The immune system serves a pivotal role in both the
initiation and progression of cancer (1,2). In
1909, Paul Ehrlich hypothesized that the immune system regulates
tumor development (3). In 1957,
Burnet (4) introduced the cancer
immunosurveillance theory, which suggests that lymphocytes serve a
role as ‘guardians’ of the body by identifying, eliminating and
killing mutated cells, thereby preventing tumor formation. However,
tumors evade immune detection through immune escape mechanisms,
leading to cancer progression.
Over the past decades, immunotherapy (therapeutic
strategies aimed at targeting and modulating the immune system) has
notably changed cancer treatment (5,6). The
United States Food and Drug Administration (FDA) has approved
numerous types of cancer immunotherapy, including immune checkpoint
inhibitors, cancer vaccines and adoptive immune cell therapy
(7–13). However, despite these advancements,
immunotherapy remains largely effective only against tumors that
are intrinsically sensitive to immune responses. A challenge is
presented by the immunosuppressive tumor microenvironment (TME),
which is characterized by regulatory immune cells [such as
regulatory T cells (Tregs) and myeloid-derived suppressor cells
(MDSCs)] and immunosuppressive cytokines (14). The TME impedes T cell infiltration
and function, thereby presenting a barrier to effective
immunotherapy. Therefore, strategies aimed at reversing immune
suppression within the TME are key for expanding the applicability
of cancer immunotherapy.
Flavonoids, which are abundant in fruits,
vegetables, tea and other plant-based foods, exhibit anti-cancer
properties, including antioxidant and anti-inflammatory activity,
induction of apoptosis, inhibition of angiogenesis and modulation
of the immune system (15–19). Flavonoids regulate immune cells,
cytokines and antigen presentation, thereby effectively reversing
the immunosuppressive TME (19,20).
These properties position flavonoids as promising adjuvants in
immunotherapy. Although the majority of studies remain at the
preclinical stage, clinical trials involving flavonoid compounds in
combination with immunotherapy have already been approved by the
FDA (Table SI). Furthermore, the
application of nanotechnology has promise in enhancing the
bioavailability and targeting of flavonoid compounds, thereby
improving their efficacy in immunotherapy (21).
The present review aimed to explore the role of
flavonoids in cancer immunotherapy, emphasizing their ability to
modulate the immune system and to reverse the immunosuppressive
TME, as well as their potential to enhance efficacy and expand
application of existing immunotherapies.
Cytotoxic lymphocytes (CTLs) serve a key role in the
immune system via identifying and eliminating cancer cells.
However, the TME exerts inhibitory effects on their function. Key
contributors to immune suppression within the TME include immune
inhibitory factors, immunosuppressive cells and immune checkpoint
pathways (22).
The TME exhibits immunosuppressive properties
through a variety of mechanisms; these include the infiltration of
immunomodulatory cell populations, such as MDSCs, tumor-associated
macrophages (TAMs), cancer-associated fibroblasts (CAFs), Tregs and
fetal-like immune and stromal cells (23,24).
Moreover, the TME is characterized by the expression of
immunosuppressive cytokines, including TGF-β, IL-10, IL-35,
chemokine ligand (CCL) 5 and C-X-C motif chemokine ligand 12 (also
known as stromal cell-derived factor 1) (25). These factors create an
immunosuppressive milieu that impairs cytotoxic T cell activity and
inhibits effective anti-tumor immune responses.
Tumor cells secrete chemokines, such as CCL22, to
recruit macrophages into the TME, which induces their polarization
into the immunosuppressive M2 phenotype via factors such as
vascular endothelial growth factor (VEGF), galectin-1,
gangliosides, TGF-β, prostaglandin E2 (PGE2) and IL-10 (26–28).
Additionally, IL-10, TGF-β and VEGF inhibit antigen presentation
mediated by dendritic cells (DCs), thereby facilitating recruitment
of Tregs into the TME and suppressing CTL activity (29,30).
Tumors induce T cell exhaustion and decrease
anti-tumor activity by upregulating immune checkpoint molecules,
including programmed cell death protein 1 (PD-1) and CTL-associated
protein 4 (CTLA-4) (31–33). For example, PD-1 binds to PD-ligand
(PD-L) 1/2, which are expressed on tumor or stromal cells, thereby
inhibiting T cell proliferation and cytokine production (34). Similarly, CTLA-4 competes with CD28
for binding to CD80/CD86, thereby decreasing T cell activation
(35,36). In addition to PD-1 and CTLA-4,
other immune checkpoints also serve roles in immune evasion and T
cell dysfunction. For example, lymphocyte activation gene 3 (LAG-3)
binds major histocompatibility complex (MHC) class II molecules,
thereby impairing antigen presentation and decreasing T cell
activation, while also enhancing the suppressive activity of Tregs,
which exacerbates immune suppression (37). The immune receptor T cell
immunoglobulin and immunoreceptor Tyrosine-based Inhibitory
Motifdomain, expressed on T cells and natural killer (NK) cells,
binds to CD155 on antigen-presenting cells (APCs), inhibiting T
cell receptor (TCR) signaling and reducing NK cell cytotoxicity,
which thereby promotes tumor immune escape (38,39).
In addition, T cell immunoglobulin and mucin domain 3 interacts
with galectin-9 and phosphatidylserine, which induces T cell
exhaustion and decreases cytokine production; this protein is often
co-expressed with PD-1 to produce a synergistic inhibitory effect
(40,41). The effects of these immune
checkpoints are not isolated; their functions are amplified by the
presence of immunosuppressive cells, including MDSCs and Tregs.
These cells secrete cytokines (TGF-β and IL-10) and express ligands
for immune checkpoint molecules, thereby intensifying immune
suppression (42). For example,
MDSCs upregulate PD-L1 expression, enhancing PD-1-mediated T cell
suppression (43), whereas Tregs
exacerbate immune inhibition by highly expressing CTLA-4 and LAG-3
(44,45).
The TME maintains a balance between tumor-promoting
and tumor-antagonistic immune cells. Tumor-promoting cells, such as
Tregs, MDSCs and M2-polarized macrophages, foster cancer
progression by inhibiting anti-tumor immunity and creating an
immunosuppressive environment (46). By contrast, tumor-antagonistic
cells, including CTLs (CD8+ T cells), NK cells, DCs and
M1-polarized macrophages, recognize and destroy cancer cells.
Flavonoids modulate these immune cells, thereby shifting the TME
from an immunosuppressive to an immune-supportive state (47).
Monocytes and macrophages serve a key role in the
immune system via the detection of pathogen-associated molecular
patterns, mediating inflammatory responses, promoting immune
killing and facilitating antigen presentation (48–50).
Macrophages recruited to the TME are known as TAMs. TAMs are
primarily classified into two types: M1 and M2. M1 macrophages are
typically pro-inflammatory and kill tumor cells, whereas M2
macrophages are associated with tissue repair and tumor progression
(51–53). In numerous types of tumor, such as
breast, lung, colorectal and liver cancer, and glioblastoma, TAMs
adopt an M2-like phenotype, which promotes tumor growth, invasion
and metastasis. M2-like TAMs stimulate cancer cell proliferation,
angiogenesis and migration, thereby contributing to tumor expansion
and spread. Moreover, TAMs engage in positive cross-talk with other
immunosuppressive cells, such as Tregs, MDSCs and CAFs, further
enhancing tumor growth and the release of growth factors (54,55).
Flavonoids reverse immune suppression through
inhibiting macrophage recruitment. Luteolin and catechin, for
example, inhibit the cytokine CCL2, which is secreted by TAMs,
thereby suppressing the recruitment of macrophages and monocytes to
the TME and inhibiting tumor progression (56,57).
Furthermore, a combination of resveratrol, curcumin and quercetin
inhibits macrophage recruitment, prevents the polarization of TAMs
into the M2 phenotype and alleviates immune suppression within the
TME (58).
Flavonoids regulate TAM polarization by modulating
key signaling pathways that are involved in macrophage
polarization, including the STAT3 and NF-κB signaling pathways. For
example, a previous study on total flavonoids from Glycyrrhiza
Radix et Rhizoma revealed that these compounds decrease STAT6
phosphorylation and enhance the expression of microRNA-155, which
inhibits M2 macrophage polarization and the expression of the M2
marker arginase-1 (Arg-1) (59).
Isoliquiritigenin suppresses M2 polarization by inhibiting the
PGE2/peroxisome proliferator-activated receptor-δ and IL-6/STAT3
signaling pathways (60).
Furthermore, baicalein notably decreases the expression of
immunosuppressive factors, including IL-10 and TGF-β, by inhibiting
the NF-κB signaling pathway, thereby suppressing M2 polarization
and promoting M1 polarization (61). Additionally, baicalin induces TAM
polarization towards the M1-like phenotype, potentially through
activating autophagy and driving transcriptional activation via the
RelB/p52 pathway (62). TriCurin,
a formulation combining curcumin with other polyphenols, shifts
TAMs from an M2 to an M1 phenotype, thereby promoting the
IL-12-dependent recruitment of NK cells and CTLs to the tumor site.
This facilitates tumor cell elimination via apoptosis (63). Furthermore, xanthohumol, a natural
product found in the female inflorescences of Humulus
lupulus, when encapsulated in poly (lactic-co-glycolic acid)
(PLGA) nanoparticles, stimulates M1 polarization in macrophages
(64).
Flavonoids have also been demonstrated to enhance
macrophage phagocytic activity: Hesperidin-loaded gold
nanoparticles increase macrophage phagocytic capacity, decrease the
secretion of pro-inflammatory cytokines and notably inhibit the
proliferation of the human MDA-MB-231 breast cancer cell line
(65). Furthermore, epicatechins
inhibit macrophage migration inhibitory factor, thereby enhancing
both the anti-inflammatory properties of macrophages and phagocytic
activity (66). Finally,
isorhamnetin (3′-O-methylquercetin) has been identified as a
compound that enhances lysosomal degradation in macrophages,
thereby increasing phagocytic capacity (67).
Apigenin inhibits the TNF-α-mediated release of CCL2
and other chemokines in breast cancer cells, thereby suppressing
the recruitment of immune-suppressive cells such as MDSCs and
decreasing MDSC-mediated immune suppression in the TME (75). In a murine breast tumor model,
epigallocatechin-3-gallate (EGCG) decreases the immunosuppressive
effects of MDSCs by downregulating the canonical signaling pathway
that includes Arg-1, iNOS, NADPH oxidase 2, NF-κB and STAT3
(76). Silymarin (milk thistle
extract) attenuates the immunosuppressive function of MDSCs by
reducing the mRNA expression levels of iNOS2 and Arg-1 and
enhancing the infiltration and efficacy of CD8+ T cells,
thereby reversing the inhibitory TME (77). Neobavaisoflavone (Neo), a natural
isoflavone first isolated from the seeds of Psoralea
corylifolia, effectively inhibits the expansion of MDSCs and
suppresses their immunosuppressive function by targeting STAT3
signaling (78). In addition, Neo
directly inhibits the growth of tumors derived from the 4T1 and
Lewis lung carcinoma (LLC) cell lines in vivo (78). Icariin and its derivative,
3,5,7-Trihydroxy-4′-methoxy-8-(3-hydroxy-3-methylbutyl)-flavone,
have been revealed to inhibit the JAK2/STAT3 pathway, downregulate
S100A8/A9 proteins and promote the differentiation of MDSCs into
immune-stimulatory macrophages and DCs (79). In a mouse model, the downregulation
of immunosuppressive factors, including IL-10, IL-6 and TNF-α, is
observed following treatment with icariin and its derivative
(79). Moreover, Chrysin (Chr), a
natural flavonoid found in honey, propolis and numerous plants,
inhibits the function of MDSCs by targeting the PI3K/AKT pathway,
thereby reversing the immunosuppressive TME (80).
Tregs primarily influence tumors through
immunosuppressive mechanisms. Tregs suppress the activity of
cytotoxic and helper T cells, inhibit anti-tumor immune responses
and induce immune tolerance within the TME (29,81,82).
Tregs limit the activation of effector T cells and DCs by releasing
immunosuppressive cytokines, including IL-10 and TGF-β (83–85).
Additionally, Tregs indirectly promote tumor growth through
fostering angiogenesis and regulating inflammation (86,87).
Moreover, the presence of Tregs within tumors is often associated
with poorer prognoses (88).
DCs serve a key role in the TME through capturing,
processing and presenting tumor-associated antigens to T cells,
thereby initiating an adaptive immune response against cancer
(95–97). They serve as a key link between
innate and adaptive immunity, activating cytotoxic T and T helper
(Th) cells. Moreover, specialized killer DCs have been demonstrated
to express various TNF family members, including Fas ligand (FasL),
TNF-related apoptosis-inducing ligand and TNF-α, which enable them
to promote tumor cell apoptosis (98,99).
