Introduction
Ulcerative colitis (UC) is a serious chronic
inflammatory intestinal disease with clinical manifestations that
include abdominal pain, diarrhea, presence of mucous, pus and blood
in the stool, and accompanied by intestinal inflammation and
intestinal mucosal tissue damage (1). Patients with UC exhibit a low
curative rate, high recurrence and long disease durations, and have
a high risk of developing colitis-related colon cancer, which
affects millions of patients worldwide (2,3).
In previous years, with the improvement of population living
standards and the aggravation of environmental pollution, the
incidence rate of UC has been increasing yearly worldwide (4). At present, the treatment of UC
includes 5-aminosalicylic acid drugs, glucocorticoids and
immunosuppressants; however, the long-term use of these drugs will
cause a variety of adverse reactions, reducing the quality of life
of patients (5). Therefore,
identifying a potential cure for UC has become a priority in recent
UC research.
Traditional Chinese Medicine has the unique
advantage of being a multi-system, multi-link and multi-target
treatment that demonstrates good therapeutic effects in refractory
diseases (6). Studies have shown
that radix sophora flavescens, artemisinin and other Traditional
Chinese Medicines are widely used in the treatment of UC (7,8).
Oridonin (Ori) is a type of natural organic compound that is
isolated from Rabdosia rubescens, which has
anti-inflammatory, antibacterial and antitumor activities (9). Ori has been demonstrated to improve
inflammation-induced bone loss in mice by inhibiting dendritic
cell-specific transmembrane protein expression (10). Ori also inhibits the macrophage
inflammatory response via the AKT-related pathway and alleviates
ischemia/reperfusion-induced renal injury (11). This suggests that Ori plays an
important role in the inflammatory response exhibited during UC. In
addition, Ori has been revealed to reduce trinitrobenzene sulfonic
acid (TNBS)-induced inflammatory post-irritable bowel syndrome via
the pregnane X receptor (PXR)/NF-κB signaling pathway (12). Furthermore, Ori derivatives
ameliorate experimental colitis by inhibiting the translocation of
activated T cells and NF-Κb (13). In China, Ori is a commonly
available over-the-counter herbal medicine for the treatment of
inflammatory diseases (14).
However, the effect of Ori on dextran sulfate sodium (DSS)-induced
murine UC and the specific underlying mechanisms have not yet been
reported.
Sirtuin-1 (Sirt1) plays an important role in UC, as
demonstrated by the results of a previous study, in which Sirt1
reduced the endoplasmic reticulum stress-mediated apoptosis of
intestinal epithelial cells in UC (15). It has also been demonstrated that
the upregulation of Sirt1 inhibits NF-κB-mediated macrophage
activation, thereby improving experimental colitis in mice
(16). Moreover, Sirt1 can be
used as a target of ginipinine to inhibit the NOD-, LRR- and pyrin
domain-containing protein 3 (NLRP3) inflammasome of macrophages,
and improve the inflammatory response in colitis (17). The current study therefore aimed
to determine whether Ori serves an important role in DSS-induced UC
by regulating Sirt1 and examined the downstream pathways involved
in this process.
Materials and methods
Ethics statement
All the animal care and experimental procedures were
performed according to the Guidelines for the Care and Use of
Laboratory Animals and the ARRIVE checklist. Appropriate measures
were taken to minimize pain and stress to the animals. The
experiments were approved by the Experimental Animal Ethics
Committee of Beihua University (approval no. BHDX2021-116; Jilin,
China).
Animal experiments
Male BALB/C mice (n=20; 18±8 g), aged 6–8 weeks were
randomly divided into the following four groups after 1 week of
adaptive feeding: i) Control, ii) DSS, iii) Ori low-dose (Ori-L)
and iv) Ori high-dose (Ori-H). A total of 5 mice were included in
each group. For the DSS, Ori-L and Ori-H groups, the mice were fed
with 3% DSS dissolved in drinking water for 7 days to induce acute
colitis. For the Ori-L and Ori-H groups specifically,
intraperitoneal injections of Ori-L (2 mg/kg) and Ori-H (10 mg/kg)
commenced on day 7 and were subsequently administered once a day
for 1 week (10,18). The control and DSS groups were
intraperitoneally injected with the same quantity of normal saline.
The body weight of the mice was recorded daily and 7 days after
treatment. The duration of the experiment was 14 days. To further
observe the colonic condition of the mice, the mice (n=20) were
euthanized, and the colon was removed. The mice were sacrificed by
cervical dislocation following anesthesia with sodium pentobarbital
(40 mg/kg intraperitoneal injection). After ensuring the mice
didn't have a heartbeat, tissues from the mice were collected for
the subsequent experiments.
Disease activity index (DAI)
Body weight, gross rectal bleeding and stool
consistency were checked daily in the UC mice. A disease activity
index (DAI) score was calculated based on a previously described
method (19) to assess the
disease severity.
Database
The STITCH database (stitch.embl.de) was used to
predict the target of Ori (20,21).
H&E staining
H&E staining was performed using a H&E
staining Kit (cat. no. C0105S; Beyotime Institute of
Biotechnology). Murine colons were obtained, fixed in 4% buffered
paraformaldehyde solution for 24 h at room temperature and embedded
in paraffin. The 5-µm thick sections were subjected to H&E
staining according to the manufacturer's instructions. The results
were visualized and images were captured under a light microscope
(magnification, ×400).
Western blotting
Total protein was extracted from murine colons using
RIPA lysis buffer (Beyotime Institute of Biotechnology).
