Introduction
Epilepsy is a complex syndrome of the nervous system
induced by the over synchronized abnormal discharge of neurons.
There are approximately 70 million patients with epilepsy (PWE)
worldwide that mainly live in low-income countries (1). In China, the number of PWE is nearly
10 million (2). From both an
individual and societal perspective, the high morbidity rate and
long therapy period of epilepsy impose an enormous burden on public
health, the economy and mental wellbeing (3–5).
However, the mechanism of epilepsy still remains unclear. In recent
decades, new scientific research has reported that the activation
of inflammatory cells and molecules, and the regulation by
decomposition of injury serve key roles in epilepsy (6).
High mobility group box protein-1 (HMGB1) is a
nuclear protein with a stable nucleic acid structure that is
produced by activated monocytes or macrophages. When cells are
exposed to certain harmful signals, HMGB-1 can be produced in the
nucleus and then transferred to the cytoplasm, where it binds to
receptors to form late glycation end products (RAGE), Toll-like
receptor 2 and Toll-like receptor 4 (TLR4) (7). Previous studies have reported that
HMGB1 is a proinflammatory agent, which is related to the
production and release of multiple pro-inflammatory factors in
numerous diseases, including interleukin-1b, tumour necrosis
factor-α and intercellular cell adhesion molecule-1, and serves an
indispensable role in maintenance of the inflammatory response in
the late stage of inflammation (8). A recent study reported that HMGB1 was
significantly overexpressed in the hippocampus in drug-induced
status epilepticus (SE) rat models, which suggested that HMGB1 was
involved in pathophysiological processes and disease progression
(9). However, the possible
involvement of HMGB1 in SE has not been previously clarified.
p38 mitogen-activated protein kinase (p38MAPK)
serves a key role in the serine-lysine MAPK family, regulating the
production of inflammatory cells and oxidative stress in numerous
diseases (10). P38MAPK can be
activated by different extracellular stimuli and successfully
converted to phosphorylated (p)-p38MAPK by specific serine or
threonine phosphorylation and is subsequently transferred from the
cytoplasm into the nucleus, where it activates the expression of
transcription factors, regulates related genes and participates in
numerous biological processes, such as tissue development, cell
reproduction or apoptosis, inflammation and cancer metastasis
(11–15). In previous studies of myocardial
ischemia-reperfusion injury, asthma and intestinal injury, HMGB1
has been reported to be associated with the p38MAPK signalling
pathway (16–18). However, it has not previously been
reported whether HMGB1 participates in the pathogenesis of epilepsy
by regulation of the p38MAPK signalling pathway.
Pilocarpine-induced epilepsy is a well-established
animal model for SE (19).
Injection of pilocarpine in rats induces SE, which triggers a
cascade of molecular and cellular events, which lead to neuronal
cell death and eventually to epilepsy. Glycyrrhizin (GL) is a
natural anti-inflammatory component and can be extracted from
liquorice, a traditional Chinese medicine, or chemically
synthesized (20). It is a direct
inhibitor of HMGB1 and binds directly to both HMG boxes. GL exerts
neuroprotective effects via the blocking of HMGB1 release into the
extracellular space through interaction with HMG boxes (21). Based on the aforementioned data, it
was hypothesized that HMGB1 participates in status epilepticus via
the p38MAPK signalling pathway and, that GL regulates epileptic
seizures by inhibition of the activity of HMGB1 and p-p38MAPK. To
evaluate this hypothesis, a lithium-pilocarpine induced epileptic
seizures rat model was established and the protein expression
levels of HMGB1 in hippocampus during epileptic seizures were
assessed. The effectiveness of GL in treatment of
lithium-pilocarpine induced status epilepticus in rats was also
evaluated.
Materials and methods
Experimental animals
A total of 50 healthy adult male Sprague-Dawley (SD)
rats, weighing 250–280 g (age, 6–8 weeks), were purchased from the
Hunan SJA Laboratory Animal Co., Ltd. All rats were maintained
separately in a room with an average of 12 h light/dark cycles at
room temperature (24±2°C and 55±5% humidity) and provided food and
water ad libitum. After the SE model had been successfully
established for 24 h, the rats were anaesthetized using 1%
pentobarbital sodium (40 mg/kg) before being sacrificed. All
efforts were made to minimize the rat's suffering and the total
number of animals used (for example, prevention of asphyxia,
pneumonia and trauma). All procedures were approved by the Animal
Care and Use Committee of Zunyi Medical University [approval no.
KLLY(A)-2020-009].
Acute LiCl-pilocarpine induced a rat
status epilepticus model
SD rats were randomly divided into four groups
(n=12) as follows: control (Con), status epilepticus (SE), 2%
dimethyl sulfoxide treatment group (DMSO + SE) and GL treatment
group (GL + SE). Lithium chloride (LiCl) and pilocarpine were
prepared using 0.9% normal saline. Rats in the SE group were
administered 127 mg/kg lithium chloride by intraperitoneal
injection and SE was induced using 40 mg/kg pilocarpine by
intraperitoneal injection after waiting for 18–20 h, the peripheral
side effect of muscarinic/M-like symptoms was prevented using
intraperitoneal injection with 1 mg/kg atropine sulfate 30 min
before the induction phase, while the control group were injected
with the same volume of 0.9% normal saline. The rat's behaviour was
then evaluated using the Racine scale (22), briefly: 0, The rats demonstrated no
abnormal reactions; 1, the rats demonstrated shaking of the
whiskers, facial convulsions and chewing but no body movement; 2,
the rats demonstrated facial twitching, cramps and nodded
involuntarily; 3, the rats demonstrated one-side paroxysmal limb
twitching; 4, the rats were standing with two forelimb twitching;
5, the rats demonstrated a drop attack including the behaviour of a
level 4 attack. Only rats with persistent severe SE (Racine scale
4–5) were used in further studies. Thirty minutes after SE
induction, pilocarpine was again injected into rats with scores
below grade 4 until the score reached at least grade 4 (20). If the number of injections exceeded
5 and still did not reach grade 4, the modelling was considered to
have failed. The number of seizures and the latency period were
recorded. SE was terminated 60 min later by intraperitoneal
injection of 5 mg/ml diazepam (1 mg/kg). If SE continued, further
injections of diazepam (maximum, n=3) were administered. If any of
the following occurred, SE was terminated earlier than 60 min:
Continuous stage 5 seizures (rearing and falling) last longer than
5 min, severe dyspnoea or hypothermia.
For rats in the GL + SE group, 100 mg/kg GL
(prepared using fresh 2% DMSO) was injected 30 min prior to
pilocarpine injection and the other groups were injected with fresh
2% DMSO in the same volume.
Western blotting
After anaesthesia using 1% pentobarbital sodium (40
mg/kg), six rats were randomly selected from each group for
extraction of the brain tissue. The rats were sacrificed by
decapitation, the brains were dissected on a cooled plate and the
hippocampus was separated from brain tissues. The collected brain
tissue samples were homogenized in a radioimmune precipitation
buffer containing 1% phenylmethylsulfonyl fluoride (PMSF) (cat no.
HY-B0496, MedChemExpress), an irreversible serine/cysteine protease
inhibitor used to prepare cell lysates. In the present study, PMSF
was used to inhibit protein degradation during protein extraction.
The concentration of the supernatant was estimated using an Instant
BCA Protein Assay Kit (cat no. ZJ101, EpiZyme, Inc.). The 30 µg per
lane protein was loaded onto SDS-PAGE gels (stacking, 5%;
separation, 10%). The samples were then transferred to PVDF
membranes and blocked using 5% skim milk powder at room temperature
for 1 h. The membranes were incubated overnight at 4°C with an
anti-HMGB1-specific antibody (1:1,300; cat no. 10829-1-AP, Wuhan
Sanying Biotechnology) and then incubated at room temperature for 2
h with HRP-conjugated Affinipure goat anti-rabbit IgG (H+L)
(1:8,000; cat no. SA00001-2, Wuhan Sanying Biotechnology). After
rinsing with an appropriate buffer, protein bands were developed
using the Western Lightning Plus ECL kit (cat no. P0018FS; Shanghai
Beyotime Institute of Biotechnology), according to the
manufacturer's protocols. Blots were imaged using ChemicDoc™
Imaging System (Bio-Rad Laboratories, Inc.). ImageJ 1.52a (National
Institutes of Health) was used to assess the greyscale values and
SPSS 19.0 (IBM Corp.) was used to analyse the data.
