Introduction
A substantial number of patients worldwide have
chronic liver disease, with some patients eventually progressing to
cirrhosis and even liver cancer. The enormous medical costs and
loss of labor force among liver disease patients impose a
significant burden on socio-economic development (1). Hepatic fibrosis (HF) is a common
pathological stage of a number of chronic liver diseases. HF is
induced by viral hepatitis, steatohepatitis, metabolic disorders,
autoimmune conditions and drug- or toxin-related hepatitis and is a
crucial influence on liver disease outcomes (2). HF is a reparative response of the
body to chronic liver injury. It is primarily characterized by an
imbalance between extracellular matrix (ECM) proliferation and
degradation in the liver, leading to abnormal fibrous connective
tissue deposition within the liver and liver structure and function
disruption (3). Increasing
evidence has suggested that HF is dynamic and reversible (4). However, there are no markedly
effective treatment options for HF. Clinically, the main approaches
to improving symptoms include lifestyle interventions, liver
protection, lipid-lowering therapies and modulating the gut
microbiota (5). To date,
definitive anti-fibrotic drugs in clinical practice are
lacking.
Hepatic stellate cell (HSC) activation is central in
the HF process (6) and is a
prerequisite for ECM production and a key step in HF formation
(7). Quiescent HSCs are
responsible for liver regeneration, immune regulation, maintaining
sinusoidal circulation and storing vitamin A, providing structural
and functional support to the liver, with minimal ECM secretion
(8). Continuous stimulation of the
liver by chronic inflammation damages Kupffer, bile duct epithelial
cells and vascular endothelial cells, which release large amounts
of inflammatory factors such as transforming growth factor beta 1
(TGF-β1), tumor necrosis factor alpha (TNF-α), platelet-derived
growth factor and connective tissue growth factor, which enter the
space of Disse and activate HSCs (9). The activated HSCs transform into
myofibroblasts, continuously proliferating and secreting large
amounts of ECM components such as collagen I/III and alpha smooth
muscle actin (α-SMA), which promote fibrous connective tissue
proliferation in the liver and lead to early HF onset (10).
Multiple signaling pathways and cytokines regulate
HF pathogenesis, among which the TGF-β1-Smad signaling pathway is a
crucial intracellular pathway (11). The TGF-β1-Smad signaling pathway is
closely related to HF occurrence and development, including HSC
activation and proliferation, ECM deposition, oxidative stress in
hepatocytes and autophagy (12).
As the pathway is significant in HF formation and progression,
blocking it can slow or even reverse HF and effectively reduce
liver cancer incidence, thereby decreasing patients' fibrogenesis
and carcinogenesis rates (13).
Traditional Chinese medicine (TCM) acts on multiple
pathways and targets, which confers a comprehensive advantage for
treating diseases with complex pathological mechanisms (14). TCM was recently demonstrated to
have good efficacy and promising prospects in HF treatment
(15). Umbelliferone (UMB;
7-hydroxycoumarin) is a natural product found in plants of the
Rutaceae and Apiaceae families. UMB exhibits various biological and
pharmacological activities, including strong antioxidant,
anti-inflammatory, anti-diabetic and anti-tumor effects (16). These effects are primarily mediated
through the inhibition of oxidative stress and inflammation. Kassim
et al (17) demonstrated
the antioxidant properties of UMB through
1,1-diphenyl-2-picrylhydrazyl radical scavenging. The authors also
reported that UMB exhibited effective anti-inflammatory activity in
an ovalbumin-induced allergic airway inflammation mouse model.
Reactive oxygen species and oxidative stress can
induce hepatocyte damage and death, directly or indirectly leading
to HF (18). Notably, UMB
inhibited the proliferation of various tumor cells and induced
their apoptosis with no significant toxicity to normal cells
(19). Therefore, exploiting the
anti-inflammatory and antioxidant properties of UMB holds
significant potential and hope for effective HF treatment or
reducing fibrosis-induced damage. However, there are few reports on
the therapeutic effects of UMB on HF and the underlying molecular
mechanisms remain undetermined. The present study established a rat
model of HF using carbon tetrachloride (CCl4) and UMB
interventions were administered in vivo and in vitro.
It investigated the improvement and reversal of HF by UMB through
fibrotic factors, oxidative stress and the TGF-β1-Smad signaling
pathway and the effects of UMB on HSC proliferation to provide
comprehensive information for improved HF treatment.
Materials and methods
Chemicals and reagents
UMB was from Huahe Medical (purity according to
HPLC, 99%). CCl4 was from Shanghai Aladdin Biochemical
Technology Co., Ltd. The enzyme-linked immunosorbent assay (ELISA)
kits for TNF-α and IL-6 were from Beijing Solarbio Science &
Technology Co., Ltd. The biochemical assay kits for aspartate
aminotransferase (AST), alanine aminotransferase (ALT),
hydroxyproline, total bile acids (TBA), total bilirubin (TBIL) and
hydroxyproline (Hyp) were from Shanghai Enzyme-linked Biotechnology
Co., Ltd. The kits for malondialdehyde (MDA), catalase (CAT),
glutathione (GSH) and superoxide dismutase (SOD) activity were from
Beijing Solarbio Science & Technology Co., Ltd. The Masson and
hematoxylin and eosin (H&E) staining kits were from Beijing
Solarbio Science & Technology Co., Ltd. TRIzol® and
the reverse transcription kit were from Thermo Fisher Scientific,
Inc. SYBR Green reagents were from Thermo Fisher Scientific, Inc.
The primers were synthesized by Beijing Qingke Biotechnology Co.,
Ltd. The Cell Counting Kit-8 (CCK-8) kit was from Thermo Fisher
Scientific, Inc. The radioimmunoprecipitation assay (RIPA) buffer
was from Beijing Solarbio Science & Technology Co., Ltd. The
bicinchoninic acid (BCA) assay kit was from Thermo Fisher
Scientific, Inc. The primary antibodies against TGF-β1, α-SMA,
phosphorylated (p)-Smad2/3, Smad2/3 and β-actin and the horseradish
peroxidase (HRP)-conjugated rabbit anti-mouse IgG secondary
antibodies were from Cell Signaling Technology, Inc. The ECL
chemiluminescence kit was from Beyotime Institute of
Biotechnology.
Apparatus
The apparatus used in this study were the PT1020
tissue processor and tissue slicer (Leica Microsystems GmbH), Axio
Scan.Z1 Slide Scanner (Zeiss GmbH), Optima XPN-100 Ultracentrifuge
(Beckman Coulter, Inc.), automatic biochemical analyzer (Hitachi
7020; Hitachi, Ltd.), Shimadzu UV-1800 UV-Vis Spectrophotometer
(Shimadzu Corporation), ABI StepOne Plus real-time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.), Tanon-5200
Chemiluminescent Imaging System (Tanon Science and Technology Co.,
Ltd.), ImageJ (Version 1.48, National Institutes of Health) and
GraphPad Prism 9.0 (Dotmatics).
Animal experiments
A total of 32 Specific pathogen-free (SPF)-grade
male Sprague-Dawley (SD) rats (age, 6 weeks; weight, 250±30 g) were
from the Inner Mongolia Medical University Experimental Animal
Center (Hohhot, China). The animals were acclimatized for one week,
at a controlled temperature of 22±2°C and a humidity level of 50±5%
in the designated facility, allowed unrestricted access to food and
water, and acclimated to alternating 12-h light and dark cycles. No
unexpected animal deaths occurred during the experiment and all
animals were sacrificed in accordance with the approved protocol at
the end of the study. Cervical dislocation was employed to ensure a
rapid and painless death. The study strictly adhered to humane
endpoint criteria to minimize animal suffering, with specific
indicators including significant weight loss (>20%), severe
behavioral abnormalities, or loss of autonomous feeding and
drinking ability. All animal experimental protocols adhered to
international ethical guidelines and were approved by the Inner
Mongolia Medical University Animal Experiment Ethics Committee
(approval no. YKD202201124).
After acclimation, all rats were weighed and
randomly divided into four groups (n=8): Control group,
CCl4-treated model group and low and high dose (50 and
100 mg/kg) of UMB co-treated group. The low dose was chosen to
reflect a suboptimal therapeutic level, while the high dose was
selected to represent a potentially maximal effective dose. A rat
model of HF was established by intraperitoneal injection of
CCl4 (3 ml/kg; 40% olive oil solution) twice a week for
8 weeks (with a double dose for the first injection) in the model
and UMB treatment groups. The control rats were given olive oil.
Starting from day 1 of week 5, UMB (50 or 100 mg/kg) dissolved in
0.5% sodium carboxymethyl cellulose was orally administered to the
rats in the UMB treatment groups once daily. The control and model
rats received equal volumes of solvent (Fig. 1A). After the final administration,
anesthesia was induced and maintained using isoflurane inhalation.
The rats were initially anesthetized with 4–5% isoflurane in an
induction chamber and then maintained with 1.5–2% isoflurane
delivered via a nose cone. Blood samples were collected from the
abdominal aortas of each group (5 ml per rat) and the liver tissues
were harvested. A portion of the liver tissue was fixed in 4%
paraformaldehyde for 24 h at 4°C and the remainder was stored at
−80°C for further analysis. After procedures, death was confirmed
by the absence of a heartbeat, cessation of breathing for at least
5 min and lack of pupillary reflexes. If necessary, sacrifice was
performed via cervical dislocation.
