Cancer remains the leading cause of premature
mortality and reduced life expectancy in a number of countries
around the world and is a heavy health burden (1). Cancer of the digestive system is one
of the most common types of malignant tumor, with esophageal,
gastric, colorectal, liver and pancreatic cancers ranking among the
top cancers of the digestive system in terms of incidence and
mortality rates (2). The majority
of types of cancer of the digestive system are in the
middle-to-late stages of progression when they are detected and
diagnosed; these patients have a poor prognosis so they have become
among the most commonly discussed public health problems (3). Epigenetics is the stable, heritable
alteration of gene function and expression levels without changes
to the nucleotide sequence and epigenetics mainly includes DNA
methylation, histone modification, noncoding RNA regulation and
chromatin remodeling (4–6). Epigenetic modifications can regulate
the biological processes of cancer and thus influence the
progression of diseases such as tumors. RNA 5-methylcytosine (m5C)
methylation is at the forefront of epitranscriptomics and is one of
the most important epigenetic modification mechanisms in RNA
posttranscriptional regulation. Dynamic RNA modifications have
emerged as key posttranscriptional regulators of genetic
information during embryonic development and disease progression
(7). Among them, RNA m5C
methylation is one of the most important epigenetic modification
mechanisms in RNA posttranscriptional regulation. m5C is a common
RNA modifier that has received widespread attention for its key
regulatory role in mRNA metabolism (8). m5C modification was first identified
in DNA and later shown to mediate RNA methylation (9). m5C modifications are one of the most
common posttranscriptional modifications of RNA, along with
N6-methyladenosine and pseudouridine (Ψ) (10) and are found in a wide variety of
RNA molecules, including transfer (t)RNAs, ribosomal (r)RNAs, mRNAs
and noncoding (nc)RNAs (11). The
level of posttranscriptional modification of RNA methylation
regulates a variety of biological processes, such as splicing,
nuclear export, stability and translation of RNA (12), which in turn affects physiological
processes such as cell differentiation, embryonic development and
learning and memory; however, it also plays an important role in
the onset and development of a number of diseases, including tumors
(13,14). In digestive system tumors, RNA m5C
modification plays a key role in the pathogenesis of esophageal,
gastric, liver and pancreatic cancers (15–28)
(Table I). However, there is no
systematic review summarizing the role of RNA m5C modification in
digestive system tumors. Therefore, the present study reviewed the
role and regulatory mechanisms of RNA m5C methylation and its
regulators in digestive system cancers, such as esophageal,
gastric, hepatocellular and pancreatic cancers, with the aim of
providing new ideas for precise tumor prevention, intervention and
potential therapeutic targets.
The m5C modification in which a methyl group is
attached to the fifth carbon of the DNA or RNA cytosine ring, is a
reversible type of epigenetic modification. This modification
process was first identified in DNA (29) and was later shown to mediate RNA
methylation and is a ubiquitous posttranscriptional modification of
RNA along with N6-methyladenosine (m6A) and Ψ (10). There are various types of RNA
methylation, such as m6A, N1-methyladenosine (m1A), m5C,
N7-methylguanosine (m7G) and 2′-O-methylribosidine (Um) (10). m6A methylation is a methylation
that occurs at the sixth N atom of adenine and is the most common
and abundant chemical modification of eukaryotic mRNAs, accounting
for ~60% of the total (30). m1A
is the result of the methylation of adenosine at position 1 and is
found mainly in tRNAs, rRNAs, mRNAs and lncRNAs (31,32).
N7-methylguanosine (m7G) is an RNA methylation that occurs at the
N7 position of guanine and accounts for ~0.4% of all guanine
residues (33). m7G methylation
occurs in mRNA, tRNA and rRNA and is catalyzed by the
methyltransferase METTL1-WDR4 complex (34,35).
Research on RNA methylation has focused mainly on m6A and less so
on m5C methylation. However, in recent years, m5C methylation has
been shown to markedly affect a variety of biological processes,
including cell proliferation, differentiation, migration and
apoptosis (36,37). As a result, research in oncology
has received increasing attention. m5C modifications exist in a
wide range of RNA molecules, including tRNAs, rRNAs, mRNAs and
ncRNAs (11). The m5C methylation
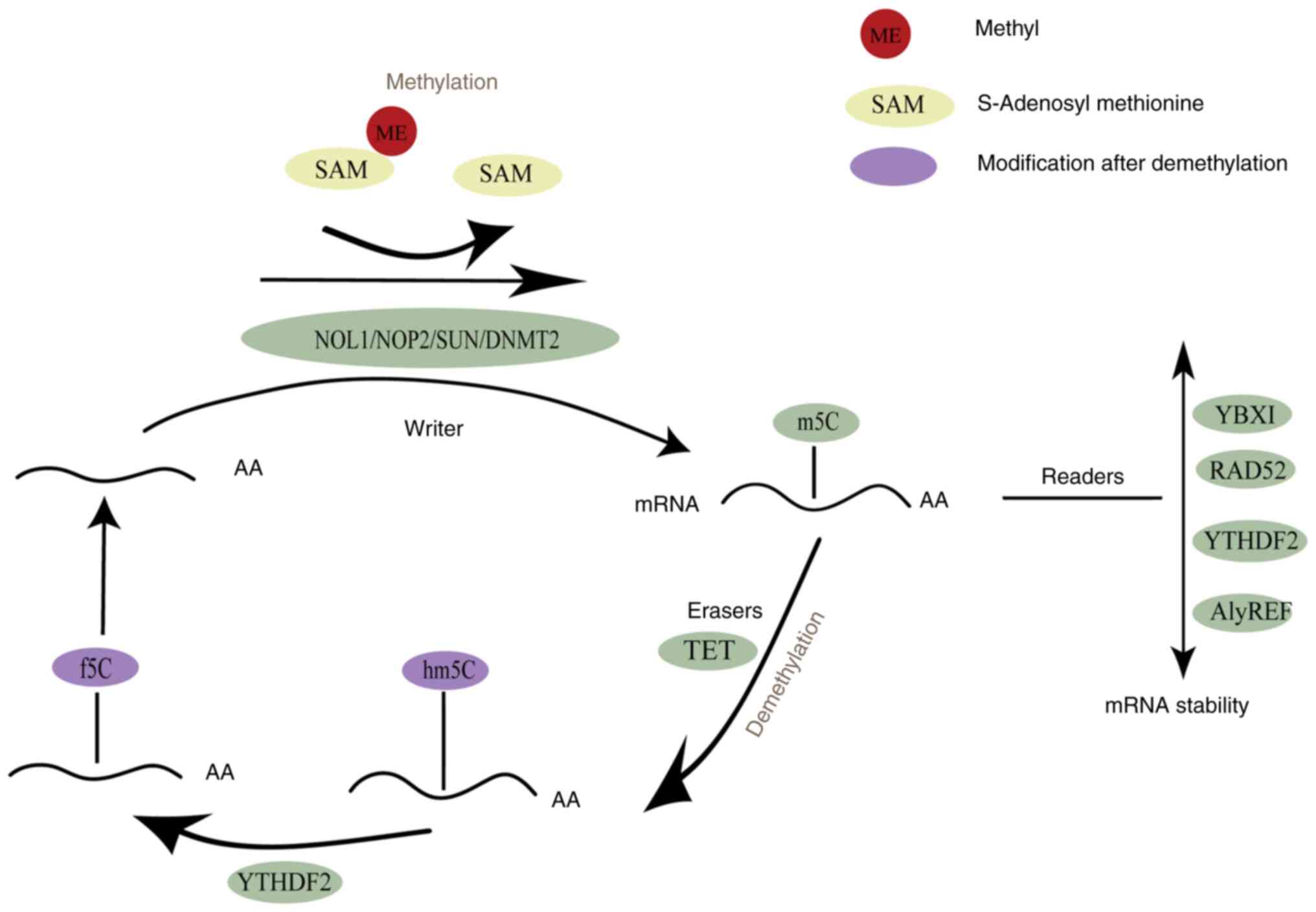
process mainly involves relevant methyltransferases (writers),
demethylases (erasers) and binding proteins (readers). The m5C
methylation uses S-adenosylmethionine (SAM) as a methyl donor.