Immunofluorescence
Rats were anaesthetized using 1% pentobarbital
sodium (40 mg/kg) and were then intracardially perfused with 0.9%
saline, followed by 4% paraformaldehyde. No heartbeat, continued
absence of spontaneous breathing for 2–3 min and no blink reflex
was considered to indicate mortality. Then, brain tissues were
isolated and stored in 4% paraformaldehyde overnight at 4°C. Brain
tissues were dehydrated using 20 and 30% sucrose solutions.
Finally, samples were cut into 10 µm sections. The sections were
repaired using microwave antigen retrieval set on high for 5 min
for a total of 3 times with citric acid buffer (pH, 6.0),
meanwhile, the membrane was permeabilized using 0.4% Triton X-100
at 37°C for 15 min. After inactivation of the endogenous enzymes
using 3% H2O2 at room temperature for 30 min,
goat serum (cat no. AR0009, Wuhan Boster Biological Technology,
Ltd.) was used to block membranes at room temperature for 20 min.
Each step before blocking with goat serum should be washed 5 min, 3
times with PBS solution. The sections were incubated overnight at
4°C with anti-HMGB1 antibody (1:100, cat no. 10829-1-AP and
66525-1-Ig, Wuhan Sanying Biotechnology), anti-neuronal nuclei
(NeuN) antibody (1:200, cat no. A11954-1, Wuhan Boster Biological
Technology, Ltd.) and anti-glial fibrillary acidic protein (GFAP)
antibody (1:300, cat no. BM0055, Wuhan Boster Biological
Technology, Ltd.). The samples were then rewarmed for 1 h at 37°C,
washed with PBS solution and incubated at 37°C for 1 h in a dark
environment with 488-conjugated anti-rabbit second fluorescent
antibody (1:200, cat no. ab150077, Abcam) and Cy3-conjugated
anti-rat second fluorescent antibody (1:200, cat no. A0507,
Beyotime Institute of Biotechnology). Sections were then washed
using PBS solution, incubated with DAPI solution for 5 min.
Finally, the sections were ready for imaging after sealing with
antifade solution (cat no. S3023, Dako, Agilent Technologies, Inc.)
at room temperature. Then the hippocampal region of the section was
assessed and imaged using laser-scanning confocal microscopy (Nikon
Corporation) within 48 h. The colocalization analyses were
performed using NIS-viewer (Nikon Corporation).
Immunohistochemistry
The rats were anaesthetized using 1% pentobarbital
sodium (40 mg/kg). After anesthetisation, the brains were removed
from cranial cavity after perfusion by saline and then 4%
paraformaldehyde according to the aforementioned protocol. The
tissues were fixed using 4% polyformaldehyde at 4°C overnight,
embedded in paraffin and sectioned to a thickness of 5 µm. Briefly,
sectioned specimens were deparaffinized using xylene for 20 min,
twice, and then rehydrated using a graded ethanol series (100, 95,
80 and 70%) for 5 min at room temperature. Antigen recovery was
performed using microwave antigen retrieval set on high for 5 min,
3 times, with citric acid buffer (pH, 6.0). After inactivation of
the endogenous enzymes using 3% H2O2 for 10
min at room temperature, sections were blocked using bovine serum
albumin (cat no. AR1006, Wuhan Boster Biological Technology, Ltd.)
for 30 min at room temperature. Then the sections were incubated at
4°C overnight with diluted primary antibodies against HMGB1 (1:200,
cat no. 10829-1-AP, Wuhan Sanying Biotechnology) and Iba1 (1:1,000,
cat no. 012-26723, FUJIFILM Wako Pure Chemical Corporation). They
were then rewarmed in an incubator at 37°C for 1.5 h. According to
the manufacturer's protocols for the immunohistochemical kit (cat.
no. PV9001, OriGene Technologies, Inc.), the sections were
incubated at 37°C for 30 min with the Bio-goat anti-rabbit IgG and
streptavidin-POD solution (1:100), then washed using PBS. Sections
were stained using DAB colour liquid (cat. no. B1072, ApplyGen
Technologies, Inc.) according to the manufacturer's protocols, in a
dark environment at room temperature and the staining reaction was
terminated when moderate tan particles were observed under the
microscope. The slices were differentiated with hydrochloric acid
alcohol differentiation solution (0.5%) for 2 sec, dehydrated for 5
min with each specific concentration of alcohol (70, 80, 95 and
100%) and neutral gum sealed after haematoxylin staining for 40 sec
at room temperature. Finally, immunohistochemical images were
captured using a CX43 light microscope (Olympus Corporation). The
protein expression levels of HMGB1 and activation of microglia were
assessed using ImageJ 1.52a (National Institutes of Health).
Transmission electron microscopy
(TEM)
Three rats in each group were anaesthetized using 1%
pentobarbital sodium (40 mg/kg) before decapitation. The CA1 region
in the hippocampus was separated, the CA1 tissue was cut into small
chunks of approximately 1 mm3 and was placed into 4%
glutaraldehyde. The samples were fixed using 2.5%
glutaraldehyde-paraformaldehyde mixture fixing solution (pH, 7.2)
for 2 h at room temperature, washed with PBS for 10 min, 3 times
and fixed in 1% osmic acid for 2 h at room temperature. Samples
were then dehydrated using an graded ethanol (50, 70, 80, 90 and
100%) for 10 min and 100% acetone for 15 min. Samples were then
embedded after soaking in epoxy resin, polymerized in an oven at
60°C for 46 h, cut into sections with a thickness of 70 nm and
stained for 15 min using 2% uranium acetate saturated alcohol
solution and lead citrate at room temperature, sequentially.
Samples were removed and placed in a ventilated place to dry
overnight and then assessed using TEM and images were captured for
evaluation.
Haematoxylin and eosin (H&E)
staining
For H&E staining, the brain tissues were
obtained using the aforementioned method used for
immunohistochemistry staining. Slices were fixed using a graded
alcohol series (100, 95, 85 and 70%) for 5 min and hydrated by
immersion in 1% hydrochloric acid alcohol for 30 sec at room
temperature. The slices were then stained using haematoxylin for 5
min and rinsed in water for 1 min at room temperature. The slices
were subsequently stained with 0.5% eosin for 3 min, and dehydrated
using a graded alcohol series (70, 85, 95 and 100%) for 5 min at
room temperature. Finally, sections were covered using a coverslip
and imaged using a HD-2700 scanning electron microscope (Hitachi
High-Technologies Corporation).
Statistical analysis
Data from the present study were analysed using SPSS
19.0 (IBM Corp.) for statistical analysis. Data are presented as
mean ± standard deviation, comparisons of >2 groups were
performed using 1-way ANOVA followed by Bonferroni's post hoc test
and P<0.05 was considered to indicate a statistically
significant difference.