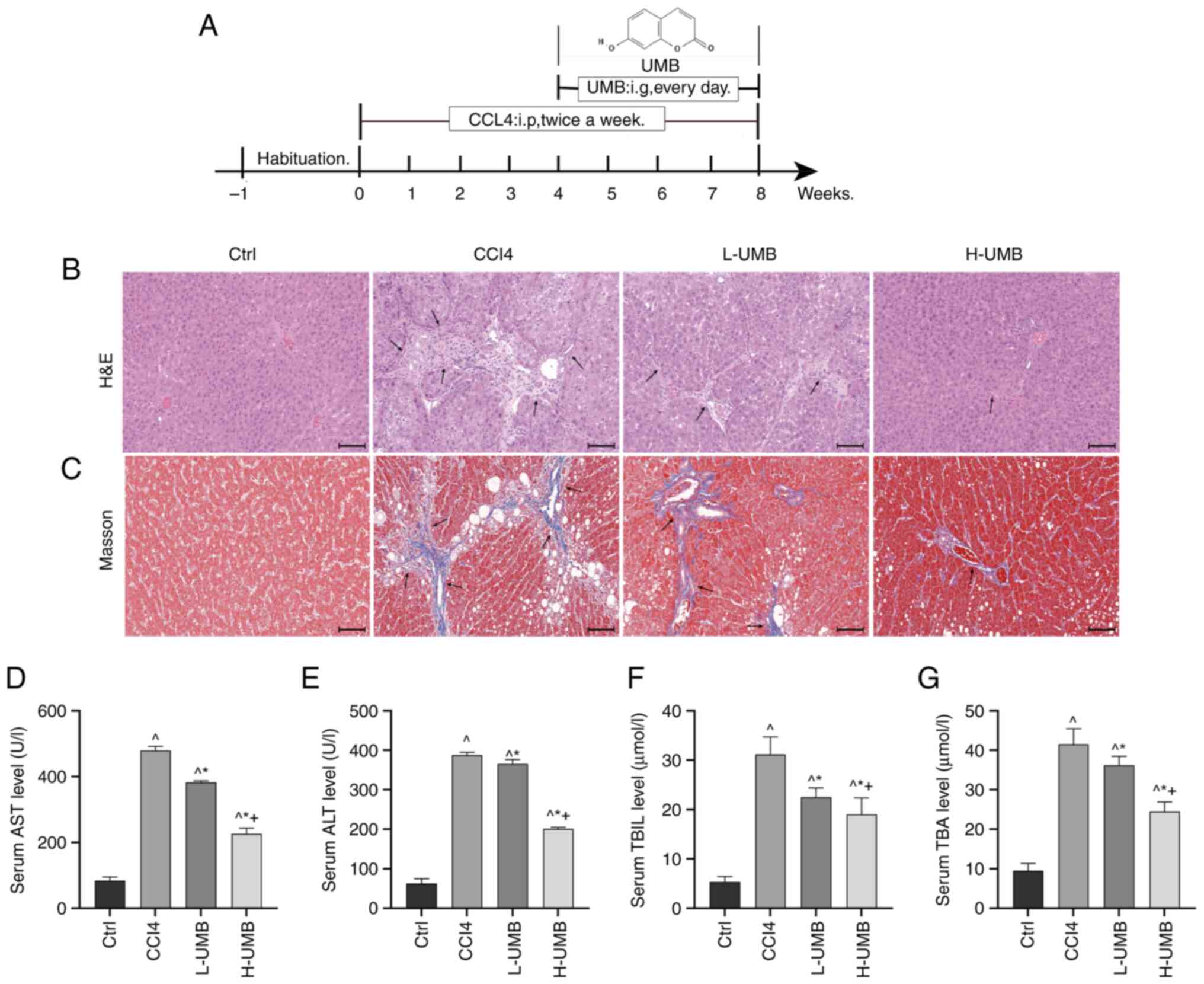 | Figure 1.Hepatoprotective effects of UMB in a
CCl4-induced rat HF model. CCl4-induced rat
HF models were treated with 50 or 100 mg/kg/day UMB. (A) The rat
model treatment diagram. SD rats were intraperitoneally injected
with CCl4 twice a week and gavaged daily with 50 or 100
mg/kg UMB from week 5 onward. All parameters were analyzed 8 weeks
later. (B) H&E and (C) Masson's trichrome staining
(magnification, ×200). Black arrows indicate damaged liver tissue
and fiber cords. Serum levels of AST (D) ALT (E) TBIL (F) and TBA
(G) Results are the mean ± SD (n=8). ^P<0.05 vs.
control group; *P<0.05 vs. CCl4 group;
+P<0.05 vs. L-UMB. UMB, Umbelliferone; HF, hepatic
fibrosis; AST, aspartate aminotransferase; ALT, alanine
aminotransferase; TBIL, total bilirubin; TBA, total bile acids;
H&E, hematoxylin and eosin; Ctrl, control group;
CCl4, carbon tetrachloride-treated group (model); L-UMB,
low-dose UMB treatment (50 mg/kg); H-UMB, high-dose UMB treatment
(100 mg/kg). |
Primary HSC isolation and culture
The rat livers were digested using collagenase IV
and pronase E. The digested cells were filtered through a 200-mesh
cell strainer to eliminate undigested tissue. The resulting cell
suspension was centrifuged at 40 × g for 3 min at 4°C and washed
three times until the supernatant became clear, thereby removing
hepatocytes and collecting the supernatant. The supernatant was
centrifuged at 500 × g for 10 min at 4°C to collect the pellet,
which was resuspended in Dulbecco's modified Eagle's medium (DMEM)
(Thermo Fisher Scientific, Inc.). The resuspended cells were
filtered again through a 200-mesh cell strainer. Then, the pellet
was resuspended in 5 ml 40% Percoll solution, overlaid with an
equal volume of 12% Percoll solution and topped with 1 ml DMEM. A
30-min centrifugation at 1,150 × g at 4°C allowed the HSCs to be
located between the phosphate-buffered saline (PBS) and 12% Percoll
layers. The isolated primary HSCs were cultured for 3 days in the
DMEM to promote spontaneous activation before being used in further
experiments.
UMB intervention of HSCs
The HSCs (6×104 cells/ml) were seeded in
96-well plates, treated with 0, 2, 5, 10, or 20 µM UMB, dissolved
in dimethyl sulfoxide (DMSO) and cultured for 72 h. The control
group was the drug-free group. After 0-, 24-, 48- and 72-h
treatment, HSC proliferation was measured using CCK-8.
Subsequently, the cells from each group were incubated with the
detection reagent at 37°C for another hour. The HSC proliferation
was measured using Shimadzu UV-1800 UV–Vis Spectrophotometer at 450
nm. The hydroxyproline concentration was determined using a
commercial kit. At the end of the experiment, the mRNA expression
of the fibrosis-related factors TGF-β1 and α-SMA was assessed using
reverse transcription-quantitative (RT-q) PCR. The relative TGF-β1,
α-SMA, p-Smad2/3 and Smad2/3 protein expression levels were
measured using western blotting.
Histopathological examination
The rat liver tissues were fixed in 4%
paraformaldehyde for 24 h at 4°C for pathological analysis.
Standard dehydration, xylene-clearing and paraffin-embedding were
conducted and 4-µm thick sections were cut with a microtome. The
liver tissue sections were deparaffinized and stained with the
H&E and Masson staining kits. Finally, the histopathological
changes in the liver tissue were observed under a light
microscope.
ELISA
Blood samples from each group were centrifuged at
4,000 × g for 10 min at 4°C to obtain serum samples. The serum
TNF-α and IL-6 concentrations were measured using Rat TNF-α ELISA
kits (cat. no. Sekr0009) and Rat IL-6 ELISA kits (cat. no.
Sekr0005). The ALT, AST, TBIL and TBA concentrations were
determined using the automated biochemical analyzer and commercial
clinical assay kits (cat. no. Ml059334, cat. no. Ml059335, cat. no.
M1224L, cat. no. M122CM48).
Appropriate amounts of liver tissue or HSCs were
obtained from each group and the Hyp concentration was measured
using a standard commercial kit (cat. no. Ml092986), which is used
to assess liver function indicators and evaluate liver function
changes. The frozen liver tissues from each group were weighed and
homogenized in 0.9 ml ice-cold saline to prepare a 10% homogenate.
The homogenate was centrifuged at 4°C for 10 min at 1,000 × g and
the supernatant was collected. The GSH, CAT, SOD and MDA levels in
the liver tissue were measured by chemichromatometry according to
the directions of the reagent kits (cat. no. BC1170, cat. no.
BC0200, cat. no. BC5165, cat. no. BC0020).
Reverse transcription-quantitative
(RT-q) PCR
Rat liver tissue or HSCs were homogenized in lysis
buffer using a tissue homogenizer. Total RNA was extracted using
TRIzol® according to the supplier's instructions. The
RNA concentration was measured and RNA purity was assessed by
measuring the optical density (OD) at 260/280 nm with a
spectrophotometer. The total RNA integrity was evaluated by agarose
gel electrophoresis. The total mRNA was reverse-transcribed into
cDNA according to the reverse transcription kit instructions.