Cytoplasmic proteins were subsequently extracted using an
extraction kit (cat. no. C500051; Sangon Biotech Co., Ltd.). Equal
quantities (40 µg) protein were separated using 12% SDS-PAGE and
transferred to PVDF membranes (cat. no. IPFL00010; MilliporeSigma).
The membranes were blocked with 5% skimmed milk at 25°C for 1 h and
incubated overnight at 4°C with the following primary antibodies
(all from Abcam): Zona occludin-1 (ZO-1; 1:1,000; cat. no.
ab221547), occludin (1:1,000; cat. no. ab216327), claudin-1
(1:1,000; cat. no. ab211737), Bcl-2 (1:1,000; cat. no. ab182858),
Bax (1:1,000; cat. no. ab32503), cleaved caspase 3 (1:1,000; cat.
no. ab214430), Sirt1 (1:1,000; cat. no. ab110304), phosphorylated
(p)-NF-κB (1:1,000; cat. no. ab239882), NF-κB (1:1,000; cat. no.
ab207297), acetyl-p53 (1:1,000; cat. no. ab183544) and GAPDH
(1:1,000; cat. no. ab8245). A secondary antibody (1:5,000; cat. no.
ab150113; Abcam) was then added and the membrane was incubated at
room temperature for 1 h. Protein expression was visualized using
ECL (Promega Corporation) and quantified using ImageJ software
(version 146; National Institutes of Health).
Myeloperoxidase (MPO), malondialdehyde
(MDA), glutathione peroxidase (GSH-Px), superoxide dismutase (SOD)
and reactive oxygen species (ROS) assays in murine colon
tissue
The colons of the mice were homogenized and
fluidized in extraction buffer phosphate buffer solution (Beyotime
Institute of Biotechnology). MPO (cat. no. A044-1-1), MDA (cat. no.
A003-1-2), GSH (cat. no. A005-1-2), SOD (cat. no. A001-3-2) and ROS
(cat. no. E004-1-1) activities were subsequently measured using
activity kits (Nanjing Jiancheng Bioengineering Institute) along
with the change in absorbance at 460 nm using a 96-well plate
reader.
ELISA
Biotinylated antibodies and enzyme-linked reaction
substrate were separately incubated at 37°C, following which the
corresponding developer and stop solution were added. Absorbance at
450 nm was measured using a microplate luminometer (Omega Bio-Tek,
Inc.). The concentrations of TNF-α (cat. no. ab208348; Abcam),
IL-1β (cat. no. ab197742; Abcam) and IL-6 (cat. no. ab222503;
Abcam) were subsequently calculated based on the appropriate
standard curve.
TUNEL assay
TUNEL staining (Beyotime Institute of Biotechnology)
was used to analyze cell apoptosis. Following fixation with 4%
paraformaldehyde for 24 h at room temperature, sections of murine
colons were rinsed in distilled water and incubated with 3%
hydrogen peroxide in methanol for 5 min at room temperature to
block endogenous peroxidase activity, following which the samples
were deparaffinized using xylene and rehydrated in a descending
ethanol series. The sections were incubated with 20 µg/ml
proteinase K (Dako; Agilent Technologies, Inc.) for 15 min at room
temperature, and TdT enzyme solution was added and incubated for 1
h at 37°C. The sections were then incubated with
streptavidin-peroxidase conjugate for 30 min at 37°C. Peroxidase
activity was demonstrated by the addition of 50 µl DAB for 10 min
at room temperature and a light microscope was used to observe
apoptosis in six randomly selected fields of view (magnification,
×200).
Statistical analysis
The data were expressed as the mean ± SD.
Differences between groups were analyzed using an unpaired
Student's t-test and one-way ANOVA with Tukey's post hoc test. SPSS
version 21 (IBM Corp.) was used for statistical analysis and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Ori improves clinical symptoms and
pathological damage in DSS-induced UC mice
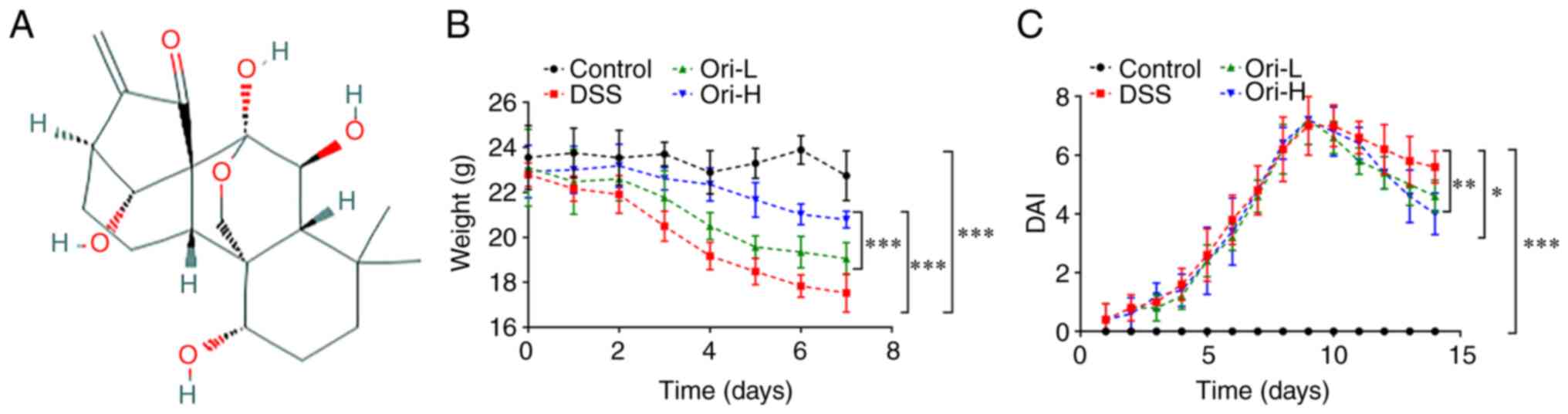
The chemical structural formula of Ori is presented
in Fig. 1A. At 7 days after the
beginning of treatment, the daily weight of mice was measured. The
results revealed that the weight of the mice in the DSS group
decreased significantly over time compared with that in the control
group. Additionally, administration of Ori induced an increase in
murine weight compared with that in the DSS group, as demonstrated
in Fig. 1B. The DAI from the mice
demonstrated that DAI was significantly higher in the DSS group
compared with that in the control group. Furthermore, after day 10,
when compared with that in the DSS group, the DAI decreased
significantly in the Ori-L and Ori-H groups, and even more
significantly in the Ori-H group (Fig. 1C).