Results
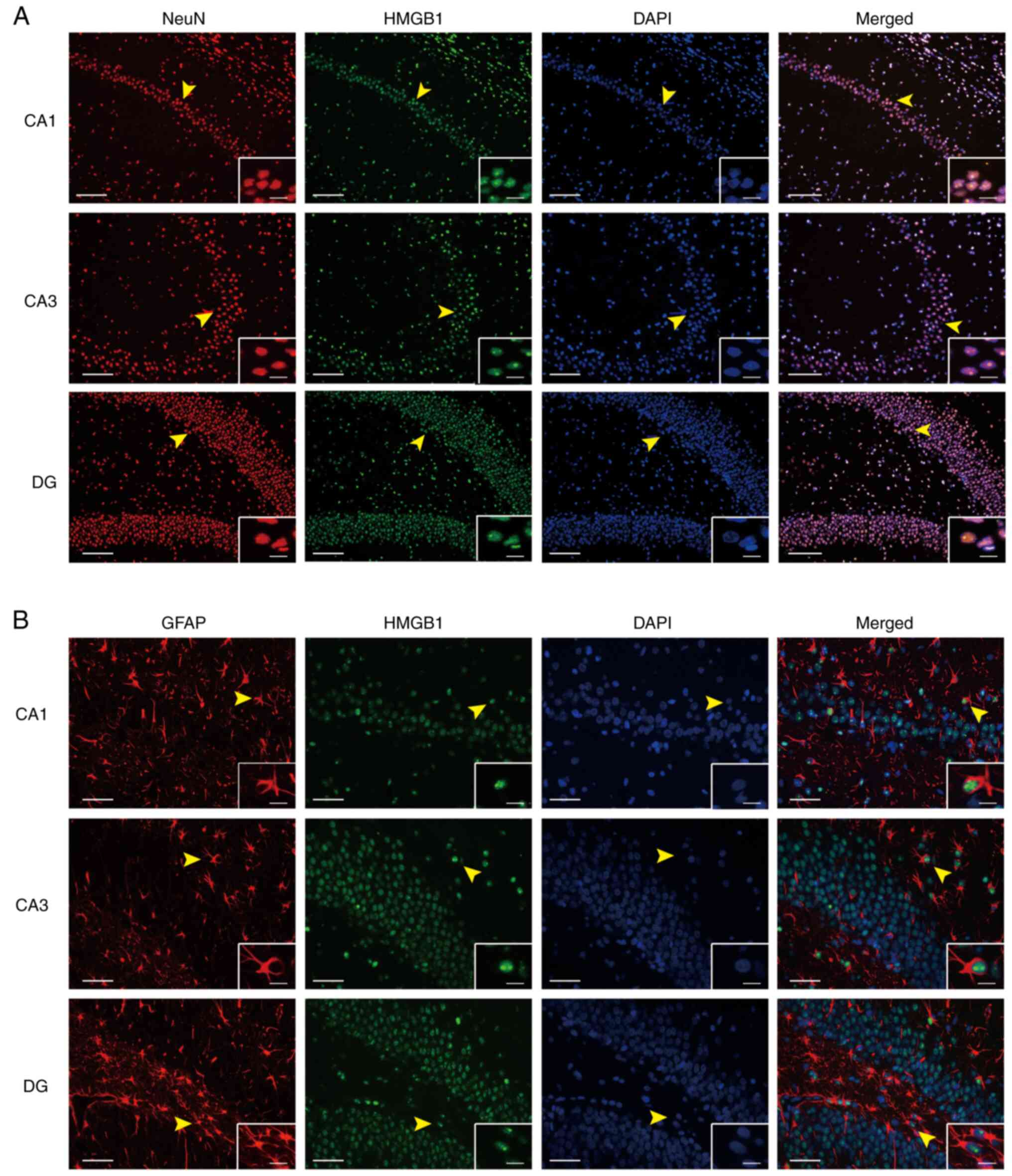
Localisation of HMGB1 in the
hippocampus of LiCl-pilocarpine induced SE rats
Evaluation of the protein expression levels of HMGB1
in the hippocampal neurons of the SE group demonstrated that HMGB1
was highly expressed in different hippocampal regions.
Immunofluorescence staining of neurons using the specific marker
NeuN, indicated that HMGB1 was co-expressed with neurons (Fig. 1A). Furthermore, immunofluorescence
staining of neurons with the specific marker GFAP demonstrated that
HMGB1 was also co-expressed with astrocytes (Fig. 1B).
HMGB1, p38MAPK and p-p38MAPK protein
expression levels in the hippocampus of LiCl-pilocarpine induced SE
rats
HMGB1, p38MAPK and p-p38MAPK protein expression
levels in the hippocampus of pilocarpine induced SE rats were
assessed using western blotting. Compared with the control group,
protein expression levels of HMGB1 were significantly upregulated
in the SE group. However, compared with SE group, the HMGB1 protein
expression level in the DMSO + SE group did not demonstrate a
significant difference. Compared with the DMSO + SE group, the
HMGB1 protein expression level in the GL + SE group was
significantly decreased (mean intensity ratio: 0.57±0.15 in the Con
group, 1.09±0.20 in the SE group, 1.08±0.20 in the DMSO + SE group
and 0.8±0.17 in the GL + SE group; Fig. 2A and B). Western blotting was also
performed to evaluate the p38MAPK and p-p38MAPK protein expression
levels in the hippocampus of pilocarpine induced SE rats. The ratio
of p-p38MAPK/p38MAPK was evaluated and demonstrated a similar trend
to the protein expression levels of HMGB1 in the hippocampus of
pilocarpine induced SE rats (mean ratio of p-p38MAPK/p38MAPK:
0.39±0.09 in the control group, 0.71±0.08 in the SE group,
0.69±0.13 in the DMSO + SE group and 0.49±0.09 in the GL + SE
group; Fig. 2C-E).
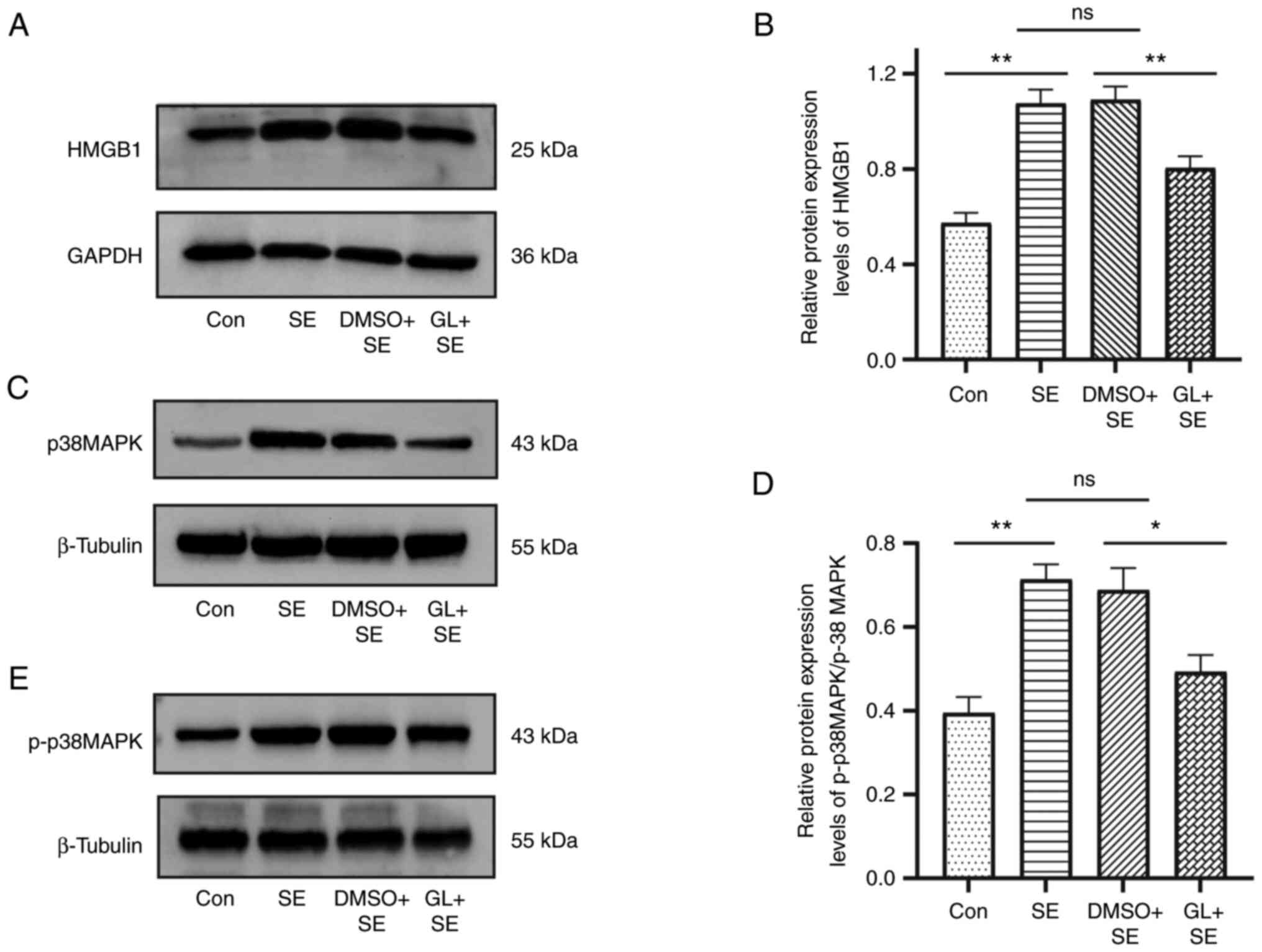 | Figure 2.GL affects the protein expression
levels of HMGB1, p38MAPK and p-p38MAPK in the hippocampus of
pilocarpine induced SE rats. Representative images of (A) HMGB1,
(C) p38MAPK and (E) p-p38MAPK protein expression levels in the
hippocampus of pilocarpine induced SE rats. Comparison of western
blotting intensity ratios of (B) HMGB1/GAPDH and (D)
p-p38MAPK/p38MAPK in the hippocampus of pilocarpine induced SE
rats. The base protein expression level of HMGB1, p38MAPK and
p-p38MAPK was assessed in the Con group. Data presented were the
mean of four technical replicates (n=6 in each group). *P<0.05
