-Dimethylacrylshikonin Leads
to ROS generation, loss of mitochondrial membrane potential, and
autophagy induction. Molecules. 23:28232018. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Yumnam S, Kang MC, Oh SH, Kwon HC, Kim JC,
Jung ES, Lee CH, Lee AY, Hwang JI and Kim SY: Downregulation of
dihydrolipoyl dehydrogenase by UVA suppresses melanoma progression
via triggering oxidative stress and altering energy metabolism.
Free Radic Biol Med. 162:77–87. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Fang J, Huang X, Yang Y, Wang X, Liang X
and Liu J: Berberine-photodynamic induced apoptosis by activating
endoplasmic reticulum stress-autophagy pathway involving CHOP in
human malignant melanoma cells. Biochem Biophys Res Commun.
552:183–190. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Hu WP, Hsu CC, Wang YC, Senadi GC, Kuo KK,
Jen JF and Wang JJ: Bis(phenylidenebenzeneamine)-1-disulfide
derivatives induce autophagy in melanoma cells through a
mitochondria-mediated pathway. Anticancer Res. 35:6075–6080.
2015.PubMed/NCBI
|
|
89
|
Ghosh S, Bishayee K and Khuda-Bukhsh AR:
Graveoline isolated from ethanolic extract of Ruta graveolens
triggers apoptosis and autophagy in skin melanoma cells: A novel
apoptosis-independent autophagic signaling pathway. Phytother Res.
28:1153–1162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Nicolau-Galmés F, Asumendi A,
Alonso-Tejerina E, Pérez-Yarza G, Jangi SM, Gardeazabal J,
Arroyo-Berdugo Y, Careaga JM, Díaz-Ramón JL, Apraiz A and Boyano
MD: Terfenadine induces apoptosis and autophagy in melanoma cells
through ROS-dependent and -independent mechanisms. Apoptosis.
16:1253–1267. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Mohammadalipour Z, Rahmati M, Khataee A
and Moosavi MA: Differential effects of N-TiO2
nanoparticle and its photo-activated form on autophagy and
necroptosis in human melanoma A375 cells. J Cell Physiol.
235:8246–8259. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Yun HR, Jo YH, Kim J, Shin Y, Kim SS and
Choi TG: Roles of autophagy in oxidative stress. Int J Mol Sci.
21:32892020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Soldevila-Barreda JJ, Romero-Canelón I,
Habtemariam A and Sadler PJ: Transfer hydrogenation catalysis in
cells as a new approach to anticancer drug design. Nat Commun.
6:65822015. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Sopha P, Ren HY, Grove DE and Cyr DM:
Endoplasmic reticulum stress-induced degradation of DNAJB12
stimulates BOK accumulation and primes cancer cells for apoptosis.
J Biol Chem. 292:11792–11803. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Coverdale JPC, Romero-Canelon I,
Sanchez-Cano C, Clarkson GJ, Habtemariam A, Wills M and Sadler PJ:
Asymmetric transfer hydrogenation by synthetic catalysts in cancer
cells. Nat Chem. 10:347–354. 2018. View Article : Google Scholar : PubMed/NCBI
|