RT-qPCR was performed using SYBR Green with an initial denaturation
for 30 sec at 95°C, followed by 40 cycles of amplification (95°C
for 5 sec and 60°C for 34 sec), according to the manufacturer's
protocol. Each sample was analyzed in triplicate. The internal
control was β-actin. The RNA expression levels were calculated
using the comparative threshold cycle (2−ΔΔCq) (20) method. Table I presents the RT-qPCR primer
sequences.
 | Table I.Primer sequences used for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences used for reverse
transcription-quantitative PCR.
| Gene | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| α-SMA |
TCCTGACCCTGAAGTATCCG |
TCTCCAGAGTCCAGCACAAT |
| Collagen I |
CTGCCGATGTCGCTATCC |
CCACAAGCGTGCTGTAGGT |
| TIMP-1 |
CCTCTGGCATCCTCTTG |
TTGATCTCATAACGCTGGT |
| MMP-1 |
ATGCTTAGCCTTCCTTT |
CACCCAAGTTGTAGTAGTTTT |
| β-Actin |
CGTTGACATCCGTAAAGACC |
GCTAGGAGCCAGGGCAGTA |
Western blotting
The rat liver tissues or HSCs were homogenized in
RIPA buffer containing protease and phosphatase inhibitors, then
centrifuged at 4°C for 15 min at 8,945 × g to obtain the
supernatant. The total protein concentration was determined using a
bicinchoninic acid (BCA) assay kit. A total of 10 µl protein
underwent sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(10% SDS-PAGE) and then were transferred to a PVDF membrane. The
membrane was blocked with skimmed milk for 2 h at 4°C, then
incubated at 4°C overnight with primary antibodies against TGF-β1
(cat. no. 3711S), p-Smad2/3 (cat. no. 12001C), and Smad2/3 (cat.
no. 12460S) (1:1,000 dilution in 3% skimmed milk) to measure the
expression of the respective proteins. The internal control was
β-actin (1:1,000; cat. no. 4970T). After washing with TBST (0.1%
Tween 20), the membrane was incubated for 1 h with secondary
antibodies (1:1,000 dilution, cat. no. 7074P2) at room temperature.
After washing with TBST, the blots were visualized using the ECL
chemiluminescence kit. The protein bands were scanned and detected
using the Tanon-5200 chemiluminescent imaging system. Finally, the
bands were quantified using ImageJ to calculate the relative
expression levels of each protein.
Statistical analysis
The data were analyzed using SPSS (version 26.0).
The measurement data are presented as the mean ± standard
deviation. Statistical significance was assessed using either the
one-way analysis of variance (ANOVA) or Student's t-test, followed
by Tukey's post-hoc multiple comparison tests to evaluate
differences among groups. P<0.05 was considered to indicate a
statistically significant difference. The statistical analyses were
performed using GraphPad Prism 9.0 (Dotmatics).
Results
UMB alleviates CCl4-induced
liver damage in the rat model
There were no unexpected fatalities in any group
throughout the study. The control rats exhibited optimal health,
characterized by agility, regular dietary patterns and normal bowel
movements. By contrast, the model rats exhibited slightly
diminished mental states and signs of irritability. The rats in the
treatment group exhibited noticeable improvements compared with the
model rats.
Liver tissue abnormalities were identified using
H&E staining (21). The
analysis revealed that the hepatocytes of the model group had a
disorganized arrangement and were accompanied by small and variably
sized round vacuoles, along with sporadic inflammatory cell
infiltration. The UMB treatment was followed by a notable
improvement in hepatocyte integrity and reduced inflammatory
infiltration. Notably, the treatment groups exhibited decreased
hepatic steatosis, necrosis and collagen deposition, suggesting a
restoration towards normal liver architecture (Fig. 1B). The Masson staining results
indicated markedly increased perivascular and interstitial fibrosis
in the CCl4-induced rats (22). UMB ameliorated these fibrotic
changes (Fig. 1C). Furthermore,
the higher dose of UMB (100 mg/kg) was more effective than the
lower dose (50 mg/kg), demonstrating that UMB markedly mitigated
the CCl4-induced liver pathology and fibrosis, providing
substantial protective and therapeutic benefits.
Effect of UMB on liver function
indicators
AST and ALT are critical biomarkers for assessing
liver function and are extensively utilized in diagnosing liver
damage in clinical settings (23).
TBIL and TBA are indicators of cholestasis, reflecting the
secretory and excretory capacities of the liver (24). The present study evaluated changes
in these liver-associated parameters in CCl4-induced
rats that received 50 and 100 mg/kg UMB. The results demonstrated a
statistically significant dose-dependent reduction in AST, ALT,
TBIL and TBA levels in the treatment groups compared with the model
group (P<0.05; Fig. 1D-G).
These results suggested that UMB effectively mitigated hepatocyte
damage and enhanced liver functionality. Unexpectedly, the data
also revealed that UMB might alleviate liver injury-associated bile
stasis.
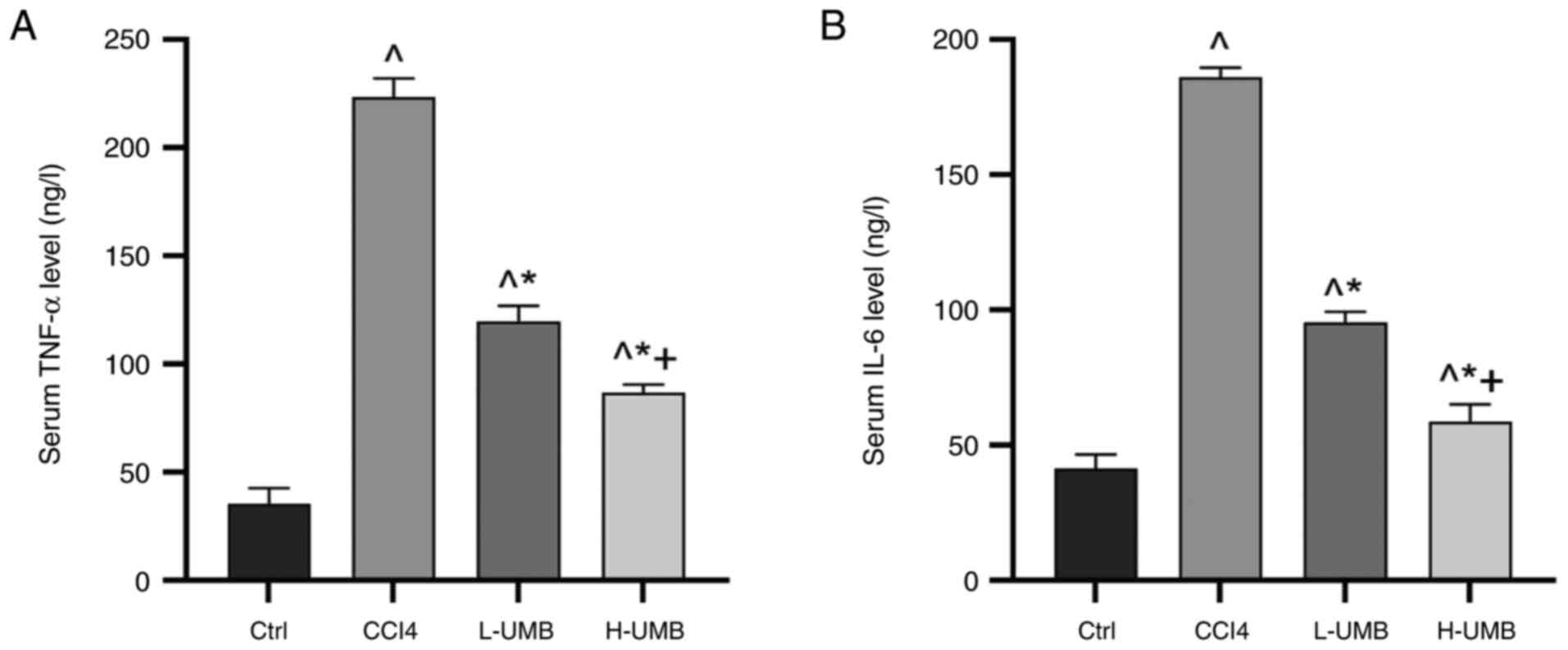
Effects of UMB on inflammation
levels
Inflammatory responses activate HSCs, which regulate
immune responses by secreting chemokines and cytokines, or
transform into myofibroblasts that produce matrix, advancing liver
fibrosis progression (25). The
inflammatory responses associated with HF in the rats was evaluated
using ELISA kits to quantify the serum TNF-α and IL-6
concentrations following UMB treatment. The results demonstrated
that UMB significantly decreased the serum TNF-α and IL-6 levels
dose-dependently compared with the model group (P<0.05; Fig. 2).
Following UMB intervention, a marked reduction in
the levels of pro-inflammatory cytokines TNF-α and IL-6 was
observed, which associated consistently with the diminished
inflammatory cell infiltration evident in liver histopathological
analysis. This concordance between cytokine modulation and
histopathological findings provided evidence supporting the
anti-inflammatory efficacy of UMB to reduce liver damage.
Effects of UMB on liver oxidative
stress
Oxidative stress is pivotal in the pathogenesis of
liver disorders, markedly contributing to hepatic injury and
fibrosis advancement (26).
Hepatocytes combat oxidative stress using enzymatic and
non-enzymatic antioxidant defenses (27). The prominent enzymatic antioxidants
include SOD and CAT, whereas GSH is a crucial non-enzymatic
antioxidant (28). Furthermore,
MDA levels indicate lipid peroxidation (29). Given its recognized antioxidant
capabilities, the present study proposed that UMB might protect rat
livers against fibrosis by leveraging these antioxidant mechanisms.