Th1 lymphocytes enhance DC-mediated tumor-killing activity via an
IFN-γ-dependent pathway (100);
by contrast, immunosuppressive cytokines (TGF-β and IL-10) and
immunosuppressive cells (MDSCs and Tregs) inhibit DC maturation and
antigen presentation (101).
NK cells recognize abnormal cells due to their
decreased MHC-I expression or via interaction with stress-induced
ligands and are activated by receptors such as killer inhibitory
and natural cytotoxicity receptors (108–111). Upon activation, NK cells form an
immune synapse with target tumor cells, releasing cytotoxic
granules containing perforin and granzymes (111). NK cells induce apoptosis via
death receptor pathways, such as the pathway involving the
interaction of Fas with FasL (112). Furthermore, NK cells secrete
cytokines such as IFN-γ and TNF-α (113,114), which exert an anti-tumor role and
stimulate other immune cells to participate in the immune
response.
The combined action of naringenin and asiatic acid
rebalances the TGF-β1/Smad3 signaling pathway in NK cells, thereby
promoting their differentiation, maturation and cytotoxicity
against cancer cells (115). The
administration of apigenin, a plant-derived flavonoid, enhances NK
cell proliferation by increasing the expression of Bcl-2 and
decreasing Bax expression (116).
Additionally, apigenin activates the JNK and ERK signaling pathways
in NK cells, leading to an upregulation of the expression of
perforin, granzyme B and the receptor NK group 2, member D (NKG2D),
thereby boosting NK cell cytotoxicity against cancer cells
(116). Apigenin promotes the
upregulation of NK cell-activating receptors (NKG2D, NKp30 and
NKp44), which enhances the expression of CD95L on NK cell surfaces,
resulting in the induction of apoptosis in hepatocellular carcinoma
(HCC) cells (117).
Baicalein and baicalin restore the sensitivity of T
cells to tumor cells through inhibiting STAT3 activity and
suppressing IFN-γ-induced expression of PD-L1 (127). Furthermore, naringenin activates
CD169+ macrophages in lymph nodes, thereby upregulating
the expression of immune-associated genes such as CD169, IL-12 and
CXCL10, leading to the recruitment of CTLs to the tumor site,
whereby their activation is promoted, leading to an enhancement of
the anti-tumor immune response (128,129). Betulin, a natural triterpene
obtained from birch bark, enhances T cell cytotoxicity against
tumors by inducing the secretion of IL-2 and IFN-γ from white blood
cells (130). Both Chr and
hesperetin notably enhance the activity of CTLs (131,132). Hesperidin and linarin
specifically stimulate Vδ1+ T cells, thereby enhancing
their functional activity (133).
Vδ1 T cells have antitumor functions, and their presence is
associated with improved patient outcomes in metastatic colorectal
cancer and lung cancer (134,135). Moreover, the flavonoid polyphenol
melafolone enhances the proliferation and effector function of
CD8+ T cells via downregulation of the immunosuppressive
factors TGF-β and PD-L1 (136).
In a study by Tian et al (137), luteolin was revealed to activate
the PI3K/AKT pathway in APCs, which allows activated APCs to
efficiently present tumor antigens to CTLs. This activation further
stimulates CTLs, thereby strengthening the antitumor immune
response. Xanthohumol enhances the cytotoxic immune response by
increasing the secretion of perforin and granzyme B and promoting a
higher ratio of CD8+ cytotoxic T cells to
CD25+ Tregs (CD8+/CD25+);
furthermore, xanthohumol shifts the immune response towards Th1
polarization by upregulating the expression of Th1 cytokines
(138).
The aforementioned studies demonstrate that
flavonoids regulate immune signaling pathways and immune cell
functions by targeting multiple pathways, including inhibition of
the NF-κB, JAK-STAT and PI3K/AKT pathways, as well as activation of
IFN-γ and TNF-α. By suppressing pro-tumor signals and enhancing
anti-tumor responses, flavonoids decrease the risk of resistance,
thereby demonstrating their notable anti-tumor potential and
offering a promising strategy to overcome the limitations of
single-target immunotherapy.
Immune checkpoint inhibitors restore immune
responses against cancer by targeting the PD-1/PD-L1 pathway;
however, immunosuppressive TMEs often hinder their efficacy. A
number of studies have demonstrated that flavonoids modulate the
expression of PD-1 and PD-L1, thereby reversing immune suppression
within the TME (127,139–152).
PD-L1 expression is regulated by two primary
signaling pathways, namely the JAK/STAT and NF-κB pathways. In the
JAK/STAT pathway, external stimuli activate cell surface receptors,
triggering JAK activation and subsequent phosphorylation of the
STAT proteins. Phosphorylated STAT proteins are translocated to the
nucleus, where they enhance PD-L1 gene transcription, thereby
increasing PD-L1 expression on the cell surface (153–155). In the NF-κB signaling pathway,
extracellular signals activate NF-κB by promoting the degradation
of inhibitory IκBs, which releases NF-κB transcription factors.
These factors enter the nucleus, where they bind specific DNA
sequences and promote PD-L1 gene transcription, resulting in
increased PD-L1 expression (156,157).
The JAK/STAT signaling pathway has a key role in
regulating PD-L1 mRNA expression. Activation of this pathway by
cytokines or growth factors leads to transcription of the PD-L1
gene, resulting in PD-L1 protein expression on the cell surface
(155,158–160). Agents that block or inhibit the
JAK/STAT pathway disrupt this cascade, preventing expression of
PD-L1. Flavonoids have potential as natural inhibitors of PD-1 and
PD-L1 expression, resulting in an enhancement of the immune
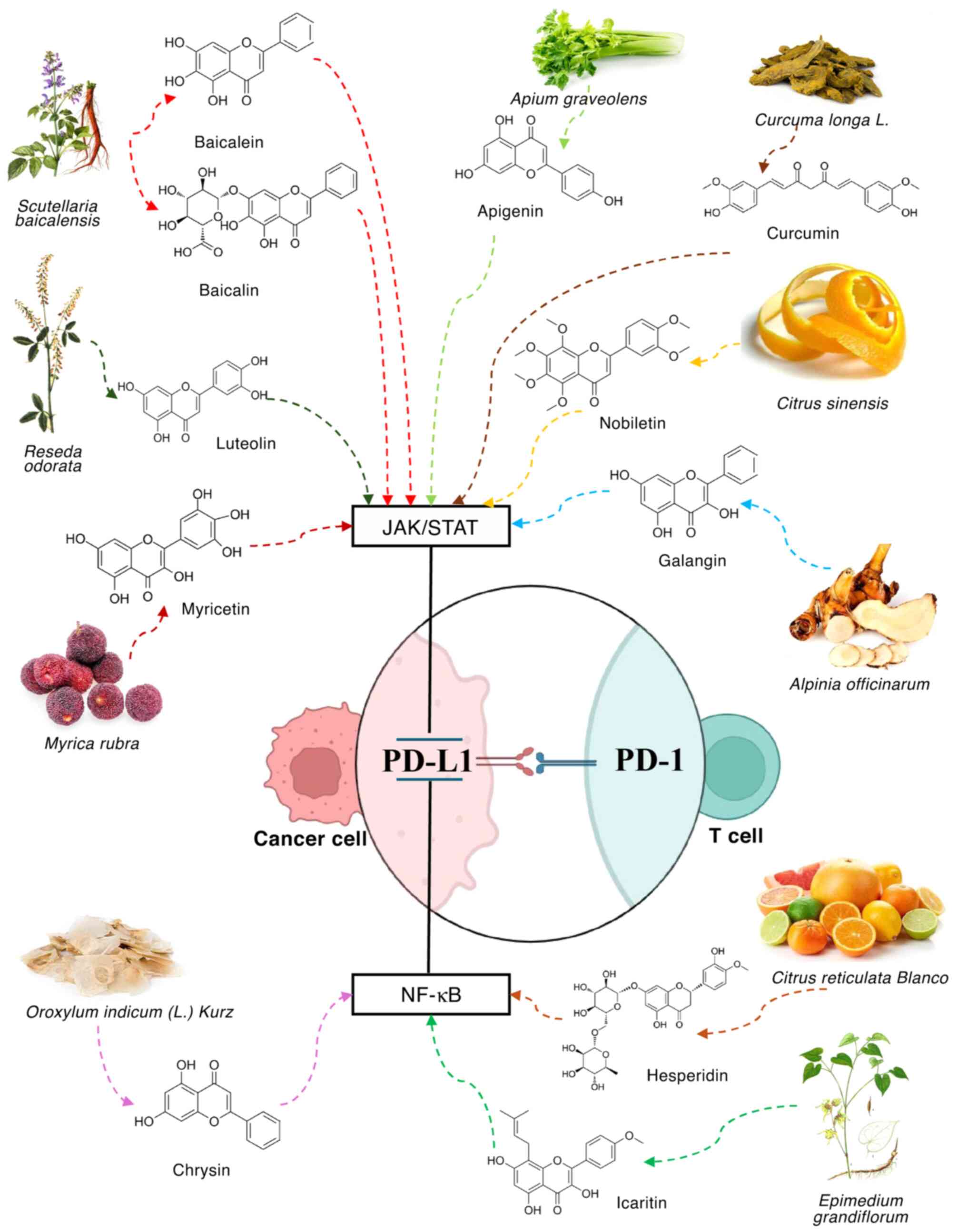
response against numerous types of cancer (Fig. 1). In SMMC-7721 and HepG2 liver
cancer cell lines, baicalein dose-dependently inhibits
IFN-γ-induced expression of PD-L1. Flow cytometric and western blot
analyses and revealed that treatment with baicalein (10 µM) and
baicalin (40 µM) considerably reduced the expression levels of
PD-L1 on the membrane surface (127). Apigenin, combined with curcumin,
inhibits the IFN-γ-induced upregulation of PD-L1 in melanoma cells,
with apigenin demonstrating a more marked inhibitory effect. At the
concentration of 30 µM, apigenin decreases expression of PD-L1, and
this effect is associated with a decrease in STAT1 phosphorylation
(139,140). In KRAS-mutant lung cancer,
luteolin and apigenin inhibit STAT3 phosphorylation and
downregulate IFN-γ-induced PD-L1 expression levels, thereby
exhibiting anticancer properties (141). Pentamethylquercetin, a methylated
quercetin derivative, inhibits expression of PD-L1 in HCC cells by
modulating IFN-γ, especially in the context of obesity, via the
IFN-γ/JAK-STAT signaling pathway (142). In addition, nobiletin, a natural
flavonoid isolated from citrus peel, inhibits PD-L1 expression
levels in non-small cell lung cancer cells via the EGFR/JAK2/STAT3
signaling pathway (143).
Myricetin, a flavonoid compound found in numerous types of plant,
fruit, vegetable and tea, interferes with the JAK/STAT/IFN
regulatory factor 1 signaling pathway activated by IFN-γ, thereby
inhibiting the transcription of PD-L1 in tumor cells (144). Galangin, a flavonoid that is
abundant in galangal and propolis, inhibits expression of PD-L1 by
blocking STAT3 activation via the JAK1/JAK2/Src pathway and
suppressing the activation of Myc via the Ras/RAF/MEK/ERK pathway
(145).
Similarly, the NF-κB signaling pathway markedly
regulates expression of PD-L1. When the NF-κB pathway is mutated or
hyperactivated, PD-L1 expression is increased (156,161). Inhibiting the NF-κB signaling
pathway decreases PD-L1 expression in numerous types of cancer
(162). One study revealed that
hesperidin inhibits breast cancer cell proliferation via the
downregulation of PD-L1 expression via inhibition of the AKT and
NF-κB signaling pathways (146).
Moreover, Chr notably downregulates PD-L1 expression levels in HCC
cells by blocking the STAT3 and NF-κB pathways (147). Additionally, Chr increases the
concentration of IL-2, stimulating T cell proliferation (147). Icaritin, an active ingredient of
the Chinese herb Epimedium, binds specific amino acids in
IκB kinase-α (IKK-α), namely Cys-46 and Cys-178, preventing the
formation and activation of the IKK complex. This inhibition
disrupts the activation of the NF-κB signaling pathway, thereby
hindering NF-κB nuclear translocation and reducing PD-L1 expression
levels (148). Treatment with
icaritin decreases PD-L1 expression levels on MDSCs and neutrophils
(149). When icaritin is combined
with immune checkpoint therapies, such as anti-PD-1/CTLA-4, it
notably increases antitumor efficacy (149,150).