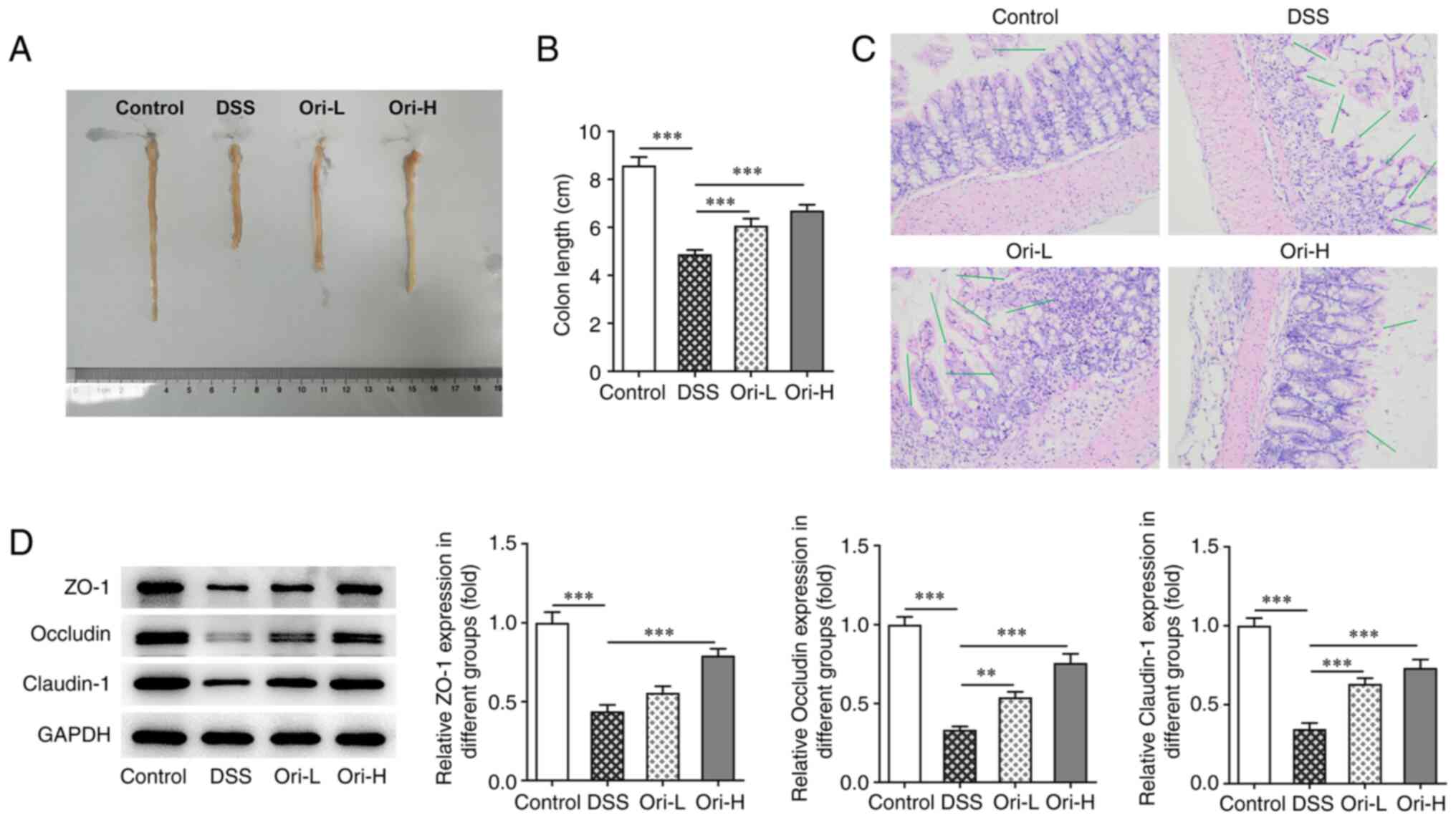
Colon length was subsequently measured in the
treated mice (Fig. 2A). As
presented in Fig. 2B, colon
length was significantly decreased in the DSS group compared with
that in the control group. Additionally, there was a significant
increase in colon length following Ori treatment compared with the
DSS group. H&E staining revealed that there were no notable
lesions in the control group, while mice in the DSS group exhibited
a number of pathological changes in their colon tissue, such as
irregular and damaged surface epithelium, no branching, twisted and
loosely arranged cup cells in deep crypts, and a large number of
scattered lymphocytes in the lamina propria accompanied by
epithelial cell loss. However, colon injury was reversed following
Ori administration (Fig. 2C). The
expression of tight junction-related proteins was subsequently
examined to measure the integrity of the colonic mucosal barrier.