and **P<0.01 vs. SE group. GL, glycyrrhizin; HMGB1, high
mobility group box protein-1; MAPK, mitogen-activated protein
kinase; p, phosphorylated; SE, status epilepticus; Con, control;
ns, not significant. |
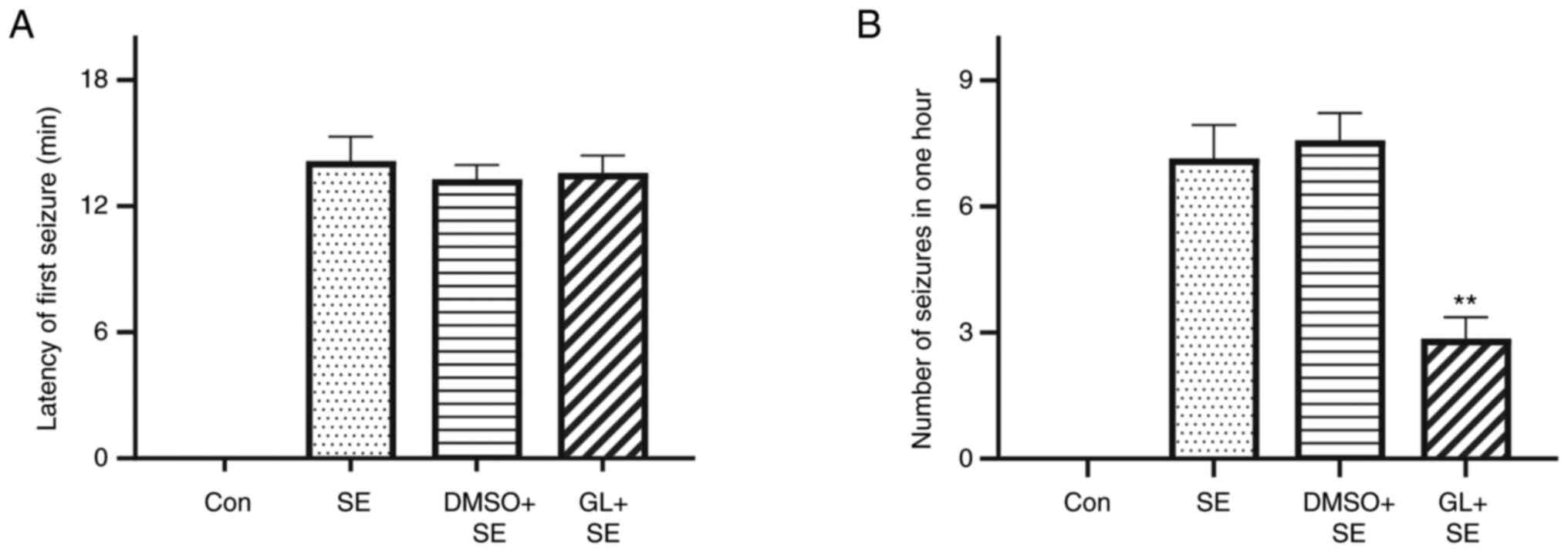
GL affects the latency of the first
seizure and the number of epileptic seizures in LiCl-pilocarpine
induced SE rats
Behavioural results indicated that the control group
had no seizures. The latency of the first seizure in the SE and
DMSO + SE groups were 14.14±3.08 and 13.29±1.9 min, respectively,
and the latency of the first seizure in the GL + SE group was
13.57±2.23 min. The number of seizures within 1 h following the
first seizure in the SE and DMSO + SE groups were 7.1±2.13 and
7.6±1.72, respectively, and the number of seizures within 1 h
following the first seizure in the GL + SE group was 2.9±1.35. The
results demonstrated no significant differences amongst the latency
or the number of seizures within 1 h between the SE and the DMSO +
SE groups. Compared with the SE and the DMSO + SE groups, the GL +
SE group demonstrated no significant effect on the seizure latency
(Table I; Fig. 3A). However, the GL + SE group did
demonstrate a significantly reduced number of seizures compared
with the SE group (Table I;
Fig. 3B).
 | Table I.Glycyrrhizin affects the latency of
the first seizure and the number of epileptic seizures in rats. |
Table I.
Glycyrrhizin affects the latency of
the first seizure and the number of epileptic seizures in rats.
| Groups | Latency of firstmer
seizure (min) | Number of seizures
in one hour |
|---|
| Con | 0 | 0 |
| SE | 14.14±3.08 | 7.1±2.13 |
| DMSO + SE | 13.29±1.90 | 7.6±1.72 |
| GL + SE | 13.57±2.23 |
2.9±1.35a |
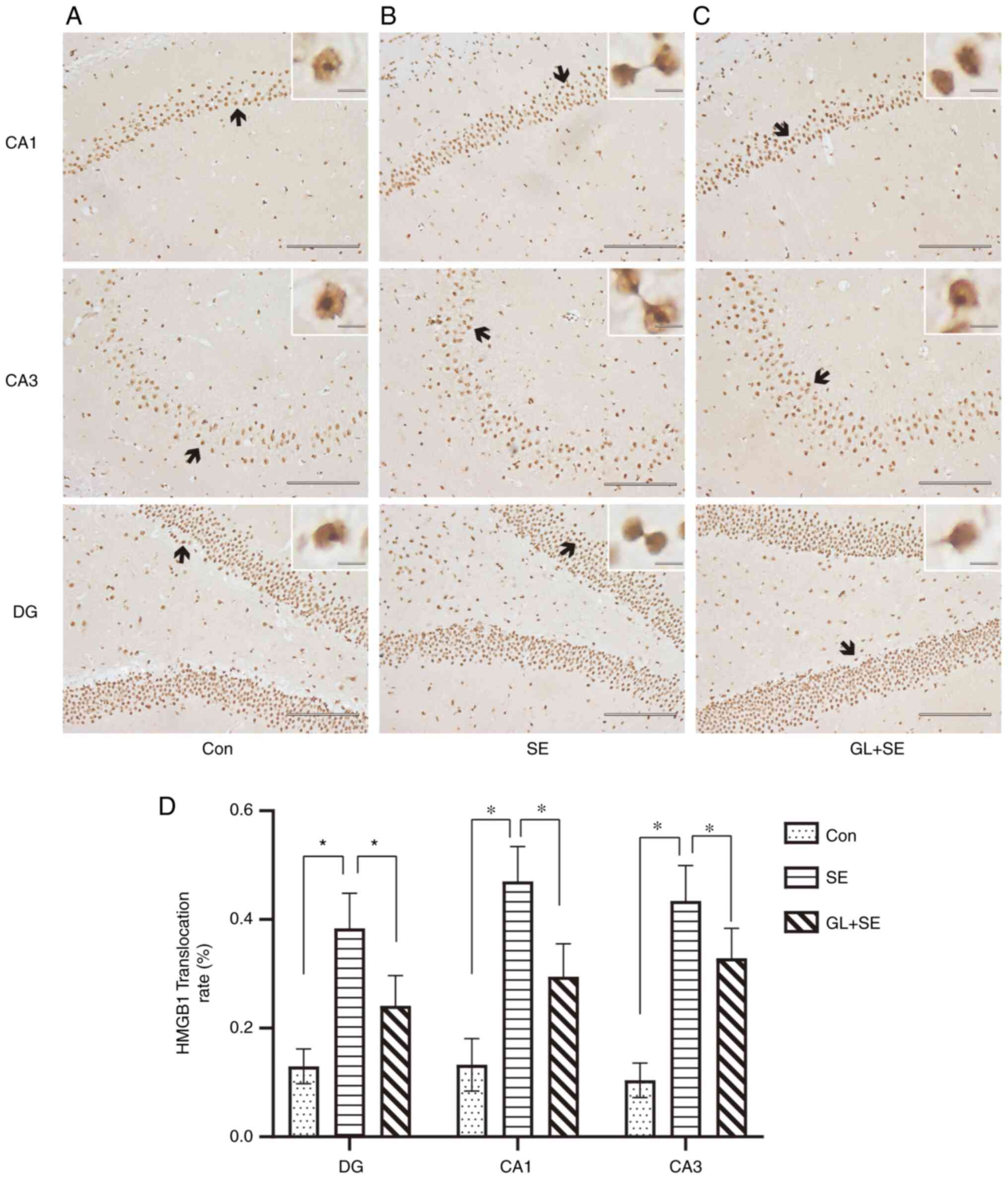
GL alters translocation and release of
HMGB1 from the nucleus of cells in the hippocampus of
LiCl-pilocarpine induced SE rats
Immunohistochemistry was used to assess the protein
expression levels and degree of translocation of HMGB1 in
hippocampal tissues. It was demonstrated that HMGB1 positive cells
in the Con group (Fig. 4A) were
mainly located in the nucleus and cytoplasm of neurons, whereas in
the SE group (Fig. 4B), nuclear
cytoplasmic translocation and extracellular release occurred in
HMGB1 positive cells. Compared with SE group, the nuclear
cytoplasmic translocation and extracellular release were markedly
inhibited in HMGB1 positive cells (Fig. 4C). The HMGB1 translocation rate
post-SE treatment was calculated (Fig.
