Introduction
The Bcl-2 protein family constitutes a key group of
regulators that maintain cellular homeostasis by modulating both
autophagy and apoptosis (1). This
family is classified into anti-apoptotic proteins, including Bcl-2,
Bcl-xl and myeloid cell leukemia 1 (Mcl-1), and pro-apoptotic
proteins, such as Bax, Bcl-2 homologous antagonist/killer (Bak) and
BH3 interacting-domain death agonist (2) (Table
I). This classification underscores their opposing roles in
cell survival and programmed cell death. Anti-apoptotic proteins
inhibit mitochondrial outer membrane permeabilization (MOMP) and
suppress caspase activation, thereby preventing apoptosis. By
contrast, pro-apoptotic proteins promote MOMP and facilitate
apoptotic cascades, leading to cell death. The dynamic equilibrium
between these determines cellular fate under stress conditions
(3–6).
 | Table I.Family members of Bcl-2. |
Table I.
Family members of Bcl-2.
| Effect on
apoptosis | Protein |
| Function | Key
interactions |
|---|
| Anti-apoptotic | Bcl-2 |
| Inhibits apoptosis,
promotes cell survival | Bax, Bak, Beclin
1 |
|
| Bcl-xl |
| Prevents apoptosis,
maintains mitochondrial integrity | Bax, Bak, Bad |
|
| Mcl-1 |
| Supports cell
survival | Bax, Bak, BH3- |
|
|
|
|
| only proteins |
|
| Bcl-w |
| Inhibits apoptosis,
supports neuronal survival | Bax, Bak |
|
| A1/Bfl-1 |
| Promotes cell
survival, especially in immune cells |
|
| Pro-apoptotic | Multi domain | Bax | Oligomerizes in the
cytosol upon activation, translocates to mitochondria to induce
MOMP and initiate apoptosis | Bcl-2, Bcl-xl,
Bak |
|
|
| Bak | Constitutively
anchored in the mitochondrial membrane; triggers MOMP upon
activation and promotes apoptosis in cooperation with Bax | Bcl-2, Bcl-xl,
Bax |
|
| BH3 only | Bid | Activates Bax and
Bak, promotes apoptosis | Bcl-2, Bcl-xl |
|
|
| Bim | Activates Bax and
Bak, inhibits Bcl-2 and Bcl-xl |
|
|
|
| Bad | Interacts with
anti-apoptotic proteins to promote apoptosis |
|
|
|
| Noxa | Targets Mcl-1 for
degradation, induces apoptosis | Mcl-1 |
|
|
| Puma | Inhibits Bcl-2
family proteins, induces apoptosis | Bcl-2, Bcl-xl,
Mcl-1 |
|
|
| Bnip3 | Regulates autophagy
and apoptosis | Beclin 1,
Bcl-2 |
|
|
| Bik | Promotes apoptosis
by interacting with anti-apoptotic proteins | Bcl-2, Bcl-xl |
Autophagy and apoptosis are key for cellular
integrity. Autophagy, a catabolic pathway, degrades and recycles
damaged organelles and misfolded proteins to sustain cell energy
levels and homeostasis (7,8). Conversely, apoptosis eliminates
irreversibly damaged or dysfunctional cells, thereby preventing
harm to the organism (9,10). While these processes are distinct,
they share regulatory molecules, including Bcl-2 family proteins,
and their interplay is key for cell decision-making under stress
conditions (11).
The role of Bcl-2 is multifaceted. Bcl-2 inhibits
autophagy by binding to Beclin 1, a key autophagy regulator,
thereby suppressing autophagic flux initiation. Simultaneously, it
prevents apoptosis by interacting with pro-apoptotic proteins such
as Bax and Bak. However, under severe or prolonged stress,
post-translational modifications (PTMs), including phosphorylation,
may disrupt these interactions, shifting the balance toward either
autophagy or apoptosis (12–14).
Dysregulation of Bcl-2-mediated pathways is implicated in various
pathological conditions: In cancer, Bcl-2 upregulation enhances
tumor survival by preventing apoptosis and suppressing autophagy,
contributing to chemotherapy resistance (15). Conversely, in neurodegenerative
disorders, impaired regulation of autophagy and apoptosis
accelerates neuronal loss. These pathological contexts highlight
the key role of Bcl-2 in coordinating the transition between
autophagy and apoptosis (16,17).
The present review aimed to provide a comprehensive
analysis of the mechanisms by which Bcl-2 regulates autophagy and
apoptosis. By examining its molecular interactions, pathological
implications and potential as a therapeutic target, the present
review aimed to elucidate the central role of Bcl-2 in cellular
homeostasis and disease pathogenesis.
Database search
The present review was conducted through a
systematic literature search in PubMed (pubmed.ncbi.nlm.nih.gov/), Google Scholar (scholar.google.com/) and Web of Science (https://www.webofscience.com/wos/). The search
covered publications from January 2014 to December 2024. The
following search terms were: ‘Bcl-2 AND autophagy’, ‘Bcl-2 AND
apoptosis’, ‘Bcl-2 AND Beclin 1’, ‘Bcl-2 AND mitophagy’, ‘Bcl-2 AND
cancer resistance’, ‘Bcl-2 AND neurodegeneration’, ‘Bcl-2
inhibitors AND therapy’ and ‘Bcl-2 AND post-translational
modifications’. Only peer-reviewed articles published in English
were selected. Eligible studies included review articles and
preclinical and clinical studies; non-peer-reviewed sources (for
example, preprints, conference abstracts, editorials) and articles
without a primary focus on Bcl-2 regulatory functions were
excluded. Reference lists of studies were manually searched to
identify additional relevant literature that may have been missed
in the initial search.
Mechanisms of Bcl-2 in regulating
autophagy
Bcl-2 and autophagy regulation
Bcl-2 regulates autophagy through its interactions
with Beclin 1 and mitochondrial quality control mechanisms
(18). The inhibition of autophagy
by Bcl-2 primarily occurs via direct binding to the BH3 domain of
Beclin 1, a key autophagy regulator (13). This interaction prevents Beclin 1
from initiating autophagosome formation, thereby restricting
autophagic activity under physiological conditions (14). However, under metabolic or
oxidative stress, PTMs, such as phosphorylation at serine 70
(Ser70), disrupt this interaction, enabling Beclin 1 to activate
autophagy (19–21) (Fig.
1).
