Introduction
Allergic rhinitis (AR) is a chronic inflammatory
disease of the nasal cavity mediated by immunoglobulin (Ig)E,
characterized by typical symptoms such as sneezing, nasal itching,
nasal congestion and rhinorrhea, which severely affect patient
quality of life (1,2). The key mechanism underlying the
pathogenesis of AR is a hypersensitivity reaction triggered by
specific allergens coming into contact with the nasal mucosa.
Allergen exposure induces the recruitment of various immune cells,
including eosinophils, mast cells, basophils and neutrophils, into
the nasal mucosa, activating epithelial cells, fibroblasts and T
helper 2 (Th2) cell responses. This leads to the release of
numerous pro-inflammatory mediators, such as IL-4, IL-5 and IL-13,
which trigger B cells to produce antigen-specific IgE, further
activating mast cells and basophils, resulting in nasal mucosal
allergy and inflammation (3,4).
Human placental extracts (HPE) refer to a range of
small bioactive molecules extracted from the human placenta
(5). Previous studies have shown
that HPE is rich in active substances, such as nutritional factors,
cytokines, DNA materials, neuropeptides, enkephalins,
polysaccharides, amino acids, proteins, lipids and trace elements
(6–8). These substances have pharmacological
effects, including enhancing the body's energy metabolism (9), promoting neurovascular growth,
combating fatty liver, exerting antioxidant effects, modulating the
immune system and exerting anti-inflammatory actions (10).
AMPK is an energy-sensing enzyme that plays a vital
role in regulating cellular metabolism and energy balance.
Activating AMPK can directly phosphorylate the p65 subunit of
NF-κB. The phosphorylated p65 subunit has a reduced ability to bind
to DNA, thereby inhibiting the transcriptional activity of NF-κB
and reducing the expression of downstream inflammation-related
genes such as tumor necrosis factor-α (TNF-α) and interleukin-6
(IL-6), thereby reducing the inflammatory response. Since AR is an
allergic inflammatory disease, activating AMPK may reduce
inflammation of the nasal mucosa and alleviate symptoms. AMPK
activation may also improve the local immune environment, thereby
reducing the severity of allergic reactions (11).
NF-κB refers to a family of transcription factors
widely present in mammalian cells, which have a key role in
regulating immune responses, inflammatory reactions, cell survival,
proliferation and differentiation. NF-κB can act on Src homology
2-containing phosphatase (SHP)1 and SHP2 signaling molecules
through various stimuli (12–14).
SHP1 is a powerful anti-inflammatory factor, and it has been
reported that the astrocytes from SHP1 gene-deficient mice exhibit
a stronger inflammatory response under stimulation by interferon
(IFN) and lipopolysaccharide (LPS) (15). SHP2 is a non-receptor protein
tyrosine phosphatase expressed in various cell types that regulates
multiple signaling pathways. The regulatory role of SHP2 may
balance inflammatory responses by inhibiting or promoting the NF-κB
pathway. For example, it has been reported that SHP2 indirectly
affects the activation level of NF-κB by regulating the activity of
RTK to regulate the intensity and duration of the inflammatory
response (16). The present study
aimed to evaluate the relationship between AMPK and NF-κB, as well
as the role of SHP1 and SHP2 in AR.
Materials and methods
Experimental animals
A total of 32 male Sprague-Dawley (SD) rats (age,
4–5 weeks; weight, 200–220 g) were purchased from Henan SKBS
Biotechnology Co., Ltd (http://www.hnskbs.com/). [animal production license
no. SCXK (Yu) 2020–0005]. All experiments were approved by the
Animal Protection and Use Committee of Hebei Medical University
[Shijiazhuang, China; approval no. Institutional Animal Care and
Use Committee (IACUC)-Hebmu-P-2024024], and were conducted in
accordance with the laboratory animal husbandry regulations and
ethical requirements of Hebei Medical University. All methods were
performed in accordance with the relevant guidelines and
regulations. and conformed to the principle of the 3Rs and ARRIVE
guidelines (17). The rats were
housed in specific pathogen-free-grade animal facilities with a
temperature of 23±1°C and relative humidity of 55±2%, with free
access to food and water. The rats were raised in a 12-h light/dark
cycle.