4D). The HMGB1 translocation rate in the SE group was
significantly higher compared with those of the Con and GL + SE
groups. The data suggested that translocation of HMGB1 from the
nucleus to the cytoplasm was significantly inhibited in the GL + SE
group (Fig. 4D) compared with the
SE group.
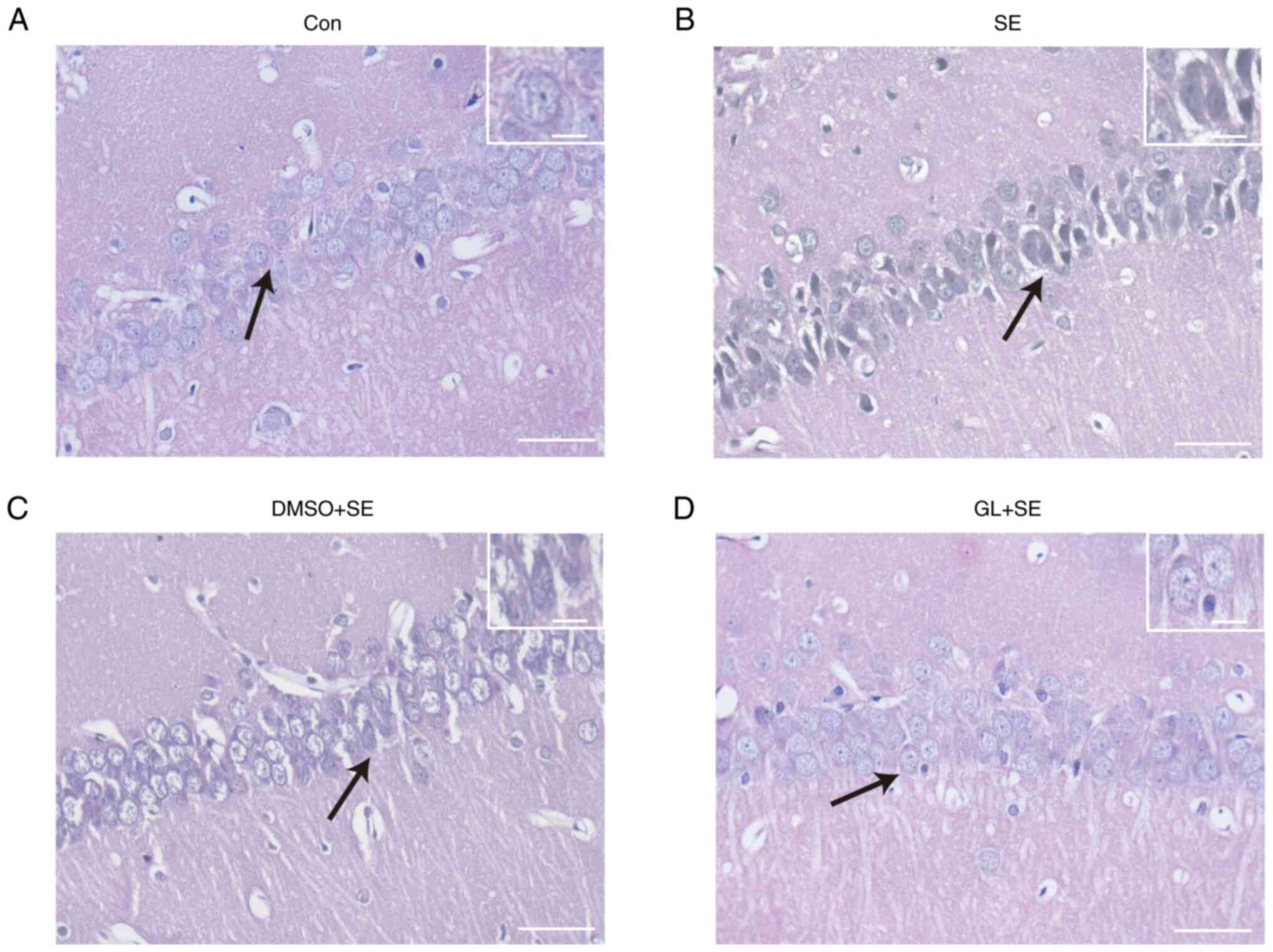
GL attenuates neuronal injury in the
hippocampal CA1 region of LiCl-pilocarpine induced SE rats
H&E staining demonstrated that hippocampal
neurons in the Con group (Fig. 5A)
were neatly arranged with clear cell contours and nucleoli, and
chromatin in the neurons was transparent and evenly distributed
around the nucleus. The neurons in the SE (Fig. 5B) and DMSO + SE groups (Fig. 5C) were irregularly arranged and
impaired, and the neurons in both groups were pyroptotic and
demonstrated cytoplasmic vacuoles. The neuronal arrangement in the
GL + SE group (Fig. 5D) was
slightly disordered, with neuronal lysis and necrosis; however, the
degree of neuronal damage was markedly less than that in SE and
DMSO + SE groups.
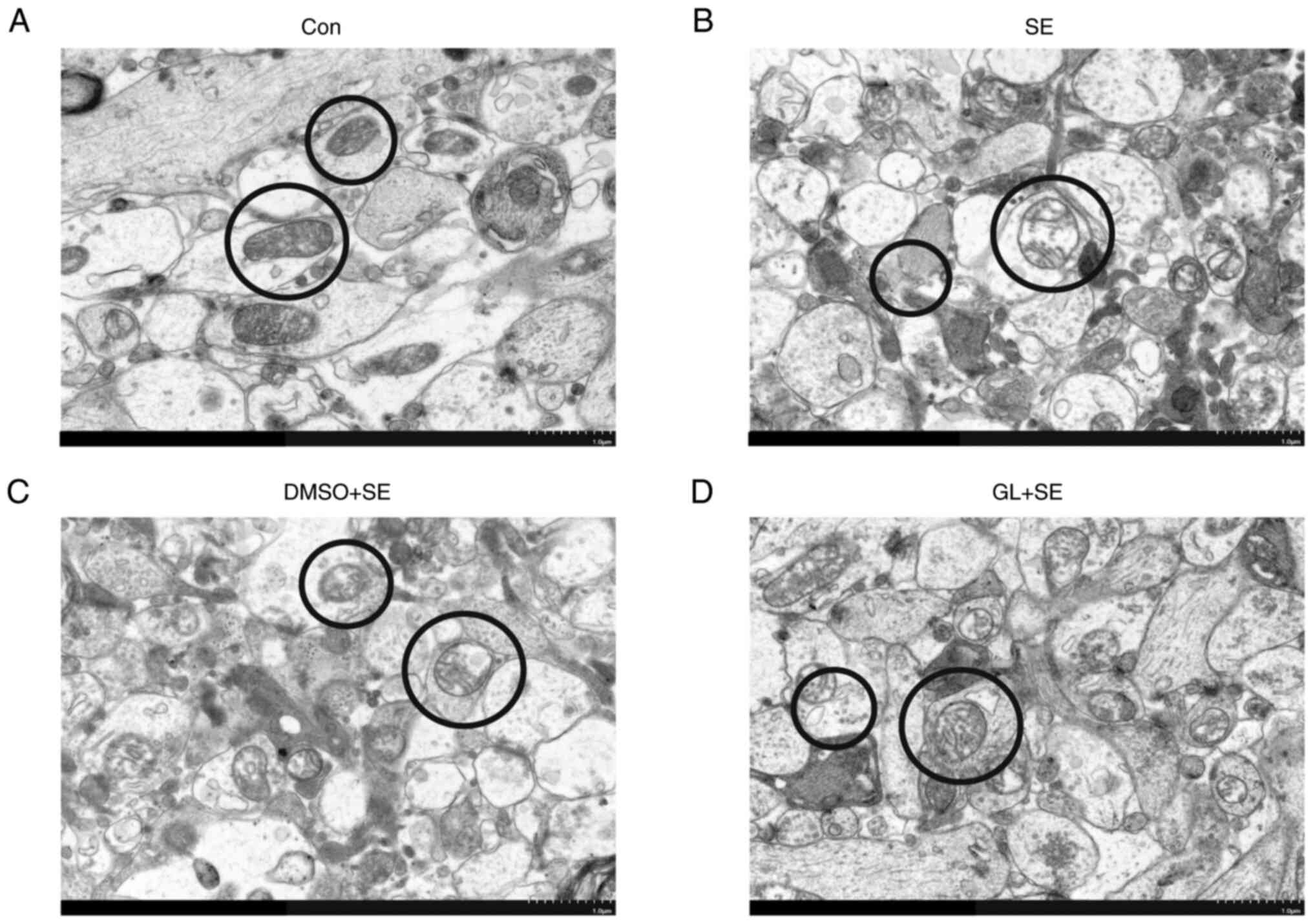
GL reduces mitochondrial damage in the
hippocampal CA1 region of LiCl-pilocarpine induced SE rats
The ultrastructure of mitochondria of neurons in the
CA1 region of rats' hippocampi were assessed using TEM. The
ultrastructure of mitochondria was clear and both the inner and
outer membranes were discoid or short rods were continuous and
intact and had a high matrix density in the Con group (Fig. 6A). The ultrastructure of
mitochondria in the other three groups were damaged to varying
degrees: The SE (Fig. 6B) and DMSO
+ SE (Fig. 6C) groups had unclear
mitochondrial structure, obvious swelling, reduced matrix density
and partial or complete degradation of inner and outer membranes.