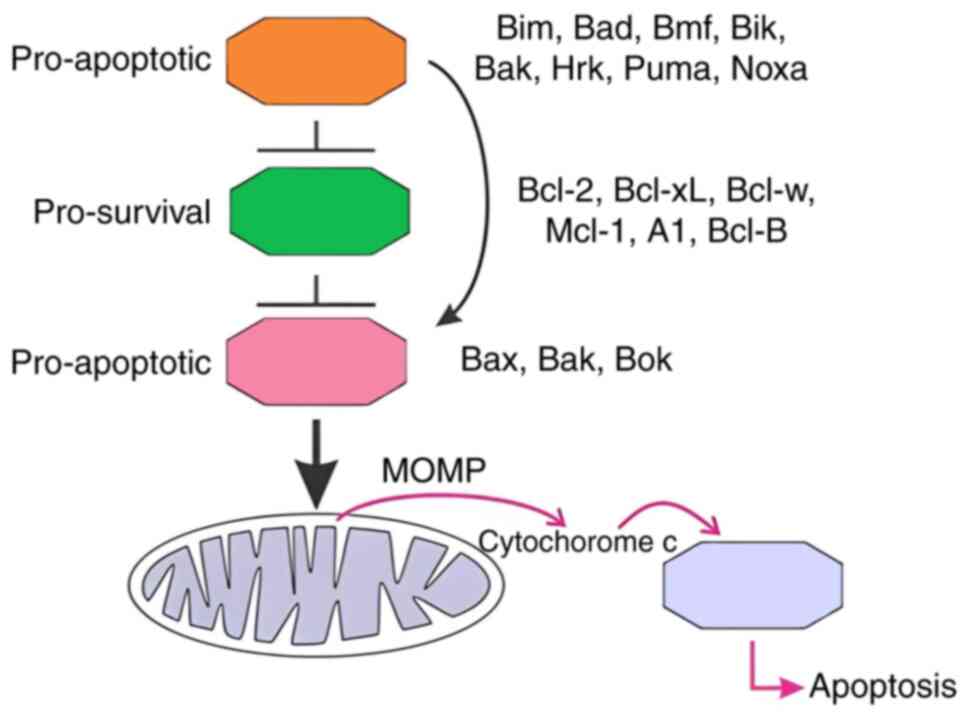 | Figure 1.Role of the Bcl-2 protein family in
regulating apoptosis. Bcl-2 family regulates cell survival or
death. BH3-only proteins (Bim, Bad, Puma) interact with and inhibit
anti-apoptotic proteins (Bcl-2, Bcl-xL), enabling activation of
pro-apoptotic effector proteins (Bax and Bak). These proteins
facilitate MOMP by forming pores in the mitochondrial outer
membrane. This results in the release of cytochrome c, which binds
apoptotic protease activating factor-1 and activates the
apoptosome, ultimately leading to apoptosis. MOMP, mitochondrial
outer membrane permeabilization; BH3-only, Bcl-2 homology 3-only;
Bcl-w, Bcl-2-like protein W; Mcl-1, myeloid cell leukemia 1; A1,
Bcl-2-related protein A1; Bok, Bcl-2-related ovarian killer; Bim,
Bcl-2-like protein 11; Bad, Bmf, Bcl-2-modifying factor; Bik, Bcl-2
interacting killer; Bak, Bcl-2 homologous antagonist/killer; Hrk,
harakiri. Diagram taken from the study by Banjara et al
(6) with permission. |
Bcl-2 regulates mitophagy, the selective degradation
of dysfunctional mitochondria. It modulates MOMP, thereby
controlling the release of mitochondrial quality control factors
such as PTEN-induced kinase 1 and Parkin (22,23).
These proteins coordinate mitochondrial tagging for degradation,
ensuring the elimination of damaged mitochondria while preventing
excessive mitophagy, which could compromise cellular bioenergetics
(24).
This dual regulatory function positions Bcl-2 as a
key integrator of cellular stress signals (Fig. 2). Under nutrient deprivation, AMPK
activation phosphorylates Bcl-2, leading to its dissociation from
Beclin 1, facilitating autophagy (25,26).
Conversely, under nutrient-rich conditions, mTOR activation
stabilizes the Bcl-2-Beclin 1 complex, thereby inhibiting autophagy
and promoting cell survival. Furthermore, oxidative stress-induced
phosphorylation of Bcl-2 determines whether the balance shifts
toward autophagy or apoptosis (27,28).
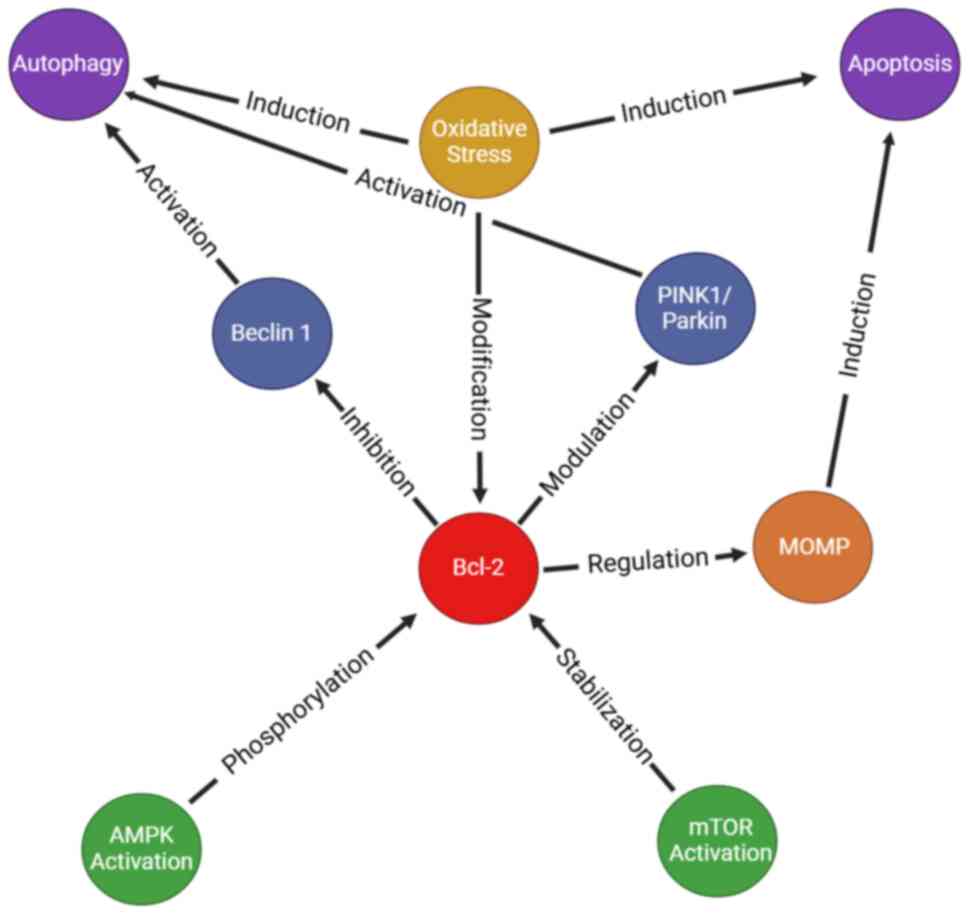 | Figure 2.Bcl-2 regulation in autophagy and
apoptosis. Bcl-2 regulates the balance between autophagy and
apoptosis by integrating cellular signals. It inhibits autophagy by
binding Beclin1, preventing autophagosome formation. Under
metabolic stress, AMPK activation phosphorylates Bcl-2, inhibiting
its interaction with Beclin 1 and promoting autophagy. mTOR
activation stabilizes Bcl-2, suppressing autophagy. Bcl-2 modulates
mitophagy by regulating PINK1 and Parkin, ensuring mitochondrial
quality control. In response to severe stress, MOMP triggers
apoptosis, with Bcl-2 acting as a key regulator. Oxidative stress
modifies Bcl-2 via post-translational modifications, influencing
the shift between autophagy and apoptosis. PINK1, PTEN-induced
kinase 1; MOMP, mitochondrial outer membrane permeabilization;
Parkin, E3 ubiquitin ligase. This image was created with BioRender.com. |
PTMs of Bcl-2
PTMs of Bcl-2 serve a key role in modulating its
interactions and function, allowing for precise regulation of
autophagy and apoptosis. Phosphorylation at residues such as Ser70
enhances dissociation of Bcl-2 from Beclin 1, thereby promoting
autophagy in response to stress, including nutrient deprivation or
oxidative damage. Conversely, phosphorylation at alternative sites
may stabilize interactions of Bcl-2 with pro-apoptotic proteins,
further suppressing apoptosis (29,30).
Caspase-mediated cleavage of Bcl-2 during apoptosis
is another key PTM, converting Bcl-2 from an anti-apoptotic protein
into a pro-apoptotic fragment that amplifies apoptotic signaling
(31,32). Additionally, ubiquitination
regulates proteasomal degradation of Bcl-2, influencing its
intracellular levels and functional activity. Sumoylation and
acetylation are key PTMs that modulate interactions of Bcl-2 with
mitochondrial and cytosolic targets, further underscoring the
complexity and context-dependent nature of its regulation (33–35)
(Fig. 3).
