Cardiovascular diseases are the main cause of
mortality globally, accounting for ~30% of annual worldwide
mortalities (1). This number is
expected to rise to ~40% by the year 2030 (2). The high mortality rate of
cardiovascular diseases indicates that further assessments are
required to understand the mechanisms underlying the development of
these diseases and identify other pharmacological agents targeting
protection against heart injuries. The P2X7 purinergic receptor
(P2X7R) has been implicated in several signaling pathways and in
the development of a variety of pathological conditions including
chronic neuropathic pain, neurodegenerative diseases, such as
multiple sclerosis, inflammatory disorders, orthopedic diseases,
such as osteoporosis and cancerous diseases such as lung cancer
(3–10). Studies have highlighted the role of
the P2X7R in the development of heart diseases, such as acute
myocardial infarction, myocardial ischemia-reperfusion injury and
myocarditis (11–13). It is interesting to note that the
importance of the P2X7R in heart injury mediated by the coronavirus
disease-2019 (COVID-19) has been highlighted by various studies
(14–16). These studies have revealed a
potential new approach that positions the P2X7R as a prognostic
cardiac biomarker and pharmacological target for the prevention and
treatment of heart injuries, which may increase survival and
improve the quality of life in patients with heart diseases. The
present review article provided a brief overview of the properties
of the P2X7R, its distribution in the heart and its pathological
role in heart diseases, with a particular focus on acute myocardial
infarction, myocardial ischemia-reperfusion injury, autoimmune
myocarditis and various types of cardiomyopathies as well as
myocardial injury induced by COVID-19.
P2X7R belongs to a family of purinergic receptors.
This family is categorized into two main groups, namely the P1 and
P2 receptors (17). P2 receptors
are subdivided into P2X receptors (P2XRs), which are ligand-gated
ion channels and P2Y receptors (P2YRs), which are G-protein coupled
receptors (18,19). A total of seven P2XR subtypes
(P2X1R-P2X7R) and eight P2YR subtypes (P2Y1R, P2Y2R, P2Y4R, P2YR6
and P2Y11R-P2Y14R) have been identified to date (20,21).
All subtypes of P2XR are non-selective cation channels activated by
exogenous adenosine triphosphate (ATP), which is triggered by the
efflux of K+ and the influx of Na+ and
Ca2+ (22). ATP is
stored intracellularly in synaptic vesicles and is released from
neuronal and non-neuronal cells in response to various stimuli such
as hypoxia, pain, infection and inflammation (23,24).
It is released into the extracellular space by vesicular exocytosis
or pore-forming channels by pannexin 1 and connexin 43 (23–26).
The release of ATP by vesicular exocytosis is a calcium-dependent
release that is dependent on an increase in intracellular calcium
concentration, whereas the ATP release through pore-forming
channels is a calcium-independent release (27).
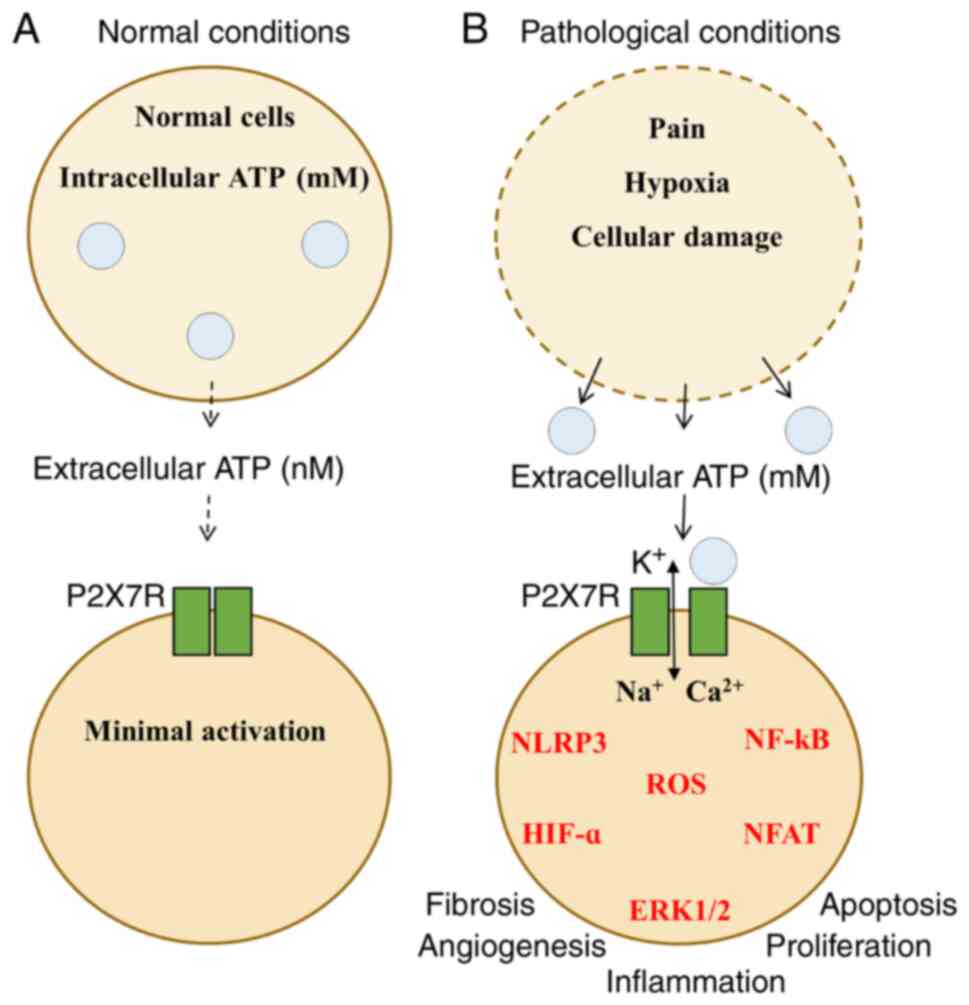
P2X7R has distinctive features that differentiate it
from the other P2XRs. One of these features is that it is activated
by ATP with a half maximal effective concentration
(EC50) value in the range of 0.1–1 mM. This range is
higher than that of the other P2XRs (EC50, 1–10 µM)
(28). Its activation requires a
massive amount of extracellular ATP, which does not usually exist
in normal cells (29). It seems
likely that P2X7Rs have low activity levels under normal conditions
(Fig. 1A). By contrast, during
certain pathological conditions, including hypoxia, inflammation,
pain, cellular damage and other stress conditions, high levels of
extracellular ATP can activate P2X7Rs and subsequent multiple
signaling pathways (30).