Through the action of methyltransferases, m5C methylation is
initiated. The methylated RNA subsequently binds to binding
proteins to exert biological effects (Fig. 1) (38). This process also involves the
action of demethylases, making it a dynamic and reversible process.
In summary, through the interaction of the aforementioned three
types of proteins, m5C methylation widely affects gene expression
and various biological processes at multiple levels, although the
specific mechanism remains unclear.
The methyltransferases of m5C are mainly composed of
the nucleolar protein NSUN (NOL1/NOP2/SUN domain family) family and
DNA methyltransferase member 2 (DNMT2) (39). DNMT2 mainly regulates the m5C
methylation of tRNAs and miRNAs and can catalyze the C38
methylation of aspartic acid transporter RNA (tRNA-Asp) (40). This process is associated with the
primary sequence and tertiary structure of tRNAs (41). In mammals, the NSUN enzyme family
is composed of NSUN1-7 (42).
However, m5C is characterized mainly by NSUN family proteins, among
which NSUN2 has been the most thoroughly studied. It is widely
acknowledged that RNA m5C is catalyzed mainly by NOP2/Sun RNA
methyltransferase family member 2 (NSUN2). As an essential m5C
‘writer’, NSUN2 participates in a broad spectrum of biological
processes (43). However, the
ultimate consequences of posttranscriptional regulation rely
largely on m5C ‘readers’. These ‘readers’ can recognize m5C
modifications and exert crucial influences on mRNA output,
stability and translation initiation (12,44).
NSUN2 is a methyltransferase that depends on two cysteine sites.
C321 catalyzes the methylation of cytosine by binding to the
cytosine pyrimidine ring and forming a covalent bond, whereas C271
mediates the release of methylated RNA. The methylation process
mediated by NSUN2 mainly involves leucine at the variable loop
swing position of the majority of tRNAs. Additionally, it
methylates mRNAs, ncRNAs and lncRNAs (42). NSUN2 can methylate cytosine through
binding to the cytosine ring and forming a covalent bond. In
addition, the intracellular localization of NSUN2 varies with
different stages of the cell cycle. Specifically, during the
G1 phase, NSUN2 is located mainly in the nucleus; during
the S phase, it is positioned between the nucleolus and
nucleoplasm; during the G2 phase, it is in the
cytoplasm; and during the M phase, it is in the centromere
(45,46). NSUN2 participates in a diverse
range of biological processes, including cell differentiation,
proliferation and migration (47–49).
In addition, it is highly expressed in numerous types of cancers,
such as gastric cancer, esophageal cancer, hepatocellular carcinoma
(HCC), pancreatic cancer, prostate cancer and kidney cancer
(15–18,50–52).
NSUN1 (NOP2) is a protein specific to the nucleolus. It can
catalyze the methylation of yeast 25SrRNA, 60S ribosomal subunit
and 26SrRNA. Additionally, it can catalyze methylation at the
cytosine 4447 position of human 28S rRNA and it stabilizes the
structure of rRNA (53). NSUN3 is
located mainly in the mitochondrial matrix within human cells. It
can recognize the anticodon loop of the mitochondrial methionine
transfer RNA (tRNA-Met) and methylate C34. In addition, it is
essential for the formation of 5-formyl-2′-cytidine (f5C) (54). The absence of NSUN3 leads to
mitochondrial dysfunction (55).
NSUN4 functions mainly on 12S rRNA in eukaryotic mitochondria and
participates in the methylation of rRNA at the C911 position
(56). It is abnormally expressed
in lung adenocarcinoma, HCC and renal clear-cell carcinoma
(26). NSUN5 contains a m5C site
at C3782 of 28S rRNA in human cervical cancer cells, which
regulates the protein translation process (57). In colorectal cancer, NSUN5 mainly
acts by modifying the second m5C methyltransferase in eukaryotic
rRNA. It is associated with ribosomes and can change the total
protein content (57) as well as
regulate the cell cycle to promote tumor development (21). NSUN6 is localized to the Golgi
apparatus in the cytoplasm of human cells and is a methylation
transferase with strong substrate properties for mRNAs; it is
enriched in the 3′UTR and associated with translational termination
(58). It methylates threonine
transporter RNA (tRNAThr) and cysteine transporter RNA (tRNACys)
(59). NSUN7 may act on eukaryotic
eRNAs (60) and is associated with
shorter survival (61,62). These related transferase enzymes
play important roles in various physiological activities of
organisms.
Demethylases mediate RNA demethylation, which is
induced by the tet methylcytosine dioxygenase (TET) family of
proteins and the nature of TET-induced m5C demethylation is to
replace the modification by catalytically promoting the oxidation
of 5mC (63,64). At present, a definitive RNA m5C
demethylase has not yet been identified, but TET2 in the TET family
of proteins can further oxidize tRNA m5C to α-ketoglutarate in
reaction to form 5-hydroxymethylcytosine (hm5C) (65). In addition, TET was found to
mediate the specific enrichment of hm5 C in intracellular tRNAs, a
process that may destabilize the m5 C-binding protein by disrupting
its binding to RNA (66,67). Thus, TET2 can mediate C oxidation
via m5C methylation of tRNA to promote translation in vitro
(67). hm5C is oxidized to f5C in
mitochondrial tRNA by α-ketoglutarate-dependent ALKB homodimeric
dioxygenase 1 (ALKBH1). However, the mechanism through which f5C is
reduced in tRNA remains unclear (68).
The binding proteins associated with m5C RNA
methylation that have been identified are the RNA methyltransferase
Aly/REF export factor (ALYREF) and Y-box binding protein 1 (YBX1).
ALYREF is a ‘reader’ protein situated in the nucleus. It directly
recognizes and binds to the m5C site within RNA, thus facilitating
the export of RNA from the nucleus to the cytoplasm (12). YBX1, a DNA/RNA-binding protein and
a m5C ‘reader’, can stabilize m5c-modified messenger ribonucleic
acid (69). It often regulates the
stability of mRNAs by specifically binding to response elements in
various mRNA transcripts, such as those encoding IL-6, VEGF and
heat shock protein 70 (70,71).
It is highly associated with tumor cell proliferation, drug
resistance, metastasis and prognosis (72). ALYREF and YBX1 can exert their
biological effects by recognizing and binding to the m5C site
(22). In addition, ALYREF can
recognize m5C-methylated mRNAs and mediate their nucleocytoplasmic
shuttling process (73). YBX1
preferentially recognizes and binds to m5C-modified mRNAs via its
cold shock protein structural domain to regulate mRNA stability in
the cytoplasm. It is overexpressed in gastric cancer tissues and is
associated with hepatic metastasis and poor prognosis in advanced
gastric cancer patients. In addition, it promotes gastric
carcinogenesis, angiogenesis and drug resistance (18,74).
It has been shown that the level of nuclear mRNA chromosomes
increases when AlYREF expression is reduced and plays the opposite
role when it is overexpressed and that this phenomenon does not
occur in m5 C-binding-deficient Al YREF types, suggesting that
AlYREF may be involved in facilitating the nuclear egress process
of mRNA by binding to the m5C-binding site of the mRNA and that
NSUN2 is involved in regulating the nuclear egress process mediated
by AlYREF. NSUN2 is involved in the regulation of AlYREF-mediated
nucleation (12).
m5C modifications in mRNAs have important
physiological functions and are involved in various biological
processes of RNA, including RNA export, translation and ribosome
assembly (75,76). It can affect the stability,
splicing and nucleocytoplasmic shuttling process of mRNAs (12). In addition, it affects
posttranscriptional gene expression and protein synthesis (18,19).
It is also involved in various biological processes, such as DNA
damage repair (77); cell
proliferation and migration (78);
and the development, differentiation and reprogramming of stem
cells (14). Among various RNA
molecules, the m5C modification of tRNAs is involved in neural
development and cell differentiation processes, the m5C
modification of rRNAs regulates oxidative stress and the m5C
modification of mRNAs is associated with the growth, development
and aging processes of organisms (42,79).