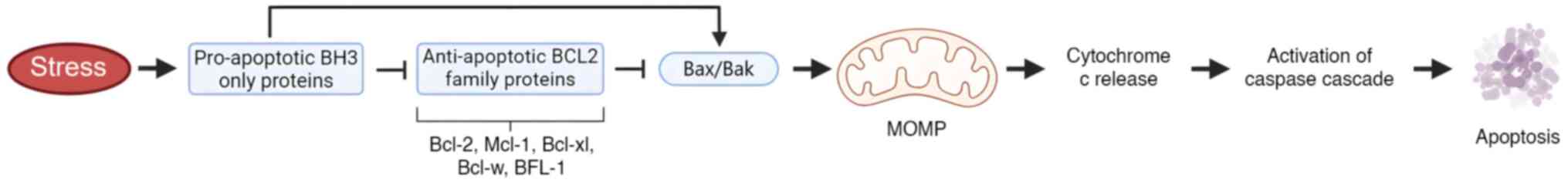 | Figure 3.Regulation of Bcl-2 via PTMs. Bcl-2
is regulated by PTMs such as phosphorylation, ubiquitination and
caspase cleavage. Phosphorylation at Ser70 promotes autophagy by
dissociating Bcl-2 from Beclin 1, while other phosphorylation sites
stabilize interactions with pro-apoptotic proteins, suppressing
apoptosis. Caspase cleavage converts Bcl-2 into a pro-apoptotic
fragment, amplifying apoptosis. Ubiquitination controls its
degradation, influencing cellular levels. PTM, post-translational
modification; MOMP, mitochondrial outer membrane permeabilization;
Mcl-1, Myeloid cell leukemia 1; Bak, Bcl-2 homologous
antagonist/killer; Bcl-w, Bcl-2-like protein W; BFL-1,
Bcl-2-related protein A1. This image was created with BioRender.com. |
Role of Bcl-2 in nutrient sensing and
metabolic regulation
Bcl-2 serves a key role in nutrient sensing and
metabolic regulation. Through its interactions with the mTOR and
AMPK pathways, Bcl-2 enables cell adaptation to nutrient
deprivation by modulating autophagy (36). During starvation, AMPK activation
phosphorylates Bcl-2, weakening its interaction with Beclin 1 and
enhancing autophagy to sustain energy production and preserve
cellular integrity (37). This
phosphorylation also facilitates the redistribution of autophagic
machinery components to damaged or stressed cell regions, ensuring
a targeted autophagic response.
Conversely, under nutrient-rich conditions, mTOR
signaling suppresses autophagy, with Bcl-2 contributing to this
suppression by stabilizing Beclin 1 in an inactive state and
reinforcing cell proliferation pathways (38,39).
Additionally, Bcl-2 serves a role in modulating lysosomal function
during autophagy, ensuring the efficient degradation and recycling
of cellular components. This multifaceted regulatory function
underscores the key role of Bcl-2 in maintaining metabolic
homeostasis and dynamically responding to fluctuating nutrient
levels (26,40,41).
Bcl-2 in endoplasmic reticulum (ER)
stress and unfolded protein response (UPR)
Under ER stress, Bcl-2 serves a key role in
modulating the UPR, an important mechanism for cell adaptation to
misfolded protein accumulation (42). At ER-mitochondria contact sites,
Bcl-2 regulates calcium signaling by controlling calcium release
from the ER to mitochondria, which is key for both ATP production
and apoptosis regulation (43).
Dysregulated calcium transfer can result in mitochondrial calcium
overload, triggering pro-apoptotic pathways.
Prolonged ER stress compromises the anti-apoptotic
functions of Bcl-2 by altering its interactions with proteins at
contact sites. Additionally, Bcl-2 directly modulates UPR pathways
through interactions with key mediators such as inositol-requiring
enzyme 1 and protein kinase R-like ER kinase, thereby influencing
the cell fate between adaptive recovery and apoptotic cell death.
Bcl-2 also contributes to stabilizing mitochondrial membrane
potential during ER stress, further underscoring its multifaceted
role in maintaining cell homeostasis under adverse conditions
(44–46).
Bcl-2 in immune cell regulation
Bcl-2 is key for the survival and function of immune
cells, including T and B lymphocytes. By inhibiting apoptosis,
Bcl-2 promotes the longevity of memory T cells, which are key for
maintaining immune memory and facilitating rapid secondary
responses to antigens (47).
Additionally, it supports the development of germinal center B
cells, enabling effective antibody diversification and
maturation.
Dysregulation of Bcl-2 in immune cells is associated
with pathologies, including autoimmune disease, where excessive
survival of autoreactive lymphocytes exacerbates immune responses,
and lymphoproliferative disorders, which are characterized by
uncontrolled immune cell proliferation (48,49).
Beyond its role in apoptosis regulation, Bcl-2 also modulates
autophagy within immune cells, aiding adaptation to metabolic and
environmental stress. This dual function in apoptosis inhibition
and autophagy regulation enables immune cells to sustain
functionality during nutrient deprivation or infection, ensuring a
robust and persistent immune response (50,51).
Bcl-2 in aging and cellular
senescence
Aging and cellular senescence are associated with
alterations in the regulation of autophagy and apoptosis,
reflecting a decline in the efficiency of cell stress response
mechanisms. The expression and activity of Bcl-2 decrease with age,
which can impair mitochondrial quality control and lead to the
accumulation of damaged organelles (52,53).
This contributes to increased oxidative stress, as reactive oxygen
species are no longer effectively neutralized, thereby exacerbating
age-associated pathologies such as neurodegeneration,
cardiovascular disease and metabolic disorders. In senescent cells,
the altered function of Bcl-2 not only affects autophagic flux but
also disrupts the balance between cell survival and programmed cell
death, resulting in chronic inflammation and tissue dysfunction
(54,55). Therapeutic strategies (such as BH3
mimetics, Bcl-2 inhibitors, caloric restriction mimetics, or gene
therapy approaches) targeting Bcl-2 in aging aim to restore the
balance between autophagy and apoptosis by enhancing mitochondrial
turnover, decreasing oxidative damage and promoting tissue
regenerative capacity. These interventions may mitigate
age-associated functional decline and enhance cell resilience in
aging populations (56,57).
Role of Bcl-2 in apoptosis
Inhibition of pro-apoptotic
proteins
Bcl-2 inhibits apoptosis by binding pro-apoptotic
proteins such as Bax and Bak, thereby preventing MOMP and the
subsequent release of cytochrome c. This inhibition blocks the
activation of caspases, the key executors of apoptosis, which
cleave cellular substrates and ultimately lead to cell death
(58,59). Activity of Bcl-2 is regulated by
its interactions with BH3-only proteins, such as Bad and Bcl-2-like
protein 11, which disrupt its anti-apoptotic function under
cellular stress (60). These
interactions are modulated by upstream signaling pathways,
including the PI3K/AKT pathway, which phosphorylates Bcl-2 to
enhance its stability and anti-apoptotic function. Furthermore,
Bcl-2 serves a key role in maintaining mitochondrial dynamics by
influencing the balance between fission and fusion (19,61).
It interacts with dynamin-related protein 1 to limit excessive
mitochondrial fragmentation, a process associated with apoptotic
signaling. Moreover, Bcl-2 regulates ER-mitochondria contact sites,
which are key for calcium signaling and apoptotic regulation,
further reinforcing its role in cell survival (62,63).