Activation of P2X7Rs opens cationic channels that facilitate the
flux of small cations, such as K+, Na+ and
Ca2+, resulting in the activation of several
intracellular signaling pathways (31). These pathways include the
activation of nucleotide-like receptor family pyrin domain member 3
(NLRP3) inflammasome, NF-κB, nuclear factor of activated T cells
(NFAT), hypoxia-inducible factor 1-alpha (HIF-1α) and ERK1/2 as
well as the formation of reactive oxygen species (ROS; Fig. 1B) (32,33).
Furthermore, sustained activation of P2X7Rs opens large pores that
facilitate the passage of large cations and organic dyes, such as
choline and ethidium, respectively, which result in apoptotic cell
death (34–36).
The NLRP3 inflammasome is the most notable response
following P2X7R activation. The binding of ATP to P2X7Rs allows
K+ efflux and Na+ and Ca2+ influx.
Low levels of intracellular K+ induce the configuration
of the NLRP3 inflammasome with apoptosis-associated speck-like
protein containing a caspase recruitment domain (ASC). This
stimulates caspase-1 that cleaves the pro-inflammatory cytokines
pro-IL-1β and pro-IL-18 to form IL-1β and IL-18, thus contributing
to a series of inflammatory responses (37). These cytokines may induce
profibrotic TGF-β1, resulting in fibrosis (38). Furthermore, the NLRP3 inflammasome
with ASC activates caspase-1 that cleaves Gasdermin-D, which forms
membrane pores and promotes the inflammatory cell death program
(39). P2X7R induces inflammation
by activation of NF-κB which increases several inflammatory
cytokine genes, such as TNF-α and IL-1β (40). Similarly, P2X7R activation promotes
NFAT function, which in turn leads to IL-2 inflammatory cytokine
secretion, downregulation of glycogen synthase kinase activity and
lymphocyte proliferation (32,41,42).
HIF-1α is also a marked response following P2X7R activation.
Ca2+ influx induced by P2X7R activation upregulates
HIF-1α through phosphoinositide 3-kinase (PI3K)/protein kinase B
(Akt)/mammalian target of rapamycin (mTOR) signaling pathway
(43,44). This association between P2X7R and
HIF-1α may promote angiogenesis by activating the vascular
endothelial growth factor (VEGF) secretion (45,46).
Furthermore, Ca2+ influx after P2X7R activation may
induce cell proliferation and migration by enhancing ERK1/2
phosphorylation and activating NF-κB transcription, which in turn
leads to the secretion of matrix metalloproteinases (MMPs)
(47,48). In addition, the P2X7R activation
may induce cell death by the formation of ROS (49). Taken together, the data indicate
that P2X7R is possibly a starting point for the activation of
several intracellular signaling pathways, which in turn cause
inflammation, fibrosis, proliferation, angiogenesis and cell death.
These multiple intracellular signaling pathways induced by the
P2X7R activity indicate that P2X7Rs may represent an attractive
target for the prevention and treatment of numerous pathological
conditions.
P2X7Rs are distributed widely throughout the body.
In the heart, P2X7Rs are found in embryonic stem cell-derived
cardiomyocytes and their activation can increase the expression
levels of several cardiac-specific genes, such as α-myosin heavy
chain (α-MHC) and α-actinin (50).
They are also expressed in epicardium-derived cells, indicating
that they play roles in embryonic cardiac growth and development
(51). P2X7Rs are mainly present
in the sinoatrial node, right atrium and left ventricular. In the
rat heart, P2X7R is highly expressed in the right atrium and left
ventricular, while in the human heart it is highly expressed in the
right atrium (52). Furthermore,
these receptors are present in cardiac muscle cells notably atrial
cardiomyocytes and other cardiac cells, such as cardiac endothelial
cells and cardiac fibroblasts (53,54).
The wide distribution of the P2X7R in cardiac cells suggests that
this receptor has a crucial role in several cardiac pathological
conditions, which are discussed in detail below.
Acute myocardial infarction (AMI), commonly called a
heart attack, is a serious condition characterized by sustained
ischemia and reduced blood flow to the heart muscle, resulting in
an accelerated death of heart muscle cells (55). Several cellular and molecular
changes occur following myocardial infarction, which can be
categorized into the inflammatory, proliferative and maturation
phases. Firstly, the inflammatory phase (the early phase within 0–4
days) is characterized by the release of ROS and pro-inflammatory
mediators as well as fibrin deposition and necrosis of
cardiomyocytes. Secondly, the proliferative phase (within ~1–2
weeks) is characterized by extracellular matrix deposition,
myofibroblast differentiation, angiogenesis and the formation of
tissue granules. Thirdly, the maturation phase (from weeks to
months), is characterized by apoptosis of myofibroblasts and the
formation of a mature scar (56–58).
A strong relationship has been noted between P2X7R
expression and AMI. P2X7Rs are upregulated in an experimental in
vivo AMI model (13,59,60).