This hypothesis was substantiated by evaluating the expression
levels of key oxidative stress-associated markers. The results of
the present study demonstrated that UMB notably decreased MDA
levels and dose-dependently increased GSH, SOD and CAT
concentrations significantly compared with the control group
(P<0.05; Fig. 3). These results
highlighted the efficacy of UMB in limiting CCl4-induced
oxidative stress in the liver and mitigating fibrosis, underscoring
its potential to preserve liver function by modulating antioxidant
enzyme activities.
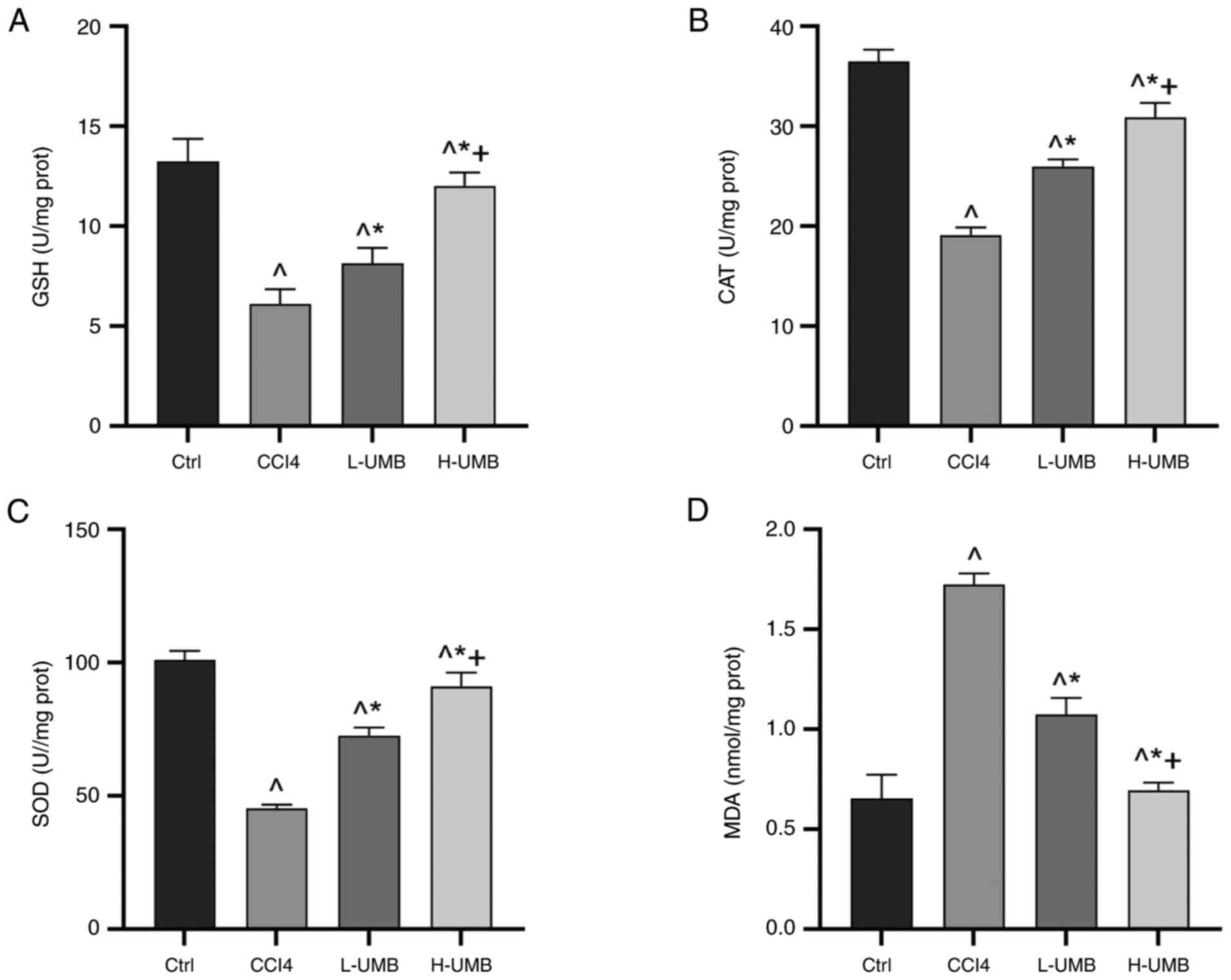 | Figure 3.Effects of UMB on liver oxidative
stress. CCl4-induced rat HF models were treated with 50
or 100 mg/kg/day UMB. Liver tissue levels of (A) GSH, (B) CAT, (C)
SOD and (D) MDA. Results are the mean ± SD (n=8).
^P<0.05 vs. control group; *P<0.05 vs.
CCl4 group; +P<0.05 vs. L-UMB. UMB,
Umbelliferone; HF, hepatic fibrosis; Ctrl, control group;
CCl4, carbon tetrachloride-treated group (model); L-UMB,
low-dose UMB treatment (50 mg/kg); H-UMB, high-dose UMB treatment
(100 mg/kg); GSH, glutathione; SOD, superoxide dismutase; CAT,
catalase; MDA, malondialdehyde. |
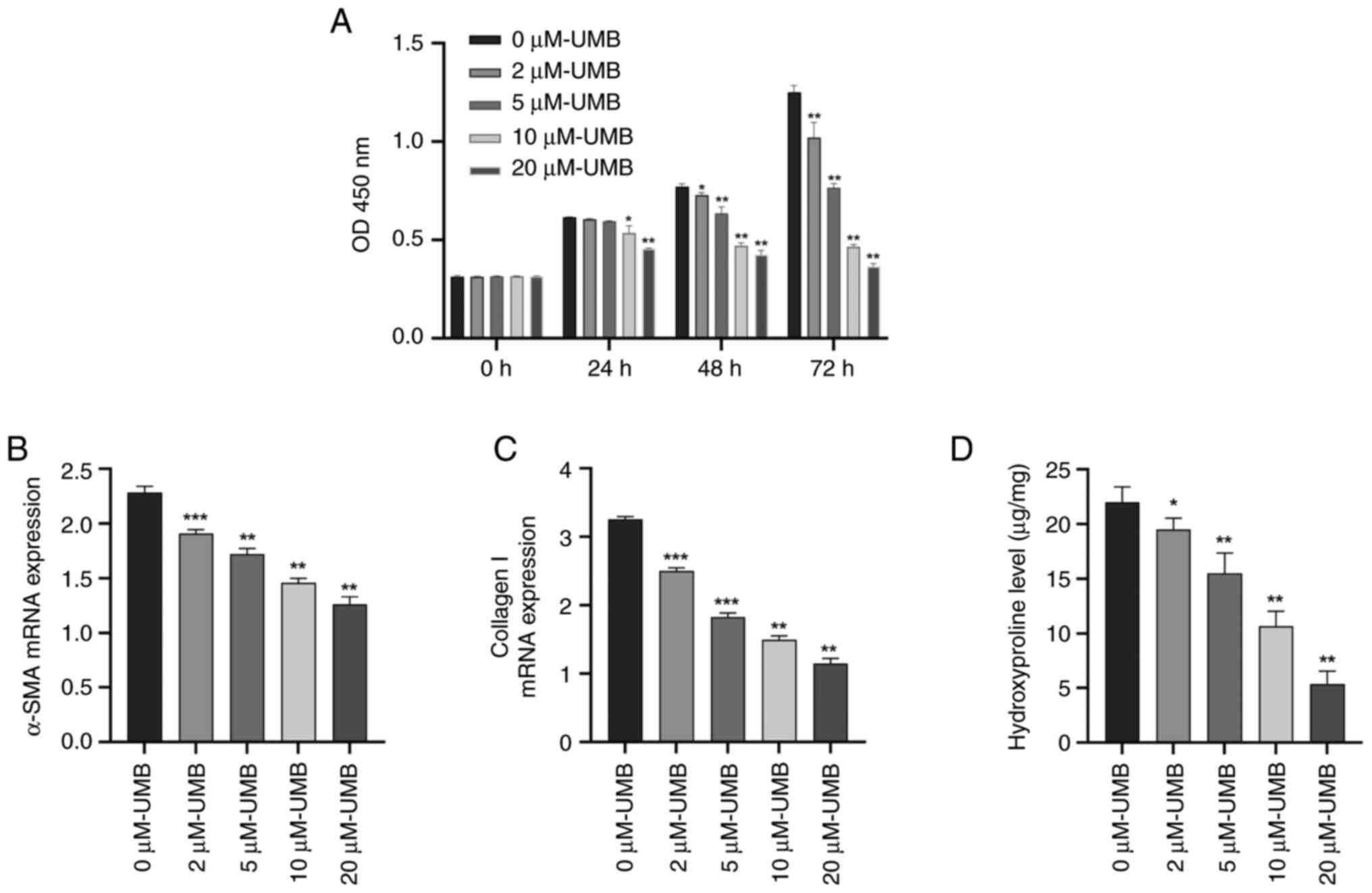
Effects of UMB on the expression of
pro-fibrotic related genes in HF rats
α-SMA and collagen I are pivotal in HSC activation
and the ensuing fibrosis process primarily through their roles in
ECM synthesis (30). Matrix
metalloproteinases (MMPs) and tissue inhibitors of
metalloproteinases (TIMPs) regulate ECM remodeling, with MMP-1 and
TIMP-1 being crucial for maintaining the balance within the liver
matrix (31). The present study
aimed to elucidate the mechanism by which UMB mitigates HF. The
quantitative analysis results of the mRNA expression of these
fibrosis-related markers indicated that α-SMA, collagen I and
TIMP-1 levels were significantly elevated relative to those of the
control group, whereas the model group had markedly reduced MMP-1
levels (P<0.05). Significantly, 50 and 100 mg/kg UMB reversed
these changes dose-dependently (P<0.05; Fig. 4A-D). Furthermore, hydroxyproline
levels, which indicate collagen deposition, were also decreased
significantly in the UMB-treated rat liver tissues, indicating
reduced fibrosis (P<0.05; Fig.