In addition, licochalcone A has been demonstrated to
inhibit the phosphorylation of eukaryotic translation initiation
factor 4E-binding protein 1, to activate the Protein Kinase R-like
endoplasmic reticulum (ER) kinase/eukaryotic initiation factor 2α
pathway and induce the generation of ROS, thereby suppressing
expression of IFN-γ-induced PD-L1 in cancer cells (151). Finally, isorhamnetin directly
targets the cell membrane receptor EGFR, thereby inhibiting the
EGFR/STAT3/PD-L1 signaling pathway, with subsequent downregulation
of PD-L1 expression levels in tumor cells (152).
Taken together, the aforementioned studies
demonstrate that flavonoids inhibit the PD-1/PD-L1 pathway by
regulating the associated signaling pathways, thereby reshaping the
immune-suppressive TME.
Flavonoids, in addition to lowering PD-1 and PD-L1
expression, offer substantial therapeutic benefits when combined
with anti-PD-1/PD-L1 therapy. This synergistic approach enhances
the immune response against cancer by decreasing inhibitory
PD-1/PD-L1 interactions through amplifying T cell activation and
cytotoxicity and favorably modulating the TME. The combination of
cryptotanshinone with low-dose anti-PD-L1 therapy exerts a
synergistic effect, effectively controlling tumor growth and
inducing long-term specific immunity against LLC in mice (163). Wu et al (164) observed that, in an HCC mouse
model, 100 mg/kg/day quercetin with the anti-PD-1 antibody
remodeled the HCC TME, thereby enhancing the efficacy of the
anti-PD-1 antibody. Furthermore, Neo increases the effectiveness of
anti-PD-1 treatment in a breast cancer 4T1 tumor model, which is
initially insensitive to immunotherapy, by inhibiting MDSCs and
modulating the TME (78). In the
4T1 model, the combination of Chr and a PD-1 inhibitor decreases
the immunosuppressive function of MDSCs, enhances T cell activity
and alleviates T cell exhaustion, outperforming single therapies in
terms of the ability to inhibit tumor growth (80).
Cancer vaccines represent a notable advancement
against cancer, serving as a cornerstone of cancer-specific active
immunotherapy. These vaccines are classified into two primary
categories: Cancer-preventive and therapeutic vaccines.
Cancer-preventive vaccines are designed to prevent the development
of cancer by targeting specific viral infections that increase
cancer risk (165,166). Human papillomavirus vaccines,
such as GARDASIL® and CERVARIX®, are
cancer-preventive vaccines (167,168). On the other hand, therapeutic
vaccines are designed to treat cancer that has already developed.
Unlike conventional treatments, such as chemotherapy and radiation,
therapeutic vaccines exert their effects by activating the immune
system to target and destroy cancer cells (169,170). For example, PROVENGE®
induces an immune response against prostate cancer cells by
stimulating CD8+ CTLs to attack the tumor (171–173).
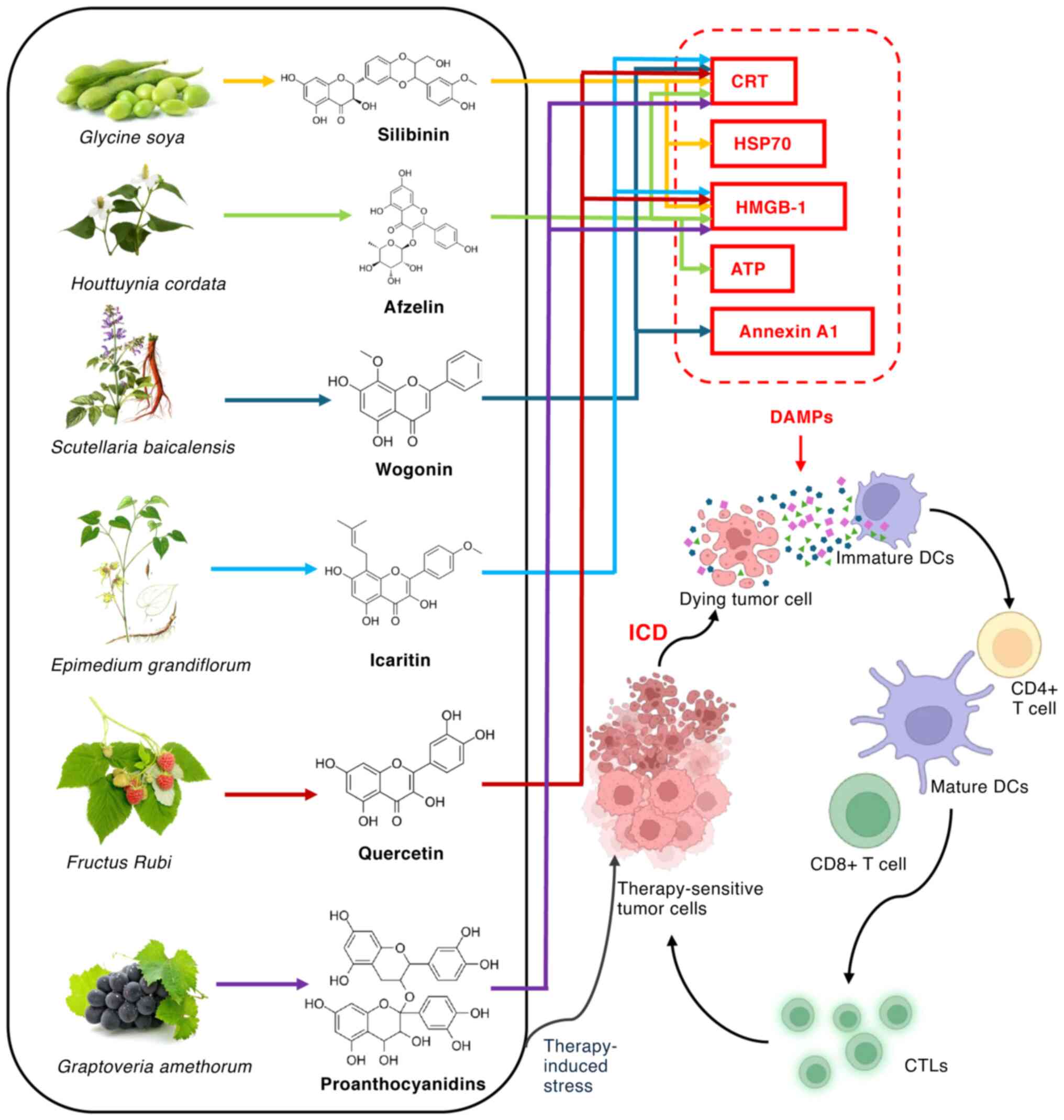
ICD is a process in which stressed or dying cells
release damage-associated molecular patterns (DAMPs) into the
extracellular space (174–176).
DAMPs include HMGB1, ATP and calreticulin (CRT). These DAMPs have a
key role in activating immune responses by modulating immune cell
functions via specific molecular pathways. For example, HMGB1 and
ATP bind pattern recognition receptors on DCs, such as toll-like
receptor (TLR)2 and 4 and the receptor for advanced glycation
end-products (RAGE), triggering DC activation (177,178). This induces DC maturation and
migration to lymph nodes, where mature DCs process antigens from
dying tumor cells and present them to T cells via MHC class I and
II molecules, thereby stimulating anti-tumor immunity (179,180). Additionally, activated DCs
upregulate co-stimulatory molecules such as CD80, CD86 and CD40,
enhancing T cell activation (96,179). ATP also binds the purinergic
receptor P2X7 on NK cells, activating them to enhance cytotoxic
activity against tumor cells via the secretion of perforin and
granzymes (181–183). Furthermore, in monocytes and
macrophages, DAMPs bind TLRs and RAGE, promoting their recruitment
to the tumor site (184).
Activated macrophages secrete pro-inflammatory cytokines such as
TNF-α, IL-1β and IL-6 via the M1 phenotype, thereby enhancing
anti-tumor immunity (185). DAMPs
also activate effector T cells, especially CTLs, inducing the
release of pro-inflammatory cytokines such as IFN-γ and
upregulating co-stimulatory molecules on tumor cells, thereby
enhancing T cell cytotoxicity and activation (186). Flavonoids enhance tumor
immunogenicity by inducing immunogenic cell death (ICD) (Fig. 2), converting cancer cells into
‘therapeutic vaccines’ that activate anti-tumor immunity without
the need for adjuvants (187).
Silymarin induces ICD in CT26 colon cancer and
B16F10 melanoma cells, as evidenced by the release of DAMPs,
including CRT, HSP70 and HMGB-1. When combined with doxorubicin,
silymarin markedly enhances this ICD response, and promotes
Th1-type immune responses by increasing the secretion of IL-12
(188). Afzelin, a flavonol
glycoside, induces ICD in lung cancer cells by activating ER stress
and promoting the release of ICD-associated molecules, including
ATP, HMGB1 and CRT (189).
Moreover, LW-213, a synthesized flavonoid, induces ICD in tumor
cells by activating ER stress and releasing DAMPs (181). These DAMPs activate APCs, leading
to DC maturation and the infiltration of CD8+ T cells
into the TME (190). Wogonin
triggers the production of ROS within tumor cells, resulting in ER
stress. ER stress induces the PI3K/AKT pathway, causing the
translocation of CRT and annexin A1 to the cell membrane (102). This allows immune cells to
recognize tumor cells. Scutellarin, a Chinese herbal medicine of
flavone glycoside origin, induces ICD in HCC, leading to a notable
increase in the levels of CRT, ATP and HMGB-1 in the extracellular
space (191). PLGA@Icaritin nanoparticles (PGLA
nanoparticles loaded with icaritin) induce the generation of ROS,
leading to subsequent mitochondrial dysfunction, including the loss
of mitochondrial membrane potential and oxidative damage to
mitochondrial DNA. This triggers the release of DAMPs from the
impaired mitochondria. DAMPs activate the immune system, resulting
in ICD within the tumor cells (192). A study of microsatellite-stable
colorectal cancer revealed that combination of quercetin and
alantolactone induces CRT translocation and HMGB1 release, thereby
inducing ICD in cancer cells (193). Furthermore, in a mouse colon
cancer model, the combination of 8 procyanidins and 2 mg/kg
mitoxantrone induces a higher level of immunogenic cell death in
CT26 tumor cells, resulting in the release of HMGB-1 and CRT. This
promotes DC maturation and enhanced T cell infiltration within the
TME, improving the efficacy of immunotherapy (194).
Flavonoids serve as adjuvants in tumor vaccines,
thereby enhancing their efficacy. Flavonoids notably enhance
antigen presentation, improving the efficacy of tumor vaccines.
Hesperetin enhances the ability of APCs to process and present
tumor antigens by activating the PI3K/AKT signaling pathway,
thereby strengthening the immune response (195). In a study of inactivated B16F10
melanoma cells, hesperetin served as an adjuvant, improving the
immune response and extending the survival of tumor-bearing mice
(195). Moreover, in a melanoma
mouse model, the flavonoid compound Chr serves as an adjuvant for
tumor vaccines by activating APCs, enhancing the function of Th1
cells and promoting CTL-mediated antitumor responses (196). Transplantation of CD8+
T cells isolated from immunized mice into tumor-bearing mice
notably prolongs survival of recipient mice (196).
Flavonoids also serve a key role in enhancing the
responses of CTLs when used as vaccine adjuvants. Luteolin serves
as an adjuvant for malignant melanoma vaccines. In a mouse model,
intramuscular injection 5×106 inactivated B16F10 cells
and 10 mg luteolin enhances the responsiveness of CTLs and
suppresses the immunosuppressive function of Tregs, thereby
inhibiting tumor growth and prolonging the survival of
tumor-bearing mice (137).
Procyanidin enhances T cell-mediated immune responses and
anti-tumor activity when used as a vaccine adjuvant by promoting
CD8+ T cell activation and cytokine secretion, which
inhibits tumor growth and prolongs survival in tumor-bearing mice
(197). In another study, the
combination of EGCG with DNA vaccination notably enhanced
tumor-specific T cell responses and improved antitumor efficacy,
exceeding the effects of either immunotherapy or EGCG alone
(198). Finally, in a mouse TC-1
tumor model, intraperitoneal injection of 25 mg/kg apigenin
combined with the E7-HSP70 DNA vaccine increases the production of
E7-specific CD8+ T cells, thereby enhancing the immune
response (199).
Adoptive cell immunotherapy, an approach in cancer
treatment, involves the extraction, modification and reinfusion of
immune cells, especially T cells, to more effectively target cancer
(200,201). However, adoptive cell
immunotherapy faces challenges such as immunosuppression, limited T
cell efficacy in vitro and high treatment costs (200,202). Flavonoid compounds with
immune-modulating properties may provide potential solutions.