In digestive system tumors, studies have shown that RNA m5C
methylation is involved in the occurrence and development of
digestive system cancers such as esophageal cancer, gastric cancer,
liver cancer and pancreatic cancer (15–18).
This may be due to the action of RNA methyltransferases, which
change the abundance of m5C modifications in the RNA of oncogenes
or tumor suppressor genes. The modification sites are further
recognized by RNA m5C-binding proteins, thereby regulating the
function and expression level of tumor-related genes. As a result,
the homeostasis of the internal environment is unbalanced,
promoting or inhibiting the formation of tumors.
Esophageal cancer (ESCC) is an aggressive tumor with
rapid growth and a high rate of lymph node metastasis (80). Esophageal squamous cell carcinoma
and adenocarcinoma are the two main histologic subtypes, of which
ESCC accounts for ~90% of cases (81). The m5C methyltransferase NSUN2 is
overexpressed in ESCC, m5C methylation is increased in ESCC tumors
and the higher the expression of NSUN2 is, the worse the prognosis
of ESCC patients (15).
Furthermore, in the NSUN2 knockout mouse model of ESCC constructed
in that study, the tumorigenesis and progression of ESCC were
inhibited. Mechanistically, NSUN2 induces m5C modification of
growth factor receptor-binding protein 2 (GRB2) and increases its
stability. This process is mediated by a novel m5C-mediated
RNA-binding protein, lin-28 homolog B (LIN28B). GRB2 transcripts
are dependent on LIN28B for stabilization and increased levels of
GRB2 activate the PI3K/AKT/ERK/MAPK signaling pathway, which
promotes esophageal squamous cell carcinoma progression (Fig. 2). These results indicate that NSUN2
indirectly activates the oncogenic PI3K/AKT and ERK/MAPK signaling
pathways through m5C, promoting the occurrence and progression of
ESCC and providing a promising targeted therapeutic strategy for
ESCC (15). Similarly, a study
showed that high expression of NSUN2 leads to an increase in the
level of m5C-modified mRNA in ESCC cells. The m5C ‘reader’ YBX1
binds to spermine oxidase (SMOX) mRNA and enhances its stability in
an NSUN2-mediated m5C-dependent manner, thereby accelerating the
proliferation and metastasis of ESCC cells (82). These findings further confirm the
m5C-mediated epigenetic regulatory mechanism and that the
YBX1/m5C-SMOX-mTOCR1 axis is involved in the occurrence and
development of ESCC. In summary, these findings suggest that NSUN2
can mediate the tumorigenesis and development of ESCC through
multiple signaling pathways. However, the specific mechanism has
not been fully elucidated, so further research is still needed.
Nevertheless, m5C methylation can serve as a potential therapeutic
target in the treatment of esophageal cancer and further research
can be conducted on the PI3K/AKT, ERK/MAPK and YBX1/m5C-SMOX-mTOCR1
axes.
GC is the fifth most common cancer globally and the
fourth leading cause of cancer-related mortality (83). Studies have demonstrated that NSUN2
is highly expressed in gastric cancer cells and tissues (16,18,84).
NSUN2 promotes the proliferation of gastric cancer cells and the
growth of tumors (16).
Cyclin-dependent kinase inhibitor 1C (CDKN1C) p57Kip2 is
a tumor suppressor (85). In
addition, p57Kip2 is also an important downstream gene
regulated by NSUN2. After NSUN2 is knocked out, the level of m5C on
the 3′UTR of p57Kip2 mRNA decreases, which undermines the mRNA
stability of p57Kip2 and downregulates its protein level and the
proliferation ability of gastric cancer cells is enhanced (20). That is, NSUN2 can promote the
proliferation of cancer cells in a m5C-dependent manner by
inhibiting p57Kip2. Hu et al (16) report that small ubiquitin-like
modifier 2 and 3 directly interact with NSUN2 by stabilizing it and
mediating its nuclear translocation to promote its oncogenic
activity. The expression of the m5C RNA methyltransferase NSUN2 is
upregulated in gastric cancer tissues, which promotes the
proliferation, migration and invasion of gastric cancer cells and
is associated with a poor prognosis. Hu et al (16) also report that PIK3R1 and PCYT1A
may be m5C target genes and that knockdown of NSUN2 reduces the m5C
of PIK3R1 and PCYT1A, decreases their expression levels and
markedly lowers RNA m5C levels in gastric cancer cells. NSUN2 has
also been shown to maintain stability and upregulate the expression
of a methylated lncRNA, NR-033928, in a m5C-dependent manner, which
correlates with poor prognosis in patients with gastric cancer
(86). Mechanistically, NSUN2
catalyzes the m5C modification of NR-033928, which promotes GC
proliferation and inhibits apoptosis by increasing glutaminase
expression (86). Li et al
(87) experimentally explored
lncRNA profiles in gastric cancer neuroinvasion (GC-NI) and
reported the upregulation of DIAPH2-AS1 in NI-positive GC tissues.
A further study revealed that DIAPH2-AS1 interacted with NSUN2 and
stabilized NSUN2 from ubiquitin-proteasome pathway-mediated
degradation. The protective effect of DIAPH2-AS1 on NSUN2 is
enhanced by m5C modification to increase the stability of NTN1
mRNA, which ultimately induces GC-NI (87). Their study reveals that in GC-NI,
DIAPH2-AS1 is a new oncogenic lncRNA and validated the
DIAPH2-AS1-NSUN2-NTNI axis as a potential NI-positive GC
therapeutic target, providing a new diagnostic biomarker. In
addition, FOXC2 antisense RNA 1 (FOXC2 antisense RNA 1, FOXC2-AS1)
is a newly identified functional lncRNA that is highly expressed in
gastric cancer tissues and cells and promotes gastric cancer cell
proliferation, migration and invasion; it is associated with poor
prognosis in gastric cancer patients (18). Specifically, FOXC2-AS1 recruits
NSUN2 to FOXC2 mRNA and increases its m5C level, followed by
binding of the m5C-binding protein YBX1 to FOXC2 mRNA to increase
FOXC2 mRNA stability. In conclusion, FOXC2-AS1 mediates the
oncogenic effects of the m5c modification of FOXC2 via NSUN2 and
YBX1 in gastric cancer cells, providing a new target for gastric
cancer therapy.
HCC is the sixth most common cancer and the third
leading cause of cancer mortality worldwide (83). Surgery is a common treatment, but
owing to late detection and easy metastasis, most patients are not
suited for surgical treatment. Therefore, research on the role of
HCC development and its mechanism has focused on finding effective
targets for the treatment of HCC and thus improving the prognosis
of HCC patients. The m5C methyltransferase NSUN2 is highly
expressed in HCC tissues (88).
Hypermethylated target genes (GRB2, AATF and RNF115) are involved
in oncogenic pathways. The expression of genes such as GRB2,
RNF115, AATF, ADAM15, RTN3 and HDGF is positively associated with
the expression of NSUN2. These findings indicate that
hypermethylated genes associated with NSUN2 are involved in the
development of tumors. Transcriptome analysis revealed that
hypermethylated genes are involved mainly in phosphokinase
signaling pathways, such as the Ras and PI3K-Akt pathways. NSUN2
affects the sensitivity of HCC cells to sorafenib by regulating the
activity of the Ras pathway (88).
NSUN4, which has been less studied, has also been reported to be
involved in the poor prognosis of HCC patients (22). ALYREF may be involved in
hepatocellular carcinogenesis by affecting the methylation levels
of target genes (23). As
aforementioned, NSUN5 is associated with lower overall survival.