4E). These outcomes suggested that UMB substantially reduced
pro-fibrotic factor expression and effectively counteracted HF
progression.
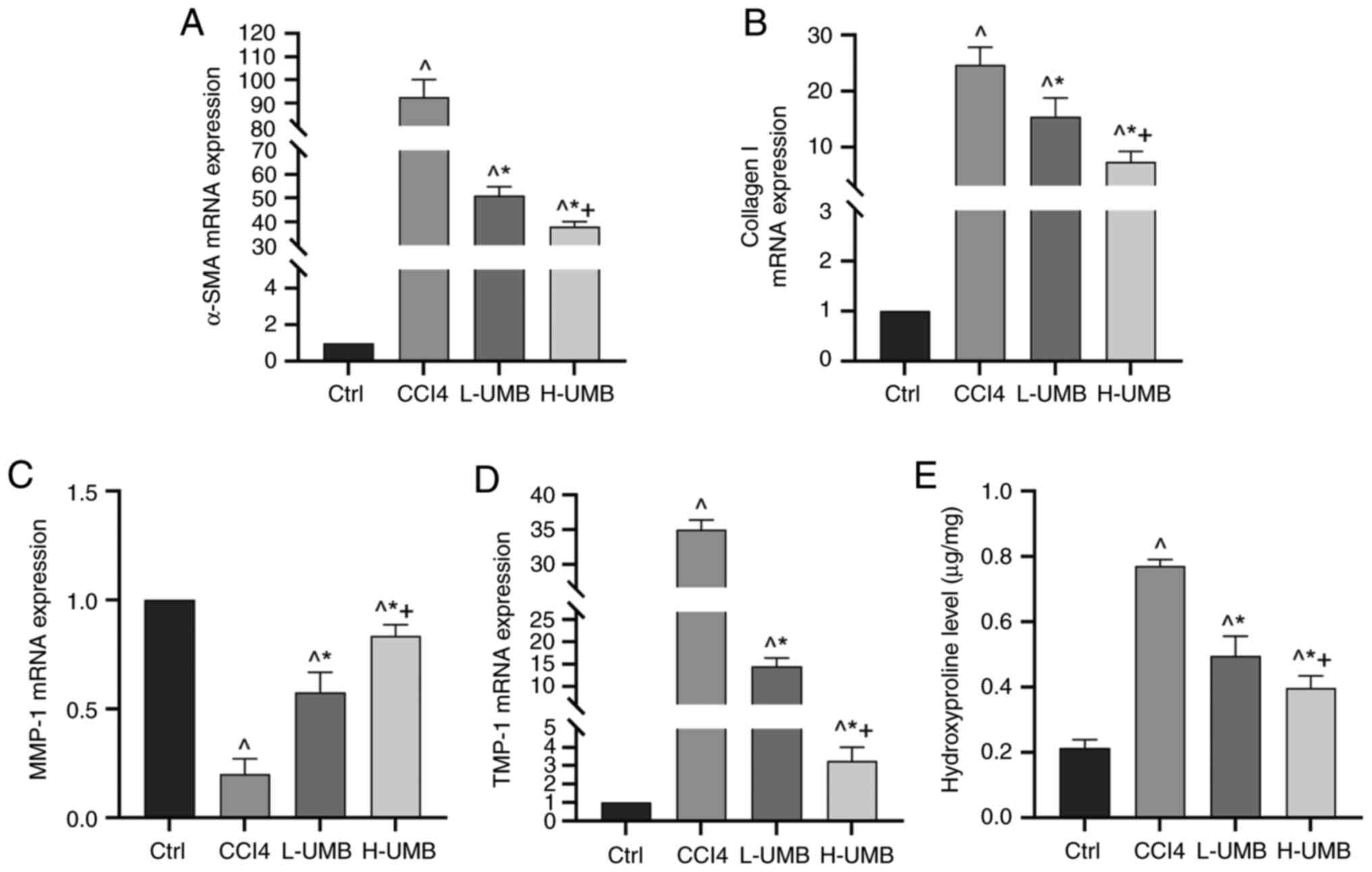 | Figure 4.Effects of UMB on expression of
pro-fibrotic related genes in HF rats. CCl4-induced rat
HF models were treated with 50 or 100 mg/kg/day UMB. mRNA
expression was detected using RT-qPCR. mRNA expression of (A)
α-SMA, (B) collagen I, (C) MMP-1 and (D) TIMP-1. (E) Hydroxyproline
expression. Results are the mean ± SD (n=8). ^P<0.05
vs. control group; *P<0.05 vs. CCl4 group;
+P<0.05 vs. L-UMB. UMB, Umbelliferone; HF, hepatic
fibrosis; RT-qPCR, reverse transcription-quantitative PCR; α-SMA,
alpha smooth muscle actin; MMP-1, matrix metalloproteinase 1;
TIMP-1, tissue inhibitors of metalloproteinase 1; Ctrl, control
group; CCl4, carbon tetrachloride-treated group (model);
L-UMB, low-dose UMB treatment (50 mg/kg); H-UMB, high-dose UMB
treatment (100 mg/kg). |
Effects of UMB on the TGF-β1-Smad2/3
pathway in HF rats
The effects of UMB on HF were assessed by
quantifying the TGF-β1, phosphorylated (p-)Smad2/3 and total
Smad2/3 protein expression levels in rat liver tissues using
western blotting. The results revealed that the model group had
markedly elevated TGF-β1 and p-Smad2/3 protein levels compared with
the control, suggesting enhanced fibrotic activity. Upon UMB
administration, there was a notable dose-dependent decrease in the
expression of these proteins, confirming the anti-fibrotic efficacy
of UMB (P<0.05). However, the UMB treatment did not
significantly alter the total Smad2/3 protein levels, indicating a
selective effect of UMB on the phosphorylated forms of Smad
proteins (P>0.05; Fig. 5).
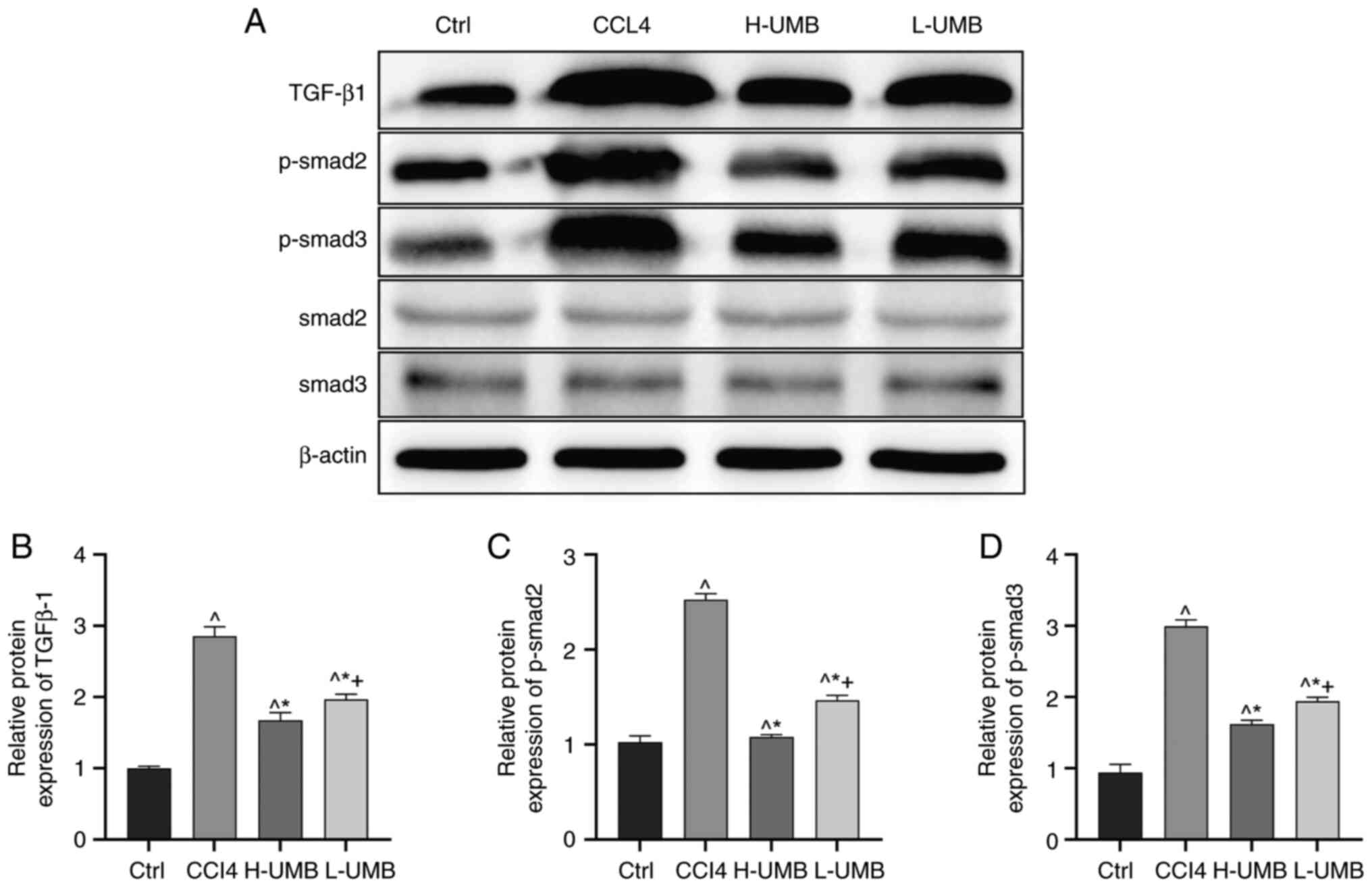 | Figure 5.Effects of UMB on the TGF-β1-Smad2/3
pathway in HF rats. CCl4-induced rat HF models were
treated with 50 or 100 mg/kg/day UMB. (A) Western blot measurement
of TGF-β1, Smad2, Smad3, p-Smad2 and p-Smad3 protein expression
levels. Quantitation of western blot analysis of (B) TGF-β1, (C)
p-Smad2 and (D) p-Smad3. Results are the mean ± SD (n=8).