Dual role in cell survival and
death
Anti-apoptotic functions of Bcl-2 are
context-dependent and associated with environmental cellular
stressors. Under mild stress, Bcl-2 effectively binds and
neutralizes pro-apoptotic proteins such as Bax and Bak, thereby
maintaining mitochondrial integrity and promoting cell survival
(64,65). However, under severe or prolonged
stress, Bcl-2 undergoes PTMs, such as phosphorylation or cleavage,
which alter its binding affinity and disrupt its interactions with
Bax and Bak (59,66). This can trigger MOMP, leading to
the release of apoptogenic factors such cytochrome c. Additionally,
the function of Bcl-2 is modulated by upstream pathways, such as
JNK signaling, which phosphorylate Bcl-2 at specific residues,
shifting the balance from survival to apoptosis. These mechanisms
highlight the dual role of Bcl-2 as a regulator of both cell
survival and programmed cell death depending on cellular context
and stress severity (67–69).
Interplay between autophagy and
apoptosis
Molecular crosstalk
Interplay between autophagy and apoptosis involves
shared regulatory proteins, including Bcl-2. By sequestering Beclin
1, Bcl-2 inhibits autophagy while promoting cell survival, thus
ensuring cell resources are preserved under stress (70,71).
This regulatory mechanism helps cells avoid autophagic
overactivation, which leads to self-digestion and cellular demise.
By contrast, when Bcl-2 binds to Bax or Bak, its anti-apoptotic
function is compromised, tipping the balance toward apoptosis
(72,73). This switch is triggered by upstream
signals, such as JNK activation or oxidative stress, which alter
the conformation of Bcl-2 and disrupt its interactions. The
modulation of this crosstalk is key for maintaining cellular
homeostasis, particularly under dynamic environmental conditions
(72,74).
Pathological implications
In cancer, Bcl-2 upregulation leads to autophagy
inhibition and resistance to apoptosis, facilitating tumor
progression by enabling cancer cells to evade programmed cell death
and adapt to metabolic stresses (75,76).
This dysregulation contributes to therapy resistance, as cancer
cells exploit Bcl-2-mediated survival pathways to withstand
chemotherapeutic and radiological insult. In neurodegenerative
diseases such as Alzheimer's and Parkinson's disease, impaired
autophagy and increased apoptosis exacerbate neuronal loss by
promoting the accumulation of damaged organelles and proteins,
further destabilizing cell homeostasis (77,78).
Targeting Bcl-2 to restore the balance between autophagy and
apoptosis holds therapeutic potential for mitigating disease
progression, enhancing cell survival and improving treatment
response in these conditions (19).
Therapeutic implications
Cancer therapy and drug
resistance
Upregulation of the anti-apoptotic protein Bcl-2
(79) contributing to chemotherapy
resistance in numerous types of cancer, particularly hematological
malignancies such as chronic lymphocytic and acute myeloid leukemia
(80). The development of Bcl-2
inhibitors, notably venetoclax, targets this resistance mechanism.
Venetoclax, a selective Bcl-2 inhibitor, has efficacy in inducing
apoptosis in cancer cells by antagonizing the function of Bcl-2
(81,82). Other Bcl-2 inhibitors, such as
navitoclax and obatoclax, have been developed. Navitoclax targets
both Bcl-2 and Bcl-xl, making it a broader inhibitor, while
obatoclax is a pan-BCL inhibitor that disrupts multiple
anti-apoptotic proteins (83).
However, clinical applications of these inhibitors are limited due
to dose-limiting toxicity, particularly thrombocytopenia associated
with navitoclax (84).
Resistance to venetoclax presents a clinical
challenge. Mechanisms underlying resistance include the
upregulation of alternative anti-apoptotic proteins, such as Mcl-1
and Bcl-xl, which compensates for Bcl-2 inhibition, thereby
sustaining cell survival (85).
Additionally, acquired mutations in the Bcl-2 gene, such as the
Gly101Val mutation, decrease venetoclax binding affinity and lead
to therapeutic resistance (86).
Recent studies have demonstrated that pathogens manipulate host
cell death pathways to promote their survival and replication,
highlighting the interplay between infectious agents and cell death
mechanisms (87–89). Furthermore, metabolic adaptations
within cancer cells may decrease their dependence on Bcl-2-mediated
survival pathways, further complicating treatment outcomes
(90).
To overcome venetoclax resistance, combination
therapeutic strategies are under investigation. One approach
involves the concurrent inhibition of both Bcl-2 and Mcl-1 to
prevent compensatory survival signaling (91). Preclinical studies have shown that
combining venetoclax with Mcl-1 inhibitors enhances apoptotic
responses in resistant cancer models (92,93).
For example, the combination of venetoclax + Mcl-1 inhibitors, such
as S63845 and AZD5991, exerts synergistic effects in preclinical
leukemia models, leading to increased cancer cell apoptosis
(94,95). Another promising therapeutic
strategy involves the integration of venetoclax, a potent Bcl-2
inhibitor, with autophagy modulators to counteract resistance
mechanisms in cancer therapy (96). Autophagy, a catabolic process
responsible for degrading and recycling intracellular components,
is upregulated in malignant cells as a cytoprotective response to
therapeutic stress (97). This
adaptive mechanism enables tumor cells to survive apoptotic
triggers induced by agents such as venetoclax (98).
Furthermore, dual inhibition strategies targeting
both Bcl-2 and autophagy-associated signaling pathways, including
the PI3K/Akt/mTOR axis, have garnered interest (99). The combination of venetoclax with
PI3K inhibitors (e.g., idelalisib) or mTOR inhibitors (e.g.,
everolimus) has shown synergistic cytotoxicity in hematologic
malignancies by concurrently blocking parallel survival pathways
and enhancing apoptotic priming (100). Autophagy, a cellular degradation
process, is co-opted by cancer cells as a survival mechanism under
therapeutic stress (101).
Inhibiting autophagy in conjunction with Bcl-2 antagonism has shown
promise in preclinical settings, suggesting a potential method to
mitigate resistance (102).
Additionally, the combination of venetoclax + PI3K/mTOR pathway
inhibitors, such as idelalisib and everolimus, has been explored to
enhance apoptotic responses by disrupting alternative survival
signals (103). These combination
approaches aim to address the adaptive resistance mechanisms that
cancer cells employ to evade venetoclax-induced apoptosis.
Neurodegenerative disorder. In neurodegenerative diseases
such as Alzheimer's and Parkinson's disease, dysregulation of Bcl-2
family proteins is implicated in neuronal cell death (104). Alterations in the expression of
Bcl-2 and associated proteins disrupt the balance between
pro-apoptotic and anti-apoptotic signals, leading to increased
neuronal vulnerability and degeneration (105). For example, decreased Bcl-2
expression or function may fail to counteract pro-apoptotic
stimuli, resulting in enhanced neuronal apoptosis (66). Conversely, upregulation of
anti-apoptotic Bcl-2 family members, such as Bcl-2, Bcl-xL, and
Mcl-1, can inhibit key apoptotic processes, leading to the
accumulation of damaged neurons. Therapeutic strategies targeting
Bcl-2 family proteins have been explored to restore the balance
between cell survival and death in neurodegenerative disorders
(106). For example,
small-molecule inhibitors of anti-apoptotic proteins such as Bcl-2
and Bcl-xL, promote the clearance of damaged neurons by enhancing
apoptosis (107). Conversely, in
diseases like Alzheimer's and Parkinson's, upregulation of
pro-survival Bcl-2 has been investigated to prevent excessive
neuronal loss and mitochondrial dysfunction (106). Modulating Bcl-2 activity to
enhance neuronal survival while preventing the accumulation of
dysfunctional cells holds promise as a potential approach for
mitigating neurodegeneration (108).
Research gaps and future directions
While progress has been made in elucidating the role
of Bcl-2 in autophagy and apoptosis, several key gaps remain.