A clinical study confirmed that P2X7R mRNA expression was
upregulated in patients with AMI (61), suggesting that P2X7Rs may be used
as predictive biomarkers in AMI. Previous studies have shown that
the upregulation of P2X7R expression in AMI is commonly accompanied
by an enhanced inflammatory response. For example, overexpression
of P2X7R following myocardial infarction increases the NLRP3
inflammasome and release the proinflammatory cytokine IL-1β
(59). Furthermore, activation of
P2X7Rs aggravated AMI injury in an animal model resulting in
increased ROS levels and vasopressin activity (60). Taken together, these studies
indicate that the P2X7R plays a key role in the pathogenesis of
myocardial infarction.
The inhibition of P2X7Rs may enhance cardiac
function and improve survival following AMI. Gao et al
(13) indicated that inhibition of
P2X7Rs with short hairpin RNA attenuated sympathetic
hyperinnervation by suppressing nerve growth factor levels. This
study further indicated that inhibition of P2X7Rs ameliorated
inflammatory infiltration by suppressing NF-κB activation and
improved cardiac dysfunction by inhibiting the AKT/ERK1/2 signaling
pathways (13). Furthermore,
Mezzaroma et al (62)
demonstrated that inhibition of P2X7Rs with small interfering RNA
prevents caspase-1 activity and ameliorates cardiac remodeling. The
role of P2X7R in AMI was confirmed by using specific P2X7R
antagonists. It was shown that the P2X7R antagonist
pyridoxalphosphate-6-azophenyl-2′,4-disulfonic acid hindered the
formation of the ASC/cryopyrin inflammasome and reduced infarct
size as well as cell death following AMI (62). In addition, the application of the
P2X7R antagonist Brilliant Blue G (BBG) attenuated sympathetic
hyperactivity and cardiac dysfunction by reducing oxidative stress
as well as vasopressinergic cell activation in AMI rats (60). These studies indicate that P2X7R is
a possible candidate for the treatment of myocardial infarction,
particularly during the early inflammatory phase. The effect of
P2X7Rs in the late phases of myocardial infarction remains to be
elucidated. Therefore, further studies are required to verify
whether the P2X7R plays a key role during the proliferation and
maturation phases following myocardial infarction.
Myocardial ischemia/reperfusion (I/R) injury is a
complex condition characterized by the restoration of blood flow to
the ischemic heart muscle (63).
P2X7R expression is upregulated in the experimental model of
myocardial I/R injury (64,65).
There is controversy regarding the ability of P2X7R to exacerbate
or reduce myocardial I/R injury. A previous study indicated that
the P2X7R promoted cardiac damage by inducing inflammatory
responses in myocardial I/R injury. In an animal model of
myocardial I/R injury, overexpression of the P2X7R was shown to
increase the activity of NF-κB and release several inflammatory
cytokines, such as IL-6, IL-8, IL-10 and TNF-α (64). However, it is still unknown whether
P2X7R inhibitors can preserve heart function in response to
myocardial I/R injury. Therefore, further assessment studies on the
effect of the P2X7R-induced inflammatory response in myocardial I/R
injury are recommended.
In contrast to these findings, other studies have
shown that the activation of the P2X7R can alleviate myocardial I/R
injury by stimulating the release of endogenous cardioprotectants.
During myocardial I/R injury, it was demonstrated that pannexin-1
interacts with P2X7Rs to form a channel. The activation of this
channel is responsible for the release of the cardioprotectants
adenosine and sphingosine 1-phosphate (11). These cardioprotectants can
attenuate mitochondrial damage, prevent myocardial apoptosis and
improve myocardial I/R injury via the activation of the PI3K/AKT
signaling pathway (66). This was
confirmed by using pannexin-1 and P2X7R antagonists in an animal
model. Vessey et al (66)
indicated that the pannexin-1 antagonist carbenoxolone and the
P2X7R antagonist BBG increases infarct size and blocks
cardioprotection in a rat experimental myocardial I/R injury model.
Furthermore, overexpression of the P2X7R following myocardial
ischemia increases ERK1/2 phosphorylation, suggesting that the
P2X7R may prevent myocardial apoptosis and attenuate cardiomyocyte
injury in response to I/R (65,67).
Considering all this evidence, the activation of P2X7R seems to be
beneficial in myocardial I/R injury, suggesting that the P2X7R
agonist is a possible target for the prevention of myocardial I/R
injury.
Autoimmune myocarditis is an inflammatory disease of
the myocardium characterized by the infiltration of inflammatory
monocytes, macrophages and CD4+ helper T cells into the
myocardium and consequently fibrosis and necrosis (68–70).
P2X7R expression is upregulated in ~50% of the experimental models
of autoimmune myocarditis, which leads to CD4+ helper T
cell and macrophage infiltration (12). A previous study using a mouse model
of autoimmune cardiomyopathy indicated that the wild-type mice
demonstrated increases IL-1β and IL-17 cytokine production.
However, the levels of these cytokines are decreased in
P2X7R−/−mice (71). In
addition, treatment of mice with autoimmune myocarditis mice with
the P2X7R antagonist A740003 improves their cardiac function by
inhibiting CD4+ helper T cell and macrophage
infiltration (12). These studies
indicated that the P2X7R is a possible target for the treatment of
autoimmune myocarditis.