NSUN5 mRNA and protein expression levels are upregulated in HCC
tissues and the overexpression of NSUN5 promotes the proliferation
and migration of HCC cells although the exact mechanism remains
unclear (21). Although the
mechanism involved in the aforementioned experimental studies has
not yet been elucidated, compared with the less-studied NSUN4 and
NSUN5, they have also been proven to be involved in the progression
of liver cells. High expression of NSUN2, NSUN4, NSUN5 and ALYREF
is associated with poor prognosis in patients with HCC and all of
these genes can be used as biomarkers for the diagnosis and
prognosis of HCC. These findings indicate that both
methyltransferases and related binding proteins involved in m5C
methylation are involved in the occurrence and development of HCC,
which provides directions for subsequent relevant research.
In their further experiments, they reported that
RPL6 closely interacts with NSUN2. GBC cells grew markedly slower
in the absence of RPL6 and grew relatively normally in the presence
of NSUN2 (26). Therefore, the
synergistic effect of NSUN2 and RPL6 promotes GB occurrence. In
summary, the function of NSUN2 in GBC provides new mechanistic
insights and targeting NSUN2 may be a potentially effective
treatment for GBC as well as a diagnostic biomarker, which, of
course, needs to be supported by more definitive clinical studies.
In any case, NSUN2 is indeed involved in the development of GBC,
which provides direction both in terms of finding inhibitors of
methylation and in terms of pathways.
CCA is a biliary epithelial malignancy. It is the
second most common type of primary HCC and accounts for ~15% of
mortalities from hepatobiliary malignancies (95). Early diagnosis of CCA is difficult
and most patients are already in the locally progressive stage or
have distant metastases when they present to the doctor (96). CCA is difficult to treat and even
if a few patients are able to undergo surgical treatment, there is
a high rate of recurrence and early local or distant metastasis
following surgery, whereas the 5-year survival rate is <10% and
the 1-year recurrence rate is ≤50% (97). Therefore, there is an urgent need
to elucidate the underlying mechanisms of CCA progression to
develop new therapeutic strategies. NF-κB-interacting lncRNA
(NKILA) is a functional lncRNA and a study showed that
dysregulation of NKILA expression is associated with the malignant
behavior of cancer cells (98).
Similarly, NKILA expression is upregulated in CCA patients and
NKILA expression is associated with advanced TNM stage, lymph node
and distant metastasis in CCA patients. NKILA promotes CCA
proliferation and metastasis both in vitro and in
vivo and this process is associated with NSUN2 and increases
its m5C level (27). NKILA
promotes CCA proliferation and metastasis both in vitro and
in vivo and this process is associated with NSUN2 and
increases its m5C level. This study suggests that NKILA functions
as an oncogenic lncRNA in regulating the growth and metastasis of
CCA and is a promising therapeutic target for CCA patients. The
current experimental study is far from sufficient and more
experimental studies are needed to elucidate the mechanism by which
NKILA promotes CCA progression through m5C methylation.
PC is a malignant tumor of the digestive system
characterized by a high degree of metastasis, ranking 12th among
the most common cancers in the world, with an increasing annual
incidence (83,99). In recent years, despite advances in
diagnosis and treatment, treatment outcomes remain suboptimal and
are the 7th leading cause of cancer-related mortality (100). High morbidity and mortality pose
a great threat to human health and have become an enormous global
burden. Therefore, the need to clarify the molecular mechanisms of
PC progression and identify promising and effective therapeutic
targets is urgent. Previous studies have demonstrated that NSUN2 is
highly expressed in PC tissues compared with normal tissues and
that its elevated expression portends a poor prognosis (100). Silencing NSUN2 reduced the
proliferation, migration and invasive ability of PC cells and
inhibited the growth and metastasis of xenograft tumors.
Conversely, the overexpression of NSUN2 promoted PC growth and
metastasis (101). TIAM2 has been
shown to promote the proliferation and migration of cancer cells
(102,103). TIAM2 is associated with poor
prognosis in PC patients, but its regulatory mechanism is unclear
(104). Thus, they identified the
downstream targets of NSUN2 by m5C sequencing and RNA sequencing
and the results revealed that NSUN2 deletion resulted in reduced
m5C modification levels, along with reduced TIAM2 mRNA expression.
Further validation revealed that NSUN2 silencing accelerated TIAM2
mRNA decay in a YBX1-dependent manner. In addition, NSUN2 exerts
its oncogenic function in part by enhancing TIAM2 transcription.
More importantly, disruption of the NSUN2/TIAM2 axis suppresses the
malignant phenotype of PC cells by blocking EMT. This study
highlights the critical function of NSUN2 in PC and provides novel
mechanistic insights into the NSUN2/TIAM2 axis as a promising
therapeutic target.
Pancreatic ductal adenocarcinoma (PDAC) is one of
the deadliest human malignancies, with a low overall 5-year
survival rate and is the seventh leading cause of cancer mortality
in men and women worldwide (83).
PDAC has no obvious symptoms in the early stage and most patients
are diagnosed with locally advanced or metastatic tumors; thus, the
mortality rate is high (105).
Therefore, it is particularly important to discover new targeted
drugs that can diagnose and treat PDAC at an early stage. There are
few studies on m5C and PDAC and there are only a few score on m5C
prognosis. Yun et al (106) obtained a negative correlation of
m5C scores with overall survival for predicting prognosis in
patients with PDAC by integrating m5C-related differentially
expressed genes. Similarly, high m5c expression has been shown to
predict poor prognosis in PDAC patients and their response to
immunotherapy (107,108). Pancreatic and pancreatic ductal
adenocarcinomas are both associated with m5C methylation and even
more clearly, in the case of pancreatic cancer, tumor growth and
development can be promoted through the NSUN2/TIAM2 axis.
Disruption of this axis inhibits tumor growth, suggesting
directions for the treatment of pancreatic cancer.
Colorectal cancer (CRC) has the third highest
incidence and the second highest mortality rate among all cancers
worldwide. Surgery is a common treatment, but most of the
treatments are unsatisfactory (83,109). Therefore, finding effective
therapeutic targets is particularly important. SnoN, also known as
SKIL, is a negative regulator of TGF-β signaling (110,111). NSUN2 is highly expressed in
colorectal cancer. Further knockdown of NSUN2 in mice revealed the
oncogenic role of NSUN2-mediated RNA m5C modification in colorectal
cancer. The methylation of NSUN2 promotes the stability of SKIL
mRNA in a YBX1-dependent manner, which ultimately activates and
upregulates the expression of TAZ, thus promoting the development
of CRC. Although few studies have investigated m5C methylation in
CRC, the NSUN2-m5C-SKIL-TAZ axis provides a clear direction for the
treatment of CRC.
m5C methylation plays a crucial role in the
development and progression of various digestive tumors, offering a
potential target for the development of novel therapeutic
strategies. However, research on m5C methylation remains in the
basic stage, lacking specific intervention strategies and
therapeutic approaches for clinical application. The following
analysis is based on existing studies and potential future research
directions.
Alterations in m5C methylation levels are closely
linked to the occurrence, progression and prognosis of numerous
digestive tumors. For instance, NSUN2 is highly expressed in
multiple cancers and is associated with a poor prognosis (15–18).
In addition, changes in the stability of m5C-modified RNA
molecules, such as lncRNAs and mRNAs, in tumor cells have paved the
way for the development of diagnostic markers. For example,
m5C-modified H19 lncRNA is associated with poor differentiation in
HCC (17), while FOXC2-AS1
promotes invasion and metastasis in gastric cancer by stabilizing
FOXC2 mRNA through m5C modification (18). These findings suggest that
m5C-modified RNA molecules could serve as potential diagnostic and
prognostic markers for the early detection and monitoring of tumor
progression.
Although no specific m5C methyltransferase
inhibitors have been developed for clinical use yet, certain drugs
such as azacitidine have been shown to inhibit cancer cell
proliferation by non-specifically inhibiting RNA and DNA
methylation (112). Future
research will need to focus on developing m5C methyltransferase
inhibitors that are specific to m5C methyltransferase, aiming to
minimize side effects on normal cells. Additionally, studies
targeting m5C demethylating enzymes, such as TET family proteins,
may also offer insights for the development of new therapeutic
agents.