The results demonstrated that the protein expression level of ZO-1,
occludin and claudin-1 decreased significantly in the DSS group
compared with that in the control group. Furthermore, compared with
that in the DSS group, the protein expression level of ZO-1,
occludin and claudin-1 in the Ori-L and Ori-H groups were reversed
(Fig. 2D). The results indicated
that Ori protected the integrity of the colonic mucosal
barrier.
Ori reduces the inflammatory response
and oxidative stress in DSS-induced UC mice
To detect colon tissue infiltration in UC-induced
mice, MPO concentration in colon tissue was detected. Compared with
that in the control group, MPO concentration in the DSS group was
significantly increased. Furthermore, MPO concentration was
inhibited in the Ori-L and Ori-H groups compared with that in the
DSS group (Fig. 3A). ELISA was
performed to detect inflammatory factor concentration in murine
colon tissue. The results revealed that, when compared with that in
the control group, TNF-α, IL-1β and IL-6 concentration was
significantly increased after DSS induction. In addition, TNF-α,
IL-1β and IL-6 concentrations were all significantly decreased
after Ori administration compared with that in the DSS group
(Fig. 3B). The concentrations of
oxidative stress-related indices, including ROS, MDA, GSH-Px and
SOD were subsequently detected. The data revealed comparable
concentration trends between ROS and MDA, and TNF-α, IL-1β and
IL-6. However, the concentration trend of GSH-Px and SOD were the
reverse of TNF-α, IL-1β and IL-6 (Fig. 3C). The results indicated that Ori
treatment reduced the inflammatory response and oxidative stress in
DSS-induced UC mice.
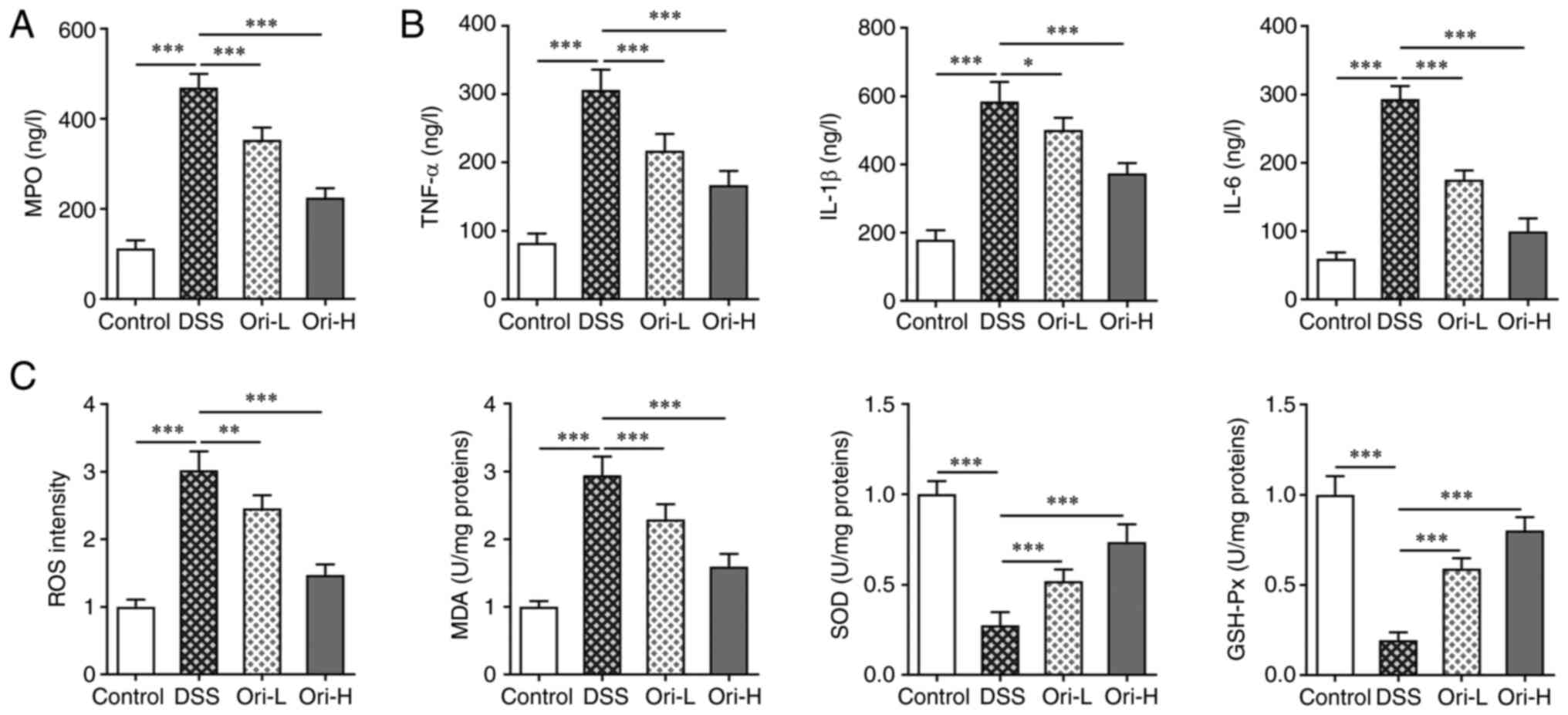 | Figure 3.Ori reduces inflammatory response and
oxidative stress in DSS-induced UC mice. ELISA kits were used to
analyze (A) MPO concentration and (B) inflammatory factors in colon
tissue. (C) Detection of proteins involved in oxidative stress were
analyzed in colon tissue. *P<0.05, **P<0.01, ***P<0.001.