In an E.G7 mouse lymphoma model, intraperitoneal
injection of 70 mg/kg/day curcumin, combined with adoptive T cell
therapy, enhances CD8+ T cell-mediated tumor
cytotoxicity (203). This effect
is mediated by modulating the TME through the blockade of
immunosuppressive factors, including TGF-β, indoleamine
2,3-dioxygenase and Tregs, thereby increasing T cell accumulation
and activity (203). Apigenin
improves the efficacy of adoptive cell immunotherapy by promoting
the activation of CTLs, enhancing antigen presentation and
inhibiting Tregs (204).
Quercetin, by contrast, enhances the sensitivity of cancer cells to
adoptive cell immunotherapy by inducing an imbalance of ROS,
mitochondrial dysfunction and apoptosis (205).
The optimization of flavonoid dosing is key for
clinical application as pharmacological effects are dose-dependent
(206). Low doses may be
ineffective, whereas high doses may lead to toxicity or side
effects. Therefore, personalized dosing regimens should be
developed based on cancer type and patient needs. For example,
baicalein inhibits expression of PD-L1 at a concentration of 10 µM
in vitro, whereas in animal studies, oral doses typically
range from 50 to 200 mg/kg (127,207). In clinical trials, quercetin is
administered at daily doses of 500–1,000 mg, demonstrating
tolerability and immune modulation (208,209). When combined with immune
checkpoint inhibitors, the dosing range for synergistic effects
should be optimized to avoid increased toxicity.
The low water solubility and rapid metabolism of
flavonoids limits their bioavailability (210–213). To enhance therapeutic efficacy,
various novel formulations have been developed, including
nanoparticle formulations, prodrug designs and sustained-release
systems (214–216). Nanoparticles, such as liposomes
and polymeric and solid lipid nanoparticles, encapsulate flavonoids
to improve stability and targeting (214). Prodrug design involves chemically
modifying flavonoids into forms that release active ingredients in
specific in vivo environments, thereby enhancing both
efficacy and safety (215).
Sustained-release systems, such as microspheres or hydrogels, allow
prolonged release of flavonoids, decreasing dosing frequency and
improving patient compliance (216).
The clinical efficacy of flavonoids is not only
influenced by pharmacological properties but is also associated
with the route of administration. A rational choice of
administration route may notably enhance drug absorption, targeting
and therapeutic effects, while minimizing the risk of adverse
reactions. Oral administration is the most common route; however,
this route is limited by poor solubility, gastrointestinal
degradation and first-pass metabolism (213). To overcome these challenges,
strategies such as nanoparticle carriers, prodrug design and
excipient improvements have been employed to enhance
bioavailability (214–216). Intravenous injection is suitable
for efficient anti-tumor treatment, with liposomes, polymeric
nanoparticles or suspensions used to improve water solubility and
plasma stability. Inhalation is ideal for treating respiratory
diseases such as lung cancer, where nebulized delivery markedly
increases pulmonary drug concentration (217,218). c. The combination of intravenous
with local injection, or oral administration with inhalation,
produces synergistic effects.
Novel drug delivery systems, especially
nanosystems, improve flavonoid bioavailability and enable targeted
tumor delivery (219). Nano-drug
delivery systems use biocompatible, surface-modifiable nanocarriers
to specifically target tumor sites, enhancing drug concentrations
at the target site, while minimizing toxic effects on normal tissue
(219–221). This approach has demonstrated
promising results in tumor targeting and anti-tumor activity
(220,221).
Nano-drug delivery systems markedly improve the
bioavailability of drugs. In a microsatellite-stable colorectal
cancer mouse model, QA-M, an innovative type of nanotherapy, uses a
unique nanodelivery system that synergistically encapsulates
quercetin and alantolactone in a 1:4 molar ratio. This system
prolongs drug circulation time and increases drug accumulation in
tumor tissue, thereby enhancing bioavailability (184). Additionally, it promotes
induction of ICD, further boosting the immune response and
contributing to more effective tumor control (193).
Nano-drug delivery systems enable the targeted
delivery of therapeutics. In a mouse melanoma model, a dual
pH-sensitive nanocarrier loaded with curcumin and anti-PD-1
antibodies enhances cancer immunotherapy. This carrier selectively
binds to circulating PD-1+ T cells, directing them to
the TME. Upon reaching the tumor site, the nanocarrier releases
anti-PD-1 antibodies, blocking PD-1 on T cells and enhancing their
anti-tumor response (222).
Moreover, curcumin inhibits the NF-κB signaling pathway, modulating
the expression of immunosuppressive factors and further boosting
the anti-tumor immune response (222).
Nano-drug delivery systems encapsulate multiple
drugs or therapeutic agents, enabling combination therapy to
enhance efficacy and decrease drug resistance. In a mouse melanoma
model, Trp2 peptide vaccine combined with curcumin-polyethylene
glycol (CUR-PEG) micelles improves the effectiveness of the
immunotherapy (223). Lu et
al (223) revealed that
CUR-PEG effectively reshapes the TME by reducing immunosuppressive
factors and increasing proinflammatory signals. This approach
strengthens CTL responses and enhances the production of IFN-γ,
thereby promoting the transition of immune-suppressive M2 to
immune-activating M1 macrophages. By decreasing the populations of
immunosuppressive cells such as MDSCs and Tregs, and
immunosuppressive molecules such as IL-6, while increasing the
levels of proinflammatory cytokines such as TNF-α and IFN-γ,
CUR-PEG effectively transforms the inhibitory TME.
In conclusion, nano-drug delivery systems offer
notable advantages in addressing the limitations of flavonoid
bioavailability. By enhancing drug stability, prolonging
circulation time and enabling precise targeted delivery, these
systems increase drug accumulation at tumor sites while minimizing
toxicity to healthy tissue. Additionally, the multifunctional
design of nanocarriers supports the co-delivery of multiple
therapeutic agents, promoting synergistic effects and mitigating
drug resistance.
Flavonoids are generally safe and well-tolerated at
standard doses. However, at high doses, they may cause mild
gastrointestinal discomfort, headache, skin reactions or slight
liver dysfunction. These side effects are typically mild and
reversible and readily resolved upon dose adjustment or
discontinuation of the treatment. Although flavonoids generally
exhibit low toxicity, prolonged or high-dose use may lead to liver
and kidney damage, drug interactions or allergic reactions,
especially in individuals with pre-existing liver or kidney
conditions or a history of allergies (224,225). There is limited evidence on
irreversible side effects, but caution is advised in individuals
with compromised liver or kidney function to avoid potential
long-term damage (224,225).
When combined with immunotherapeutic agents such as
PD-1/PD-L1 inhibitors or CTLA-4 inhibitors, flavonoids may induce
side effects. Although they enhance the efficacy of immunotherapy
by modulating the immune microenvironment and inhibiting
immune-suppressive factors (such as TGF-β and IL-10), flavonoids
may also result in excessive immune activation, potentially
increasing the risk of autoimmune diseases such as rheumatoid
arthritis or systemic lupus erythematosus. Additionally, by
amplifying the anti-tumor immune response, flavonoids may also
trigger a cytokine storm (78,80,152,163).
Flavonoids inhibit the secretion of
immunosuppressive factors, promote the release of anti-tumor immune
factors, decrease the number and function of immunosuppressive
cells and enhance effector T cell activity, contributing to the
reversal of the immunosuppressive microenvironment (47). Moreover, flavonoids can
considerably enhance the efficacy of cancer immunotherapies,
including cancer vaccines, immune checkpoint inhibitors and
adoptive cell immunotherapy (228).
Although flavonoids have potential in enhancing the
efficacy of immunotherapy, their effective application faces
several challenges. First, tumor heterogeneity notably impacts the
effectiveness of flavonoids (228). Tumors from different patients
typically exhibit notable variation in terms of genetic mutations,
immune cell infiltration and the TME, which can lead to differences
in the responses to flavonoids across tumor types or individuals
(229). Secondly, the role of
flavonoids in immune signaling pathways requires further
investigation. For example, the JAK/STAT, NF-κB and PI3K/AKT/mTOR
signaling pathways are hypothesized to serve key roles in
immune-regulatory effects (153–157); however, the underlying mechanisms
remain unclear (127,139,146,147). A deeper understanding of how
these pathways are modulated by flavonoids may optimize their
therapeutic outcomes. To the best of our knowledge, there is
currently a lack of clinical data on the use of flavonoids in
cancer immunotherapy. Although flavonoids have antitumor effects in
animal models, their effective application in clinical settings
still requires clinical trial data. Therefore, future studies
should focus on validating the use of flavonoids in clinical
trials.
In future, the application of high-throughput
technologies, such as single-cell sequencing, may enable in-depth
analysis of the dynamic changes in immune cells within the TME,
thereby revealing the underlying mechanisms of tumor escape and
their interactions with flavonoids to support precision medicine
(230). Simultaneously,
innovative drug delivery systems, including nanotechnology,
liposomes and polymeric nanoparticles, may improve the
bioavailability, targeted delivery and accumulation of flavonoids
within the TME, thereby enhancing anti-tumor efficacy and
decreasing toxicity (229–221).
Personalized treatment strategies may tailor flavonoid-based
therapy according to the genomic features of a patient, TME and
immune response differences, to improve efficacy and minimize
adverse effects. Furthermore, long-term efficacy and safety
assessment of flavonoids should become a research priority,
focusing on potential toxicity, drug interactions and effects on
normal tissue to ensure the safety and sustainability of clinical
applications. Clinical trials evaluating the combination of
flavonoids with immune checkpoint inhibitors should also be
performed to assess their potential in terms of enhancing immune
responses, improving therapeutic outcomes and prolonging survival.
Finally, biomarker detection may elucidate the underlying
mechanisms and therapeutic prospects, thereby advancing flavonoids
as an effective adjunctive therapeutic strategy.
In conclusion, flavonoids have promise in cancer
immunotherapy by reshaping the TME and enhancing the capacity of
the immune system to combat cancer. Advanced technologies such as
metabolomics, single-cell sequencing, innovative drug delivery
systems and computer-aided design are needed to develop targeted
antitumor immunotherapeutic agents.
Not applicable.
The present was supported by Shandong Provincial Health
Commission (grant no. Z-2023064).
Not applicable.
CY wrote the manuscript. GW conceived the study and
reviewed the manuscript. Both authors have read and approved the
final manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Kumar H: Cancer and immunity: Who is
shaping whom? Int Rev Immunol. 40:317–318. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bosch F and Rosich L: The contributions of
Paul Ehrlich to pharmacology: A tribute on the occasion of the
centenary of his Nobel Prize. Pharmacology. 82:171–179. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burnet M: Cancer; a biological approach.
I. The processes of control. Br Med J. 1:779–786. 1957. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kennedy LB and Salama AKS: A review of
cancer immunotherapy toxicity. CA Cancer J Clin. 70:86–104. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Y and Zhang Z: The history and
advances in cancer immunotherapy: Understanding the ch
aracteristics of tumor-infiltrating immune cells and their
therapeutic implications. Cell Mol Immunol. 17:807–821. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hargadon KM, Johnson CE and Williams CJ:
Immune checkpoint blockade therapy for cancer: An overview of
FDA-approved immune checkpoint inhibitors. Int Immunopharmacol.
62:29–39. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
O'Leary MC, Lu X, Huang Y, Lin X, Mahmood
I, Przepiorka D, Gavin D, Lee S, Liu K, George B, et al: FDA
approval summary: Tisagenlecleucel for treatment of patients with
relapsed or refractory B-cell precursor acute lymphoblastic
leukemia. Clin Cancer Res. 25:1142–1146. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bouchkouj N, Kasamon YL, de Claro RA,
George B, Lin X, Lee S, Blumenthal GM, Bryan W, McKee AE and Pazdur
R: FDA approval summary: Axicabtagene ciloleucel for relapsed or
refractory large B-cell Lymphoma. Clin Cancer Res. 25:1702–1708.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheever MA and Higano CS: PROVENGE
(Sipuleucel-T) in prostate cancer: The first FDA-approved the
rapeutic cancer vaccine. Clin Cancer Res. 17:3520–3526. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Centers for Disease Control and Prevention
(CDC), . FDA licensure of quadrivalent human papillomavirus vaccine
(HPV4, Gardasil) for use in males and guidance from the Advisory
Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly
Rep. 59:630–632. 2010.PubMed/NCBI
|
|
12
|
Kirby T: FDA approves new upgraded
Gardasil 9. Lancet Oncol. 16:e562015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Centers for Disease Control and Prevention
(CDC), . FDA licensure of bivalent human papillomavirus vaccine
(HPV2, Cervarix) for use in females and updated HPV vaccination
recommendations from the Advisory Committee on Immunization
Practices (ACIP). MMWR Morb Mortal Wkly Rep. 59:626–629.