In recent years, significant advancements have been
made in the application of m5C methylation in tumor immunotherapy
(113). For example, Segovia
et al (114) successfully
induced apoptosis and immunogenic cell death in cancer cells using
a combination of m5C inhibitors and immune checkpoint inhibitors.
Another study revealed that m5C-modified mRNAs reprogram
tumor-associated macrophages or anticancer T cells, inducing
antitumor immunity and promoting tumor regression (115). Currently, the m5C-associated risk
score is an independent prognostic factor for patients with colon
cancer and the score can be used to predict the prognosis,
immunotherapy response and drug sensitivity of colon cancer
patients (116). In addition, the
methyltransferase complex component RBM15B- and the m6A ‘card
reader’ IGFBP2-mediated glutathione peroxidase 4 may be novel
modulators of cancer immunotherapy through activation of the cyclic
GMP-AMP synthase-interferon signaling pathway in colorectal
adenocarcinoma, which has emerged as a novel modulator of cancer
immunotherapy (117). These
findings imply that m5C methylation modification could be a
potential target for immunotherapy and future studies could further
explore its application in other digestive tumors. Regarding future
research directions, they can concentrate on clinical data
integration. They should strengthen the integration with clinical
medical data and verify the diagnostic and prognostic value of m5C
methylation markers through large-scale clinical sample analysis.
Drug development is another important direction. Developing
specific m5C methyltransferase and demethylase inhibitors and
exploring their application in clinical treatment are essential.
Multiomics research can also be applied. By combining
transcriptomics, proteomics and metabolomics, we can
comprehensively elucidate the mechanism of m5C methylation in
tumorigenesis and development. Further translational medicine
research can be conducted to explore the application of m5C
methylation in personalized medicine, providing theoretical support
for precision therapy.
The present study reviewed the role of m5C
methylation in digestive system tumors and its potential as a
therapeutic target. Although m5C methylation has been shown to play
a significant role in the development of a number of digestive
system tumors, such as esophageal squamous cell carcinoma, HCC,
breast cancer and thyroid cancer, current research is still in the
basic stage, lacking specific intervention strategies and
therapeutic approaches for clinical application. Future studies
need to strengthen the integration with clinical medical data,
develop specific m5C methylation inhibitors and explore their
potential application in tumor immunotherapy. Through these
efforts, m5C methylation is expected to become a new target for the
diagnosis and treatment of digestive system tumors, providing a new
direction for improving patient prognosis.
Not applicable.
The present study was supported by grants from the National
Natural Science Foundation of China (grant nos. 81960507, 82073087
and 82160112), the Science and Technology Bureau fund of Zunyi City
[grant no. ZUN SHI KE HE HZ ZI (2019)93-Hao], the Science and
Technology Plan Project of Guizhou Province [grant nos. QIAN KE HE
JI CHU-ZK(2021)YI BAN451 and QIAN KE HE LH ZI(2017)7095 HAO] and
Collaborative Innovation Center of Chinese Ministry of Education
(2020–39).
Not applicable.
LZ and JY made substantial contributions to the
conception and design of the present study. SY, GW, JA, HJ and BT
were involved in revising the manuscript critically for important
intellectual content. Data authentication is not applicable. All
authors read and approved the final manuscript for publication.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Cao W, Chen HD, Yu YW, Li N and Chen WQ:
Changing profiles of cancer burden worldwide and in China: A
secondary analysis of the global cancer statistics 2020. Chin Med J
(Engl). 134:783–791. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu C, Yang S, Zhang Y, Wang C, Du D, Wang
X, Liu T and Liang G: Emerging roles of N6-methyladenosine
demethylases and its interaction with environmental toxicants in
digestive system cancers. Cancer Manag Res. 13:7101–7114. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Y: Modern epigenetics methods in
biological research. Methods. 187:104–113. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luo C, Hajkova P and Ecker JR: Dynamic DNA
methylation: In the right place at the right time. Science.
361:1336–1340. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stepanov AI, Besedovskaia ZV, Moshareva
MA, Lukyanov KA and Putlyaeva LV: Studying chromatin epigenetics
with fluorescence microscopy. Int J Mol Sci. 23:89882022.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roundtree IA, Evans ME, Pan T and He C:
Dynamic RNA modifications in gene expression regulation. Cell.
169:1187–1200. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hussain S: The emerging roles of
cytosine-5 methylation in mRNAs. Trends Genet. 37:498–500. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dubin DT and Stollar V: Methylation of
sindbis virus ‘26S’ messenger RNA. Biochem Biophys Res Commun.
66:1373–1379. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Motorin Y, Lyko F and Helm M:
5-methylcytosine in RNA: Detection, enzymatic formation and
biological functions. Nucleic Acids Res. 38:1415–1430. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu L, Song B, Ma J, Song Y, Zhang SY,
Tang Y, Wu X, Wei Z, Chen K, Su J, et al: Bioinformatics approaches
for deciphering the epitranscriptome: Recent progress and emerging
topics. Comput Struct Biotechnol J. 18:1587–1604. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang X, Yang Y, Sun BF, Chen YS, Xu JW,
Lai WY, Li A, Wang X, Bhattarai DP, Xiao WS, et al:
5-methylcytosine promotes mRNA export-NSUN2 as the
methyltransferase and ALYREF as an m5C reader. Cell Res.
27:606–625. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang M, Song J, Yuan W, Zhang W and Sun
Z: Roles of RNA methylation on tumor immunity and clinical
implications. Front Immunol. 12:6415072021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zou F, Tu R, Duan B, Yang Z, Ping Z, Song
X, Chen S, Price A, Li H, Scott A, et al: Drosophila YBX1 homolog
YPS promotes ovarian germ line stem cell development by
preferentially recognizing 5-methylcytosine RNAs. Proc Natl Acad
Sci USA. 117:3603–3609. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Su J, Wu G, Ye Y, Zhang J, Zeng L, Huang
X, Zheng Y, Bai R, Zhuang L, Li M, et al: NSUN2-mediated RNA
5-methylcytosine promotes esophageal squamous cell carcinoma
progression via LIN28B-dependent GRB2 mRNA stabilization. Oncogene.
40:5814–5828. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu Y, Chen C, Tong X, Chen S, Hu X, Pan B,
Sun X, Chen Z, Shi X, Hu Y, et al: NSUN2 modified by SUMO-2/3
promotes gastric cancer progression and regulates mRNA m5C
methylation. Cell Death Dis. 12:8422021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun Z, Xue S, Zhang M, Xu H, Hu X, Chen S,
Liu Y, Guo M and Cui H: Aberrant NSUN2-mediated m5C
modification of H19 lncRNA is associated with poor differentiation
of hepatocellular carcinoma. Oncogene. 39:6906–6919. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yan J, Liu J, Huang Z, Huang W and Lv J:
FOXC2-AS1 stabilizes FOXC2 mRNA via association with NSUN2 in
gastric cancer cells. Hum Cell. 34:1755–1764. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Y, Li J, Luo M, Zhou C, Shi X, Yang W,
Lu Z, Chen Z, Sun N and He J: Novel long noncoding RNA NMR promotes
tumor progression via NSUN2 and BPTF in esophageal squamous cell
carcinoma. Cancer Lett. 430:57–66. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mei L, Shen C, Miao R, Wang JZ, Cao MD,
Zhang YS, Shi LH, Zhao GH, Wang MH, Wu LS and Wei JF: RNA
methyltransferase NSUN2 promotes gastric cancer cell proliferation
by repressing p57Kip2 by an m5C-dependent
manner. Cell Death Dis. 11:2702020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang XW, Wu LY, Liu HR, Huang Y, Qi Q,
Zhong R, Zhu L, Gao CF, Zhou L, Yu J and Wu HG: NSUN5 promotes
progression and predicts poor prognosis in hepatocellular
carcinoma. Oncol Lett. 24:4392022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
He Y, Yu X, Li J, Zhang Q, Zheng Q and Guo
W: Role of m5C-related regulatory genes in the diagnosis
and prognosis of hepatocellular carcinoma. Am J Trans Res.