Cardiomyopathy is a disorder of the cardiac muscle
characterized by structural and functional changes of
cardiomyocytes (72). A total of
two types of cardiomyopathies have been identified, primary or
secondary. Primary cardiomyopathies can be divided into three main
classes as follows: Genetic (hypertrophic cardiomyopathy),
mixed-genetic and non-genetic (dilated cardiomyopathy) and acquired
(inflammatory cardiomyopathy). Secondary cardiomyopathies are
usually associated with a variety of systemic disorders (diabetes)
and toxicity of specific drugs (chemotherapy) (72). Cardiac fibrosis is a key feature of
various cardiomyopathies and is defined as an excess deposition of
the extracellular matrix including type I collagen by cardiac
fibroblasts (73). It is notable
that activation of P2X7Rs by the P2X7R agonist BzATP elevates the
protein expression of profibrotic markers, including TGF-β1,
connective tissue growth factor (CTGF) and α-smooth muscle actin
(α-SMA) in neonatal rat cardiac fibroblasts (67). This indicates that that P2X7Rs
participate in the development of cardiac fibrosis (74).
An association between P2X7 expression and
cardiomyopathy has been shown since overexpression of this receptor
has been observed in various forms of cardiomyopathy. It has been
demonstrated that P2X7Rs are upregulated in mouse models of
experimental diabetes (68) and
experimental dilated cardiomyopathies (75). Overexpression of P2X7Rs in animal
cardiomyopathy models is commonly accompanied by cardiac
hypertrophy, fibrosis and apoptosis. For example, overexpression of
P2X7R in a mouse model of diabetic cardiomyopathy increases cardiac
hypertrophy markers, such as atrial natriuretic peptide and
β-myosin heavy chain, fibrosis markers, such as collagen I and
TGF-β1 and apoptosis markers, such as caspase 3 and Bax (75). A genetic study demonstrated an
association between P2X7R expression and cardiomyopathy. In humans,
the single nucleotide polymorphism of P2X7R (E186K) that results in
loss of function is associated with hypertrophic cardiomyopathy
(76).
Inhibition of P2X7R can attenuate cardiac fibrosis
in cardiomyopathies. Cardiac fibrosis markers including collagen I,
CTGF, α-SMA and TGF-β1 are reduced in a P2X7R knockdown model. This
was supported by using P2X7R antagonists in an animal model of
cardiomyopathy. The application of the P2X7R antagonist BBG
attenuated cardiac fibrosis by inhibiting the NLRP3/IL-1β signaling
pathway (74). The application of
the P2X7R antagonist A438079 ameliorates cardiac hypertrophy,
fibrosis and apoptosis by inhibiting the PKCβ/ERK signaling pathway
(75). These data demonstrate that
P2X7Rs have a potential role in the prevention and/or treatment of
various forms of cardiomyopathy.
COVID-19 is a respiratory viral infection
characterized by the excessive and sustained production of
inflammatory cytokines, the so-called cytokine storm (15,77).
This disease can cause serious injuries in various organ systems,
including the cardiovascular system. Viral myocarditis is the most
common form of heart injury mediated by COVID-19 (78). Arrhythmia and myocardial infarction
have also been reported as heart injuries in patients with COVID-19
(79,80). There is growing evidence that
demonstrates the importance of purinergic receptors, particularly
the P2X7R, in COVID-19 (81). It
is important to note that the P2X7R is hyperactivated in patients
infected with the COVID-19 viral strain. This hyperactivity can
cause myocardial injury by the activation of several intracellular
signaling pathways. The first possible pathway is the cytokine
storm. During this viral infection, the P2X7R is stimulated by high
levels of ATP resulting in NLRP3 inflammasome activation and
considerable inflammatory cytokine production. These cytokines
contribute to the cardiac inflammatory responses that may cause
AMI, viral myocarditis and arrhythmia (14,81).
The second possible pathway involves the angiotensin-converting
enzyme (ACE) II. During COVID-19 infection, the P2X7R triggers
pathways associated with the action of the
renin-angiotensin-aldosterone system (RAAS) (81). The main effector molecule in the
RAAS is angiotensin II, which causes vasoconstriction, cardiac
hypertrophy and apoptosis. This molecule is upregulated in several
pathological conditions including cardiovascular diseases. In fact,
ACE generates angiotensin II from angiotensin I, while ACE2
inhibits the activity of angiotensin II by transferring it to
angiotensin 1–7. Therefore, ACE2 has a cardioprotective effect
against myocardial injury (82).
It has been demonstrated that during the COVID-19 viral infection,
the virus enters human cells by binding to ACE2 resulting in the
downregulation of the ACE2 signaling pathway, which can potentially
cause myocardial injury (83–85).
Another possible pathway is that of VEGF. Hyperactivity of P2X7Rs
following COVID-19 has been shown to increase VEGF production
(15). This may stimulate
angiogenesis in cardiac cells which in turn leads to cardiac
hypertrophy. Collectively, these studies indicate that the P2X7R
plays a major role in myocardial injury caused by COVID-19. It can
be hypothesized that inhibition of P2X7R is a promising therapeutic
target for the prevention or treatment of cardiac injuries in
patients with COVID-19.
In conclusion, the aforementioned studies have shown
that the P2X7R plays an essential role in the development of
several heart diseases. The majority of the studies have
demonstrated that the activation of the P2X7R promotes the
development of AMI, autoimmune myocarditis and various types of
cardiomyopathies including diabetic cardiomyopathy, dilated
cardiomyopathy and hypertrophic cardiomyopathy. Activation of the
P2X7R during COVID-19 infection may also enhance the process of
myocardial injury via the activation of several intracellular
signaling pathways. Based on this evidence, it is likely that the
P2X7R can be used as a prognostic indicator for the detection of
various heart diseases. Overall, P2X7R inhibitors appear to be a
promising therapeutic target for the prevention or treatment of
heart diseases, as these inhibitors have been shown to have
primarily anti-inflammatory effects against AMI and myocarditis, as
well as antifibrotic and antiapoptotic effects in the case of
cardiomyopathies. This view is supported by a recent review that
draws attention to purinergic receptors as therapeutic targets for
the treatment of cardiovascular disease (86). However, it is important to note the
efficacy of P2X7R inhibitors during the progression of heart
disease when these inhibitors are used in clinical cardiology, as
they may not have the same beneficial effects in different types of
heart disease. This has been observed in experimental models of
myocardial infarction, the use of P2X7R inhibitors may enhance
cardiac function and significantly improve survival in the early
inflammatory phase following myocardial infarction (13,60,62).