Moreover, in the GL + SE group (Fig.
6D), part of mitochondrial structure was broken and swollen,
and part of membrane was damaged; however, the degree of damage was
less than that in the SE and DMSO + SE groups.
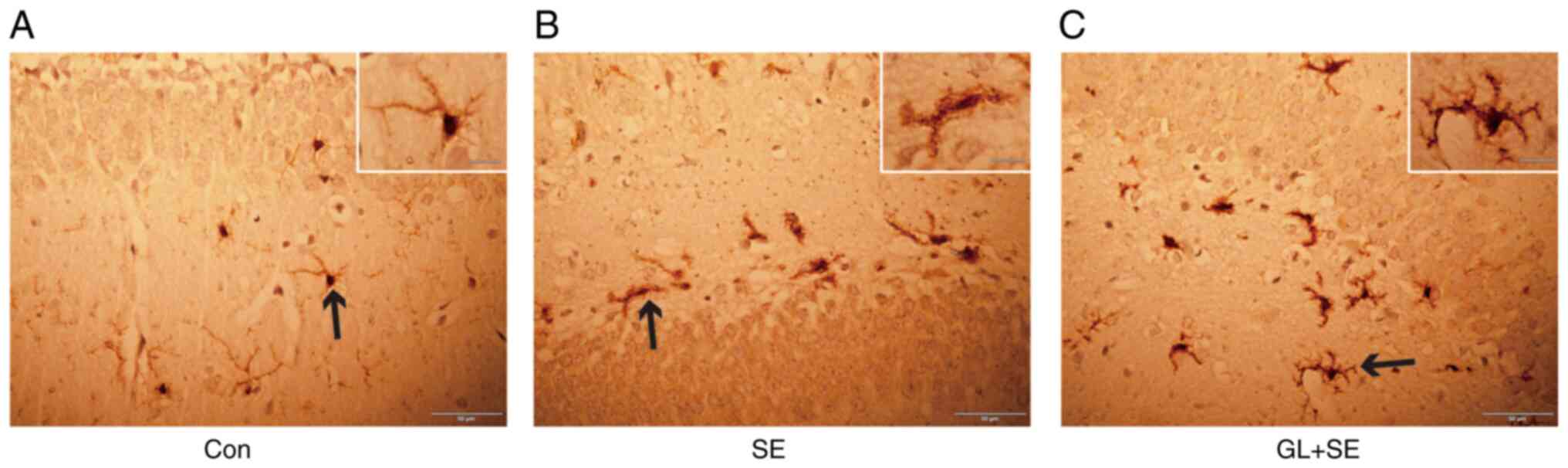
GL alters microglial activation in the
hippocampus of LiCl-pilocarpine induced SE rats
The extent of glial activation was assessed using
immunohistochemical staining with Iba-1, which specifically
labelled microglia cells. In the hippocampus, it was demonstrated
that microglia in the Con group (Fig.
7A) were characterized by small cell size, dense cytoplasm,
multiple elongated dendrites and phagocytosis at rest. Compared
with those in the Con group, microglia in SE group (Fig. 7B) were in the activation stage and
mainly manifested with cell body enlargement, cytoplasm dilution,
dendrite thickening and shortening, and phagocytosis. Compared with
the Con group, although the cell body was still enlarged, the
dendrites were shortened and partially thickened in the GL + SE
group (Fig. 7C) which indicated
that GL markedly inhibited the activation of microglia.
Discussion
Numerous studies have reported the pathogenesis of
epilepsy, including but not limited to axon sprouting, dendritic
morphology, inflammatory cell infiltration, glial cell
proliferation, vascular growth, and degenerative changes (23–28),
which demonstrated great potential for the treatment of epilepsy.
However, the exact pathogenic mechanisms of epilepsy remain
unclear.
HMGB1 is a new and attractive intervention target
for epilepsy. Although understanding of HMGB1 has increased, little
is known about the exact mechanism by which HMGB1 participates in
the development of epileptic seizures. Previous studies have
reported that HMGB1 participated in the development of epilepsy
mainly by interaction with RAGE (29) or TLR4 (30), damage to the blood-brain barrier
and the induction of the inflammatory cascade. Furthermore, HMGB1
has been reported to contribute significantly to the inflammatory
response and is associated with sterile inflammation and,
autoimmune and neurodegenerative diseases (31). During the development of epilepsy,
HMGB1 translocates from the nucleus to the cytoplasm after injury
and is released into the extracellular space to act as a
proinflammatory factor which induces an inflammatory response
(32). Because the CA1 region of
hippocampus is particularly vulnerable to hypoxia and ischemia, the
present study focused on the regulation of the HMGB1/p38MAPK
signalling pathway by GL. The lack of CA3 and dentate gyrus regions
in parts of the study is a limitation of the present study which
requires further evaluation in the future. The present study
demonstrated that among LiCl-pilocarpine-induced acute epileptic
rats, HMGB1 protein was significantly upregulated after seizures.
In the control group, the immunohistochemical results illustrated
that HMGB1-positive cells mainly existed in the neuronal nucleus
and markedly reduced numbers existed in the cytoplasm. For a total
of 24 h following seizures, HMGB1-positive cells underwent nuclear
translocation to the cytoplasm and some HMGB1 was released into the
extracellular space. Evaluation of immunofluorescence labelled
HMGB1, demonstrated that HMGB1 was expressed in both hippocampal
neurons and astrocytes. Furthermore, different experimental methods
were used to demonstrate that HMGB1 may participate in the
development of epileptic seizures, which was in agreement with
previously reported studies.
HMGB1, like other inflammatory agents, may induce
epileptic seizures by damaging the blood-brain barrier (BBB). HMGB1
can induce and maintain BBB injury through the regulation of
endothelial tight junctions and the base membrane or promote
epileptic seizures through the regulation of the neuronal
excitability and epileptic threshold during epilepsy (32,33).
During seizures, BBB damage may facilitate the invasion of the
brain parenchyma by HMGB1 and other inflammatory molecules, which
may aggravate epileptic seizures (34). The BBB can be damaged by
extracellular potassium influx, which may depolarize the neurons
directly or cause other serious consequences, such as injury to the
potassium buffer capacity and activation of glial cells, which then
induce inflammation, synaptogenesis, the injury of mitochondrial
dynamic balance function and finally induce SE (35,36).