12:912–922. 2020.
|
|
23
|
Xue C, Gu X, Zheng Q, Shi Q, Yuan X, Su Y,
Jia J, Jiang J, Lu J and Li L: ALYREF mediates RNA m5C
modification to promote hepatocellular carcinoma progression.
Signal Transduct Target Ther. 8:1302023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen SY, Chen KL, Ding LY, Yu CH, Wu HY,
Chou YY, Chang CJ, Chang CH, Wu YN, Wu SR, et al: RNA bisulfite
sequencing reveals NSUN2-mediated suppression of epithelial
differentiation in pancreatic cancer. Oncogene. 41:3162–3176. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang R, Liang X, Wang H, Guo M, Shen H,
Shi Y, Liu Q, Sun Y, Yang L and Zhan M: The RNA methyltransferase
NSUN6 suppresses pancreatic cancer development by regulating cell
proliferation. EBioMedicine. 63:1031952021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao Y, Wang Z, Zhu Y, Zhu Q, Yang Y, Jin
Y, Zhang F, Jiang L, Ye Y, Li H, et al: NOP2/Sun RNA
methyltransferase 2 promotes tumor progression via its interacting
partner RPL6 in gallbladder carcinoma. Cancer Sci. 110:3510–3519.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zheng H, Zhu M, Li W, Zhou Z and Wan X:
m5C and m6A modification of long noncoding
NKILA accelerates cholangiocarcinoma progression via the
miR-582-3p-YAP1 axis. Liver Int. 42:1144–1157. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yin H, Huang Z, Niu S, Ming L, Jiang H, Gu
L, Huang W, Xie J, He Y and Zhang C: 5-Methylcytosine
(m5C) modification in peripheral blood immune cells is a
novel non-invasive biomarker for colorectal cancer diagnosis. Front
Immunol. 13:9679212022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zin'kovskaia GG, Berdyshev GD and
Vaniushin BF: Tissue-specific decrease and change in the character
of DNA methylation in cattle with aging. Biokhimiia. 43:1883–1892.
1978.(In Russian). PubMed/NCBI
|
|
30
|
Deng X, Qing Y, Horne D, Huang H and Chen
J: The roles and implications of RNA m6A modification in
cancer. Nat Rev Clin Oncol. 20:507–526. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou H, Rauch S, Dai Q, Cui X, Zhang Z,
Nachtergaele S, Sepich C, He C and Dickinson BC: Evolution of a
reverse transcriptase to map N1-methyladenosine in human
messenger RNA. Nat Methods. 16:1281–1288. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li J, Zhang H and Wang H:
N1-methyladenosine modification in cancer biology:
Current status and future perspectives. Comput Struct Biotechnol J.
20:6578–6585. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen Y, Lin H, Miao L and He J: Role of
N7-methylguanosine (m7G) in cancer. Trends Cell Biol.
32:819–824. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pandolfini L, Barbieri I, Bannister AJ,
Hendrick A, Andrews B, Webster N, Murat P, Mach P, Brandi R, Robson
SC, et al: METTL1 Promotes let-7 MicroRNA processing via m7G
Methylation. Mol Cell. 74:1278–1290. e92019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lin S, Liu Q, Lelyveld VS, Choe J, Szostak
JW and Gregory RI: Mettl1/Wdr4-Mediated m7G tRNA
methylome is required for normal mRNA translation and embryonic
stem cell self-renewal and differentiation. Mol Cell. 71:244–255.
e52018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang Q, Liu F, Chen W, Miao H, Liang H,
Liao Z, Zhang Z and Zhang B: The role of RNA m5C
modification in cancer metastasis. Int J Biol Sci. 17:3369–3380.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li M, Tao Z, Zhao Y, Li L, Zheng J, Li Z
and Chen X: 5-methylcytosine RNA methyltransferases and their
potential roles in cancer. J Transl Med. 20:2142022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang R, Ding L, Lin Y, Luo W, Xu Z, Li W,
Lu Y, Zhu Z, Lu Z, Li F, et al: The quiet giant: Identification,
effectors, molecular mechanism, physiological and pathological
function in mRNA 5-methylcytosine modification. Int J Biol Sci.
20:6241–6254. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nombela P, Miguel-Lopez B and Blanco S:
The role of m6A, m5C and ψ RNA modifications
in cancer: Novel therapeutic opportunities. Mol Cancer. 20:182021.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang ZX, Li J, Xiong QP, Li H, Wang ED
and Liu RJ: Position 34 of tRNA is a discriminative element for
m5C38 modification by human DNMT2. Nucleic Acids Res.
49:13045–13061. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li H, Zhu D, Wu J, Ma Y, Cai C, Chen Y,
Qin M and Dai H: New substrates and determinants for tRNA
recognition of RNA methyltransferase DNMT2/TRDMT1. RNA Biol.
18:2531–2545. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bohnsack KE, Hobartner C and Bohnsack MT:
Eukaryotic 5-methylcytosine (m5C) RNA
Methyltransferases: Mechanisms, cellular functions, and links to
disease. Genes (Basel). 10:1022019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kong W, Biswas A, Zhou D, Fiches G,
Fujinaga K, Santoso N and Zhu J: Nucleolar protein NOP2/NSUN1
suppresses HIV-1 transcription and promotes viral latency by
competing with Tat for TAR binding and methylation. PLoS Pathog.
16:e10084302020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen X, Li A, Sun BF, Yang Y, Han YN, Yuan
X, Chen RX, Wei WS, Liu Y, Gao CC, et al: 5-methylcytosine promotes
pathogenesis of bladder cancer through stabilizing mRNAs. Nat Cell
Biol. 21:978–990. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hong B, Brockenbrough JS, Wu P and Aris
JP: Nop2p is required for pre-rRNA processing and 60S ribosome
subunit synthesis in yeast. Mol Cell Biol. 17:378–388. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sakita-Suto S, Kanda A, Suzuki F, Sato S,
Takata T and Tatsuka M: Aurora-B regulates RNA methyltransferase
NSUN2. Mol Biol Cell. 18:1107–1117. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xing J, Yi J, Cai X, Tang H, Liu Z, Zhang
X, Martindale JL, Yang X, Jiang B, Gorospe M and Wang W: NSun2
promotes cell growth via elevating cyclin-dependent kinase 1
translation. Mol Cell Biol. 35:4043–4052. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sun Z, Xue S, Xu H, Hu X, Chen S, Yang Z,
Yang Y, Ouyang J and Cui H: Effects of NSUN2 deficiency on the mRNA
5-methylcytosine modification and gene expression profile in HEK293
cells. Epigenomics. 11:439–453. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sajini AA, Choudhury NR, Wagner RE,
Bornelov S, Selmi T, Spanos C, Dietmann S, Rappsilber J, Michlewski
G and Frye M: Loss of 5-methylcytosine alters the biogenesis of
vault-derived small RNAs to coordinate epidermal differentiation.
Nat Commun. 10:25502019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kar SP, Beesley J, Amin Al Olama A,
Michailidou K, Tyrer J, Kote-Jarai Z, Lawrenson K, Lindstrom S,
Ramus SJ, Thompson DJ, et al: Genome-wide meta-analyses of breast,
ovarian, and prostate cancer association studies identify multiple
new susceptibility loci shared by at least two cancer types. Cancer
Discov. 6:1052–1067. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li H, Jiang H, Huang Z, Chen Z and Chen N:
Prognostic value of an m5C RNA methylation
regulator-related signature for clear cell renal cell carcinoma.