First, the precise mechanisms by which the PTMs of Bcl-2, such as
phosphorylation, ubiquitination and sumoylation, modulate its dual
role in different cell contexts require further exploration. For
example, specific PTMs may have contrasting effects on its
interactions with Beclin 1 vs. pro-apoptotic proteins such as Bax
and Bak.
Second, the tissue-specific roles of Bcl-2 in
regulating the autophagy-apoptosis axis remain largely unexplored
(109). Variations in Bcl-2
expression and its interactions with cellular machinery across
tissues may provide insights into why certain diseases, such as
neurodegeneration and cancer, exhibit distinct pathological
profiles associated with this protein.
Third, the interplay between Bcl-2 and emerging
regulatory pathways, such as non-coding RNAs (for example,
microRNAs and long non-coding RNAs), presents a promising but
underexplored area (110). These
molecules may modulate activity of Bcl-2 indirectly, influencing
autophagic and apoptotic responses (111).
Additionally, the potential for combination
therapies that simultaneously target anti-apoptotic and
autophagy-suppressive roles of Bcl-2 has yet to be fully realized.
Preclinical models should evaluate the efficacy of dual inhibitors
or combinations of Bcl-2 inhibitors with autophagy modulators in
cancer, neurodegenerative disease and aging-associated conditions.
Finally, advances in single-cell analysis and imaging technologies
should be leveraged to study the real-time dynamics of Bcl-2 in
coordinating autophagy and apoptosis at the cell and subcellular
levels. Such approaches may uncover transient interactions and
compartmentalized functions of Bcl-2 that are not apparent in bulk
analyses.
Future research is key for developing precise,
context-specific therapeutic strategies targeting Bcl-2. Future
studies should identify additional PTMs of Bcl-2 that regulate its
dual role and tissue-specific roles of Bcl-2 in different diseases
and develop combination therapies targeting both autophagy and
apoptosis.
Conclusion
Bcl-2 is a key regulator of the autophagy-apoptosis
axis, orchestrating cellular responses to stress and maintaining
homeostasis. Its dual role as an inhibitor of apoptosis and
modulator of autophagy underscores its key importance in cellular
fate determination. By interacting with Beclin 1, Bax, Bak and
other molecular partners, Bcl-2 regulates autophagic flux and
apoptotic pathways to adapt to dynamic cellular environments.
In pathological contexts, such as cancer and
neurodegenerative disease, dysregulation of the functions of Bcl-2
contributes to disease progression, making it a valuable
therapeutic target. Targeting Bcl-2 using inhibitors or combination
therapies that modulate both autophagy and apoptosis is a promising
avenue for innovative treatment strategies. Furthermore, research
on the interactions if Bcl-2 with non-coding RNAs, PTMs and
tissue-specific roles has increased understanding of its full
regulatory network (112).
Future therapeutic interventions should develop
precise, context-specific strategies to facilitate advancements in
cancer therapy and neurodegeneration management. A deeper
understanding of the regulation of Bcl-2 and its interplay with
other cellular pathways is key for advancing biomedical research
and improving patient outcomes.
Acknowledgements
Not applicable.
Funding
Funding: Not funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
AAP conceived and designed the study, performed the
literature review and wrote the manuscript. Data authentication is
not applicable. The author has read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, artificial
intelligence tools were used to improve the readability and
language of the manuscript or to generate images, and subsequently,
the authors revised and edited the content produced by the
artificial intelligence tools as necessary, taking full
responsibility for the ultimate content of the present
manuscript.
References
|
1
|
Zheng C, Liu T, Liu H and Wang J: Role of
BCL-2 family proteins in apoptosis and its regulation by nutrients.
Curr Protein Pept Sci. 21:799–806. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
D'Aguanno S and Del Bufalo D: Inhibition
of Anti-Apoptotic Bcl-2 proteins in preclinical and clinical
studies: Current overview in cancer. Cells. 9:12872020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aslam M, Kanthlal SK and Panonummal R:
Peptides: A supercilious candidate for activating intrinsic
apoptosis by targeting mitochondrial membrane permeability for
cancer therapy. Int J Pept Res Ther. 27:2883–2893. 2021. View Article : Google Scholar
|
|
4
|
Wolf P, Schoeniger A and Edlich F:
Pro-apoptotic complexes of BAX and BAK on the outer mitochondrial
membrane. Biochim Biophys Acta Mol Cell Res. 1869:1193172022.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shahar N and Larisch S: Inhibiting the
inhibitors: Targeting anti-apoptotic proteins in cancer and therapy
resistance. Drug Resist Updat. 52:1007122020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Banjara S, Suraweera CD, Hinds MG and
Kvansakul M: The Bcl-2 family: ancient origins, conserved
structures, and divergent mechanisms. Biomolecules. 10:1282020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang L, Klionsky DJ and Shen HM: The
emerging mechanisms and functions of microautophagy. Nat Rev Mol
Cell Biol. 24:186–203. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kumar R, Chhikara BS, Gulia K and Chhillar
M: Cleaning the molecular machinery of cells via proteostasis,
proteolysis and endocytosis selectively, effectively, and
precisely: Intracellular self-defense and cellular perturbations.
Mol Omics. 17:11–28. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alqahtani SM, Alassiri HA, Alshahrani MSM,
Alahmari NA, Alshahrani NZS, Alqahtani AA and Alqahtani AM:
Cellular responses to ionizing radiation: Mechanisms of DNA repair
and mutation. JICRCR. 7:2299–2315. 2024.
|
|
10
|
Bertheloot D, Latz E and Franklin BS:
Necroptosis, pyroptosis and apoptosis: An intricate game of cell
death. Cell Mol Immunol. 18:1106–1121. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bernardi P, Gerle C, Halestrap AP, Jonas
EA, Karch J, Mnatsakanyan N and Soukas AA: Identity, structure, and
function of the mitochondrial permeability transition pore:
controversies, consensus, recent advances, and future directions.
Cell Death Differ. 30:1869–1885. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Z, Wang Q, Zhang H, Wang S, Ma X and
Wang H: Golm1 facilitates the CaO2-DOPC-DSPE200-PEI-CsPbBr3
QDs-induced apoptotic death of hepatocytes through the stimulation
of mitochondrial autophagy and mitochondrial reactive oxygen
species production through interactions with P53/Beclin-1/Bcl-2.
Chem Biol Interact. 398:1110762024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zając A, Maciejczyk A, Sumorek-Wiadro J,
Filipek K, Deryło K, Langner E, Pawelec J, Wasiak M, Ścibiorski M,
Rzeski W, et al: The role of Bcl-2 and Beclin-1 complex in
‘switching’ between apoptosis and autophagy in human glioma cells
upon LY294002 and sorafenib treatment. Cells. 12:26702023.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Prerna K and Dubey VK: Beclin1-mediated
interplay between autophagy and apoptosis: New understanding. Int J
Biol Macromol. 204:258–273. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rosa N, Speelman-Rooms F, Parys JB and
Bultynck G: Modulation of Ca2+ signaling by antiapoptotic Bcl-2
versus Bcl-xL: From molecular mechanisms to relevance for cancer
cell survival. Biochim Biophys Acta Rev Cancer. 1877:1887912022.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saleem S: Apoptosis, autophagy, necrosis
and their multi galore crosstalk in neurodegeneration.
Neuroscience. 469:162–174. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sukumaran P, Nascimento Da Conceicao V,
Sun Y, Ahamad N, Saraiva LR, Selvaraj S and Singh BB: Calcium
signaling regulates autophagy and apoptosis. Cells. 10:21252021.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dong X, Liang Q, Pan YZ, Wang X, Kuo YC,
Chiang WC, Zhang X, Williams NS, Rizo J, Levine B and De Brabander
JK: Novel Bcl-2 inhibitors selectively disrupt the
autophagy-specific Bcl-2-Beclin 1 protein-protein interaction. ACS
Med Chem Lett. 13:1510–1516. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu YP, Zhang S, Xin YF, Gu LQ, Xu XZ,
Zhang CD and You ZQ: Evidence for the mechanism of Shenmai
injection antagonizing doxorubicin-induced cardiotoxicity.