Experimental materials
Fetal bovine serum (FBS) and DMEM were obtained from
Gibco; Thermo Fisher Scientific, Inc. Ovalbumin (OVA) was purchased
from MilliporeSigma. HPE was obtained from Handan Kangye
Pharmaceutical Co., Ltd. The ELISA kit includes Interleukin-1β
(IL-1β) (PI303, Beyotime), Interferon-β (IFNβ) (E-EL-R0545,
Elabscience), Interferon-α (IFNα) (E-EL-R3036, Elabscience),
malondialdehyde (MDA) (S0131S, Beyotime), superoxide dismutase
(SOD) (E-EL-R1424, Elabscience), Immunoglobulin E (IgE)
(E-EL-R0517, Elabscience), Immunoglobulin G1 (IgG1) (88-50500-88,
Thermo Fisher Scientific), Immunoglobulin G2a (IgG2a) (E-EL-R2415,
Elabscience) and glutathione (GSH) (E-EL-0026, Elabscience). The
AMPK inhibitor dorsomorphin 2HCl and Transwell chambers were
obtained from Beijing Baiolaibo Technology Co., Ltd. The SHP2/SHP1
inhibitor NSC-87877 and the STING agonist DMXAA were purchased from
MedChemExpress. Western blot electrophoresis equipment was
purchased from Shanghai Sevier Technology Co., Ltd (https://www.servier.com.cn/).
Kyoto encyclopedia of genes and
genomes (KEGG) pathway analysis of key biological pathways
The AR dataset GSE44037 (18) was downloaded from the Gene
Expression Omnibus (GEO) database (URL: http://example.com/gse44037); this dataset includes
five AR samples and six healthy control samples. For data
preprocessing, R software (version 4.0.3.; http://www.r-project.org) was used to normalize RNA
sequencing data using log2 transformation Microarray data from the
GEO dataset were background corrected, normalized and averaged
using robust multi-array analysis. The processed data were then
batch-effect corrected using the ComBat (https://bioconductor.org/packages/release/bioc/html/sva.html)
method. The AR dataset GSE44037 included five samples of patients
with AR-related disorders and six samples of healthy controls. The
limma package (https://bioconductor.org/packages/release/bioc/html/limma.html)
was utilized for identifying differentially expressed genes (DEGs)
between the AR samples and healthy controls; DEGs between these
groups were identified with |log2 fold change|>1 and P<0.05.
P-values were adjusted for multiple hypotheses using the
Benjamini-Hochberg method. Gene Ontology (GO) and KEGG pathway
enrichment analyses were performed using the ClusterProfiler
package (version 3.18.1; http://bioconductor.org/packages/release/bioc/html/clusterProfiler.html).
GO analysis identified enriched cellular component (CC), molecular
function (MF) and biological process (BP) terms, whereas KEGG
pathway analysis identified key biological pathways. Spearman
correlation analysis was performed to assess the correlation among
DEGs. This analysis helps to identify relationships between the
expression levels of DEGs in the context of AR-related disorders
and healthy controls.
Construction of a rat model of AR
A total of 32 male rats were randomly divided into
the following four groups (n=8 rats/group): Sham, model, model +
HPE and model + HPE + AMPK inhibitor groups. Modeling of AR in rats
was performed according to a previous study (19). The AR rat model was established as
follows: i) Sensitization phase: OVA solution [0.3 mg OVA and 30 mg
Al(OH)3 in 1 ml 0.9% saline] was administered
intraperitoneally on days 1, 3, 5, 7, 9, 11 and 13, totaling seven
injections. ii) Challenge phase: On days 15–17, a 50 mg/ml OVA
solution (50 mg OVA in 1 ml 0.9% saline) was administered
intranasally, 20 µl/rat; and on days 18–21, a 100 mg/ml OVA
solution (100 mg OVA in 1 ml 0.9% saline) was administered
intranasally, 20 µl/rat. The sham group received the same volume of
saline or Al(OH)3 solution.
Once the AR model was established, interventions
with HPE or an AMPK inhibitor were administered. The model + HPE
group received intraperitoneal injections of 5 ml/kg HPE solution,
from days 28–46. The model + HPE + AMPK inhibitor group received
intravenous injections of 10 mg/kg AMPK inhibitor solution and 5
ml/kg HPE solution intraperitoneally, from days 28 to 46. Follow-up
experiments were conducted after 46 days. At least once a day, the
health of the rats was thoroughly inspected, including skin, fur,
eyes, nose, mouth, ears, limbs, tail and abdomen. The rats were
weighed weekly to monitor growth and development or disease
progression. Food and water consumption was checked daily, and rats
were observed for behavior, including activity levels, social
interactions, eating and drinking habits, and exploratory
behavior.
After subsequent H&E experiments and ELISA
tests, the rats were euthanized using CO2 (30% vol/min
CO2). The rats were exposed to CO2 until they
lost consciousness and were maintained in that environment for 20
min to confirm death. The exposure time was 1–3 min. CO2
was delivered into a well-sealed chamber of an appropriate size for
the rats to ensure the CO2 concentration met the
specified standard. The behavior of the rats was monitored to
ensure they quickly lost consciousness. During the euthanasia
process, a quiet, softly lit room was used, avoiding a noisy
environment and strong light stimulation, and warm mats or towels
were used to wrap the rats to ensure they were comfortable; rough
movements were avoided during the operation. The staff performing
euthanasia were professionally trained and familiar with the
operation procedure, and were able to complete the operation
quickly and effectively, reducing the pain and fear of the animals.