^P<0.05 vs. control group; *P<0.05 vs.
CCl4 group; +P<0.05 vs. L-UMB. UMB,
Umbelliferone; Ctrl, control group; CCl4, carbon
tetrachloride-treated group (model); L-UMB, low-dose UMB treatment
(50 mg/kg); H-UMB, high-dose UMB treatment (100 mg/kg); p-,
phosphorylated; TGF-β1, transforming growth factor beta 1. |
UMB inhibits HSC activation and
proliferation in vitro
Considering the pivotal role of HSCs in HF onset and
advancement, the present study explored whether the beneficial
effects of UMB on reducing HF might be due to its capability of
suppressing HSC activation. The effects of UMB on HSC proliferation
were assessed using the CCK-8 assay, which indicated that UMB
significantly limited HSC proliferation in a time- and
dose-dependent manner (P<0.05). Notably, the proliferation rates
in the 2 and 5 µM UMB-treated groups did not significantly diverge
from those of the control group (0 µM) at the 24-h mark, indicating
no substantial effect at these lower concentrations (P>0.05;
Fig. 6A). The UMB treatment
dose-dependently suppressed the α-SMA and collagen I mRNA levels of
fibrogenic genes in the HSCs (P<0.05) (Fig. 6B and C). Conversely, an increased
UMB concentration corresponded with a significant downtrend in HSC
hydroxyproline levels (P<0.05; Fig.
6D). This trend underscored the potential of UMB to effectively
mitigate ECM accumulation, thereby impeding HSC proliferation.
Effects of UMB on the TGF-β1-Smad2/3
pathway in HSCs
TGF-β1 is a powerful pro-fibrotic factor essential
for activating HSCs, a process pivotal to HF development (32). The present study aimed to delve
deeper into the mechanisms through which UMB impedes HSC activation
and the resultant fibrosis. Specifically, the present study
examined the modulatory effects of UMB on the TGF-β1 signaling
pathway. It explored the effects of UMB on the expression of
critical proteins in HSCs by administering different doses over
different durations. The results demonstrated that the TGF-β1 and
p-Smad2/3 expression levels in the HSCs paralleled those in the
liver tissue, demonstrating a significant dose-dependent response
(P<0.05). Conversely, the total Smad2/3 expression levels were
not statistically significantly altered (P>0.05; Fig. 7). These results suggested that UMB
potentially modulates the HSC activation state by influencing
TGF-β1 and p-Smad2/3 expression, which may be pivotal in HF
progression.
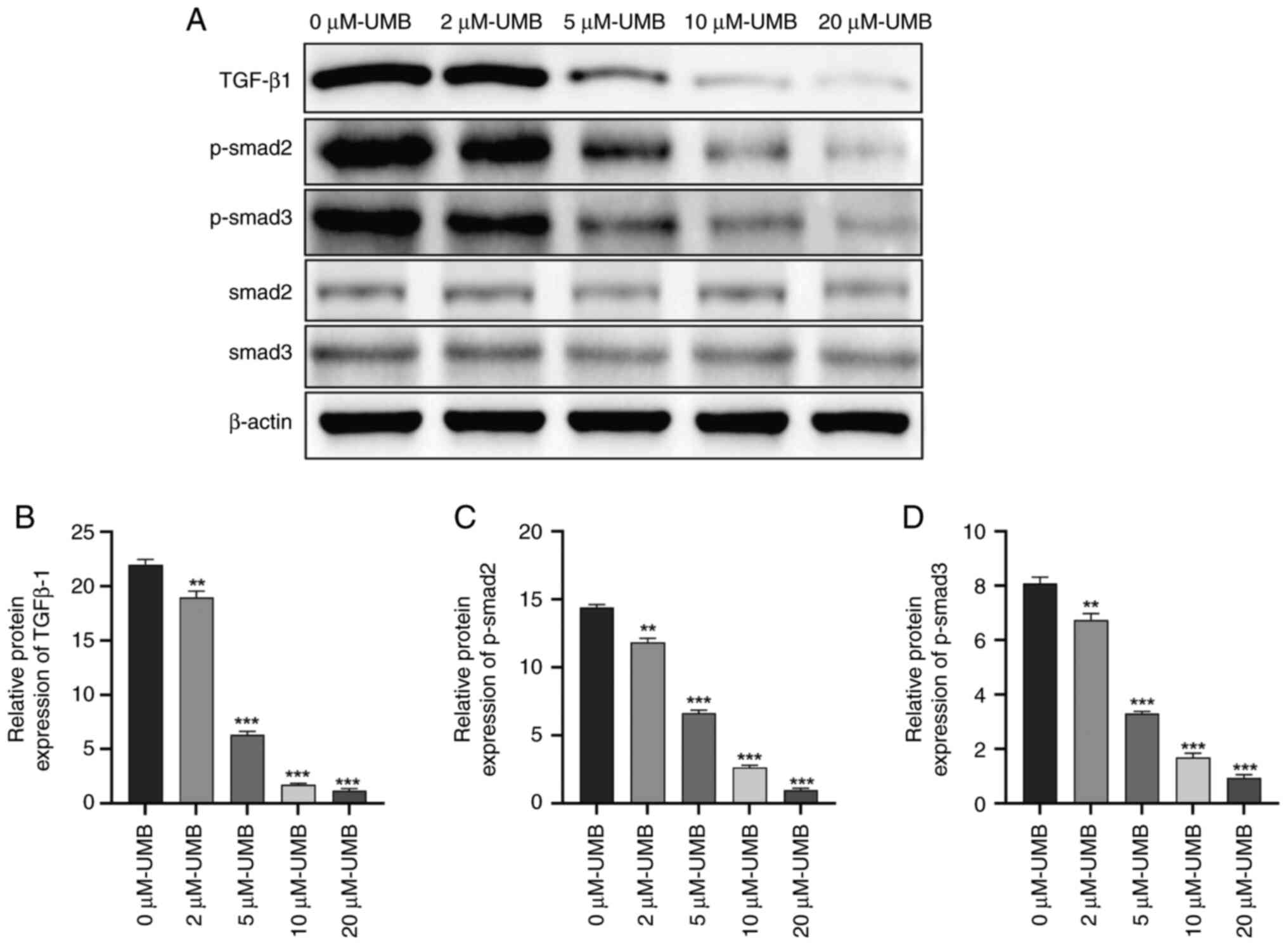 | Figure 7.Effects of UMB on the TGF-β1-Smad2/3
pathway in HSCs. HSCs were treated with 0, 2, 5, 10, or 20 µM UMB
dissolved in DMSO and cultured for 72 h. (A) Western blot
measurement of TGF-β1, Smad2, Smad3, p-Smad2 and p-Smad3 protein
expression levels. Quantitation of western blot analysis of (B)
TGF-β1, (C) p-Smad2 and (D) p-Smad3. Results are the mean ± SD
(n=3). **P<0.01, ***P<0.001 compared with 0 µM UMB. UMB,
Umbelliferone; HSCs, hepatic stellate cells; TGF-β1, transforming
growth factor beta 1; p-, phosphorylated. |
Discussion
HF is a common pathological progression in a number
of chronic liver diseases, where timely and effective intervention
is pivotal for treating these diseases and preventing the onset of
cirrhosis and liver cancer (33).
In various chronic liver conditions, fibrosis is reversible before
it advances to severe cirrhosis. HSCs are specialized mesenchymal
cells in the liver that are crucial in normal physiological and
pathological processes (34). HSCs
triggered by external stimuli transdifferentiate into
myofibroblasts, which significantly contribute to ECM accumulation,
leading to HF (35). UMB is a
natural coumarin from Rutaceae and Apiaceae plants that has
demonstrated efficacy in treating a range of acute and chronic
conditions due to its wide-ranging biological and pharmacological
properties (16). Nevertheless,
its effectiveness against HF has been relatively underexplored. The
present study investigated the protective effects of UMB on
CCl4-induced HF in rats and its mechanisms on primary
cultured HSCs.