Phytomedicine. 88:1535972021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li S, Li X, Liang H, Yu K, Zhai J, Xue M,
Luo Z, Zheng C and Zhang H: SARS-CoV-2 ORF7a blocked autophagy flux
by intervening in the fusion between autophagosome and lysosome to
promote viral infection and pathogenesis. J Med Virol.
95:e292002023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li S, Zhang H, Li W, Zhai J, Li X and
Zheng C: The role of SARS-CoV-2 ORF7a in autophagy flux disruption:
implications for viral infection and pathogenesis. Autophagy.
20:1449–1451. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kataoka T: Biological properties of the
BCL-2 family protein BCL-RAMBO, which regulates apoptosis,
mitochondrial fragmentation, and mitophagy. Front Cell Dev Biol.
10:10657022022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
King LE, Hohorst L and García-Sáez AJ:
Expanding roles of BCL-2 proteins in apoptosis execution and
beyond. J Cell Sci. 136:jcs2607902023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Iorio R, Celenza G and Petricca S:
Mitophagy: Molecular mechanisms, new concepts on parkin activation
and the emerging role of AMPK/ULK1 axis. Cells. 11:302021.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moyzis AG, Lally NS, Liang W, Najor RH and
Gustafsson ÅB: Mcl-1 differentially regulates autophagy in response
to changes in energy status and mitochondrial damage. Cells.
11:14692022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li YY, Qin ZH and Sheng R: The multiple
roles of autophagy in neural function and diseases. Neurosci Bull.
40:363–382. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
El-Wetidy MS, Ahmad R, Rady I, Helal H,
Rady MI, Vaali-Mohammed MA, Al-Khayal K, Traiki TB and Abdulla MH:
Urolithin A induces cell cycle arrest and apoptosis by inhibiting
Bcl-2, increasing p53-p21 proteins and reactive oxygen species
production in colorectal cancer cells. Cell Stress Chaperones.
26:473–493. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Redza-Dutordoir M and Averill-Bates DA:
Interactions between reactive oxygen species and autophagy: Special
issue: Death mechanisms in cellular homeostasis. Biochim Biophys
Acta Mol Cell Res. 1868:1190412021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shu F, Xiao H, Li QN, Ren XS, Liu ZG, Hu
BW, Wang HS, Wang H and Jiang GM: Epigenetic and post-translational
modifications in autophagy: Biological functions and therapeutic
targets. Signal Transduct Target Ther. 8:322023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun X, Cao S, Mao C, Sun F, Zhang X and
Song Y: Post-translational modifications of p65: State of the art.
Front Cell Dev Biol. 12:14175022024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hussar P: Apoptosis regulators bcl-2 and
caspase-3. Encyclopedia. 2:1624–1636. 2022. View Article : Google Scholar
|
|
32
|
Green DR: The mitochondrial pathway of
apoptosis part II: The BCL-2 protein family. Cold Spring Harb
Perspect Biol. 14:a0410462022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Iksen Witayateeraporn W, Hardianti B and
Pongrakhananon V: Comprehensive review of Bcl-2 family proteins in
cancer apoptosis: Therapeutic strategies and promising updates of
natural bioactive compounds and small molecules. Phytother Res.
38:2249–2275. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sharma A and Trivedi AK: Regulation of
apoptosis by E3 ubiquitin ligases in ubiquitin proteasome system.
Cell Biol Int. 44:721–734. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cui J and Placzek WJ: Post-transcriptional
regulation of anti-apoptotic BCL2 family members. Int J Mol Sci.
19:3082018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bednarczyk M, Dąbrowska-Szeja N, Łętowski
D, Dzięgielewska-Gęsiak S, Waniczek D and Muc-Wierzgoń M:
Relationship between dietary nutrient intake and autophagy-related
genes in obese humans: A narrative review. Nutrients. 16:40032024.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xi H, Wang S, Wang B, Hong X, Liu X, Li M,
Shen R and Dong Q: The role of interaction between autophagy and
apoptosis in tumorigenesis (Review). Oncol Rep. 48:2082022.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jin C, Zhu M, Ye J, Song Z, Zheng C and
Chen W: Autophagy: Are amino acid signals dependent on the mTORC1
pathway or independent? Curr Issues Mol Biol. 46:8780–8793. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kma L and Baruah TJ: The interplay of ROS
and the PI3K/Akt pathway in autophagy regulation. Biotechnol Appl
Biochem. 69:248–264. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Patra S, Patil S, Klionsky DJ and Bhutia
SK: Lysosome signaling in cell survival and programmed cell death
for cellular homeostasis. J Cell Physiol. 238:287–305. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kench U, Sologova S, Smolyarchuk E,
Prassolov V and Spirin P: Pharmaceutical agents for targeting
autophagy and their applications in clinics. Pharmaceuticals
(Basel). 17:13552024. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Prasad V and Greber UF: The endoplasmic
reticulum unfolded protein response-homeostasis, cell death and
evolution in virus infections. FEMS Microbiol Rev. 45:fuab0162021.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Morris JL, Gillet G, Prudent J and
Popgeorgiev N: Bcl-2 family of proteins in the control of
mitochondrial calcium signalling: An old chap with new roles. Int J
Mol Sci. 22:37302021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Casas-Martinez JC, Samali A and McDonagh
B: Redox regulation of UPR signalling and mitochondrial ER contact
sites. Cell Mol Life Sci. 81:2502024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Naim S and Kaufmann T: The multifaceted
roles of the BCL-2 family member BOK. Front Cell Dev Biol.
8:5743382020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Larrañaga-SanMiguel A, Bengoa-Vergniory N
and Flores-Romero H: Crosstalk between mitochondria-ER contact
sites and the apoptotic machinery as a novel health meter. Trends
Cell Biol. 35:33–45. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhao S, Zhang Y, Lu X, Ding H, Han B, Song
X, Miao H, Cui X, Wei S, Liu W, et al: CDC20 regulates the cell
proliferation and radiosensitivity of P53 mutant HCC cells through
the Bcl-2/Bax pathway. Int J Biol Sci. 17:3608–3621. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mancuso S, Mattana M, Carlisi M, Santoro M
and Siragusa S: Effects of B-cell lymphoma on the immune system and
immune recovery after treatment: The paradigm of targeted therapy.
Int J Mol Sci. 23:33682022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zeng H, Kong X, Zhang H, Chen Y, Cai S,
Luo H and Chen P: Inhibiting DNA methylation alleviates cigarette
smoke extract-induced dysregulation of Bcl-2 and endothelial
apoptosis. Tob Induc Dis. 18:512020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Şimşek H, Akaras N, Gür C, Küçükler S and
Kandemir FM: Beneficial effects of Chrysin on Cadmium-induced
nephrotoxicity in rats: Modulating the levels of Nrf2/HO-1,
RAGE/NLRP3, and Caspase-3/Bax/Bcl-2 signaling pathways. Gene.
875:1475022023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gulia S, Chandra P and Da A: The prognosis
of cancer depends on the interplay of autophagy, apoptosis, and
anoikis within the tumor microenvironment. Cell Biochem Biophys.