2010.PubMed/NCBI
|
|
14
|
Kalathil SG and Thanavala Y: High
immunosuppressive burden in cancer patients: A major hurdle for
cancer immunotherapy. Cancer Immunol Immunother. 65:813–819. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ren W, Qiao Z, Wang H, Zhu L and Zhang L:
Flavonoids: Promising anticancer agents. Med Res Rev. 23:519–534.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Raffa D, Maggio B, Raimondi MV, Plescia F
and Daidone G: Recent discoveries of anticancer flavonoids. Eur J
Med Chem. 142:213–228. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ravishankar D, Rajora AK, Greco F and
Osborn HM: Flavonoids as prospective compounds for anti-cancer
therapy. Int J Biochem Cell Biol. 45:2821–2831. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Duan N, Hu X, Zhou R, Li Y, Wu W and Liu
N: A review on dietary flavonoids as modulators of the tumor
microenvironment. Mol Nutr Food Res. 67:e22004352023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sudhakaran M, Sardesai S and Doseff AI:
Flavonoids: New frontier for immuno-regulation and breast cancer
control. Antioxidants (Basel). 8:1032019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, Zhang Q, Chen Y, Liang CL, Liu H,
Qiu F and Dai Z: Antitumor effects of immunity-enhancing
traditional Chinese medicine. Biomed Pharmacother. 121:1095702020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu L, Jin Y, Song M, Zhao Y and Zhang H:
When natural compounds meet nanotechnology: Nature-Inspired
nanomedicines for cancer immunotherapy. Pharmaceutics. 14:15892022.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wilky BA: Immune checkpoint inhibitors:
The linchpins of modern immunotherapy. Immunol Rev. 290:6–23. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Czajka-Francuz P, Prendes MJ, Mankan A,
Quintana Á, Pabla S, Ramkissoon S, Jensen TJ, Peiró S, Severson EA,
Achyut BR, et al: Mechanisms of immune modulation in the tumor
microenvironment and impl ications for targeted therapy. Front
Oncol. 13:12006462023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Currenti J, Mishra A, Wallace M, George J
and Sharma A: Immunosuppressive mechanisms of oncofetal
reprogramming in the tumor microenvironment: Implications in
immunotherapy response. Biochem Soc Trans. 51:597–612.
2023.PubMed/NCBI
|
|
25
|
Li Y, Xiang S, Pan W, Wang J, Zhan H and
Liu S: Targeting tumor immunosuppressive microenvironment for
pancreatic cancer immunotherapy: Current research and future
perspective. Front Oncol. 13:11668602023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pollard JW: Tumour-educated macrophages
promote tumour progression and metastasis. Nat Rev Cancer. 4:71–78.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kimura S, Nanbu U, Noguchi H, Harada Y,
Kumamoto K, Sasaguri Y and Nakayama T: Macrophage CCL22 expression
in the tumor microenvironment and implications for survival in
patients with squamous cell carcinoma of the tongue. J Oral Pathol
Med. 48:677–685. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yeung OW, Lo CM, Ling CC, Qi X, Geng W, Li
CX, Ng KT, Forbes SJ, Guan XY, Poon RT, et al: Alternatively
activated (M2) macrophages promote tumour growth and inv asiveness
in hepatocellular carcinoma. J Hepatol. 62:607–616. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tie Y, Tang F, Wei YQ and Wei XW:
Immunosuppressive cells in cancer: Mechanisms and potential
therapeuti c targets. J Hematol Oncol. 15:612022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Khazaie K and von Boehmer H: The impact of
CD4+CD25+ Treg on tumor specific CD8+ T cell cytotoxicity and
cancer. Semin Cancer Biol. 16:124–136. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rotte A: Combination of CTLA-4 and PD-1
blockers for treatment of cancer. J Exp Clin Cancer Res.
38:2552019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang H, Dai Z, Wu W, Wang Z, Zhang N,
Zhang L, Zeng WJ, Liu Z and Cheng Q: Regulatory mechanisms of
immune checkpoints PD-L1 and CTLA-4 in cancer. J Exp Clin Cancer
Res. 40:1842021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wojtukiewicz MZ, Rek MM, Karpowicz K,
Górska M, Polityńska B, Wojtukiewicz AM, Moniuszko M, Radziwon P,
Tucker SC and Honn KV: Inhibitors of immune checkpoints-PD-1,
PD-L1, CTLA-4-new opportunities for cancer patients and a new
challenge for internists and general practitioners. Cancer
Metastasis Rev. 40:949–982. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zak KM, Grudnik P, Magiera K, Dömling A,
Dubin G and Holak TA: Structural biology of the immune checkpoint
receptor PD-1 and its ligands PD-L1/PD-L2. Structure. 25:1163–1174.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kennedy A, Waters E, Rowshanravan B, Hinze
C, Williams C, Janman D, Fox TA, Booth C, Pesenacker AM, Halliday
N, et al: Differences in CD80 and CD86 transendocytosis reveal CD86
as a key target for CTLA-4 immune regulation. Nat Immunol.
23:1365–1378. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chikuma S: CTLA-4, an essential
immune-checkpoint for T-cell activation. Curr Top Microbiol
Immunol. 410:99–126. 2017.PubMed/NCBI
|
|
37
|
Goldberg MV and Drake CG: LAG-3 in cancer
immunotherapy. Curr Top Microbiol Immunol. 344:269–278.
2011.PubMed/NCBI
|
|
38
|
Chauvin JM and Zarour HM: TIGIT in cancer
immunotherapy. J Immunother Cancer. 8:e0009572020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tang W, Chen J, Ji T and Cong X: TIGIT, a
novel immune checkpoint therapy for melanoma. Cell Death Dis.
14:4662023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tang R, Rangachari M and Kuchroo VK:
Tim-3: A co-receptor with diverse roles in T cell exhaustion and
tolerance. Semin Immunol. 42:1013022019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kane LP: Regulation of Tim-3 function by
binding to phosphatidylserine. Biochem J. 478:3999–4004. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Haist M, Stege H, Grabbe S and Bros M: The
functional crosstalk between myeloid-derived suppressor cells and
regulatory T cells within the immunosuppressive tumor
microenvironment. Cancers (Basel). 13:2102021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lu C, Redd PS, Lee JR, Savage N and Liu K:
The expression profiles and regulation of PD-L1 in tumor-induced
myeloid-derived suppressor cells. Oncoimmunology. 5:e12471352016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sasidharan Nair V and Elkord E: Immune
checkpoint inhibitors in cancer therapy: A focus on T-regulatory
cells. Immunol Cell Biol. 96:21–33. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Malinga NZ, Siwele SC, Steel HC, Kwofie
LLI, Meyer PWA, Smit T, Anderson R, Rapoport BL and Kgokolo MCM:
Systemic levels of the soluble co-inhibitory immune checkpoints,
CTLA-4, LAG-3, PD-1/PD-L1 and TIM-3 are markedly increased in basal
cell carcinoma. Transl Oncol. 19:1013842022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu Y and Cao X: Immunosuppressive cells
in tumor immune escape and metastasis. J Mol Med (Berl).
94:509–522. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Martínez G, Mijares MR and De Sanctis JB:
Effects of flavonoids and its derivatives on immune cell responses.
Recent Pat Inflamm Allergy Drug Discov. 13:84–104. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Taylor PR, Martinez-Pomares L, Stacey M,
Lin HH, Brown GD and Gordon S: Macrophage receptors and immune
recognition. Annu Rev Immunol. 23:901–944. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nikitina E, Larionova I, Choinzonov E and
Kzhyshkowska J: Monocytes and macrophages as viral targets and
reservoirs. Int J Mol Sci. 19:28212018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wynn TA, Chawla A and Pollard JW:
Macrophage biology in development, homeostasis and disease. Nature.
496:445–455. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sica A and Mantovani A: Macrophage
plasticity and polarization: In vivo veritas. J Clin Invest.
122:787–795. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Locati M, Curtale G and Mantovani A:
Diversity, mechanisms, and significance of macrophage plasticity.
Annu Rev Pathol. 15:123–147. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Aras S and Zaidi MR: TAMeless traitors:
Macrophages in cancer progression and metastasis. Br J Cancer.
117:1583–1591. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Shen Y, Chen JX, Li M, Xiang Z, Wu J and
Wang YJ: Role of tumor-associated macrophages in common digestive
system malign ant tumors. World J Gastrointest Oncol. 15:596–616.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Khan SU, Khan MU, Azhar Ud Din M, Khan IM,
Khan MI, Bungau S and Hassan SSU: Reprogramming tumor-associated
macrophages as a unique approach to target tumor immunotherapy.
Front Immunol. 14:11664872023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Choi HJ, Choi HJ, Chung TW and Ha KT:
Luteolin inhibits recruitment of monocytes and migration of Lewis
lung carcinoma cells by suppressing chemokine (C-C motif) ligand 2
express ion in tumor-associated macrophage. Biochem Biophys Res
Commun. 470:101–106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tripathi DK, Nagar N, Kumar V, Joshi N,
Roy P and Poluri KM: Gallate moiety of catechin is essential for
inhibiting CCL2 chemokine-mediated monocyte recruitment. J Agric
Food Chem. 71:4990–5005. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Li C, Xu Y, Zhang J, Zhang Y, He W, Ju J,
Wu Y and Wang Y: The effect of resveratrol, curcumin and quercetin
combination on immuno-suppression of tumor microenvironment for
breast tumor-bearing mice. Sci Rep. 13:132782023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jiang YX, Chen Y, Yang Y, Chen XX and
Zhang DD: Screening Five Qi-Tonifying Herbs on M2 phenotype
macrophages. Evid Based Complement Alternat Med. 2019:95493152019.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhao H, Zhang X, Chen X, Li Y, Ke Z, Tang
T, Chai H, Guo AM, Chen H and Yang J: Isoliquiritigenin, a
flavonoid from licorice, blocks M2 macrophage polarization in
colitis-associated tumorigenesis through downregulating PGE2 and
IL-6. Toxicol Appl Pharmacol. 279:311–321. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
He S, Wang S, Liu S, Li Z, Liu X and Wu J:
Baicalein potentiated M1 macrophage polarization in cancer through
Tar geting PI3Kγ/NF-κB signaling. Front Pharmacol. 12:7438372021.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Tan HY, Wang N, Man K, Tsao SW, Che CM and
Feng Y: Autophagy-induced RelB/p52 activation mediates
tumour-associated macrophage repolarisation and suppression of
hepatocellular carcinoma by natural compound baicalin. Cell Death
Dis. 6:e19422015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Mukherjee S, Hussaini R, White R, Atwi D,
Fried A, Sampat S, Piao L, Pan Q and Banerjee P: TriCurin, a
synergistic formulation of curcumin, resveratrol, and epicatechin
gallate, repolarizes tumor-associated macrophages and triggers an
immune response to cause suppression of HPV+ tumors. Cancer Immunol
Immunother. 67:761–774. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Fonseca M, Macedo AS, Lima SAC, Reis S,
Soares R and Fonte P: Evaluation of the antitumour and
antiproliferative effect of xanthohumol-loaded PLGA nanoparticles
on melanoma. Materials (Basel). 14:64212021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sulaiman GM, Waheeb HM, Jabir MS, Khazaal
SH, Dewir YH and Naidoo Y: Hesperidin loaded on gold nanoparticles
as a drug delivery system for a successful biocompatible,
anti-cancer, anti-inflammatory and phagocytosis inducer model. Sci
Rep. 10:93622020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Dickerhof N, Magon NJ, Tyndall JDA, Kettle
AJ and Hampton MB: Potent inhibition of macrophage migration
inhibitory factor (MIF) by m yeloperoxidase-dependent oxidation of
epicatechins. Biochem J. 462:303–314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Sakai M, Ohnishi K, Masuda M, Ohminami H,
Yamanaka-Okumura H, Hara T and Taketani Y: Isorhamnetin, a
3′-methoxylated flavonol, enhances the lysosomal prote olysis in
J774.1 murine macrophages in a TFEB-independent manner. Biosci
Biotechnol Biochem. 84:1221–1231. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Greten TF, Manns MP and Korangy F: Myeloid
derived suppressor cells in human diseases. Int Immunopharmacol.
11:802–807. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Li BH, Garstka MA and Li ZF: Chemokines
and their receptors promoting the recruitment of myeloid-de rived
suppressor cells into the tumor. Mol Immunol. 117:201–215. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ozga AJ, Chow MT and Luster AD: Chemokines
and the immune response to cancer. Immunity. 54:859–874. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Lindau D, Gielen P, Kroesen M, Wesseling P
and Adema GJ: The immunosuppressive tumour network: Myeloid-derived
suppressor cells, regulatory T cells and natural killer T cells.