Previous studies (36,37)
have demonstrated that UMB intervention promoted the normalization
of liver cell arrangement in fibrotic rats, accompanied by reduced
fibrous tissue and inflammatory cell infiltration. Furthermore,
administering 50 and 100 mg/kg UMB in the present study effectively
decreased the serum TNF-α, IL-6, ALT, AST, TBIL and TBA levels in
the fibrotic models. These observations indicated that UMB may
reduce inflammation in liver tissues and mitigate
fibrosis-associated damage. Consequently, these results underscored
the potential of UMB to slow HF progression and highlighted its
significant hepatoprotective effects.
ECM deposition is the hallmark of HF and is pivotal
in disease progression. Under pathological conditions, TGF-β1
overexpression catalyzes ECM accumulation (38). In this context, α-SMA and collagen
I are critical biomarkers for assessing ECM deposition (39). The interplay between MMPs and their
inhibitors is essential for ECM remodeling, with MMP-1 and TIMP-1
being particularly significant (40). MMP-1 is a principal enzyme in
collagen I and III degradation and facilitates ECM breakdown
(41). Strategic modulation that
increases MMP-1 and decreases TIMP-1 markedly promotes ECM
degradation, mitigating HF in rat models (42). In the present study, the fibrotic
model rats exhibited elevated levels of pro-fibrotic factors
(TGF-β1, α-SMA and TIMP-1) and notably reduced MMP-1 expression.
Conversely, these trends were reversed in the UMB-treated groups.
In summary, the findings demonstrated that UMB exerts a
dose-dependent reduction in the expression of fibrotic gene
markers. For example, curcumin appears to directly inhibit HSCs
activation, as evidenced by its ability to downregulate key
fibrotic markers such as α-SMA and collagen I, consistent with
previous studies (25). In
addition, Berberine may also modulate the hepatic microenvironment
through its anti-inflammatory properties, reducing the release of
pro-inflammatory cytokines and regulating Kupffer cell activity,
thereby indirectly attenuating HSC activation (17). These dual mechanisms highlight the
multifaceted anti-fibrotic potential of UMB, suggesting that its
therapeutic effects are probably mediated through a combination of
direct actions on HSCs and broader modulation of the liver
microenvironment.
Oxidative stress represents an imbalance between
oxidative mechanisms and antioxidant processes in the body,
contributing significantly to the development of conditions such as
atherosclerosis and fibrosis (43). Oxidative stress is pivotal in HF,
where excess free radicals inflict chemical damage on lipids,
proteins and carbohydrates (44).
This damage disrupts cellular metabolism, leading to liver cell
injury and fibrosis progression (45). The present study delved into the
effects of oxidative stress on HF by analyzing oxidative stress
markers. The rats with fibrosis exhibited reduced GSH, CAT and SOD
levels and markedly increased MDA, which indicated lipid
peroxidation caused by free radicals. Conversely, UMB markedly
increased antioxidant levels and significantly reduced MDA levels,
demonstrating its potential protective effects against
CCl4-induced HF. These results underscored the
antioxidative properties of UMB in counteracting HF.
TGF-β1 is pivotal in promoting fibrosis and is
closely linked to HF onset and progression. As downstream effectors
of TGF-β1, Smad2/3 proteins enhance TGF-β1-induced HF through their
phosphorylation processes. TGF-β1 activates the Smad protein
signaling cascade upon binding to its specific receptors on the
liver cell surface (45). This
activation triggers apoptosis and accelerates ECM synthesis while
concurrently inhibiting its degradation, thereby intensifying HF
(11). Consequently, inhibiting
the TGF-β1-Smad signaling pathway presents a dual therapeutic
advantage: it reduces ECM formation and attenuates HF (46). The present study revealed that the
NF-κB pathway acted in concert with TGF-β1 signaling and
pharmacological inhibition of NF-κB markedly attenuated
TGF-β1-mediated hepatic fibrosis (47). In addition, there is functional
interplay between autophagy and TGF-β1 signaling, showing that
augmented autophagy alleviated TGF-β1-driven hepatic fibrosis
through the clearance of impaired organelles and protein aggregates
(48). The experimental results
supported this mechanism, demonstrating significantly elevated
TGF-β1 and p-Smad2/3 levels in the model group, which UMB treatment
substantially reversed. The reduction in p-Smad2/3 levels primarily
reflected the inhibition of the TGF-β1 pathway. However, UMB can
modulate inflammatory pathways, oxidative stress and other fibrotic
signaling cascades, which may synergistically contribute to its
overall antifibrotic effects.
Upon liver damage, the injured epithelial cells and
fibrotic tissues activate HSCs, prompting their transformation into
a myofibroblast phenotype that produces significant quantities of
ECM (49). This shift from
balanced ECM synthesis to degradation fosters scar tissue
development, culminating in HF (50). In vitro studies have
demonstrated that UMB inhibits HSC proliferation and reduces
hydroxyproline production, decreasing the TGF-β1, α-SMA, collagen I
and p-Smad2/3 expression levels in the supernatant of these cells.
This evidence suggests that UMB effectively prevents TGF-β1
activation, halts HSC conversion into contractile myofibroblasts
and suppresses ECM component secretion (51). Consequently, UMB appears to
mitigate inflammatory damage in liver cells, inhibit HSC activation
and decrease fibrogenic factor synthesis and secretion by
inhibiting the TGF-β1-Smad signaling pathway, thereby offering a
promising intervention for HF.
The present study demonstrated that UMB effectively
reversed HF by modulating the TGF-β1-Smad signaling pathway and its
associated factors, underscoring its capacity to halt fibrosis
through several pathways and targets. The mechanism by which UMB
intervenes in HF appears to involve TGF-β1-Smad pathway regulation
through the inhibition of Smad2/3 protein phosphorylation and mRNA
expression. Consequently, this inhibition suppresses HSC activation
and proliferation, manages collagen metabolism and decreases
oxidative stress in liver tissue. These regulatory effects
contribute to reversing HF progression, offering innovative
perspectives and methodologies for TCM treatments and the
development of new clinical drugs.
Acknowledgements
Not applicable.
Funding
The present study was funded by The Natural Science Foundation
of China (grant nos. 82160794 and 82160703); Major Project of
Natural Science Foundation of Inner Mongolia Autonomous Region
(grant no. 2023ZD15); Science and Technology Program of the Joint
Fund of Scientific Research for the Public Hospitals of Inner
Mongolia Academy of Medical Sciences (grant nos. 2024GLLH0290 and
2024GLLH0404); Program for Young Talents of Science and Technology
in Universities of Inner Mongolia Autonomous Region (grant no.
NJYT23114); Key Program of Inner Mongolia Medical University (grant
no. YKD2022ZD013); Health Science and Technology Program of Inner
Mongolia Health Commission (grant nos. 202201238 and 202202158);
PhD Initial Funding Project of the Affiliated Hospital of Inner
Mongolia Medical University (grant no. NYFY BS 202120); Hohhot
Municipal Health and Wellness Young Talent Technology Project
(grant nos. 2023012); Inner Mongolia Autonomous Region Health and
Wellness Traditional Chinese Medicine (Mongolian Medicine)
Technology Program Project (grant no. ZMY2023201); Standardization
Project of Mongolian Medicine in Inner Mongolia Autonomous Region
(grant no. 2023MB020); Inner Mongolia Autonomous Region Traditional
Chinese Medicine (Mongolian Medicine) Young and Middle-aged Leading
Talent Cultivation Project (grant no. 2022RC011); Hohhot Municipal
Science and Technology Program Project (grant no. 2023SHE24);
Special Fund for Science and Technology: Central Guidance Fund for
Local Science and Technology Development (grant no.
ZY20200071).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LB and LW conceived and designed the research; LL,
ZD, YZ, ZS and WY performed the experiments and analyzed the data.
LB drafted the manuscript. LL and ZD confirm the authenticity of
all the raw data. All authors read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
All experiments in the present study were approved
by The Ethics Committee of Inner Mongolia Medical University
(Hohhot, China; approval no. YKD202201124).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HF
|
hepatic fibrosis
|
|
HSCs
|
hepatic stellate cells
|
|
UMB
|
Umbelliferone
|
|
CCl4
|
carbon tetrachloride
|
|
ECM
|
extracellular matrix
|
|
TGF-β1
|
transforming growth factor beta 1
|
|
TNF-α
|
tumor necrosis factor alpha
|
|
α-SMA
|
alpha smooth muscle actin
|
|
TCM
|
Traditional Chinese medicine
|
|
IL
|
interleukin
|
|
AST
|
aspartate aminotransferase
|
|
ALT
|
alanine aminotransferase
|
|
TBA
|
total bile acids
|
|
TBIL
|
total bilirubin
|
|
MDA
|
malondialdehyde
|
|
CAT
|
catalase
|
|
GSH
|
glutathione
|
|
SOD
|
superoxide dismutase
|
|
MMP-1
|
matrix metalloproteinase 1
|
|
TIMP-1
|
tissue inhibitors of metalloproteinase
1
|
References
|
1
|
Pimpin L, Cortez-Pinto H, Negro F,
Corbould E, Lazarus JV, Webber L and Sheron N; EASL HEPAHEALTH
Steering Committee, : Burden of liver disease in Europe:
Epidemiology and analysis of risk factors to identify prevention
policies. J Hepatol. 69:718–735. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Caligiuri A, Gentilini A, Pastore M, Gitto
S and Marra F: Cellular and molecular mechanisms underlying liver
fibrosis regression. Cells. 10:27592021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dawood RM, El-Meguid MA, Salum GM and El
Awady MK: Key players of hepatic fibrosis. J Interferon Cytokine
Res. 40:472–489. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khanam A, Saleeb PG and Kottilil S:
Pathophysiology and treatment options for hepatic fibrosis: Can it
be completely cured? Cells. 10:10972021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Friedman SL: Hepatic fibrosis-overview.