Successful euthanasia was confirmed 20 min after the heartbeat of
the rats could no longer be detected. Euthanasia was preceded by
gas anesthesia with 4.5% isoflurane to reduce anxiety in the rats.
This euthanasia procedure followed the guidelines of the IACUC or
Animal Protection and Use Committee of Hebei Medical University. In
addition, if the rats showed loss of appetite and depression, the
experiment was terminated and the rats were sacrificed. The
procedures used in the present study minimized animal suffering,
and the bodies of the rats were properly disposed of after the
process.
Hematoxylin and eosin (H&E)
staining of morphological changes in nasal mucosa
The rats were first induced under gas anesthesia
with 4.5% isoflurane and then anesthesia was maintained with 2%
isoflurane. After anesthetizing the rats, the nasal mucosa tissues
were isolated, fixed with 4% paraformaldehyde for 24 h at room
temperature, dehydrated with ethanol and embedded in paraffin.
Paraffin-embedded sections (5 µm) were deparaffinized twice at room
temperature using xylene for 5 min each time, in 100, 90, 80 and
70% ethanol for 5 min each, and in distilled water for 5 min. The
distilled water was removed, and the sample was stained with
hematoxylin for 15 min at room temperature. Subsequently, the
hematoxylin was removed with running water and differentiation was
carried out at room temperature with 1% hydrochloric acid in
alcohol for 3 sec, followed by washing with water. The sample was
then immersed in running water to remove the blue color for 10 min.
One drop of 0.5% eosin was added, and the sample was stained for 1
min at room temperature. Subsequently, dehydration was carried out
with 80, 90, 95 and 100% ethanol, two xylene washes (5 min each)
were carried out and the tissue was left to dry at room
temperature. A drop of neutral gum was then added to the tissue and
the cover glass was sealed at room temperature. After 24 h, the
tissue was observed under an optical microscope and images were
captured.
ELISA
Rats were anesthetized with 4.5% isoflurane gas to
induce anesthesia and then maintained with 2% isoflurane to collect
1.5 ml of blood from the abdominal aorta, after which, serum was
separated by centrifugation. The serum was centrifuged at 1,200 × g
for 10 min. separated to obtain serum. placed at 4°C for 30 min.
The rats were then euthanized using CO2 at a flow rate
of 30% container volume/min, and euthanasia was verified once the
rats had not been breathing for 20 min. Interleukin-1β (IL-1β)
(PI303, Beyotime), Interferon-β (IFNβ) (E-EL-R0545, Elabscience),
Interferon-α (IFNα) (E-EL-R3036, Elabscience), malondialdehyde
(MDA) (S0131S, Beyotime), superoxide dismutase (SOD) (E-EL-R1424,
Elabscience), Immunoglobulin E (IgE) (E-EL-R0517, Elabscience),
Immunoglobulin G1 (IgG1) (88-50500-88, Thermo Fisher Scientific),
Immunoglobulin G2a (IgG2a) (E-EL-R2415, Elabscience) and
glutathione (GSH) (E-EL-0026, Elabscience) levels were measured
using the ELISA kits according to the manufacturer's
instructions.
Western blot analysis of protein
expression in the nasal mucosa
Nasal mucosa tissues were lysed with pre-cooled RIPA
lysing solution (Beyotime Institute of Biotechnology; cat. no.
P0013B; 89% RIPA+10% 10X cocktail+1% PMSF) and protein
concentration was determined by BCA assay. Proteins (20 µg of
protein was added to each lane) were separated by SDS-PAGE on 12%
gels and were transferred to PVDF membranes. The membranes were
blocked with 5% non-fat milk at room temperature for 2 h, and were
then incubated overnight with primary antibodies (p-AMPK (Abcam;
cat. no. ab133448; 1:1,000), t-AMPK (Abcam; cat. no. ab32047;
1:1,000), p-STING (Abcam; cat. no. ab318182; 1:1,000), t-STING
(Abcam; cat. no. ab227128; 1:1,000), p-TBK1 (CST, cat. no. 5483;
1:1,000), t-TBK1 (CST, cat. no. 38066; 1:1,000), p-SHP1 (CST, cat.
no. 47696; 1:1,000), t-SHP1 (CST, cat. no. 26516; 1:1,000), p-SHP2
(CST, cat. no. 5431; 1:1,000), t-SHP2 (CST, cat. no. 3397;
1:1,000), NOX4 (Beyotime, AF1498; 1:1,000), NLRP3 (Abcam; cat. no.
ab263899; 1:1,000), IFNβ (Beyotime Institute of Biotechnology; cat.
no. AF7170; 1:1,000), GAPDH (Abcam; cat. no. ab181602; 1:1,000))
and with secondary antibodies (Goat anti-rabbit IgG H&L (HRP),
Abcam; cat. no. ab6721; 1:2,000) for 1 h at room temperature. ECL
detection kit (Beyotime Institute of Biotechnology; cat. no.