DSS, dextran sulfate sodium; UC, ulcerative colitis; Ori, oridonin;
H, high-dose; L, low-dose; MPO, myeloperoxidase; ROS, reactive
oxygen species; SOD, superoxide dismutase; MDA, malondialdehyde;
GSH-Px, glutathione peroxidase. |
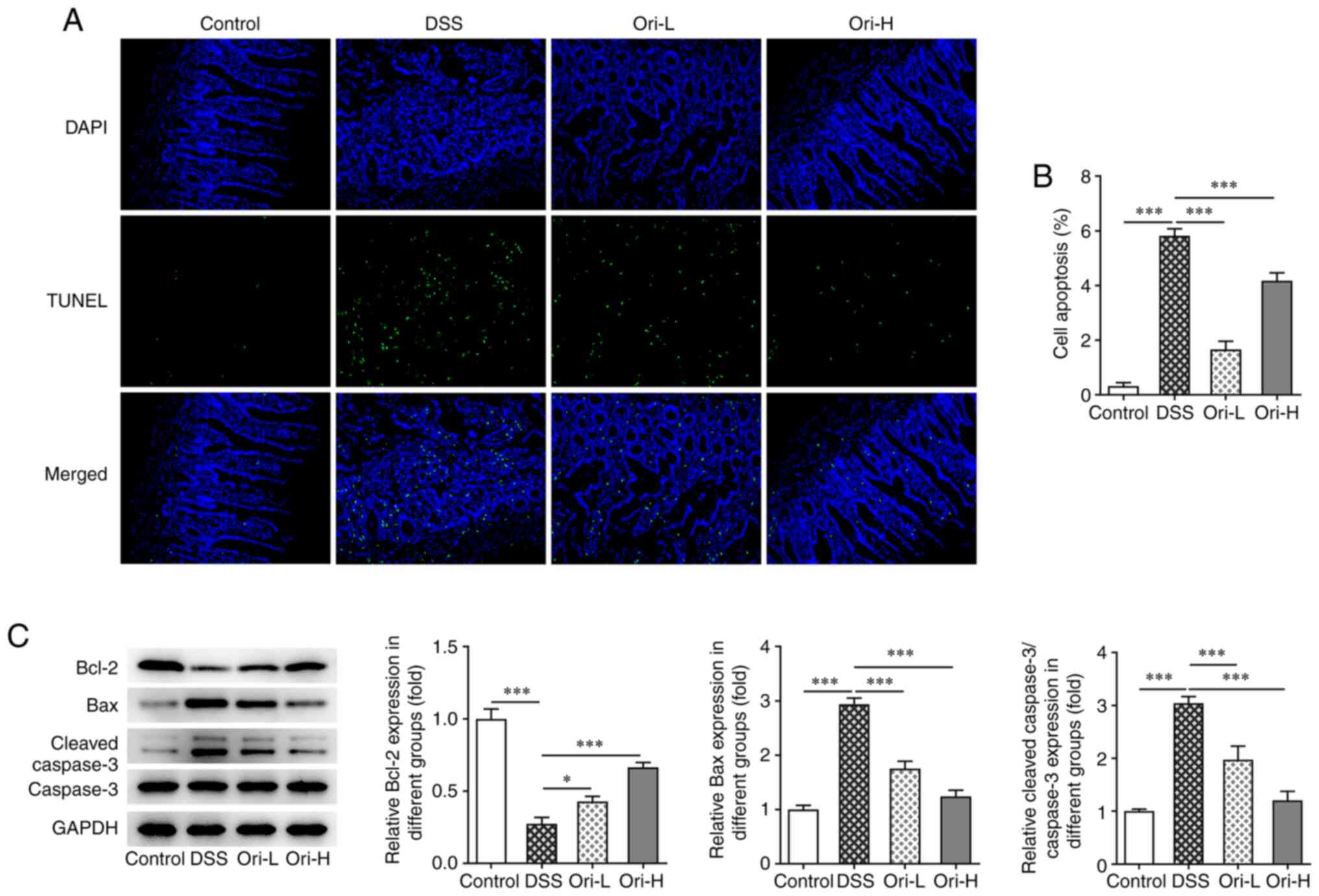
Ori inhibits intestinal mucosa cell
apoptosis in DSS-induced UC mice
A TUNEL assay was performed to detect cell
apoptosis. The results revealed that, compared with that in the
control group, apoptosis was significantly increased in the DSS
group (Fig. 4A and B).
Furthermore, decreased Bcl-2 and increased Bax and cleaved caspase
3 (Fig. 4C) protein expression
levels were observed. After Ori administration, apoptosis was
reversed, Bcl-2 protein expression levels were increased and Bax,
and cleaved caspase 3 protein expression levels were decreased.