On the other hand, the use of P2X7R inhibitors may increase infarct
size and abrogate the cardioprotective effects of adenosine and
sphingosine 1-phosphate in myocardial I/R injury (66). To develop a complete profile of
P2X7R in the setting of heart diseases, additional experimental
studies are required to elucidate the effects of P2X7R inhibitors
on other common cellular and molecular features associated with
heart diseases. For example, the effects of P2X7R inhibitors can be
examined on cardiac fibroblast proliferation, myofibroblast
differentiation, angiogenesis and scar formation following
myocardial infarction. In addition, further studies are required to
demonstrate the effect of P2X7R inhibitors on the COVID-19
signaling pathways associated with myocardial injury.
Not applicable.
Funding: No funding was received.
Data sharing is not applicable to this article, as
no data sets were generated or analyzed during the current
study.
AD conducted the literature research, wrote the
manuscript and designed the figure. AA, TA and NA contributed to
the writing and revisions of the manuscript. Data authentication is
not applicable. All authors read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
WHO, . Cardiovascular Diseases (CVDs).
Fact sheet. WHO; Geneva: 2021
|
|
2
|
Mathers CD and Loncar D: Projections of
global mortality and burden of disease from 2002 to 2030. PLoS Med.
3:e4422006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takenouchi T, Sekiyama K, Sekigawa A,
Fujita M, Waragai M, Sugama S, Iwamaru Y, Kitani H and Hashimoto M:
P2X7 receptor signaling pathway as a therapeutic target for
neurodegenerative diseases. Arch Immunol Ther Exp (Warsz).
58:91–96. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang WJ, Zhu ZM and Liu ZX: The role and
pharmacological properties of the P2X7 receptor in neuropathic
pain. Brain Res Bull. 155:19–28. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alves LA, Bezerra RJS, Faria RX, Ferreira
LG and da Silva Frutuoso V: Physiological roles and potential
therapeutic applications of the P2X7 receptor in inflammation and
pain. Molecules. 18:10953–10972. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li Q, Zhu X, Song W, Peng X and Zhao R:
The P2X7 purinergic receptor: A potential therapeutic target for
lung cancer. J Cancer Res Clin Oncol. 146:2731–2741. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang C, Shi S, Su Y, Tong JS and Li L:
P2X7R promotes angiogenesis and tumour-associated macrophage
recruitment by regulating the NF-κB signalling pathway in
colorectal cancer cells. J Cell Mol Med. 24:10830–10841. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Giannuzzo A, Saccomano M, Napp J,
Ellegaard M, Alves F and Novak I: Targeting of the P2X7 receptor in
pancreatic cancer and stellate cells. Int J Cancer Res.
139:2540–2552. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang H, He YM, Lin MM, Wang Y, Zhang X,
Liang L and He X: P2X7Rs: New therapeutic targets for osteoporosis.
Purinergic Signal. Feb 2–2022.(Epub ahead of print). View Article : Google Scholar
|
|
10
|
Grygorowicz T, Strużyńska L, Sulkowski G,
Chalimoniuk M and Sulejczak D: Temporal expression of P2X7
purinergic receptor during the course of experimental autoimmune
encephalomyelitis. Neurochem Int. 57:823–829. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vessey DA, Li L and Kelley M:
Pannexin-I/P2X 7 purinergic receptor channels mediate the release
of cardioprotectants induced by ischemic pre-and postconditioning.
J Cardiovasc Pharmacol Ther. 15:190–195. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zempo H, Sugita Y, Ogawa M, Watanabe R,
Suzuki J and Isobe M: A P2X7 receptor antagonist attenuates
experimental autoimmune myocarditis via suppressed myocardial CD4+
T and macrophage infiltration and NADPH oxidase 2/4 expression in
mice. Heart Vessels. 30:527–533. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao H, Yin J, Shi Y, Hu H, Li X, Xue M,
Cheng W, Wang Y, Li X, Li Y, et al: Targeted P2X7R shRNA delivery
attenuates sympathetic nerve sprouting and ameliorates cardiac
dysfunction in rats with myocardial infarction. Cardiovasc Ther.
35:2017. View Article : Google Scholar
|
|
14
|
Dos Anjos F, Simões JLB, Assmann CE,
Carvalho FB and Bagatini MD: Potential therapeutic role of
purinergic receptors in cardiovascular disease mediated by
SARS-CoV-2. J Immunol Res. 2020:86320482020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Di Virgilio F, Tang Y, Sarti AC and
Rossato M: A rationale for targeting the P2X7 receptor in
Coronavirus disease 19. Br J Pharmacol. 177:4990–4994. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Batista Simões JL, Sobierai LD, Pereira
SM, Rodrigues dos Santos MV and Bagatini MD: Therapeutic potential
of P2X7 purinergic receptor modulation in the main organs affected
by the COVID-19 Cytokine Storm. Curr Pharm Des. 28:1798–1814. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Burnstock G: A basis for distinguishing
two types of purinergic receptor. Cell Membrane Receptors for Drugs
and Hormone: A Multidisciplinary Approach. 107–118. 1978.
|
|
18
|
Burnstock G and Kennedy C: Is there a
basis for distinguishing two types of P2-purinoceptor? Gen
Pharmacol. 16:433–440. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abbracchio MP and Burnstock G:
Purinoceptors: Are there families of P2X and P2Y purinoceptors?