Previous studies have reported that HMGB1 was released into the
central and peripheral bloodstream after rats were treated with
pilocarpine, which may contribute to the development of epilepsy by
facilitation of BBB damage and activation of inflammatory agents
(37). The present study
demonstrated that microglia were activated during epileptic
seizures and were mainly characterized by enlarged cell bodies and,
thicker and shorter dendrites. The mitochondria in hippocampal
neurons were significantly changed, mainly characterized by oedema,
membrane breakdown and reduced matrix density. These changes
suggested that the mitochondria were severely damaged. The
aforementioned results were in agreement with previously reported
research.
The MAPK signal transduction pathway serves an
important role in the occurrence and development of nervous system
diseases; furthermore, inhibition of the MAPK signalling pathway
can significantly reduce the damage of nerve cells (38). The JNK and p38 signalling pathways,
as the two most important signal transduction pathways of MAPK, are
mainly involved in the regulation of cell proliferation,
differentiation, apoptosis, necrosis, and the cell cycle (39,40).
The present study demonstrated that p38MAPK was activated in the
status epilepticus model, accompanied by different degrees of
neuronal loss and hippocampal sclerosis. Therefore, it was
hypothesised GL could inhibit the p38MAPK signalling pathway by the
suppression of HMGB1, which could reduce neuronal damage and change
epilepsy progression after seizures. The present study demonstrated
that intraperitoneal injection of GL 30 min before the seizures
significantly reduced the number of seizures but had no significant
influence on the latency of the first seizure, which may have been
associated with GL inhibition of the translocation and release of
HMGB1. GL has anti-inflammatory, hepatoprotective and
neuroprotective effects (41–43).
It was previously reported that GL produced neuroprotective effects
in the postischemic brain as well as in the kainic acid induced
epileptic model in rats (44,45).
Furthermore, GL was also reported to have prevented excitotoxic
effects on primary cultures (46).
The mechanism of GL in the protection against neuron injury and
mitochondrial injury have been previously reported to include
suppression of oxidative stress and proinflammatory cytokines
(44). Certain studies reported
that GL could combine with HMGB1 directly, which obstructed the
interaction between the key serine residue of HMGB1 and protein
kinase C, which thereby inhibited the phosphorylation of HMGB1 and
the subsequent translocation of HMGB1 (47), which was in agreement with the
results of the present study. Western blotting was used to assess
the protein expression levels of p38MAPK and p-p38MAPK after
injection with GL to inhibit HMGB1. The results of the present
study demonstrated that the protein expression levels of p38MAPK
and p-p38MAPK were significantly downregulated in the hippocampal
tissue 24 h after seizures, which indicated that HMGB1 participated
in epileptic seizures via the p38MAPK signalling pathway. The
present study also demonstrated that activated microglia were
inhibited after HMGB1 downregulation and previous studies reported
that one of the mechanisms by which p38MAPK promoted inflammation
progression was to regulate the activation of microglia (48), which indirectly indicated that
HMGB1 may regulate the participation of the p38MAPK signalling
pathway in epileptic seizures.
In summary, the results of the present study
demonstrated that HMGB1 protein expression levels were
significantly upregulated during epileptic seizures. After
glycyrrhizin-specific inhibition of HMGB1, hippocampal neuron and
mitochondrial injury can be alleviated, p38MAPK and p-p38MAPK
protein expression levels were markedly downregulated and
microglial activation was inhibited, which markedly reduced the
number of epileptic seizures in one hour. The mechanism may be
through regulation of the p38MAPK signalling pathway by HMGB1 and
participation in epileptogenesis. However, there are certain
limitations to the present study: The study only used a single dose
and route of administration (intraperitoneal injection of 100
mg/kg) of GL in rats and lacked cellular experiments; furthermore,
the possibility of the involvement of other inflammatory signalling
pathways in this study cannot be excluded and these could have
interfered with the results. Further study of the effect of
different doses and routes of GL administration of its influence on
the paroxysm of epileptic seizures is required. Furthermore, the
detailed mechanism of HMGB1 activation of neuron injury and
astrocyte activation needs to be assessed using cell culture
experiments.
A better understanding of the exact mechanisms by
which HMGB1 participates in epileptic seizures is of great
significance for the treatment of epilepsy. A new medicine that
targets HMGB1, which could be put into use in the neuroscience
field would provide a novel pathway for epilepsy therapy.
In conclusion, GL can alleviate neuronal injury in
the CA1 regions of the hippocampus and prevent HMGB1 translocation
from the nucleus into the cytoplasm in this area. GL may exert
neuroprotective effects through the suppression of the expression
of p38MAPK and p-p38MAPK. The present study increases understanding
of the mechanism by which HMGB1 may participate in status
epilepticus and lay a foundation for the exploration of the
possible use of GL as a new antiseizure drug.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Zunyi City Science and
Technology Foundation [grant no. (2018)64], The Zunyi Medical
College Neurological Graduate Workstation (grant no. GZZ2017004),
The Science and Technology Project in Guizhou Province [grant no.
ZK (2022) General 656] and The Science and Technology Project of
Guizhou Health Commission (grant no. gzwkj2021.017 and
gzwjkj2020-1-010).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZCX and ZXX conceived and designed the study. ZL,
MX, LHZ and HQZ performed the experiments. MX and HQZ performed
statistical analysis, ZL, MX and HQZ wrote the manuscript. ZL, MX,
LHZ, ZCX and ZXX reviewed and edited the manuscript. All authors
read and approved the final manuscript. ZL and MX confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
All experimental procedures were approved by The
Animal Care and Use Committee of Zunyi Medical University (approval
no. KLLY(A)-2020-009).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mallok A, Vaillant JD, Soto MT,
Viebahn-Hänsler R, Viart Mde L, Pérez AF, Cedeño RI and Fernández
OS: Ozone protective effects against PTZ-induced generalized
seizures are mediated by reestablishment of cellular redox balance
and A1 adenosine receptors. Neurol Res. 37:204–210. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ding D, Zhou D, Sander JW, Wang W, Li S
and Hong Z: Epilepsy in China: Major progress in the past two
decades. Lancet Neurol. 20:316–326. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shangguan Y, Xu X, Ganbat B, Li Y, Wang W,
Yang Y, Lu X, Du C, Tian X and Wang X: CNTNAP4 impacts epilepsy
through GABAA receptors regulation: Evidence from temporal lobe
epilepsy patients and mouse models. Cereb Cortex. 28:3491–3504.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu X, Shangguan Y, Lu S, Wang W, Du C,
Xiao F, Hu Y, Luo J, Wang L, He C, et al: Tubulin β-III modulates
seizure activity in epilepsy. J Pathol. 242:297–308. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao Y, Li X, Zhang K, Tong T and Cui R:
The progress of epilepsy after stroke. Curr Neuropharmacol.
16:71–78. 2018.PubMed/NCBI
|
|
6
|
Rana A and Musto AE: The role of
inflammation in the development of epilepsy. J Neuroinflammation.
15:1442018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang QW, Wang JZ, Li JC, Zhou Y, Zhong Q,
Lu FL and Xiang J: High-mobility group protein box-1 and its
relevance to cerebral ischemia. J Cereb Blood Flow Metab.
30:243–254. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang H, Wang H and Andersson U: Targeting
inflammation driven by HMGB1. Front Immunol. 11:4842020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li YJ, Wang L, Zhang B, Gao F and Yang CM:
Glycyrrhizin, an HMGB1 inhibitor, exhibits neuroprotective effects
in rats after lithium-pilocarpine-induced status epilepticus. J
Pharm Pharmacol. 71:390–399. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He Y, She H, Zhang T, Xu H, Cheng L, Yepes
M, Zhao Y and Mao Z: p38 MAPK inhibits autophagy and promotes
microglial inflammatory responses by phosphorylating ULK1. J Cell
Biol. 217:315–328. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moreno-Cugnon L, Arrizabalaga O, Llarena I
and Matheu A: Elevated p38MAPK activity promotes neural stem cell
aging. Aging (Albany NY). 12:6030–6036. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yeung YT, Aziz F, Guerrero-Castilla A and
Arguelles S: Signaling pathways in inflammation and
anti-inflammatory therapies. Curr Pharm Des. 24:1449–1484. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Martínez-Limón A, Joaquin M, Caballero M,
Posas F and de Nadal E: The p38 pathway: From biology to cancer
therapy. Int J Mol Sci. 21:19132020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gaestel M: MAPK-activated protein kinases
(MKs): Novel insights and challenges. Front Cell Dev Biol.