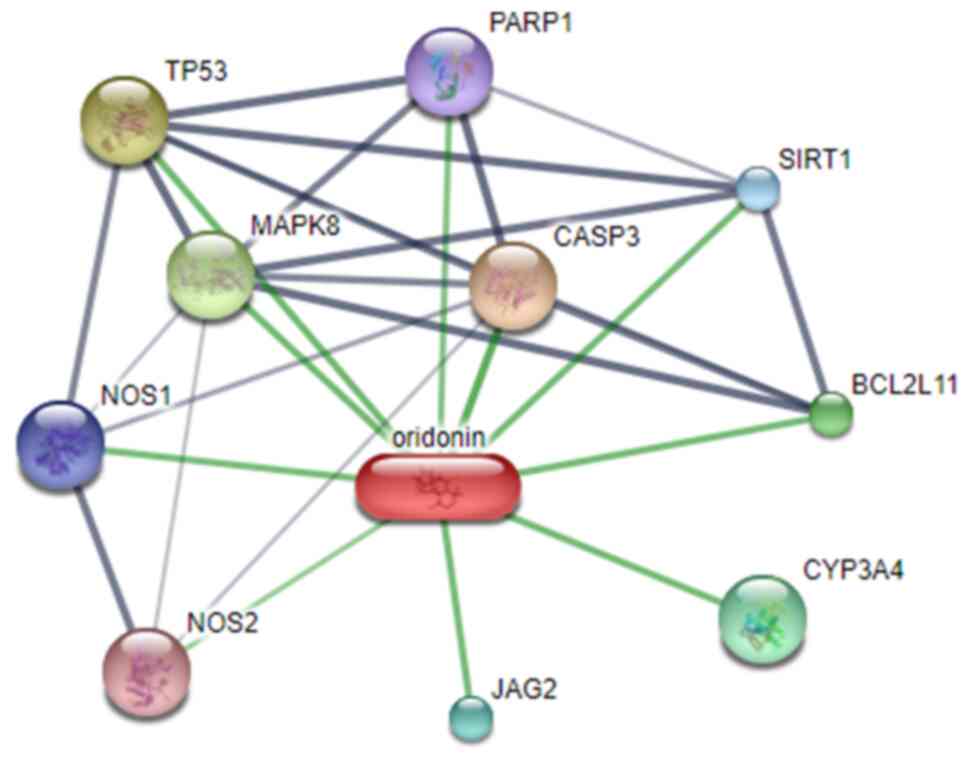
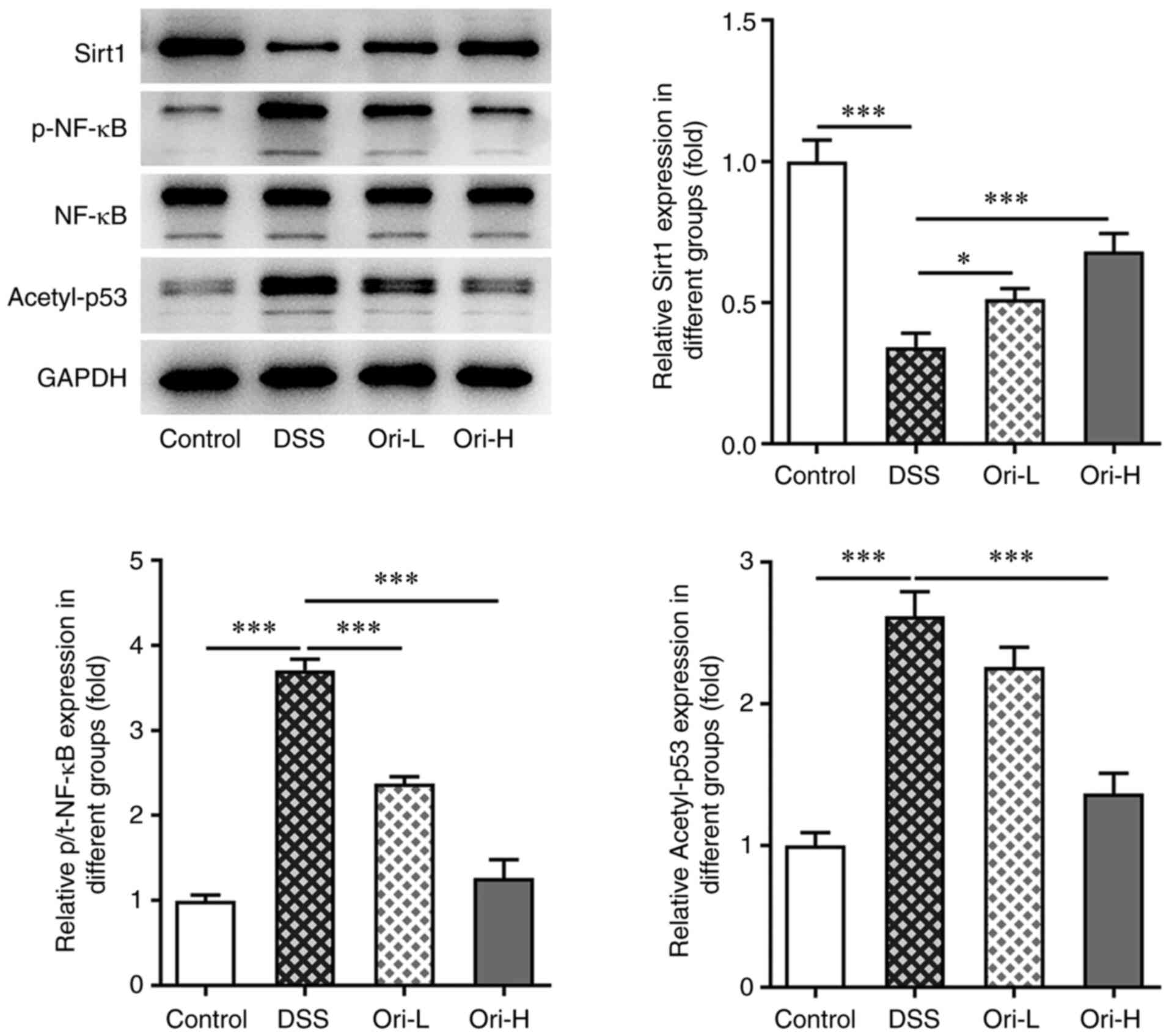
Ori affects the Sirt1/NF-κB/p53
signaling pathway
The STITCH database predicted that Sirt1 was a
potential target of Ori (Fig. 5).