Pharmacol Ther. 64:445–475. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
North RA: Molecular physiology of P2X
receptors. Physiol Rev. 82:1013–1067. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abbracchio MP, Burnstock G, Boeynaems JM,
Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C,
Jacobson KA and Weisman GA: International Union of Pharmacology
LVIII: Update on the P2Y G protein-coupled nucleotide receptors:
From molecular mechanisms and pathophysiology to therapy. Pharmacol
Rev. 58:281–341. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Burnstock G and Verkhratsky A: Receptors
for purines and pyrimidines. Springer Berlin; Heidelberg: 2012,
View Article : Google Scholar
|
|
23
|
Bodin P and Burnstock G: Purinergic
signalling: ATP release. Neurochem Res. 26:959–969. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dosch M, Gerber J, Jebbawi F and Beldi G:
Mechanisms of ATP release by inflammatory cells. Int J Mol Sci.
19:12222018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sawada K, Echigo N, Juge N, Miyaji T,
Otsuka M, Omote H, Yamamoto A and Moriyama Y: Identification of a
vesicular nucleotide transporter. Proc Natl Acad Sci USA.
105:5683–5686. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Junger WG: Immune cell regulation by
autocrine purinergic signalling. Nat Rev Immunol. 11:201–212. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiong Y, Sun S, Teng S, Jin M and Zhou Z:
Ca2+-dependent and Ca2+-independent ATP
release in astrocytes. Front Mol Neurosci. 11:2242018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abbracchio MP, Burnstock G, Verkhratsky A
and Zimmermann H: Purinergic signalling in the nervous system: An
overview. Trends Neurosci. 32:19–29. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kuzmin AI, Lakomkin VL, Kapelko VI and
Vassort G: Interstitial ATP level and degradation in control and
postmyocardial infarcted rats. Am J Physiol. 275:C766–C771. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang LH, Baldwin JM, Roger S and Baldwin
SA: Insights into the molecular mechanisms underlying mammalian
P2X7 receptor functions and contributions in diseases, revealed by
structural modeling and single nucleotide polymorphisms. Front
Pharmacol. 4:552013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Burnstock G and Kennedy C: P2X receptors
in health and disease. Adv Pharmacol. 61:333–372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Adinolfi E, Giuliani AL, De Marchi E,
Pegoraro A, Orioli E and Di Virgilio F: The P2X7 receptor: A main
player in inflammation. Biochem Pharmacol. 151:234–244. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Andrejew R, Oliveira-Giacomelli Á, Ribeiro
DE, Glaser T, Arnaud-Sampaio VF, Lameu C and Ulrich H: The P2X7
receptor: Central hub of brain diseases. Front Mol Neurosci.
13:1242020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Virginio C, MacKenzie A, Rassendren FA,
North RA and Surprenant A: Pore dilation of neuronal P2X receptor
channels. Nat Neurosci. 2:315–321. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wiley JS, Sluyter R, Gu BJ, Stokes L and
Fuller SJ: The human P2X7 receptor and its role in innate immunity.
Tissue Antigens. 78:321–332. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Alves LA, de Melo Reis RA, de Souza CA, de
Freitas MS, Teixeira PC, Neto Moreira Ferreira D and Xavier RF: The
P2X7 receptor: Shifting from a low-to a high-conductance channel-an
enigmatic phenomenon? Biochim Biophys Acta. 1838:2578–2587. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bartlett R, Stokes L and Sluyter R: The
P2X7 receptor channel: Recent developments and the use of P2X7
antagonists in models of disease. Pharmacol Rev. 66:638–675. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Artlett CM: The role of the NLRP3
inflammasome in fibrosis. Open Rheumatol J. 6:80–86. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu X, Zhang Z, Ruan J, Pan Y, Magupalli
VG, Wu H and Lieberman J: Inflammasome-activated gasdermin D causes
pyroptosis by forming membrane pores. Nature. 535:153–158. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ferrari D, Wesselborg S, Bauer MK and
Schulze-Osthoff K: Extracellular ATP activates transcription factor
NF-kappaB through the P2Z purinoreceptor by selectively targeting
NF-kappaB p65 (RelA). J Cell Biol. 139:1635–1643. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ferrari D, Stroh C and Schulze-Osthoff K:
P2X7/P2Z purinoreceptor-mediated activation of transcription factor
NFAT in microglial cells. J Biol Chem. 274:13205–13210. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yip L, Woehrle T, Corriden R, Hirsh M,
Chen Y, Inoue Y, Ferrari V, Insel PA and Junger WG: Autocrine
regulation of T-cell activation by ATP release and P2X7 receptors.
FASEB J. 23:1685–1693. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang Y, Cheng H, Li W, Wu H and Yang Y:
Highly-expressed P2X7 receptor promotes growth and metastasis of
human HOS/MNNG osteosarcoma cells via PI3K/Akt/GSK3β/β-catenin and
mTOR/HIF1α/VEGF signaling. Int J Cancer. 145:1068–1082. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Langhner E, Taghavi P, Chiles K, Mahon PC
and Semenza GL: HEER2 (neu) signaling increase the rate of hypoxia
inducible factor 1-alpha (HIF-1-alpha) synthesis: Novel mechanism
for HIF-mediated vascular endothelial growth factor expression. Mol
Cell Bioi. 21:3995–4004. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Amoroso F, Capece M, Rotondo A, Cangelosi
D, Ferracin M, Franceschini A, Raffaghello L, Pistoia V, Varesio L
and Adinolfi E: The P2X7 receptor is a key modulator of the
PI3K/GSK3β/VEGF signaling network: Evidence in experimental
neuroblastoma. Oncogene. 34:5240–5251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hill LM, Gavala ML, Lenertz LY and Bertics
PJ: Extracellular ATP may contribute to tissue repair by rapidly
stimulating purinergic receptor X7-dependent vascular endothelial
growth factor release from primary human monocytes. J Immunol.