3:882016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang HW, Ding JD, Zhang ZS, Zhao SS, Duan
KY, Zhu BQ, Zhao WF, Chai ZT and Liu XW: Critical role of p38 in
spinal cord injury by regulating inflammation and apoptosis in a
rat model. Spine (Phila Pa 1976). 45:E355–E363. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang DY, Zhang AX, Zhou YH, Wang LH and
Yao HC: Protection of intravenous HMGB1 on myocardial ischemia
reperfusion injury. Int J Cardiol. 184:280–282. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liang Y, Hou C, Kong J, Wen H, Zheng X, Wu
L, Huang H and Chen Y: HMGB1 binding to receptor for advanced
glycation end products enhances inflammatory responses of human
bronchial epithelial cells by activating p38 MAPK and ERK1/2. Mol
Cell Biochem. 405:63–71. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu S, Chen HZ, Xu ZD, Wang F, Fang H,
Bellanfante O and Chen XL: Sodium butyrate inhibits the production
of HMGB1 and attenuates severe burn plus delayed
resuscitation-induced intestine injury via the p38 signaling
pathway. Burns. 45:649–658. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Delgado-Escueta AV, Wasterlain C, Treiman
DM and Porter RJ: Status epilepticus: Summary. Adv Neurol.
34:537–541. 1983.PubMed/NCBI
|
|
20
|
Musumeci D, Roviello GN and Montesarchio
D: An overview on HMGB1 inhibitors as potential therapeutic agents
in HMGB1-related pathologies. Pharmacol Ther. 141:347–357. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mollica L, De Marchis F, Spitaleri A,
Dallacosta C, Pennacchini D, Zamai M, Agresti A, Trisciuoglio L,
Musco G and Bianchi ME: Glycyrrhizin binds to high-mobility group
box 1 protein and inhibits its cytokine activities. Chem Biol.
14:431–441. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Racine RJ: Modification of seizure
activity by electrical stimulation. II. Motor seizure.
Electroencephalogr Clin Neurophysiol. 32:281–294. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luo Z, Wang J, Tang S, Zheng Y, Zhou X,
Tian F and Xu Z: Dynamic-related protein 1 inhibitor eases
epileptic seizures and can regulate equilibrative nucleoside
transporter 1 expression. BMC Neurol. 20:3532020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Godale CM and Danzer SC: Signaling
pathways and cellular mechanisms regulating mossy fiber sprouting
in the development of epilepsy. Front Neurol. 9:2982018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rossini L, De Santis D, Mauceri RR,
Tesoriero C, Bentivoglio M, Maderna E, Maiorana A, Deleo F, de
Curtis M, Tringali G, et al: Dendritic pathology, spine loss and
synaptic reorganization in human cortex from epilepsy patients.
Brain. 144:251–265. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vezzani A, Balosso S and Ravizza T:
Neuroinflammatory pathways as treatment targets and biomarkers in
epilepsy. Nat Rev Neurol. 15:459–472. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao XF, Liao Y, Alam MM, Mathur R,
Feustel P, Mazurkiewicz JE, Adamo MA, Zhu XC and Huang Y:
Microglial mTOR is neuronal protective and antiepileptogenic in the
pilocarpine model of temporal lobe epilepsy. J Neurosci.
40:7593–7608. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ogaki A, Ikegaya Y and Koyama R: Vascular
abnormalities and the role of vascular endothelial growth factor in
the epileptic brain. Front Pharmacol. 11:202020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sarkis RA, Goksen Y, Mu Y, Rosner B and
Lee JW: Cognitive and fatigue side effects of anti-epileptic drugs:
An analysis of phase III add-on trials. J Neurol. 265:2137–2142.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Maroso M, Balosso S, Ravizza T, Liu J,
Bianchi ME and Vezzani A: Interleukin-1 type 1 receptor/Toll-like
receptor signalling in epilepsy: The importance of IL-1beta and
high-mobility group box 1. J Intern Med. 270:319–326. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Paudel YN, Semple BD, Jones NC, Othman I
and Shaikh MF: High mobility group box 1 (HMGB1) as a novel
frontier in epileptogenesis: From pathogenesis to therapeutic
approaches. J Neurochem. 151:542–557. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Iori V, Maroso M, Rizzi M, Iyer AM,
Vertemara R, Carli M, Agresti A, Antonelli A, Bianchi ME, Aronica
E, et al: Receptor for advanced glycation endproducts is
upregulated in temporal lobe epilepsy and contributes to
experimental seizures. Neurobiol Dis. 58:102–114. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nishibori M, Wang D, Ousaka D and Wake H:
High mobility group box-1 and blood-brain barrier disruption.
Cells. 9:26502020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fu L, Liu K, Wake H, Teshigawara K,
Yoshino T, Takahashi H, Mori S and Nishibori M: Therapeutic effects
of anti-HMGB1 monoclonal antibody on pilocarpine-induced status
epilepticus in mice. Sci Rep. 7:11792017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Devinsky O, Vezzani A, Najjar S, De
Lanerolle NC and Rogawski MA: Glia and epilepsy: Excitability and
inflammation. Trends Neurosci. 36:174–184. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gorter JA, van Vliet EA and Aronica E:
Status epilepticus, blood-brain barrier disruption, inflammation,
and epileptogenesis. Epilepsy Behav. 49:13–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Walker LE, Frigerio F, Ravizza T, Ricci E,
Tse K, Jenkins RE, Sills GJ, Jorgensen A, Porcu L, Thippeswamy T,
et al: Molecular isoforms of high-mobility group box 1 are
mechanistic biomarkers for epilepsy. J Clin Invest. 129:21662019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kaminska B, Gozdz A, Zawadzka M,
Ellert-Miklaszewska A and Lipko M: MAPK signal transduction
underlying brain inflammation and gliosis as therapeutic target.
Anat Rec (Hoboken). 292:1902–1913. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
de Los Reyes Corrales T, Losada-Pérez M
and Casas-Tintó S: JNK pathway in CNS pathologies. Int J Mol Sci.
22:38832021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wei TH and Hsieh CL: Effect of acupuncture
on the p38 signaling pathway in several nervous system diseases: A
systematic review. Int J Mol Sci. 21:46932020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pastorino G, Cornara L, Soares S,
Rodrigues F and Oliveira MBPP: Liquorice (Glycyrrhiza glabra): A
phytochemical and pharmacological review. Phytother Res.
32:2323–2339. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Asl MN and Hosseinzadeh H: Review of
pharmacological effects of Glycyrrhiza sp. and its bioactive
compounds. Phytother Res. 22:709–724. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ahmed-Farid OA, Haredy SA, Niazy RM,
Linhardt RJ and Warda M: Dose-dependent neuroprotective effect of
oriental phyto-derived glycyrrhizin on experimental neuroterminal
norepinephrine depletion in a rat brain model. Chem Biol Interact.
308:279–287. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kim SW, Jin Y, Shin JH, Kim ID, Lee HK,
Park S, Han PL and Lee JK: Glycyrrhizic acid affords robust
neuroprotection in the postischemic brain via anti-inflammatory
effect by inhibiting HMGB1 phosphorylation and secretion. Neurobiol
Dis. 46:147–156. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Luo L, Jin Y, Kim ID and Lee JK:
Glycyrrhizin attenuates kainic acid-induced neuronal cell death in
the mouse hippocampus. Exp Neurobiol. 22:107–115. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cherng JM, Lin HJ, Hung MS, Lin YR, Chan
MH and Lin JC: Inhibition of nuclear factor kappaB is associated
with neuroprotective effects of glycyrrhizic acid on
glutamate-induced excitotoxicity in primary neurons. Eur J
Pharmacol. 547:10–21. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sakamoto R, Okano M, Takena H and Ohtsuki
TK: Inhibitory effect of glycyrrhizin on the phosphorylation and
DNA-binding abilities of high mobility group proteins 1 and 2 in
vitro. Biol Pharm Bull. 24:906–911. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Giovannini MG, Scali C, Prosperi C,
Bellucci A, Vannucchi MG, Rosi S, Pepeu G and Casamenti F:
Beta-amyloid-induced inflammation and cholinergic hypofunction in
the rat brain in vivo: Involvement of the p38MAPK pathway.
Neurobiol Dis. 11:257–274. 2002. View Article : Google Scholar : PubMed/NCBI
|