Toxicology. 254:120–129. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ezhilarasan D, Sokal E and Najimi M:
Hepatic fibrosis: It is time to go with hepatic stellate
cell-specific therapeutic targets. Hepatobiliary Pancreat Dis Int.
17:192–197. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang Y, Deng X and Liang J: Modulation of
hepatic stellate cells and reversibility of hepatic fibrosis. Exp
Cell Res. 352:420–426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang X, Zeng Y, Zhao L, Xu Q, Miao D and
Yu F: Targeting hepatic stellate cell death to reverse hepatic
fibrosis. Curr Drug Targets. 24:568–583. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Albanis E and Friedman SL: Hepatic
fibrosis. Pathogenesis and principles of therapy. Clin Liver Dis.
5:315–334. v–vi. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brenner DA, Waterboer T, Choi SK,
Lindquist JN, Stefanovic B, Burchardt E, Yamauchi M, Gillan A and
Rippe RA: New aspects of hepatic fibrosis. J Hepatol. 32 (1
Suppl):S32–S38. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gressner AM, Weiskirchen R, Breitkopf K
and Dooley S: Roles of TGF-beta in hepatic fibrosis. Front Biosci.
7:d793–d807. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu HH, Chen DQ, Wang YN, Feng YL, Cao G,
Vaziri ND and Zhao YY: New insights into TGF-β/Smad signaling in
tissue fibrosis. Chem Biol Interact. 292:76–83. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu F, Liu C, Zhou D and Zhang L:
TGF-β/SMAD pathway and its regulation in hepatic fibrosis. J
Histochem Cytochem. 64:157–167. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Smith ME and Bauer-Wu S: Traditional
Chinese medicine for cancer-related symptoms. Semin Oncol Nurs.
28:64–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shan L, Liu Z, Ci L, Shuai C, Lv X and Li
J: Research progress on the anti-hepatic fibrosis action and
mechanism of natural products. Int Immunopharmacol. 75:1057652019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kornicka A, Balewski Ł, Lahutta M and
Kokoszka J: Umbelliferone and its synthetic derivatives as suitable
molecules for the development of agents with biological activities:
A review of their pharmacological and therapeutic potential.
Pharmaceuticals (Basel). 16:17322023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kassim NK, Rahmani M, Ismail A, Sukari MA,
Ee GC, Nasir NM and Awang K: Antioxidant activity-guided separation
of coumarins and lignan from Melicope glabra (Rutaceae). Food Chem.
139:87–92. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hassanein EHM, Hader HF, Elmansy RA,
Seleem HS, Elfiky M, Mohammedsaleh ZM, Ali FEM and Abd-Elhamid TH:
Umbelliferone alleviates hepatic ischemia/reperfusion-induced
oxidative stress injury via targeting Keap-1/Nrf-2/ARE and
TLR4/NF-κB-p65 signaling pathway. Environ Sci Pollut Res Int.
28:67863–67879. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu SM, Hu DH and Zhang JJ: Umbelliferone
exhibits anticancer activity via the induction of apoptosis and
cell cycle arrest in HepG2 hepatocellular carcinoma cells. Mol Med
Rep. 12:3869–3873. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vázquez JJ: Ground-glass hepatocytes:
light and electron microscopy. Characterization of the different
types. Histol Histopathol. 5:379–386. 1990.PubMed/NCBI
|
|
22
|
Iezzoni JC: Diagnostic histochemistry in
hepatic pathology. Semin Diagn Pathol. 35:381–389. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sharma P: Value of liver function tests in
cirrhosis. J Clin Exp Hepatol. 12:948–964. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Farooqui N and Elhence A: Shalimar: A
current understanding of bile acids in chronic liver disease. J
Clin Exp Hepatol. 12:155–173. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Koyama Y and Brenner DA: Liver
inflammation and fibrosis. J Clin Invest. 127:55–64. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Finkel T: Oxidant signals and oxidative
stress. Curr Opin Cell Biol. 15:247–254. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sánchez-Valle V, Chávez-Tapia NC, Uribe M
and Méndez-Sánchez N: Role of oxidative stress and molecular
changes in liver fibrosis: A review. Curr Med Chem. 19:4850–4860.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Foyer CH and Noctor G: Redox homeostasis
and antioxidant signaling: A metabolic interface between stress
perception and physiological responses. Plant Cell. 17:1866–1875.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tsikas D: Assessment of lipid peroxidation
by measuring malondialdehyde (MDA) and relatives in biological
samples: Analytical and biological challenges. Anal Biochem.
524:13–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Y, Fan W, Link F, Wang S and Dooley S:
Transforming growth factor β latency: A mechanism of cytokine
storage and signalling regulation in liver homeostasis and disease.
JHEP Rep. 4:1003972022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Naim A, Pan Q and Baig MS: Matrix
metalloproteinases (MMPs) in liver diseases. J Clin Exp Hepatol.
7:367–372. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dewidar B, Meyer C, Dooley S and
Meindl-Beinker AN: TGF-β in hepatic stellate cell activation and
liver fibrogenesis-updated 2019. Cells. 8:14192019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dhar D, Baglieri J, Kisseleva T and
Brenner DA: Mechanisms of liver fibrosis and its role in liver
cancer. Exp Biol Med (Maywood). 245:96–108. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tsuchida T and Friedman SL: Mechanisms of
hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol.
14:397–411. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang M, Serna-Salas S, Damba T, Borghesan
M, Demaria M and Moshage H: Hepatic stellate cell senescence in
liver fibrosis: Characteristics, mechanisms and perspectives. Mech
Ageing Dev. 199:1115722021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mahmoud AM, Hozayen WG, Hasan IH, Shaban E
and Bin-Jumah M: Umbelliferone ameliorates CCl4-induced liver
fibrosis in rats by upregulating PPARγ and attenuating oxidative
stress, inflammation, and TGF-β1/Smad3 signaling. Inflammation.
42:1103–1116. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sim MO, Lee HI, Ham JR, Seo KI, Kim MJ and
Lee MK: Anti-inflammatory and antioxidant effects of umbelliferone
in chronic alcohol-fed rats. Nutr Res Pract. 9:364–369. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang M and Zhang ST: Cells in fibrosis
and fibrotic diseases. Front Immunol. 11:11422020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schuppan D, Ashfaq-Khan M, Yang AT and Kim
YO: Liver fibrosis: Direct antifibrotic agents and targeted
therapies. Matrix Biol. 68–69. 435–451. 2018.
|
|
40
|
Cui N, Hu M and Khalil RA: Biochemical and
biological attributes of matrix metalloproteinases. Prog Mol Biol
Transl Sci. 147:1–73. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Arakaki PA, Marques MR and Santos MC:
MMP-1 polymorphism and its relationship to pathological processes.
J Biosci. 34:313–320. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Iredale JP: Tissue inhibitors of
metalloproteinases in liver fibrosis. Int J Biochem Cell Biol.
29:43–54. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gorrini C, Harris IS and Mak TW:
Modulation of oxidative stress as an anticancer strategy. Nat Rev
Drug Discov. 12:931–947. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li S, Tan HY, Wang N, Zhang ZJ, Lao L,
Wong CW and Feng Y: The role of oxidative stress and antioxidants
in liver diseases. Int J Mol Sci. 16:26087–26124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Baghy K, Iozzo RV and Kovalszky I:
Decorin-TGFβ axis in hepatic fibrosis and cirrhosis. J Histochem
Cytochem. 60:262–268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Meng XM, Nikolic-Paterson DJ and Lan HY:
TGF-β: the master regulator of fibrosis. Nat Rev Nephrol.
12:325–338. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang K, Fang S, Liu Q, Gao J, Wang X, Zhu
H, Zhu Z, Ji F, Wu J, Ma Y, et al: TGF-β1/p65/MAT2A pathway
regulates liver fibrogenesis via intracellular SAM. EBioMedicine.
42:458–469. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Filali-Mouncef Y, Hunter C, Roccio F,
Zagjou S, Dupont N, Primard C, Proikas-Cezann T and Reggiori F: The
ménage à trois of autophagy, lipid droplets and liver disease.
Autophagy. 18:50–72. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
De Oliveira da Silva B, Ramos LF and
Moraes KCM: Molecular interplays in hepatic stellate cells:
Apoptosis, senescence, and phenotype reversion as cellular
connections that modulate liver fibrosis. Cell Biol Int.
41:946–959. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tacke F and Weiskirchen R: Update on
hepatic stellate cells: Pathogenic role in liver fibrosis and novel
isolation techniques. Expert Rev Gastroenterol Hepatol. 6:67–80.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Weiskirchen R and Tacke F: Cellular and
molecular functions of hepatic stellate cells in inflammatory
responses and liver immunology. Hepatobiliary Surg Nutr. 3:344–363.
2014.PubMed/NCBI
|