Cancer Manag Res. 13:6673–6687. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Okamoto M, Hirata S, Sato S, Koga S, Fujii
M, Qi G, Ogawa I, Takata T, Shimamoto F and Tatsuka M: Frequent
increased gene copy number and high protein expression of tRNA
(cytosine-5-)-methyltransferase (NSUN2) in human cancers. DNA Cell
Biol. 31:660–671. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liao H, Gaur A, McConie H, Shekar A, Wang
K, Chang JT, Breton G and Denicourt C: Human NOP2/NSUN1 regulates
ribosome biogenesis through non-catalytic complex formation with
box C/D snoRNPs. Nucleic Acids Res. 50:10695–10716. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Delaunay S, Pascual G, Feng B, Klann K,
Behm M, Hotz-Wagenblatt A, Richter K, Zaoui K, Herpel E, Münch C,
et al: Mitochondrial RNA modifications shape metabolic plasticity
in metastasis. Nature. 607:593–603. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Paramasivam A, Meena AK, Venkatapathi C,
Pitceathly RDS and Thangaraj K: Novel biallelic NSUN3 variants
cause early-onset mitochondrial encephalomyopathy and seizures. J
Mol Neurosci. 70:1962–1965. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Metodiev MD, Spahr H, Loguercio Polosa P,
Meharg C, Becker C, Altmueller J, Habermann B, Larsson NG and
Ruzzenente B: NSUN4 is a dual function mitochondrial protein
required for both methylation of 12S rRNA and coordination of
mitoribosomal assembly. PLoS Genet. 10:e10041102014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Heissenberger C, Liendl L, Nagelreiter F,
Gonskikh Y, Yang G, Stelzer EM, Krammer TL, Micutkova L, Vogt S,
Kreil DP, et al: Loss of the ribosomal RNA methyltransferase NSUN5
impairs global protein synthesis and normal growth. Nucleic Acids
Res. 47:11807–11825. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Selmi T, Hussain S, Dietmann S, Heiß M,
Borland K, Flad S, Carter JM, Dennison R, Huang YL, Kellner S, et
al: Sequence- and structure-specific cytosine-5 mRNA methylation by
NSUN6. Nucleic Acids Res. 49:1006–1022. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Haag S, Warda AS, Kretschmer J, Gunnigmann
MA, Hobartner C and Bohnsack MT: NSUN6 is a human RNA
methyltransferase that catalyzes formation of m5C72 in specific
tRNAs. RNA. 21:1532–1543. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Aguilo F, Li S, Balasubramaniyan N, Sancho
A, Benko S, Zhang F, Vashisht A, Rengasamy M, Andino B, Chen CH, et
al: Deposition of 5-methylcytosine on enhancer RNAs enables the
coactivator function of PGC-1α. Cell Rep. 14:479–492. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Khosronezhad N, Hosseinzadeh Colagar A and
Mortazavi SM: The Nsun7 (A11337)-deletion mutation, causes
reduction of its protein rate and associated with sperm motility
defect in infertile men. J Assist Reprod Genet. 32:807–815. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Sato K, Tahata K and Akimoto K: Five genes
associated with survival in patients with lower-grade gliomas were
identified by information-theoretical analysis. Anticancer Res.
40:2777–2785. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
He YF, Li BZ, Li Z, Liu P, Wang Y, Tang Q,
Ding J, Jia Y, Chen Z, Li L, et al: Tet-mediated formation of
5-carboxylcytosine and its excision by TDG in mammalian DNA.
Science. 333:1303–1307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Tahiliani M, Koh KP, Shen Y, Pastor WA,
Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L and
Rao A: Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in
mammalian DNA by MLL partner TET1. Science. 324:930–935. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ito S, Shen L, Dai Q, Wu SC, Collins LB,
Swenberg JA, He C and Zhang Y: Tet proteins can convert
5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine.
Science. 333:1300–1303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhao LY, Song J, Liu Y, Song CX and Yi C:
Mapping the epigenetic modifications of DNA and RNA. Protein Cell.
11:792–808. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Shen H, Ontiveros RJ, Owens MC, Liu MY,
Ghanty U, Kohli RM and Liu KF: TET-mediated 5-methylcytosine
oxidation in tRNA promotes translation. J Biol Chem.
296:1000872021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Yin X and Xu Y: Structure and function of
TET enzymes. Adv Exp Med Biol. 945:275–302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lyabin DN, Eliseeva IA and Ovchinnikov LP:
YB-1 protein: Functions and regulation. Wiley Interdiscip Rev RNA.
5:95–110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Kang S, Lee TA, Ra EA, Lee E, Choi Hj, Lee
S and Park B: Differential control of interleukin-6 mRNA levels by
cellular distribution of YB-1. PLoS One. 9:e1127542014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Coles LS, Bartley MA, Bert A, Hunter J,
Polyak S, Diamond P, Vadas MA and Goodall GJ: A multi-protein
complex containing cold shock domain (Y-box) and polypyrimidine
tract binding proteins forms on the vascular endothelial growth
factor mRNA. Potential role in mRNA stabilization. Eur J Biochem.
271:648–660. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Bates M, Boland A, McDermott N and
Marignol L: YB-1: The key to personalised prostate cancer
management? Cancer Lett. 490:66–75. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wang JZ, Zhu W, Han J, Yang X, Zhou R, Lu
HC, Yu H, Yuan WB, Li PC, Tao J, et al: The role of the
HIF-1α/ALYREF/PKM2 axis in glycolysis and tumorigenesis of bladder
cancer. Cancer Commun (Lond). 41:560–575. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Yang Y, Wang L, Han X, Yang WL, Zhang M,
Ma HL, Sun BF, Li A, Xia J, Chen J, et al: RNA 5-methylcytosine
facilitates the maternal-to-zygotic transition by preventing
maternal mRNA decay. Mol Cell. 75:1188–1202. e112019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Shi H, Chai P, Jia R and Fan X: Novel
insight into the regulatory roles of diverse RNA modifications:
Re-defining the bridge between transcription and translation. Mol
Cancer. 19:782020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Trixl L and Lusser A: The dynamic RNA
modification 5-methylcytosine and its emerging role as an
epitranscriptomic mark. Wiley Interdiscip Rev RNA. 10:e15102019.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Chen H, Yang H, Zhu X, Yadav T, Ouyang J,
Truesdell SS, Tan J, Wang Y, Duan M, Wei L, et al: m5C
modification of mRNA serves a DNA damage code to promote homologous
recombination. Nat Commun. 11:28342020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Xue S, Xu H, Sun Z, Shen H, Chen S, Ouyang
J, Zhou Q, Hu X and Cui H: Depletion of TRDMT1 affects
5-methylcytosine modification of mRNA and inhibits HEK293 cell
proliferation and migration. Biochem Biophys Res Commun. 520:60–66.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Xue C, Zhao Y and Li L: Advances in RNA
cytosine-5 methylation: Detection, regulatory mechanisms,
biological functions and links to cancer. Biomark Res. 8:432020.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Morgan E, Soerjomataram I, Rumgay H,
Coleman HG, Thrift AP, Vignat J, Laversanne M, Ferlay J and Arnold
M: the global landscape of esophageal squamous cell carcinoma and
esophageal adenocarcinoma incidence and mortality in 2020 and
projections to 2040: New estimates from GLOBOCAN 2020.
Gastroenterology. 163:649–658. e22022. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Smyth EC, Lagergren J, Fitzgerald RC,
Lordick F, Shah MA, Lagergren P and Cunningham D: Oesophageal
cancer. Nat Rev Dis Primers. 3:170482017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Liu L, Chen Y, Zhang T, Cui G, Wang W,
Zhang G, Li J, Zhang Y, Wang Y, Zou Y, et al: YBX1 promotes
esophageal squamous cell carcinoma progression via m5C-dependent
SMOX mRNA stabilization. Adv Sci (Weinh). 11:e23023792024.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Xiang S, Ma Y, Shen J, Zhao Y, Wu X, Li M,
Yang X, Kaboli PJ, Du F, Ji H, et al: m5C RNA
methylation primarily affects the ErbB and PI3K-Akt signaling
pathways in gastrointestinal cancer. Front Mol Biosci.
7:5993402020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zhang E, He X, Yin D, Han L, Qiu M, Xu T,
Xia R, Xu L, Yin R and De W: Increased expression of long noncoding
RNA TUG1 predicts a poor prognosis of gastric cancer and regulates
cell proliferation by epigenetically silencing of p57. Cell Death
Dis. 7:e21092016. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Fang L, Huang H, Lv J, Chen Z, Lu C, Jiang
T, Xu P, Li Y, Wang S, Li B, et al: m5C-methylated lncRNA NR_033928
promotes gastric cancer proliferation by stabilizing GLS mRNA to
promote glutamine metabolism reprogramming. Cell Death Dis.