Immunology. 138:105–115. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Law AMK, Valdes-Mora F and Gallego-Ortega
D: Myeloid-derived suppressor cells as a therapeutic target for
cancer. Cells. 9:5612020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ohl K and Tenbrock K: Reactive oxygen
species as regulators of MDSC-mediated immune suppression. Front
Immunol. 9:24992018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hatziioannou A, Alissafi T and Verginis P:
Myeloid-derived suppressor cells and T regulatory cells in tumors:
Unr aveling the dark side of the force. J Leukoc Biol. 102:407–421.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Bauer D, Redmon N, Mazzio E and Soliman
KF: Apigenin inhibits TNFα/IL-1α-induced CCL2 release through
IKBK-epsilon signaling in MDA-MB-231 human breast cancer cells.
PLoS One. 12:e01755582017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Xu P, Yan F, Zhao Y, Chen X, Sun S, Wang Y
and Ying L: Green tea polyphenol EGCG Attenuates MDSCs-mediated
immunosuppression through canonical and non-canonical pathways in a
4T1 murine breast cancer model. Nutrients. 12:10422020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Wu T, Liu W, Guo W and Zhu X: Silymarin
suppressed lung cancer growth in mice via inhibiting
myeloid-derived suppressor cells. Biomed Pharmacother. 81:460–467.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Guo J, Shen Y, Hu S, Rui T, Liu J and Yuan
Y: Neobavaisoflavone inhibits antitumor immunosuppression via
myeloid-der ived suppressor cells. Int Immunopharmacol.
111:1091032022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhou J, Wu J, Chen X, Fortenbery N,
Eksioglu E, Kodumudi KN, Pk EB, Dong J, Djeu JY and Wei S: Icariin
and its derivative, ICT, exert anti-inflammatory, anti-tumor e
ffects, and modulate myeloid derived suppressive cells (MDSCs)
functio ns. Int Immunopharmacol. 11:890–898. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Li Y, Yang R, Huang X, Chen C, Dou D, Wang
Q, Wu X, Liu H and Sun T: Chrysin targets myeloid-derived
suppressor cells and enhances tumour response to anti-PD-1
immunotherapy. Clin Transl Med. 12:e10192022. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Sugiyama D, Hinohara K and Nishikawa H:
Significance of regulatory T cells in cancer immunology and
immunotherapy. Exp Dermatol. 32:256–263. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Tay C, Tanaka A and Sakaguchi S:
Tumor-infiltrating regulatory T cells as targets of cancer
immunotherapy. Cancer Cell. 41:450–465. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Moreau JM, Velegraki M, Bolyard C,
Rosenblum MD and Li Z: Transforming growth factor-β1 in regulatory
T cell biology. Sci Immunol. 7:eabi46132022. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Beissert S, Schwarz A and Schwarz T:
Regulatory T cells. J Invest Dermatol. 126:15–24. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Chen X, Du Y, Lin X, Qian Y, Zhou T and
Huang Z: CD4+CD25+ regulatory T cells in tumor immunity. Int
Immunopharmacol. 34:244–249. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Facciabene A, Motz GT and Coukos G:
T-regulatory cells: Key players in tumor immune escape and
angiogenesis. Cancer Res. 72:2162–2171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Hosseinalizadeh H, Rabiee F, Eghbalifard
N, Rajabi H, Klionsky DJ and Rezaee A: Regulating the regulatory T
cells as cell therapies in autoimmunity an d cancer. Front Med
(Lausanne). 10:12442982023. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wilke CM, Wu K, Zhao E, Wang G and Zou W:
Prognostic significance of regulatory T cells in tumor. Int J
Cancer. 127:748–758. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Han XY, Xu N, Yuan JF, Wu H, Shi HL, Yang
L and Wu XJ: Total flavonoids of astragalus inhibit activated
CD4[Formula: See text] T cells and regulate differentiation of
Th17/Th1/Treg cells in exper imental autoimmune encephalomyelitis
mice by JAK/STAT and NF[Formula: See text]B signaling pathways. Am
J Chin Med. 51:1233–1248. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Fujiki T, Shinozaki R, Udono M and
Katakura Y: Identification and functional evaluation of polyphenols
that induce re gulatory T cells. Nutrients. 14:28622022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Dandawate S, Williams L, Joshee N, Rimando
AM, Mittal S, Thakur A, Lum LG and Parajuli P: Scutellaria extract
and wogonin inhibit tumor-mediated induction of T(reg) cells via
inhibition of TGF-β1 activity. Cancer Immunol Immunother.
61:701–711. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Du G, Jin L, Han X, Song Z, Zhang H and
Liang W: Naringenin: A potential immunomodulator for inhibiting
lung fibrosis a nd metastasis. Cancer Res. 69:3205–3212. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Feng Z, Hao W, Lin X, Fan D and Zhou J:
Antitumor activity of total flavonoids from Tetrastigma hemsleyanum
Diels et Gilg is associated with the inhibition of regulatory T
cells in mice. Onco Targets Ther. 7:947–956. 2014.PubMed/NCBI
|
|
94
|
Chen S, Li R, Chen Y, Chou CK, Zhang Z,
Yang Y, Liao P, Wang Q and Chen X: Scutellarin enhances anti-tumor
immune responses by reducing TNFR2-expressing CD4+Foxp3+ regulatory
T cells. Biomed Pharmacother. 151:1131872022. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Gardner A and Ruffell B: Dendritic cells
and cancer immunity. Trends Immunol. 37:855–865. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Del Prete A, Salvi V, Soriani A,
Laffranchi M, Sozio F, Bosisio D and Sozzani S: Dendritic cell
subsets in cancer immunity and tumor antigen sensing. Cell Mol
Immunol. 20:432–447. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Xiao Z, Wang R, Wang X, Yang H, Dong J, He
X, Yang Y, Guo J, Cui J and Zhou Z: Impaired function of dendritic
cells within the tumor microenvironment. Front Immunol.
14:12136292023. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Wesa AK and Storkus WJ: Killer dendritic
cells: Mechanisms of action and therapeutic implicati ons for
cancer. Cell Death Differ. 15:51–57. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Chauvin C and Josien R: Dendritic cells as
killers: Mechanistic aspects and potential roles. J Immunol.
181:11–16. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
LaCasse CJ, Janikashvili N, Larmonier CB,
Alizadeh D, Hanke N, Kartchner J, Situ E, Centuori S, Har-Noy M,
Bonnotte B, et al: Th-1 lymphocytes induce dendritic cell tumor
killing activity by an IF N-γ-dependent mechanism. J Immunol.
187:6310–6317. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Mittal SK and Roche PA: Suppression of
antigen presentation by IL-10. Curr Opin Immunol. 34:22–27. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Xiao W, Wu K, Yin M, Han S, Ding Y, Qiao
A, Lu G, Deng B, Bo P and Gong W: Wogonin inhibits tumor-derived
regulatory molecules by suppressing STA T3 signaling to promote
tumor immunity. J Immunother. 38:167–184. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Yang Y, Li XJ, Chen Z, Zhu XX, Wang J,
Zhang LB, Qiang L, Ma YJ, Li ZY, Guo QL and You QD: Wogonin induced
calreticulin/annexin A1 exposure dictates the immunogenicity of
cancer cells in a PERK/AKT dependent manner. PLoS One.
7:e508112012. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Bandyopadhyay S, Romero JR and
Chattopadhyay N: Kaempferol and quercetin stimulate
granulocyte-macrophage colony-stimulating factor secretion in human
prostate cancer cells. Mol Cell Endocrinol. 287:57–64. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Wang L, Zeng W, Wang L, Wang Z, Yin X, Qin
Y, Zhang F, Zhang C and Liang W: Naringenin enhances the antitumor
effect of therapeutic vaccines by promoting antigen
cross-presentation. J Immunol. 204:622–631. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Liu BC, Qiu Y, Zhao RD, Han X, Yun FY and
Tui X: Digital gene expression profiling of dendritic cells treated
with Seabuckthorn favones. Chin J Microbiol Immunol. 37:840–848.
2017.
|
|
107
|
Verna G, Liso M, Cavalcanti E, Bianco G,
Di Sarno V, Santino A, Campiglia P and Chieppa M: Quercetin
administration suppresses the cytokine storm in myeloid and
plasmacytoid dendritic cells. Int J Mol Sci. 22:83492021.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Sawicki MW, Dimasi N, Natarajan K, Wang J,
Margulies DH and Mariuzza RA: Structural basis of MHC class I
recognition by natural killer cell receptors. Immunol Rev.
181:52–65. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Cerwenka A and Lanier LL: Ligands for
natural killer cell receptors: Redundancy or specificity. Immunol
Rev. 181:158–169. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Gianchecchi E, Delfino DV and Fierabracci
A: Natural killer cells: Potential biomarkers and therapeutic
target in A utoimmune diseases? Front Immunol. 12:6168532021.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Portale F and Di Mitri D: NK cells in
cancer: Mechanisms of dysfunction and therapeutic potentia l. Int J
Mol Sci. 24:95212023. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Prager I and Watzl C: Mechanisms of
natural killer cell-mediated cellular cytotoxicity. J Leukoc Biol.
105:1319–1329. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Alspach E, Lussier DM and Schreiber RD:
Interferon γ and its important roles in promoting and inhibiting
spontaneous and therapeutic cancer immunity. Cold Spring Harb
Perspect Biol. 11:a0284802019. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Boehm U, Klamp T, Groot M and Howard JC:
Cellular responses to interferon-gamma. Annu Rev Immunol.
15:749–795. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Lian GY, Wang QM, Tang PM, Zhou S, Huang
XR and Lan HY: Combination of asiatic acid and naringenin modulates
NK cell anti-canc er immunity by rebalancing Smad3/Smad7 signaling.
Mol Ther. 26:2255–2266. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Feng YB, Chen L, Chen FX, Yang Y, Chen GH,
Zhou ZH and Xu CF: Immunopotentiation effects of apigenin on NK
cell proliferation and killing pancreatic cancer cells. Int J
Immunopathol Pharmacol. 37:39463202311611742023. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Lee HH and Cho H: Apigenin increases
natural killer cytotoxicity to human hepatocellular carcinoma
expressing HIF-1α through high interaction of CD95/CD95L. J
Microbiol Biotechnol. 32:397–404. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Pathni A, Özçelikkale A, Rey-Suarez I, Li
L, Davis S, Rogers N, Xiao Z and Upadhyaya A: Cytotoxic T
lymphocyte activation signals modulate cytoskeletal dynamics and
mechanical force generation. Front Immunol. 13:7798882022.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Hay ZLZ and Slansky JE: Granzymes: The
molecular executors of immune-mediated cytotoxicity. Int J Mol Sci.
23:18332022. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Weigelin B and Friedl P: T cell-mediated
additive cytotoxicity-death by multiple bullets. Trends Cancer.
8:980–987. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Zeytun A, Hassuneh M, Nagarkatti M and
Nagarkatti PS: Fas-Fas ligand-based interactions between tumor
cells and tumor-specif ic cytotoxic T lymphocytes: A lethal two-way
street. Blood. 90:1952–1959. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Tanaka H, Yoshizawa H, Yamaguchi Y, Ito K,
Kagamu H, Suzuki E, Gejyo F, Hamada H and Arakawa M: Successful
adoptive immunotherapy of murine poorly immunogenic tumor with
specific effector cells generated from gene-modified tumor-primed
lymph node cells. J Immunol. 162:3574–3582. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Péguillet I, Milder M, Louis D,
Vincent-Salomon A, Dorval T, Piperno-Neumann S, Scholl SM and Lantz
O: High numbers of differentiated effector CD4 T cells are found in
patients with cancer and correlate with clinical response after
neoadjuvant therapy of breast cancer. Cancer Res. 74:2204–2216.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Magombedze G, Reddy PBJ, Eda S and Ganusov
VV: Cellular and population plasticity of helper CD4(+) T cell
responses. Front Physiol. 4:2062013. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Wang LX, Shu S, Disis ML and Plautz GE:
Adoptive transfer of tumor-primed, in vitro-activated, CD4+ T
effector cells (TEs) combined with CD8+ TEs provides intratumoral
TE prolifera tion and synergistic antitumor response. Blood.
109:4865–4876. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Bourgeois C, Rocha B and Tanchot C: A role
for CD40 expression on CD8+ T cells in the generation of CD8+ T
cell memory. Science. 297:2060–2063. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Ke M, Zhang Z, Xu B, Zhao S, Ding Y, Wu X,
Wu R, Lv Y and Dong J: Baicalein and baicalin promote antitumor
immunity by suppressing PD-L1 expression in hepatocellular
carcinoma cells. Int Immunopharmacol. 75:1058242019. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Kawaguchi S, Kawahara K, Fujiwara Y,
Ohnishi K, Pan C, Yano H, Hirosue A, Nagata M, Hirayama M, Sakata
J, et al: Naringenin potentiates anti-tumor immunity against oral
cancer by inducing lymph node CD169-positive macrophage activation
and cytotoxic T cell infiltration. Cancer Immunol Immunother.