The current study also revealed that the protein expression levels
of Sirt1, p-NF-κB and acetyl-p53 were significantly increased in
the DSS group compared with that in the control group, indicating
that the Sirt1/NF-κB/p53 pathway was activated in DSS-induced UC
mice. The protein expressions levels of Sirt1, p-NF-κB and
acetyl-p53 were reversed after Ori-L and Ori-H administration
(Fig. 6). These results suggested
that the activated Sirt1/NF-κB/p53 signaling pathway in DSS-induced
UC mice was blocked by Ori.
Discussion
UC is a major type of inflammatory bowel disease. In
recent years, the incidence rate of UC has been increasing yearly
and its pathogenesis and, treatment have become a focus of research
(5). At present, a variety of
modeling methods have been used to induce UC animals, among which
the DSS chemical method has been widely used, as it produces
similar effects to that of human UC (22). In the current study, DSS was used
in the present study to induce UC in mice and then related
indicators of UC were detected in mice after DSS induction. The
results revealed that the DAI in mice significantly increased,
while colon length decreased, and colon lesions became apparent. In
addition, inflammatory response and oxidative stress levels in the
colon tissues of DSS-induced mice were significantly increased. The
results indicated that UC occurred in DSS-induced mice.
A previous study has demonstrated that Ori
alleviates TNBS-induced inflammatory post-irritable bowel syndrome
via the PXR/NF-κB signaling pathway (12). In addition, Ori protects against
experimental murine brain injury by inhibiting the NLRP3
inflammasome (18). Ori has also
exerted protective effects against lipopolysaccharide-induced acute
lung injury by modulating nuclear factor erythroid 2-related factor
2 (Nrf2)-mediated oxidative stress and Nrf2-independent NLRP3, and
NF-κB pathways (23). It has
therefore been suggested that Ori plays a role in inflammatory
bowel disease. The current study demonstrated that Ori
significantly increased the length of the colon in DSS-induced UC
mice and improved pathological colon injury. In addition, Ori
reduced the levels of the inflammatory response and oxidative
stress, while inhibiting apoptosis in DSS-induced UC mice.
Under normal conditions, the intestinal tract
functions as a complete barrier to prevent pathogenic antigens from
invading the body and maintaining normal bodily function. However,
when intestinal epithelial tight junction proteins are damaged,
various pathogenic microorganisms, within the lumen, invade into
the body, causing mucosal inflammation and triggering the immune
response (24). The present study
detected the distribution and expression of specific tight
junction-related proteins, including ZO-1, occludin and claudin-1,
in murine colons to determine the integrity of the colonic
intestinal barrier. The results revealed that Ori treatment
significantly increased the protein expression level of ZO-1,
occludin and claudin-1 in the colon tissue of DSS-induced UC mice.
The results suggested that Ori protected the integrity of the
colonic mucosal barrier.
The STITCH database predicted that Sirt1 was a
potential target of Ori. The present study also determined that the
expression level of Sirt1 in the colon tissues of mice increased
after Ori was administered to the DSS-treated group. The protein
expression levels of p-NF-κB and acetyl-p53 were also increased,
which are downstream proteins of Sirt1. It has been previously
demonstrated that the inhibition of NF-κB-mediated macrophage
activation by Sirt1 upregulation can improve experimental colitis
in mice (16). These results
suggest that Sirt1 plays an important role in UC. The results of
the present study preliminarily concluded that Ori may ameliorate
UC in mice by targeting the Sirt1/NF-κB/p53 pathway. However, these
results should be further verified using pathway inhibitors in
future experiments. Ori can improve irritable bowel syndrome
induced by TNBS; however, there is a lack of research into Ori in
DSS-induced UC and the targeting of Ori on Sirt1 in UC; therefore,
to the best of our knowledge, for the first time, the present study
provided preliminary results.
There are also limitations in the present study. The
experiments into the mechanism of Ori on UC are deficient. The
mechanism involved will be further investigated in future studies
using Sirt1/NF-κB/p53 pathway inhibitors. In addition, we have
discussed the effect of Ori in UC animal models but have not
verified it in UC cell experiments. We will further verify it in UC
cell experiments in the following experiments.
In conclusion, the present study demonstrated that
Ori alleviated DSS-induced inflammatory response, oxidative stress
and intestinal mucosal apoptosis in UC mice possibly via the
Sirt1/NF-κB/p53 pathway. The results may provide a strong research
basis for the clinical treatment of UC with Ori.
Acknowledgements
Not applicable.
Funding
Funding: Not applicable.
Availability of data and materials
The analyzed datasets used and/or generated during
the present study are available from the corresponding author on
reasonable request.
Authors' contributions
DW contributed to the conception and design of this
study. MW performed the experiments, collected the data and
performed statistical analysis with the help of BX and LL. MW
drafted the manuscript, which was corrected and revised by DW. DW
and MW confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All animal procedures were performed according to
the NIH Guide for the Care and Use of Laboratory Animals approved
by the Experimental Animal Ethics Committee of Beihua University
(approval no. BHDX2021-116; Jilin, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Huppertz-Hauss G, Hoivik ML,
Jelsness-Jorgensen LP, Opheim R, Henriksen M, Høie O, Hovde Ø,
Kempski-Monstad I, Solberg IC, Jahnsen J, et al: Fatigue in a
population-based cohort of patients with inflammatory bowel disease
20 years after diagnosis: The IBSEN study. Scand J Gastroenterol.