81:621–658. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Fernandes MGF, Luo JXX, Cui QL, Perlman K,
Pernin F, Yaqubi M, Hall JA, Dudley R, Srour M, Couturier CP, et
al: Age-related injury responses of human oligodendrocytes to
metabolic insults: link to BCL-2 and autophagy pathways. Commun
Biol. 4:202021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tarantini S, Balasubramanian P, Delfavero
J, Csipo T, Yabluchanskiy A, Kiss T, Nyúl-Tóth Á, Mukli P, Toth P,
Ahire C, et al: Treatment with the BCL-2/BCL-xL inhibitor senolytic
drug ABT263/Navitoclax improves functional hyperemia in aged mice.
Geroscience. 43:2427–2440. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kong ASY, Maran S and Loh HS: Navigating
the interplay between BCL-2 family proteins, apoptosis, and
autophagy in colorectal cancer. Advances in Cancer
Biology-Metastasis. 11:1001262024. View Article : Google Scholar
|
|
55
|
Yang D, He L, Ma S, Li S, Zhang Y, Hu C,
Huang J, Xu Z, Tang D and Chen Z: Pharmacological targeting of
Bcl-2 induces caspase 3-mediated cleavage of HDAC6 and regulates
the autophagy process in colorectal cancer. Int J Mol Sci.
24:66622023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Andreotti DZ, Silva JDN, Matumoto AM,
Orellana AM, De Mello PS and Kawamoto EM: Effects of physical
exercise on autophagy and apoptosis in aged brain: Human and animal
studies. Front Nutr. 7:942020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Cao Z, Tian K, Ran Y, Zhou H, Zhou L, Ding
Y and Tang X: Beclin-1: A therapeutic target at the intersection of
autophagy, immunotherapy, and cancer treatment. Front Immunol.
15:15064262024. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Chota A, George BP and Abrahamse H:
Interactions of multidomain pro-apoptotic and anti-apoptotic
proteins in cancer cell death. Oncotarget. 12:1615–1626. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Qian S, Wei Z, Yang W, Huang J, Yang Y and
Wang J: The role of BCL-2 family proteins in regulating apoptosis
and cancer therapy. Front Oncol. 12:9853632022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Saddam M, Paul SK, Habib MA, Fahim MA,
Mimi A, Islam S, Paul B and Helal MMU: Emerging biomarkers and
potential therapeutics of the BCL-2 protein family: The apoptotic
and anti-apoptotic context. Egypt J Med Hum Genet. 25:122024.
View Article : Google Scholar
|
|
61
|
Kapoor I, Bodo J, Hill BT, Hsi ED and
Almasan A: Targeting BCL-2 in B-cell malignancies and overcoming
therapeutic resistance. Cell Death Dis. 11:9412020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Li Y, Guo M, Niu S, Shang M, Chang X, Sun
Z, Zhang R, Shen X and Xue Y: ROS and DRP1 interactions accelerate
the mitochondrial injury induced by polystyrene nanoplastics in
human liver HepG2 cells. Chem Biol Interact. 379:1105022023.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Jenner A, Peña-Blanco A, Salvador-Gallego
R, Ugarte-Uribe B, Zollo C, Ganief T, Bierlmeier J, Mund M, Lee JE,
Ries J, et al: DRP1 interacts directly with BAX to induce its
activation and apoptosis. EMBO J. 41:e1085872022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Radika PR, Chandrasekaran D, Mahila S and
Muninathan N: Role of reactive oxygen species and apoptotic genes
in bad obstetric history. Bulletin of Pure and Applied
Sciences-Zoology. 43:917–929. 2024.
|
|
65
|
Mustafa M, Ahmad R, Tantry IQ, Ahmad W,
Siddiqui S, Alam M, Abbas K, Moinuddin Hassan MI, Habib S and Islam
S: Apoptosis: A comprehensive overview of signaling pathways,
morphological changes, and physiological significance and
therapeutic implications. Cells. 13:18382024. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Hartman ML and Czyz M: BCL-G: 20 years of
research on a non-typical protein from the BCL-2 family. Cell Death
Differ. 30:1437–1446. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Gupta R, Ambasta RK and Pravir Kumar:
Autophagy and apoptosis cascade: Which is more prominent in
neuronal death? Cell Mol Life Sci. 78:8001–8047. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Sitthisuk P, Innajak S, Poorahong W,
Samosorn S, Dolsophon K and Watanapokasin R: Effect of acacia
concinna extract on apoptosis induction associated with endoplasmic
reticulum stress and modulated intracellular signaling pathway in
human colon HCT116 cancer cells. Nutrients. 16:37642024. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kortam MA, Ali BM and Fathy N: The
deleterious effect of stress-induced depression on rat liver:
Protective role of resveratrol and dimethyl fumarate via inhibiting
the MAPK/ERK/JNK pathway. J Biochem Mol Toxicol. 35:e226272021.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Turk M, Tatli O, Alkan HF, Ozfiliz Kilbas
P, Alkurt G and Dinler Doganay G: Co-chaperone bag-1 plays a role
in the autophagy-dependent cell survival through Beclin 1
interaction. Molecules. 26:8542021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Abu-Baih RH, Abu-Baih DH, Abdel-Hafez SMN
and Fathy M: Activation of SIRT1/Nrf2/HO-1 and Beclin-1/AMPK/mTOR
autophagy pathways by eprosartan ameliorates testicular dysfunction
induced by testicular torsion in rats. Sci Rep. 14:125662024.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Rosa N, Ivanova H, Wagner LE II, Kale J,
La Rovere R, Welkenhuyzen K, Louros N, Karamanou S, Shabardina V,
Lemmens I, et al: Bcl-xL acts as an inhibitor of IP3R
channels, thereby antagonizing Ca2+-driven apoptosis.
Cell Death Differ. 29:788–805. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Cauwelier C, de Ridder I and Bultynck G:
Recent advances in canonical versus non-canonical
Ca2+-signaling-related anti-apoptotic Bcl-2 functions
and prospects for cancer treatment. Biochim Biophys Acta Mol Cell
Res. 1871:1197132024. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Sharma M: Interplay between autophagy and
apoptosis in cancer: Mechanisms and therapeutic implications. In
role of autophagy and reactive oxygen species in cancer treatment:
Principles and Current Strategies. 235–254. 2024. View Article : Google Scholar
|
|
75
|
Yu Y, Liu B, Li X, Lu D, Yang L, Chen L,
Li Y, Cheng L, Lv F, Zhang P, et al: ATF4/CEMIP/PKCα promotes
anoikis resistance by enhancing protective autophagy in prostate
cancer cells. Cell Death Dis. 13:462022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Usman RM, Razzaq F, Akbar A, Farooqui AA,
Iftikhar A, Latif A, Hassan H, Zhao J, Carew JS, Nawrocki ST and
Anwer F: Role and mechanism of autophagy-regulating factors in
tumorigenesis and drug resistance. Asia Pac J Clin Oncol.
17:193–208. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Nolte EM, Joubert AM, Lafanechère L and
Mercier AE: Radiosensitization of breast cancer cells with a
2-methoxyestradiol analogue affects DNA damage and repair signaling
in vitro. Int J Mol Sci. 24:35922023. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Bittencourt TL, da Silva Prata RB, de
Andrade Silva BJ, de Mattos Barbosa MG, Dalcolmo MP and Pinheiro
RO: Autophagy as a target for drug development of skin ınfection
caused by mycobacteria. Front Immunol. 12:6742412021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
O'Neill J, Manion M, Schwartz P and
Hockenbery DM: Promises and challenges of targeting Bcl-2
anti-apoptotic proteins for cancer therapy. Biochim Biophys Acta.
1705:43–51. 2004.PubMed/NCBI
|
|
80
|
Perini GF, Ribeiro GN, Pinto Neto JV,
Campos LT and Hamerschlak N: BCL-2 as therapeutic target for
hematological malignancies. J Hematol Oncol. 11:652018. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Cao Q, Wu X, Zhang Q, Gong J, Chen Y, You
Y, Shen J, Qiang Y and Cao G: Mechanisms of action of the BCL-2
inhibitor venetoclax in multiple myeloma: A literature review.