185:3028–3034. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tafani M, Schito L, Pellegrini L,
Villanova L, Marfe G, Anwar T, Rosa R, Indelicato M, Fini M, Pucci
B and Russo MA: Hypoxia-increased RAGE and P2X7R expression
regulates tumor cell invasion through phosphorylation of Erk1/2 and
Akt and nuclear translocation of NF-{kappa}B. Carcinogenesis.
32:1167–1175. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ji Z, Xie Y, Guan Y, Zhang Y, Cho KS, Ji M
and You Y: Involvement of P2X7 receptor in proliferation and
migration of human glioma cells. Biomed Res Int. 2018:85913972018.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bartlett R, Yerbury JJ and Sluyter R: P2X7
receptor activation induces reactive oxygen species formation and
cell death in murine EOC13 microglia. Mediators Inflamm.
2013:2718132013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mazrouei S, Sharifpanah F, Bekhite MM,
Figulla HR, Sauer H and Wartenberg M: Cardiomyogenesis of embryonic
stem cells upon purinergic receptor activation by ADP and ATP.
Purinergic Signal. 11:491–506. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hesse J, Leberling S, Boden E, Friebe D,
Schmidt T, Ding Z, Dieterich P, Deussen A, Roderigo C, Rose CR, et
al: CD73-derived adenosine and tenascin-C control cytokine
production by epicardium-derived cells formed after myocardial
infarction. FASEB J. 31:3040–3053. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Musa H, Tellez JO, Chandler NJ, Greener
ID, Mączewski M, Mackiewicz U, Beresewicz A, Molenaar P, Boyett MR
and Dobrzynski H: P2 purinergic receptor mRNA in rat and human
sinoatrial node and other heart regions. Naunyn Schmiedebergs Arch
Pharmacol. 379:541–549. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Barth K, Pfleger C, Linge A, Sim JA,
Surprenant A, Steinbronn N, Strasser RH and Kasper M: Increased
P2X7R expression in atrial cardiomyocytes of caveolin-1 deficient
mice. Histochem. Cell Biol. 134:31–38. 2010.PubMed/NCBI
|
|
54
|
Gentile D, Natale M, Lazzerini PE,
Capecchi PL and Laghi-Pasini F: The role of P2X7 receptors in
tissue fibrosis: A brief review. Purinergic Signal. 11:435–440.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Thygesen K, Alpert JS, Jaffe AS, Simoons
ML, Chaitman BR, White HD; Joint ESC/ACCF/AHA/WHF Task Force for
Universal Definition of Myocardial Infarction; Authors/Task Force
Members Chairpersons, ; Thygesen K, Alpert JS, et al: Third
universal definition of myocardial infarction. J Am Coll Cardiol.
60:1581–1598. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liehn EA, Postea O, Curaj A and Marx N:
Repair after myocardial infarction, between fantasy and reality:
The role of chemokines. J Am Coll Cardiol. 58:2357–2362. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Forte E, Furtado MB and Rosenthal N: The
interstitium in cardiac repair: Role of the immune-stromal cell
interplay. Nat Rev Cardiol. 15:601–616. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ferrini A, Stevens MM, Sattler S and
Rosenthal N: Toward regeneration of the heart: Bioengineering
strategies for immunomodulation. Front Cardiovasc Med. 6:262019.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yin J, Wang Y, Hu H, Li X, Xue M, Cheng W,
Wang Y, Li X, Yang N, Shi Y and Yan S: P2X7 receptor inhibition
attenuated sympathetic nerve sprouting after myocardial infarction
via the NLRP3/IL-1β pathway. J Cell Mol Med. 21:2695–2710. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cheng W, Sun Y, Wu Q, Ooi K, Feng Y, Xia C
and Zhu D: Paraventricular nucleus P2X7 receptors aggravate acute
myocardial infarction injury via ROS-induced vasopressin-V1b
activation in rats. Neurosci Bull. 37:641–656. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Shi XX, Zheng KC, Shan PR, Zhang L, Wu SJ
and Huang ZQ: Elevated circulating level of P2X7 receptor is
related to severity of coronary artery stenosis and prognosis of
acute myocardial infarction. Cardiol J. 28:453–459. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Mezzaroma E, Toldo S, Farkas D, Seropian
IM, Van Tassell BW, Salloum FN, Kannan HR, Menna AC, Voelkel NF and
Abbate A: The inflammasome promotes adverse cardiac remodeling
following acute myocardial infarction in the mouse. Proc Natl Acad
Sci USA. 108:19725–19730. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Frank A, Bonney M, Bonney S, Weitzel L,
Koeppen M and Eckle T: Myocardial ischemia reperfusion injury: From
basic science to clinical bedside. Semin Cardiothorac Vasc Anesth.
16:123–132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Gu M, Zheng AB, Jin J, Cui Y, Zhang N, Che
ZP, Wang Y, Zhan J and Tu WJ: Cardioprotective effects of genistin
in rat myocardial ischemia-reperfusion injury studies by regulation
of P2X7/NF-κB pathway. Evid Based Complement Alternat Med.
2016:53812902016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Tu G, Zou L, Liu S, Wu B, Lv Q, Wang S,
Xue Y, Zhang C, Yi Z, Zhang X, et al: Long noncoding NONRATT021972
siRNA normalized abnormal sympathetic activity mediated by the
upregulation of P2X7 receptor in superior cervical ganglia after
myocardial ischemia. Purinergic Signal. 12:521–535. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Vessey DA, Li L and Kelley M: Ischemic
preconditioning requires opening of pannexin-1/P2X7 channels not
only during preconditioning but again after index ischemia at full
reperfusion. Mol Cell Biochem. 351:77–84. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wang Y: Mitogen-activated protein kinases
in heart development and diseases. Circulation. 116:1413–1423.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Magnani JW and Dec GW: Myocarditis:
Current trends in diagnosis and treatment. Circulation.