52:351–358. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Snider AJ, Bialkowska AB, Ghaleb AM, Yang
VW, Obeid LM and Hannun YA: Murine model for colitis-associated
cancer of the colon. Methods Mol Biol. 1438:245–254. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pandey A, Shen C and Man SM: Inflammasomes
in colitis and colorectal cancer: Mechanism of action and
therapies. Yale J Biol Med. 92:481–498. 2019.PubMed/NCBI
|
|
4
|
Beamish LA, Osornio-Vargas AR and Wine E:
Air pollution: An environmental factor contributing to intestinal
disease. J Crohns Colitis. 5:279–286. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kucharzik T, Koletzko S, Kannengiesser K
and Dignass A: Ulcerative colitis-diagnostic and therapeutic
algorithms. Dtsch Arztebl Int. 117:564–574. 2020.PubMed/NCBI
|
|
6
|
Wang J, Wong YK and Liao F: What has
traditional Chinese medicine delivered for modern medicine? Expert
Rev Mol Med. 20:e42018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yan YX, Shao MJ, Qi Q, Xu YS, Yang XQ, Zhu
FH, He SJ, He PL, Feng CL, Wu YW, et al: Artemisinin analogue SM934
ameliorates DSS-induced mouse ulcerative colitis via suppressing
neutrophils and macrophages. Acta Pharmacol Sin. 39:1633–1644.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen M, Ding Y and Tong Z: Efficacy and
safety of sophora flavescens (Kushen) based traditional Chinese
medicine in the treatment of ulcerative colitis: Clinical evidence
and potential mechanisms. Front Pharmacol. 11:6034762020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang S, Zhong Z, Wan J, Tan W, Wu G, Chen
M and Wang Y: Oridonin induces apoptosis inhibits migration and
invasion on highly-metastatic human breast cancer cells. Am J Chin
Med. 41:177–196. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zou BH, Tan YH, Deng WD, Zheng JH, Yang Q,
Ke MH, Ding ZB and Li XJ: Oridonin ameliorates inflammation-induced
bone loss in mice via suppressing DC-STAMP expression. Acta
Pharmacol Sin. 42:744–754. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yan Y, Tan RZ, Liu P, Li JC, Zhong X, Liao
Y, Lin X, Wei C and Wang L: Oridonin alleviates IRI-induced kidney
injury by inhibiting inflammatory response of macrophages via
AKT-related pathways. Med Sci Monit. 26:e9211142020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shao YY, Guo Y, Feng XJ, Liu JJ, Chang ZP,
Deng GF, Xu D, Gao JP and Hou RG: Oridonin attenuates TNBS-induced
post-inflammatory irritable bowel syndrome via PXR/NF-κB signaling.
Inflammation. 44:645–658. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu QQ, Wang HL, Chen K, Wang SB, Xu Y, Ye
Q and Sun YW: Oridonin derivative ameliorates experimental colitis
by inhibiting activated T-cells and translocation of nuclear
factor-kappa B. J Dig Dis. 17:104–112. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma Z, Hu C and Zhang Y: Therapeutic effect
of Rabdosia rubescens aqueous extract on chronic pharyngitis and
its safety. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 36:170–173.
2011.(In Chinese). PubMed/NCBI
|
|
15
|
Ren MT, Gu ML, Zhou XX, Yu MS, Pan HH, Ji
F and Ding CY: Sirtuin 1 alleviates endoplasmic reticulum
stress-mediated apoptosis of intestinal epithelial cells in
ulcerative colitis. World J Gastroenterol. 25:5800–5813. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang K, Li YF, Lv Q, Li XM, Dai Y and Wei
ZF: Bergenin acting as an agonist of PPARgamma, ameliorates
experimental colitis in mice through improving expression of SIRT1,
and Therefore Inhibiting NF-κB-mediated macrophage activation.
Front Pharmacol. 8:9812017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pu Z, Liu Y, Li C, Xu M, Xie H and Zhao J:
Using network pharmacology for systematic understanding of
geniposide in ameliorating inflammatory responses in colitis
through suppression of NLRP3 inflammasome in macrophage by
AMPK/Sirt1 dependent signaling. Am J Chin Med. 48:1693–1713. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yan C, Yan H, Mao J, Liu Y, Xu L, Zhao H,
Shen J, Cao Y, Gao Y, Li K and Jin W: Neuroprotective effect of
oridonin on traumatic brain injury via inhibiting NLRP3
inflammasome in experimental mice. Front Neurosci. 14:5571702020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chaudhary G, Mahajan UB, Goyal SN, Ojha S,
Patil CR and Subramanya SB: Protective effect of Lagerstroemia
speciosa against dextran sulfate sodium induced ulcerative colitis
in C57BL/6 mice. Am J Transl Res. 9:1792–1800. 2017.PubMed/NCBI
|
|
20
|
Szklarczyk D, Franceschini A, Wyder S,
Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos
A, Tsafou KP, et al: STRING v10: Protein-protein interaction
networks, integrated over the tree of life. Nucleic Acids Res.
43:D447–D452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Szklarczyk D, Santos A, von Mering C,
Jensen LJ, Bork P and Kuhn M: STITCH 5: Augmenting protein-chemical
interaction networks with tissue and affinity data. Nucleic Acids
Res. 44(D1): D380–D384. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oh SY, Cho KA, Kang JL, Kim KH and Woo SY:
Comparison of experimental mouse models of inflammatory bowel
disease. Int J Mol Med. 33:333–340. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang H, Lv H, Li H, Ci X and Peng L:
Oridonin protects LPS-induced acute lung injury by modulating
Nrf2-mediated oxidative stress and Nrf2-independent NLRP3 and NF-κB
pathways. Cell Commun Signal. 17:622019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang J, Zhang C, Guo C and Li X: Chitosan
ameliorates DSS-induced ulcerative colitis mice by enhancing
intestinal barrier function and improving microflora. Int J Mol
Sci. 20:57512019. View Article : Google Scholar : PubMed/NCBI
|