71:2127–2139. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Maatouk M, Elgueder D, Mustapha N, Chaaban
H, Bzéouich IM, Loannou I, Kilani S, Ghoul M, Ghedira K and
Chekir-Ghedira L: Effect of heated naringenin on immunomodulatory
properties and cellular antioxidant activity. Cell Stress
Chaperones. 21:1101–1109. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Pfarr K, Danciu C, Arlt O, Neske C,
Dehelean C, Pfeilschifter JM and Radeke HH: Simultaneous and dose
dependent melanoma cytotoxic and immune stimulat ory activity of
betulin. PLoS One. 10:e01188022015. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Sassi A, Maatouk M, El Gueder D, Bzéouich
IM, Abdelkefi-Ben Hatira S, Jemni-Yacoub S, Ghedira K and
Chekir-Ghedira L: Chrysin, a natural and biologically active
flavonoid suppresses tumor growth of mouse B16F10 melanoma cells:
In vitro and in vivo study. Chem Biol Interact. 283:10–19. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Sassi A, Mokdad Bzéouich I, Mustapha N,
Maatouk M, Ghedira K and Chekir-Ghedira L: Immunomodulatory
potential of hesperetin and chrysin through the cellular and
humoral response. Eur J Pharmacol. 812:91–96. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Yonekawa M, Shimizu M, Kaneko A, Matsumura
J and Takahashi H: Suppression of R5-type of HIV-1 in
CD4+ NKT cells by Vδ1+ T cells activated by
flavonoid glycosides, hesperidin and linarin. Sci Rep. 9:75062019.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Bruni E, Cimino MM, Donadon M, Carriero R,
Terzoli S, Piazza R, Ravens S, Prinz I, Cazzetta V, Marzano P, et
al: Intrahepatic CD69+Vδ1 T cells re-circulate in the blood of
patients with metastatic colorectal cancer and limit tumor
progression. J Immunother Cancer. 10:e0045792022. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Wu Y, Biswas D, Usaite I, Angelova M,
Boeing S, Karasaki T, Veeriah S, Czyzewska-Khan J, Morton C, Joseph
M, et al: A local human Vδ1 T cell population is associated with
survival in non small-cell lung cancer. Nat Cancer. 3:696–709.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Tang H, Liu Y, Wang C, Zheng H, Chen Y,
Liu W, Chen X, Zhang J, Chen H, Yang Y and Yang J: Inhibition of
COX-2 and EGFR by melafolone improves Anti-PD-1 therapy through
vascular normalization and PD-L1 downregulation in lung cancer. J
Pharmacol Exp Ther. 368:401–413. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Tian L, Wang S, Jiang S, Liu Z, Wan X,
Yang C, Zhang L, Zheng Z, Wang B and Li L: Luteolin as an adjuvant
effectively enhances CTL anti-tumor response in B16F10 mouse model.
Int Immunopharmacol. 94:1074412021. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Zhang W, Pan Y, Gou P, Zhou C, Ma L, Liu
Q, Du Y, Yang J and Wang Q: Effect of xanthohumol on Th1/Th2
balance in a breast cancer mouse model. Oncol Rep. 39:280–288.
2018.PubMed/NCBI
|
|
139
|
Xu L, Zhang Y, Tian K, Chen X, Zhang R, Mu
X, Wu Y, Wang D, Wang S, Liu F, et al: Apigenin suppresses PD-L1
expression in melanoma and host dendritic ce lls to elicit
synergistic therapeutic effects. J Exp Clin Cancer Res. 37:2612018.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Coombs MRP, Harrison ME and Hoskin DW:
Apigenin inhibits the inducible expression of programmed death
ligand 1 by human and mouse mammary carcinoma cells. Cancer Lett.
380:424–433. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Jiang ZB, Wang WJ, Xu C, Xie YJ, Wang XR,
Zhang YZ, Huang JM, Huang M, Xie C, Liu P, et al: Luteolin and its
derivative apigenin suppress the inducible PD-L1 expr ession to
improve anti-tumor immunity in KRAS-mutant lung cancer. Cancer
Lett. 515:36–48. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Li Z, Gao WQ, Wang P, Wang TQ, Xu WC, Zhu
XY and Liu H: Pentamethylquercetin inhibits hepatocellular
carcinoma progression and adipocytes-induced PD-L1 expression via
IFN-γ signaling. Curr Cancer Drug Targets. 20:868–874. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Sp N, Kang DY, Lee JM and Jang KJ:
Mechanistic insights of anti-immune evasion by nobiletin through
regul ating miR-197/STAT3/PD-L1 signaling in non-small cell lung
cancer (NSC LC) cells. Int J Mol Sci. 22:98432021. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Chen YC, He XL, Qi L, Shi W, Yuan LW,
Huang MY, Xu YL, Chen X, Gu L, Zhang LL and Lu JJ: Myricetin
inhibits interferon-γ-induced PD-L1 and IDO1 expression in l ung
cancer cells. Biochem Pharmacol. 197:1149402022. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Zhong Y, Li MY, Han L, Tai Y, Cao S, Li J,
Zhao H, Wang R, Lv B, Shan Z, et al: Galangin inhibits programmed
cell death-ligand 1 expression by suppres sing STAT3 and MYC and
enhances T cell tumor-killing activity. Phytomedicine.
116:1548772023. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Kongtawelert P, Wudtiwai B, Shwe TH,
Pothacharoen P and Phitak T: Inhibitory effect of hesperidin on the
expression of programmed death ligand (PD-L1) in breast cancer.
Molecules. 25:2522020. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Rong W, Wan N, Zheng X, Shi G, Jiang C,
Pan K, Gao M, Yin Z, Gao ZJ and Zhang J: Chrysin inhibits
hepatocellular carcinoma progression through suppressing programmed
death ligand 1 expression. Phytomedicine. 95:1538672022. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Mo D, Zhu H, Wang J, Hao H, Guo Y, Wang J,
Han X, Zou L, Li Z, Yao H, et al: Icaritin inhibits PD-L1
expression by Targeting Protein IκB Kinase α. Eur J Immunol.
51:978–988. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Hao H, Zhang Q, Zhu H, Wen Y, Qiu D, Xiong
J, Fu X, Wu Y, Meng K and Li J: Icaritin promotes tumor T-cell
infiltration and induces antitumor immu nity in mice. Eur J
Immunol. 49:2235–2244. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Dongye Z, Wu X, Wen Y, Ding X, Wang C,
Zhao T, Li J and Wu Y: Icaritin and intratumoral injection of CpG
treatment synergistically promote T cell infiltration and antitumor
immune response in mice. Int Immunopharmacol. 111:1090932022.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Yuan LW, Jiang XM, Xu YL, Huang MY, Chen
YC, Yu WB, Su MX, Ye ZH, Chen X, Wang Y and Lu JJ: Licochalcone A
inhibits interferon-gamma-induced programmed death-ligand 1 in lung
cancer cells. Phytomedicine. 80:1533942021. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Mei C, Zhang X, Zhi Y, Liang Z, Xu H, Liu
Z, Liu Y, Lyu Y and Wang H: Isorhamnetin regulates programmed death
ligand-1 expression by suppressing the EGFR-STAT3 signaling pathway
in canine mammary tumors. Int J Mol Sci. 25:6702024. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Li L, Zhang Y, Hu W, Zou F, Ning J, Rao T,
Ruan Y, Yu W and Cheng F: MTHFD2 promotes PD-L1 expression via
activation of the JAK/STAT signal ling pathway in bladder cancer. J
Cell Mol Med. 27:2922–2936. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Iwasaki T, Kohashi K, Toda Y, Ishihara S,
Yamada Y and Oda Y: Association of PD-L1 and IDO1 expression with
JAK-STAT pathway activation in soft-tissue leiomyosarcoma. J Cancer
Res Clin Oncol. 147:1451–1463. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Doi T, Ishikawa T, Okayama T, Oka K,
Mizushima K, Yasuda T, Sakamoto N, Katada K, Kamada K, Uchiyama K,
et al: The JAK/STAT pathway is involved in the upregulation of
PD-L1 expressi on in pancreatic cancer cell lines. Oncol Rep.
37:1545–1554. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Antonangeli F, Natalini A, Garassino MC,
Sica A, Santoni A and Di Rosa F: Regulation of PD-L1 expression by
NF-κB in cancer. Front Immunol. 11:5846262020. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Betzler AC, Theodoraki MN, Schuler PJ,
Döscher J, Laban S, Hoffmann TK and Brunner C: NF-κB and its role
in checkpoint control. Int J Mol Sci. 21:39492020. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Li C, Yang F, Wang R, Li W, Maskey N,
Zhang W, Guo Y, Liu S, Wang H and Yao X: CALD1 promotes the
expression of PD-L1 in bladder cancer via the JAK/S TAT signaling
pathway. Ann Transl Med. 9:14412021. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Wang Y, Zhang X, Xie X, Chen W, Li M, Diao
D and Dang C: Obesity and metabolic syndrome related macrophage
promotes PD-L1 expre ssion in TNBC through IL6/JAK/STAT pathway and
can be reversed by telm isartan. Cancer Biol Ther. 21:1179–1190.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Padmanabhan S, Gaire B, de Leon D, Vancura
A and Vancurova I: Interferon-γ induces PD-L1 expression in ovarian
cancer cells by JAK/STAT1 signaling. FASEB J 34 (S1). 1. 2020.
View Article : Google Scholar
|
|
161
|
Liu M, Wei F, Wang J, Yu W, Shen M, Liu T,
Zhang D, Wang Y, Ren X and Sun Q: Myeloid-derived suppressor cells
regulate the immunosuppressive functions of
PD-1−PD-L1+ Bregs through
PD-L1/PI3K/AKT/NF-κB axis in breast cancer. Cell Death Dis.
12:4652021. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Hu M, Yang J, Qu L, Deng X, Duan Z, Fu R,
Liang L and Fan D: Ginsenoside Rk1 induces apoptosis and
downregulates the expression of PD-L1 by targeting the NF-κB
pathway in lung adenocarcinoma. Food Funct. 11:456–471. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Liu S, Han Z, Trivett AL, Lin H, Hannifin
S, Yang D and Oppenheim JJ: Cryptotanshinone has curative dual
anti-proliferative and immunotherapeutic effects on mouse Lewis
lung carcinoma. Cancer Immunol Immunother. 68:1059–1071. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Wu R, Xiong J, Zhou T, Zhang Z, Huang Z,
Tian S and Wang Y: Quercetin/Anti-PD-1 antibody combination therapy
regulates the gut microbiota, impacts macrophage immunity and
reshapes the hepatocellular carcinoma tumor microenvironment. Front
Biosci (Landmark Ed). 28:3272023. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Cuzick J: Preventive therapy for cancer.
Lancet Oncol. 18:e472–e482. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Buonaguro L, Petrizzo A, Tornesello ML and
Buonaguro FM: Translating tumor antigens into cancer vaccines. Clin
Vaccine Immunol. 18:23–34. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Harper DM: Currently approved prophylactic
HPV vaccines. Expert Rev Vaccines. 8:1663–1679. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Cheng L, Wang Y and Du J: Human
papillomavirus vaccines: An updated review. Vaccines (Basel).
8:3912020. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Brower V: Approval of provenge seen as
first step for cancer treatment vaccines. J Natl Cancer Inst.
102:1108–1110. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Ogi C and Aruga A: Clinical evaluation of
therapeutic cancer vaccines. Hum Vaccin Immunother. 9:1049–1057.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Pao SC, Chu MT and Hung SI: Therapeutic
vaccines targeting neoantigens to induce T-cell immunity against
cancers. Pharmaceutics. 14:8672022. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Cho HI and Celis E: Optimized peptide
vaccines eliciting extensive CD8 T-cell responses with therapeutic
antitumor effects. Cancer Res. 69:9012–9019. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Stegmann T, Wiekmeijer AS, Kwappenberg K,
van Duikeren S, Bhoelan F, Bemelman D, Beenakker TJM, Krebber WJ,
Arens R and Melief CJM: Enhanced HPV16 E6/E7+ tumor eradication via
induction of tumor-specific T cells by therapeutic vaccination with
virosomes presenting synthetic long peptides. Cancer Immunol
Immunother. 72:2851–2864. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Fucikova J, Kepp O, Kasikova L, Petroni G,
Yamazaki T, Liu P, Zhao L, Spisek R, Kroemer G and Galluzzi L:
Detection of immunogenic cell death and its relevance for cancer
therapy. Cell Death Dis. 11:10132020. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Radogna F and Diederich M: Stress-induced
cellular responses in immunogenic cell death: Implications for
cancer immunotherapy. Biochem Pharmacol. 153:12–23. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Ahmed A and Tait SWG: Targeting
immunogenic cell death in cancer. Mol Oncol. 14:2994–3006. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Patra S, Roy PK, Dey A and Mandal M:
Impact of HMGB1 on cancer development and therapeutic insights
focused on CNS malignancy. Biochim Biophys Acta Rev Cancer.