113:876–890. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Fung G, Luo H, Qiu Y, Yang D and McManus
B: Myocarditis. Circ Res. 118:496–514. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Amoah BP, Yang H, Zhang P, Su Z and Xu H:
Immunopathogenesis of myocarditis: The interplay between cardiac
fibroblast cells, dendritic cells, macrophages and CD 4+ T cells.
Scand J Immunol. 82:1–9. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Martinez CG, Zamith-Miranda D, Da Silva
MG, Ribeiro KC, Brandão IT, Silva CL, Diaz BL, Bellio M, Persechini
PM and Kurtenbach E: P2×7 purinergic signaling in dilated
cardiomyopathy induced by auto-immunity against muscarinic M2
receptors: Autoantibody levels, heart functionality and cytokine
expression. Sci Rep. 5:169402015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Maron BJ, Towbin JA, Thiene G,
Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB;
American Heart Association, ; et al: Contemporary definitions and
classification of the cardiomyopathies: An American Heart
Association Scientific Statement from the Council on Clinical
Cardiology, Heart Failure and Transplantation Committee; Quality of
Care and Outcomes Research and Functional Genomics and
Translational Biology Interdisciplinary Working Groups; and Council
on Epidemiology and Prevention. Circulation. 113:1807–1816. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Eijgenraam TR, Silljé HHW and de Boer RA:
Current understanding of fibrosis in genetic cardiomyopathies.
Trends Cardiovasc Med. 30:353–361. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhou J, Tian G, Quan Y, Li J, Wang X, Wu
W, Li M and Liu X: Inhibition of P2X7 purinergic receptor
ameliorates cardiac fibrosis by suppressing NLRP3/IL-1β pathway.
Oxid Med Cell Longev. 2020:79562742020. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Huang S, Wang W, Li L, Wang T, Zhao Y, Lin
Y, Huang W, Wang Y and Huang Z: P2X7 receptor deficiency
ameliorates STZ-induced cardiac damage and remodeling through PKCβ
and ERK. Front Cell Dev Biol. 9:6920282021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Biswas A, Raza A, Das S, Kapoor M,
Jayarajan R, Verma A, Shamsudheen KV, Murry B, Seth S, Bhargava B,
et al: Loss of function mutation in the P2X7, a ligand-gated ion
channel gene associated with hypertrophic cardiomyopathy.
Purinergic Signal. 15:205–210. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Whitworth J: COVID-19: A fast evolving
pandemic. Trans R Soc Trop Med Hyg. 114:241–248. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Siripanthong B, Nazarian S, Muser D, Deo
R, Santangeli P, Khanji MY, Cooper LT Jr and Chahal CAA:
Recognizing COVID-19-related myocarditis: The possible
pathophysiology and proposed guideline for diagnosis and
management. Heart Rhythm. 17:1463–1471. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kang Y, Chen T, Mui D, Ferrari V, Jagasia
D, Scherrer-Crosbie M, Chen Y and Han Y: Cardiovascular
manifestations and treatment considerations in COVID-19. Heart.
106:1132–1141. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Guzik TJ, Mohiddin SA, Dimarco A, Patel V,
Savvatis K, Marelli-Berg FM, Madhur MS, Tomaszewski M, Maffia P,
D'Acquisto F, et al: COVID-19 and the cardiovascular system:
Implications for risk assessment, diagnosis, and treatment options.
Cardiovasc Res. 116:1666–1687. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ribeiro DE, Oliveira-Giacomelli Á, Glaser
T, Arnaud-Sampaio VF, Andrejew R, Dieckmann L, Baranova J, Lameu C,
Ratajczak MZ and Ulrich H: Hyperactivation of P2X7 receptors as a
culprit of COVID-19 neuropathology. Mol Psychiatry. 26:1044–1059.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Jiang F, Yang J, Zhang Y, Dong M, Wang S,
Zhang Q, Liu FF, Zhang K and Zhang C: Angiotensin-converting enzyme
2 and angiotensin 1–7: Novel therapeutic targets. Nat Rev Cardiol.
11:413–426. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, Guo
L, Guo R, Chen T, Hu J, et al: Characterization of spike
glycoprotein of SARS-CoV-2 on virus entry and its immune
cross-reactivity with SARS-CoV. Nat Commun. 11:16202020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Nishiga M, Wang DW, Han Y, Lewis DB and Wu
JC: COVID-19 and cardiovascular disease: From basic mechanisms to
clinical perspectives. Nat Rev Cardiol. 17:543–558. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
De Mello WC and Danser AH: Angiotensin II
and the heart: On the intracrine renin-angiotensin system.
Hypertension. 35:1183–1188. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Wernly B and Zhou Z: More purinergic
receptors deserve attention as therapeutic targets for the
treatment of cardiovascular disease. Am J Physiol Heart Circ
Physiol. 319:H723–H729. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wagner JA and Kelly RB: Topological
organization of proteins in an intracellular secretory organelle:
The synaptic vesicle. Proc Natl Acad Sci USA. 76:4126–4130. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Bours MJ, Swennen EL, Di Virgilio F,
Cronstein BN and Dagnelie PC: Adenosine 5′-triphosphate and
adenosine as endogenous signaling molecules in immunity and
inflammation. Pharmacol Ther. 112:358–404. 2006. View Article : Google Scholar : PubMed/NCBI
|