Front Pharmacol. 14:12919202023. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Vervloessem T, Kerkhofs M, La Rovere RM,
Sneyers F, Parys JB and Bultynck G: Bcl-2 inhibitors as anti-cancer
therapeutics: The impact of and on calcium signaling. Cell Calcium.
70:102–116. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Choi J, Bogenberger JM and Tibes R:
Targeting apoptosis in acute myeloid leukemia: Current status and
future directions of BCL-2 inhibition with venetoclax and beyond.
Targ Oncol. 15:147–162. 2020. View Article : Google Scholar
|
|
84
|
Puglisi M, Molife LR, de Jonge MJ, Khan
KH, Doorn L, van Forster MD, Blanco M, Gutierrez M, Franklin C,
Busman T, et al: A phase I study of the safety, pharmacokinetics
and efficacy of navitoclax plus docetaxel in patients with advanced
solid tumors. Future Oncol. 17:2747–2758. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Castelli G, Pelosi E and Testa U: Emerging
therapies for acute myelogenus leukemia patients targeting
apoptosis and mitochondrial metabolism. Cancers (Basel).
11:2602019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Blombery P, Anderson MA, Gong JN, Thijssen
R, Birkinshaw RW, Thompson ER, Teh CE, Nguyen T, Xu Z, Flensburg C,
et al: Acquisition of the recurrent Gly101Val mutation in BCL2
confers resistance to venetoclax in patients with progressive
chronic lymphocytic leukemia. Cancer Discov. 9:342–353. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wanford JJ, Hachani A and Odendall C:
Reprogramming of cell death pathways by bacterial effectors as a
widespread virulence strategy. Infect Immun. 90:e00614212022.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Zhang G, Wang J, Zhao Z, Xin T, Fan X,
Shen Q, Raheem A, Lee CR, Jiang H and Ding J: Regulated necrosis, a
proinflammatory cell death, potentially counteracts pathogenic
infections. Cell Death Dis. 13:6372022. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Yuan J and Ofengeim D: A guide to cell
death pathways. Nat Rev Mol Cell Biol. 25:379–395. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Gao J, Wang Q, Tang YD, Zhai J, Hu W and
Zheng C: When ferroptosis meets pathogenic infections. Trends
Microbiol. 31:468–479. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Jin P, Jiang J, Zhou L, Huang Z, Nice EC,
Huang C and Fu L: Mitochondrial adaptation in cancer drug
resistance: Prevalence, mechanisms, and management. J Hematol
Oncol. 15:972022. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Li Y, Lee HH, Jiang VC, Che Y, McIntosh J,
Jordan A, Vargas J, Zhang T, Yan F, Simmons ME, et al: Potentiation
of apoptosis in drug-resistant mantle cell lymphoma cells by MCL-1
inhibitor involves downregulation of inhibitor of apoptosis
proteins. Cell Death Dis. 14:7142023. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Valko Z, Megyesfalvi Z, Schwendenwein A,
Lang C, Paku S, Barany N, Ferencz B, Horvath-Rozsas A, Kovacs I,
Schlegl E, et al: Dual targeting of BCL-2 and MCL-1 in the presence
of BAX breaks venetoclax resistance in human small cell lung
cancer. Br J Cancer. 128:1850–1861. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Nwosu GO, Ross DM, Powell JA and Pitson
SM: Venetoclax therapy and emerging resistance mechanisms in acute
myeloid leukaemia. Cell Death Dis. 15:4132024. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Tatarata QZ, Wang Z and Konopleva M: BCL-2
inhibition in acute myeloid leukemia: Resistance and combinations.
Expert Rev Hematol. 17:935–946. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Cabrera-Serrano AJ, Sánchez-Maldonado JM,
González-Olmedo C, Carretero-Fernández M, Díaz-Beltrán L,
Gutiérrez-Bautista JF, García-Verdejo FJ, Gálvez-Montosa F,
López-López JA, García-Martín P, et al: Crosstalk between autophagy
and oxidative stress in hematological malignancies: Mechanisms,
implications, and therapeutic potential. Antioxidants. 14:2642025.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Yoshida GJ: Therapeutic strategies of drug
repositioning targeting autophagy to induce cancer cell death: From
pathophysiology to treatment. J Hematol Oncol. 10:672017.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Grant S: Rational combination strategies
to enhance venetoclax activity and overcome resistance in
hematologic malignancies. Leuk Lymphoma. 59:1292–1299. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Xu Z, Han X, Ou D, Liu T, Li Z, Jiang G,
Liu J and Zhang J: Targeting PI3K/AKT/mTOR-mediated autophagy for
tumor therapy. Appl Microbiol Biotechnol. 104:575–587. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Yue X, Chen Q and He J: Combination
strategies to overcome resistance to the BCL2 inhibitor venetoclax
in hematologic malignancies. Cancer Cell Int. 20:5242020.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Al-Odat OS, Guirguis DA, Schmalbach NK,
Yao G, Budak-Alpdogan T, Jonnalagadda SC and Pandey MK: Autophagy
and apoptosis: current challenges of treatment and drug resistance
in multiple myeloma. Int J Mol Sci. 24:6442022. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Mishra R, Zokaei Nikoo M, Veeraballi S and
Singh A: Venetoclax and hypomethylating agent combination in
myeloid malignancies: Mechanisms of synergy and challenges of
resistance. Int J Mol Sci. 25:4842023. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Mowers EE, Sharifi MN and Macleod KF:
Autophagy in cancer metastasis. Oncogene. 36:1619–1630. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Xu J, Dong X, Huang DC, Xu P, Zhao Q and
Chen B: Current advances and future strategies for BCL-2
inhibitors: Potent weapons against cancers. Cancers (Basel).
15:49572023. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Bazhanova ED and Kozlov AA: Role of
apoptosis-related proteins P53 and Bcl-2 in the pathogenesis of
nervous system diseases. J Evol Biochem Phys. 60:1475–1489. 2024.
View Article : Google Scholar
|
|
106
|
Singh R, Letai A and Sarosiek K:
Regulation of apoptosis in health and disease: The balancing act of
BCL-2 family proteins. Nat Rev Mol Cell Biol. 20:175–193. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Li M, Wang D, He J, Chen L and Li H:
Bcl-XL: A multifunctional anti-apoptotic protein. Pharmacol Res.
151:1045472020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Murumulla L and Challa S: Role of
Apoptosis in Neurodegeneration: Therapeutic targets and strategies.
In apoptosis and human health: Understanding mechanistic and
therapeutic potential Singapore: Springer Nature Singapore; pp.
231–249. 2024
|
|
109
|
Perrotta C, Cattaneo MG, Molteni R and De
Palma C: Autophagy in the regulation of tissue differentiation and
homeostasis. Front Cell Dev Biol. 8:6029012020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Liu M, Jiang H and Momeni MR: Epigenetic
regulation of autophagy by non-coding RNAs and exosomal non-coding
RNAs in colorectal cancer: A narrative review. Int J Biol Macromol.
273((Pt 2)): 1327322024. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Lindqvist LM, Heinlein M, Huang DC and
Vaux DL: Prosurvival Bcl-2 family members affect autophagy only
indirectly, by inhibiting Bax and Bak. Proc Natl Acad Sci USA.
111:8512–8517. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
He B, Zhao Z, Cai Q, Zhang Y, Zhang P, Shi
S, Xie H, Peng X, Yin W, Tao Y and Wang X: miRNA-based biomarkers,
therapies, and resistance in Cancer. Int J Biol Sci. 16:2628–2647.
2020. View Article : Google Scholar : PubMed/NCBI
|

