1879:1891052024. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Patidar A, Selvaraj S, Sarode A, Chauhan
P, Chattopadhyay D and Saha B: DAMP-TLR-cytokine axis dictates the
fate of tumor. Cytokine. 104:114–123. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Nace G, Evankovich J, Eid R and Tsung A:
Dendritic cells and damage-associated molecular patterns:
Endogenous danger signals linking innate and adaptive immunity. J
Innate Immun. 4:6–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Lee SM, Kim P, You J and Kim EH: Role of
damage-associated molecular pattern/cell death pathways in
vaccine-induced immunity. Viruses. 13:23402021. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Hou W, Zhang Q, Yan Z, Chen R, Zeh Iii HJ,
Kang R, Lotze MT and Tang D: Strange attractors: DAMPs and
autophagy link tumor cell death and immunity. Cell Death Dis.
4:e9662013. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Woo SR, Corrales L and Gajewski TF: Innate
immune recognition of cancer. Annu Rev Immunol. 33:445–474. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Tsuchiya K: Switching from apoptosis to
pyroptosis: Gasdermin-elicited inflammation and antitumor immunity.
Int J Mol Sci. 22:4262021. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Pandolfi F, Altamura S, Frosali S and
Conti P: Key role of DAMP in inflammation, cancer, and tissue
repair. Clin Ther. 38:1017–1028. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Helm O, Held-Feindt J, Grage-Griebenow E,
Reiling N, Ungefroren H, Vogel I, Krüger U, Becker T, Ebsen M,
Röcken C, et al: Tumor-associated macrophages exhibit pro-and
anti-inflammatory properties by which they impact on pancreatic
tumorigenesis. Int J Cancer. 135:843–861. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Shalapour S and Karin M: Pas de deux:
Control of anti-tumor immunity by cancer-associated inflammation.
Immunity. 51:15–26. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Galluzzi L, Vacchelli E, Bravo-San Pedro
JM, Buqué A, Senovilla L, Baracco EE, Bloy N, Castoldi F, Abastado
JP, Agostinis P, et al: Classification of current anticancer
immunotherapies. Oncotarget. 5:12472–12508. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Jafari S, Heydarian S, Lai R, Mehdizadeh
Aghdam E and Molavi O: Silibinin induces immunogenic cell death in
cancer cells and enhances the induced immunogenicity by
chemotherapy. Bioimpacts. 13:51–61. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Xia L, Xu X, Li M, Zhang X and Cao F:
Afzelin induces immunogenic cell death against lung cancer by
targeting NQO2. BMC Complement Med Ther. 23:3812023. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Zhu MY, Wang T, Wang HD, Wang HZ, Chen HY,
Zhang S, Guo YJ, Li H and Hui H: LW-213 induces immunogenic tumor
cell death via ER stress mediated by lysosomal TRPML1. Cancer Lett.
577:2164352023. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Li L, Zou Y, Wang L, Yang L, Li Y, Liao A,
Chen Z, Yu Z, Guo J and Han S: Nanodelivery of scutellarin induces
immunogenic cell death for treating hepatocellular carcinoma. Int J
Pharm. 642:1231142023. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Xiao Y, Yao W, Lin M, Huang W, Li B, Peng
B, Ma Q, Zhou X and Liang M: Icaritin-loaded PLGA nanoparticles
activate immunogenic cell death and facilitate tumor recruitment in
mice with gastric cancer. Drug Deliv. 29:1712–1725. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Zhang J, Shen L, Li X, Song W, Liu Y and
Huang L: Nanoformulated codelivery of quercetin and alantolactone
promotes an antitumor response through synergistic immunogenic cell
death for microsatellite-stable colorectal cancer. ACS Nano.
13:12511–12524. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Qian Y, Mao J, Leng X, Zhu L, Rui X, Jin
Z, Jiang H, Liu H, Zhang F, Bi X, et al: Co-delivery of
proanthocyanidin and mitoxantrone induces synergistic immunogenic
cell death to potentiate cancer immunotherapy. Biomater Sci.
10:4549–4560. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Jiang S, Wang S, Zhang L, Tian L, Li L,
Liu Z, Dong Q, Lv X, Mu H, Zhang Q and Wang B: Hesperetin as an
adjuvant augments protective anti-tumour immunity res ponses in
B16F10 melanoma by stimulating cytotoxic CD8+ T cells. Scand J
Immunol. 91:e128672020. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Lu R, Wang S, Jiang S, Li C, Wang Y, Li L,
Wang Y, Ma G, Qiao H, Leng Z, et al: Chrysin enhances antitumour
immunity response through the IL-12-STAT4 signal pathway in the
B16F10 melanoma mouse model. Scand J Immunol. 96:e131772022.
View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Zhang L, Wang S, Liu Z, Zhang L, Wang S
and Wang B: Procyanidin, a kind of biological flavonoid, induces
protective anti-tumor immunity and protects mice from lethal B16F10
challenge. Int Immunopharmacol. 47:251–258. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Kang TH, Lee JH, Song CK, Han HD, Shin BC,
Pai SI, Hung CF, Trimble C, Lim JS, Kim TW and Wu TC:
Epigallocatechin-3-gallate enhances CD8+ T cell-mediated antitumor
immunity induced by DNA vaccination. Cancer Res. 67:802–811. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Chuang CM, Monie A, Wu A and Hung CF:
Combination of apigenin treatment with therapeutic HPV DNA
vaccination generates enhanced therapeutic antitumor effects. J
Biomed Sci. 16:492009. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Olson DJ and Odunsi K: Adoptive cell
therapy for nonhematologic solid tumors. J Clin Oncol.
41:3397–3407. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Dudley ME and Rosenberg SA:
Adoptive-cell-transfer therapy for the treatment of patients with
cancer. Nat Rev Cancer. 3:666–675. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Du S, Yan J, Xue Y, Zhong Y and Dong Y:
Adoptive cell therapy for cancer treatment. Exploration (Beijing).
3:202100582023. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Chang YF, Chuang HY, Hsu CH, Liu RS,
Gambhir SS and Hwang JJ: Immunomodulation of curcumin on adoptive
therapy with T cell functiona limaging in mice. Cancer Prev Res
(Phila). 5:444–452. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Nozhat Z, Heydarzadeh S, Memariani Z and
Ahmadi A: Chemoprotective and chemosensitizing effects of apigenin
on cancer therapy. Cancer Cell Int. 21:5742021. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Li M, Fan J, Hu M, Xu J, He Z and Zeng J:
Quercetin enhances 5-fluorouracil sensitivity by regulating the
autophagic flux and inducing drp-1 mediated mitochondrial
fragmentation in colorectal cancer cells. Curr Mol Pharmacol. Feb
27–2024.(Epub ahead of print).
|
|
206
|
Vazhappilly CG, Alsawaf S, Mathew S, Nasar
NA, Hussain MI, Cherkaoui NM, Ayyub M, Alsaid SY, Thomas JG, Cyril
AC, et al: Pharmacodynamics and safety in relation to dose and
response of plant flavonoids in treatment of cancers.
Inflammopharmacology. 33:11–47. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Liu H, Dong Y, Gao Y, Du Z, Wang Y, Cheng
P, Chen A and Huang H: The fascinating effects of baicalein on
cancer: A review. Int J Mol Sci. 17:16812016. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Yang F, Song L, Wang H, Wang J, Xu Z and
Xing N: Quercetin in prostate cancer: Chemotherapeutic and
chemopreventive effects, mechanisms and clinical application
potential. Oncol Rep. 33:2659–2668. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Mirazimi SMA, Dashti F, Tobeiha M, Shahini
A, Jafari R, Khoddami M, Sheida AH, EsnaAshari P, Aflatoonian AH,
Elikaii F, et al: Application of quercetin in the treatment of
gastrointestinal cancers. Front Pharmacol. 13:8602092022.
View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Dahiya A, Majee C, Mazumder R, Priya N and
Atriya A: Insight into the glycosylation methods of the flavonoids
as an approach to enhance its bioavailability and pharmacological
activities. Ind J Pharm Edu Res. 57:354–371. 2023. View Article : Google Scholar
|
|
211
|
Li C, Dai T, Chen J, Chen M, Liang R, Liu
C, Du L and McClements DJ: Modification of flavonoids: Methods and
influences on biological activities. Crit Rev Food Sci Nutr.
63:10637–10658. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Gopikrishna A, Girigoswami A and
Girigoswami K: Controlled drug delivery systems for improved
efficacy and bioavailability of flavonoids. J Achiev Mater Manuf
Eng. 116:49–60. 2023.
|
|
213
|
Naeem A, Ming Y, Pengyi H, Jie KY, Yali L,
Haiyan Z, Shuai X, Wenjing L, Ling W, Xia ZM, et al: The fate of
flavonoids after oral administration: A comprehensive overview of
its bioavailability. Crit Rev Food Sci Nutr. 62:6169–6186. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Bilia AR, Piazzini V, Risaliti L, Vanti G,
Casamonti M, Wang M and Bergonzi MC: Nanocarriers: A successful
tool to increase solubility, stability and optimise bioefficacy of
natural constituents. Curr Med Chem. 26:4631–4656. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Alizadeh SR, Savadkouhi N and Ebrahimzadeh
MA: Drug design strategies that aim to improve the low solubility
and poor bioavailability conundrum in quercetin derivatives. Expert
Opin Drug Discov. 18:1117–1132. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Pecorini G, Ferraro E and Puppi D:
Polymeric systems for the controlled release of flavonoids.
Pharmaceutics. 15:6282023. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Lee WH, Loo CY, Ong HX, Traini D, Young PM
and Rohanizadeh R: Synthesis and characterization of inhalable
flavonoid nanoparticle for lung cancer cell targeting. J Biomed
Nanotechnol. 12:371–386. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Loo CY, Traini D, Young PM, Parumasivam T
and Lee WH: Pulmonary delivery of curcumin and quercetin
nanoparticles for lung cancer-Part 1: Aerosol performance
characterization. J Drug Deliv Sci Technol. 86:1046462023.
View Article : Google Scholar
|
|
219
|
Hong L, Li W, Li Y and Yin S:
Nanoparticle-based drug delivery systems targeting cancer cell
surfaces. RSC Adv. 13:21365–21382. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Batool S, Sohail S, Ud Din F, Alamri AH,
Alqahtani AS, Alshahrani MA, Alshehri MA and Choi HG: A detailed
insight of the tumor targeting using nanocarrier drug delivery
system. Drug Deliv. 30:21838152023. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Chen D, Liu X, Lu X and Tian J:
Nanoparticle drug delivery systems for synergistic delivery of
tumor therapy. Front Pharmacol. 14:11119912023. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Xiao Z, Su Z, Han S, Huang J, Lin L and
Shuai X: Dual pH-sensitive nanodrug blocks PD-1 immune checkpoint
and uses T cells to deliver NF-κB inhibitor for antitumor
immunotherapy. Sci Adv. 6:eaay77852020. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Lu Y, Miao L, Wang Y, Xu Z, Zhao Y, Shen
Y, Xiang G and Huang L: Curcumin micelles remodel tumor
microenvironment and enhance vaccine activity in an advanced
melanoma model. Mol Ther. 24:364–374. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Tiwari P and Mishra KP: Role of
plant-derived flavonoids in cancer treatment. Nutr Cancer.
75:430–449. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Kadhum WR, Ramaiah P, Tayyib NA, Hjazi A,
Kahhharov AJ, Alkhafaji AT, Al-Dami FH, Ridha BM, Alsalamy AH and
Alwave M: Novel and potential therapy options for a range of cancer
diseases: Using flavonoid. Pathol Res Pract. Nov 29–2023.(Epub
ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Galati G and O'Brien PJ: Potential
toxicity of flavonoids and other dietary phenolics: Significance
for their chemopreventive and anticancer properties. Free Radic
Biol Med. 37:287–303. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Havsteen BH: The biochemistry and medical
significance of the flavonoids. Pharmacol Ther. 96:67–202. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Dong S, Guo X, Han F, He Z and Wang Y:
Emerging role of natural products in cancer immunotherapy. Acta
Pharm Sin B. 12:1163–1185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Khatib S, Pomyen Y, Dang H and Wang XW:
Understanding the cause and consequence of tumor heterogeneity.
Trends Cancer. 6:267–271. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Xiao F, Farag MA, Xiao J, Yang X, Liu Y,
Shen J and Lu B: The influence of phytochemicals on cell
heterogeneity in chronic inflammation-associated diseases: The
prospects of single cell sequencing. J Nutr Biochem.
108:1090912022. View Article : Google Scholar : PubMed/NCBI
|