14:5202023. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Li Y, Xia Y, Jiang T, Chen Z, Shen Y, Lin
J, Xie L, Gu C, Lv J, Lu C, et al: Long noncoding RNA DIAPH2-AS1
promotes neural invasion of gastric cancer via stabilizing NSUN2 to
enhance the m5C modification of NTN1. Cell Death Dis. 14:2602023.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Song D, An K, Zhai W, Feng L, Xu Y, Sun R,
Wang Y, Yang YG, Kan Q and Tian X: NSUN2-mediated mRNA
m5C modification regulates the progression of
hepatocellular carcinoma. Genomics Proteomics Bioinformatics.
21:823–833. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Sharma A, Sharma KL, Gupta A, Yadav A and
Kumar A: Gallbladder cancer epidemiology, pathogenesis and
molecular genetics: Recent update. World J Gastroenterol.
23:3978–3998. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Misra S, Chaturvedi A, Misra NC and Sharma
ID: Carcinoma of the gallbladder. Lancet Oncol. 4:167–176. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Li M, Zhang Z, Li X, Ye J, Wu X, Tan Z,
Liu C, Shen B, Wang XA, Wu W, et al: Whole-exome and targeted gene
sequencing of gallbladder carcinoma identifies recurrent mutations
in the ErbB pathway. Nat Genet. 46:872–876. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Maurya SK, Tewari M, Mishra RR and Shukla
HS: Genetic aberrations in gallbladder cancer. Surg Oncol.
21:37–43. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Bai D, Zhang J, Xiao W and Zheng X:
Regulation of the HDM2-p53 pathway by ribosomal protein L6 in
response to ribosomal stress. Nucleic Acids Res. 42:1799–1811.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Banales JM, Marin JJG, Lamarca A,
Rodrigues PM, Khan SA, Roberts LR, Cardinale V, Carpino G, Andersen
JB, Braconi C, et al: Cholangiocarcinoma 2020: The next horizon in
mechanisms and management. Nat Rev Gastroenterol Hepatol.
17:557–588. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ramirez-Merino N, Aix SP and Cortes-Funes
H: Chemotherapy for cholangiocarcinoma: An update. World J
Gastrointest Oncol. 5:171–176. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Banales JM, Cardinale V, Carpino G,
Marzioni M, Andersen JB, Invernizzi P, Lind GE, Folseraas T, Forbes
SJ, Fouassier L, et al: Expert consensus document:
Cholangiocarcinoma: Current knowledge and future perspectives
consensus statement from the European Network for the Study of
Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol.
13:261–280. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Chen Y, Li Z, Chen X and Zhang S: Long
non-coding RNAs: From disease code to drug role. Acta Pharm Sin B.
11:340–354. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
GBD 2017 Pancreatic Cancer Collaborators,
. The global, regional, and national burden of pancreatic cancer
and its attributable risk factors in 195 countries and territories,
1990–2017: A systematic analysis for the Global Burden of Disease
Study 2017. Lancet Gastroenterol Hepatol. 4:934–947. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
McGuigan A, Kelly P, Turkington RC, Jones
C, Coleman HG and McCain RS: Pancreatic cancer: A review of
clinical diagnosis, epidemiology, treatment and outcomes. World J
Gastroenterol. 24:4846–4861. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zhang G, Liu L, Li J, Chen Y, Wang Y,
Zhang Y, Dong Z, Xue W, Sun R and Cui G: NSUN2 stimulates tumor
progression via enhancing TIAM2 mRNA stability in pancreatic
cancer. Cell Death Discov. 9:2192023. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Chen JS, Su IJ, Leu YW, Young KC and Sun
HS: Expression of T-cell lymphoma invasion and metastasis 2 (TIAM2)
promotes proliferation and invasion of liver cancer. Int J Cancer.
130:1302–1313. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Cooke M, Kreider-Letterman G, Baker MJ,
Zhang S, Sullivan NT, Eruslanov E, Abba MC, Goicoechea SM,
García-Mata R and Kazanietz MG: FARP1, ARHGEF39, and TIAM2 are
essential receptor tyrosine kinase effectors for Rac1-dependent
cell motility in human lung adenocarcinoma. Cell Rep.
37:1099052021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Jiang B, Zhou L, Lu J, Wang Y, Liu C, Zhou
W and Guo J: Elevated TIAM2 expression promotes tumor progression
and is associated with unfavorable prognosis in pancreatic cancer.
Scand J Gastroenterol. 56:59–67. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Edwards BK, Brown ML, Wingo PA, Howe HL,
Ward E, Ries LA, Schrag D, Jamison PM, Jemal A, Wu XC, et al:
Annual report to the nation on the status of cancer, 1975–2002,
featuring population-based trends in cancer treatment. J Natl
Cancer Inst. 97:1407–1427. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Yun D, Yang Z, Zhang S, Yang H, Liu D,
Grutzmann R, Pilarsky C and Britzen-Laurent N: An m5C methylation
regulator-associated signature predicts prognosis and therapy
response in pancreatic cancer. Front Cell Dev Biol. 10:9756842022.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Yuan H, Liu J, Zhao L, Wu P, Chen G, Chen
Q, Shen P, Yang T, Fan S, Xiao B and Jiang K: Prognostic risk model
and tumor immune environment modulation of m5C-Related LncRNAs in
pancreatic ductal adenocarcinoma. Front Immunol. 12:8002682021.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Liu X, Wang D, Han S, Wang F, Zang J, Xu C
and Dong X: Signature of m5C-Related lncRNA for prognostic
prediction and immune responses in pancreatic cancer. J Oncol.
2022:74677972022.PubMed/NCBI
|
|
109
|
Zhao H, Ming T, Tang S, Ren S, Yang H, Liu
M, Tao Q and Xu H: Wnt signaling in colorectal cancer: Pathogenic
role and therapeutic target. Mol Cancer. 21:1442022. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Deheuninck J and Luo K: Ski and SnoN,
potent negative regulators of TGF-beta signaling. Cell Res.
19:47–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Pan D, Zhu Q and Luo K: SnoN functions as
a tumour suppressor by inducing premature senescence. EMBO J.
28:3500–3513. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Schaefer M, Hagemann S, Hanna K and Lyko
F: Azacytidine inhibits RNA methylation at DNMT2 target sites in
human cancer cell lines. Cancer Res. 69:8127–8132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Zhang Y and Zhang Z: The history and
advances in cancer immunotherapy: Understanding the characteristics
of tumor-infiltrating immune cells and their therapeutic
implications. Cell Mol Immunol. 17:807–821. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Segovia C, San Jose-Eneriz E,
Munera-Maravilla E, Martinez-Fernandez M, Garate L, Miranda E,
Vilas-Zornoza A, Lodewijk I, Rubio C, Segrelles C, et al:
Inhibition of a G9a/DNMT network triggers immune-mediated bladder
cancer regression. Nat Med. 25:1073–1081. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Zhang F, Parayath NN, Ene CI, Stephan SB,
Koehne AL, Coon ME, Holland EC and Stephan MT: Genetic programming
of macrophages to perform anti-tumor functions using targeted mRNA
nanocarriers. Nat Commun. 10:39742019. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
He R, Man C, Huang J, He L, Wang X, Lang Y
and Fan Y: Identification of RNA methylation-related lncRNAs
signature for predicting hot and cold tumors and prognosis in colon
cancer. Front Genet. 13:8709452022. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Chen B, Hong Y, Zhai X, Deng Y, Hu H, Tian
S, Zhang Y, Ren X, Zhao J and Jiang C: m6A and m5C modification of
GPX4 facilitates anticancer immunity via STING activation. Cell
Death Dis. 14:8092023. View Article : Google Scholar : PubMed/NCBI
|