P0018M) was used to visualize the protein bands. Greyscale analysis
was performed using GAPDH as a loading control. ImageJ was used to
analyze the bands (National Institutes of Health, 1.8.0).
Cell model construction and
grouping
A 15-ml conical tube containing 3 ml complete
culture medium [10% fetal bovine serum (Beyotime Institute of
Biotechnology; cat. no. C0226) and 1% penicillin/streptomycin
solution (Beyotime Institute of Biotechnology; cat. no. C0222)] was
prepared for the macrophages obtained from 5 normal rats (these 5
rats were separated from the 32 rats that were divided into four
groups.). Bronchoalveolar lavage (BAL) buffer (PBS + EDTA) was
heated to 37°C in a water bath, taking care to maintain the
temperature throughout the process. Anesthesia was first induced in
rats using 4.5% isoflurane and then maintained using 2% isoflurane.
After the rats were fully anesthetized, they were transferred to a
CO2 euthanasia chamber and CO2 was introduced
at 30% chamber volume/min; the concentration was gradually
increased until the rats stopped breathing. The rats continued to
be exposed to CO2 for 1 min to ensure that the rats were
dead. Subsequently, the skin, chest cavity, and muscles of the rats
were excised using a dissection tool, and the lungs and trachea
were exposed to avoid cutting or rupturing blood vessels. A pair of
fine scissors was used to make a small incision in the upper part
of the trachea just below the larynx; the downward-facing part of
the trachea was left intact without cutting the entire trachea. A
slightly blunt 18-G cannula was inserted through the incision, and
the cannula was inserted deeper into the trachea toward the lungs
taking care not to damage the lung tissue. A 1-ml syringe of warm
BAL buffer was attached to the inserted cannula, the buffer was
injected and the cannula was secured in position. Subsequently, the
plunger was pulled to collect the BAL solution from the syringe.
The collected BAL solution was filtered through a 70-µm cell
strainer into a 15-ml tube containing 3 ml complete medium. The
supernatant was removed by centrifugation at 300 × g and 4°C for 5
min. Residual erythrocytes were lysed by adding 1 ml lysogeny
solution (Beyotime Institute of Biotechnology; cat. no. C3702) and
incubating for 2 min at room temperature. An appropriate amount of
complete medium was added to the tube, lysis was terminated, the
cells were collected by centrifugation (300 × g for 5 min at room
temperature) and the supernatant was removed (20). The collected alveolar macrophages
were cultured in vitro. Alveolar macrophages were cultured
in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin
(Gibco; Thermo Fisher Scientific, Inc.). The cells were incubated
at 37°C, 5% CO2 and 95% humidity. Alveolar macrophages
were identified using immunofluorescence.
Macrophages were divided into the following six
groups: i) Negative control (NC) group: Macrophages without
treatment; ii) LPS and IFN-γ group: Macrophages treated with 1
µg/ml LPS and 20 ng/ml IFN-γ; iii) LPS + IFN-γ + HPE group:
Macrophages treated with LPS (1 µg/ml), IFN-γ (20 ng/ml) and HPE
(40 ng/ml); iv) LPS + IFN-γ + HPE + AMPK inhibitor group:
Macrophages treated with LPS, IFN-γ, HPE and the AMPK inhibitor
dorsomorphin 2HCl (5 µM); v) LPS + IFN-γ + HPE + NSC-87877 group:
Macrophages treated with LPS, IFN-γ, HPE and the dual inhibitor
NSC-87877 (5 µM); vi) LPS + IFN-γ + HPE + DMXAA group: Macrophages
treated with LPS, IFN-γ, HPE and the STING agonist DMXAA (100
µg/ml). The above reagents were treated for 24 h at room
temperature.
The protein expression levels in these cell groups
were assessed by western blotting, as aforementioned, and the
levels of IL-1β, IFNβ, IFNα, MDA, SOD and GSH in the culture
supernatant of the cells groups were measured using ELISA kits as
aforementioned.
Immunofluorescence staining
First, coverslips were washed and sterilized, and
were then placed in a 24-well plate, inoculated with a macrophage
suspension of appropriate density (~1×105 cells/ml), and
incubated for 48 h at 37°C with 5% CO2 until the cells
adhered to the wall. Next, the cells were washed with PBS, fixed
with 4% paraformaldehyde for 15 min at room temperature and washed
again with PBS. Finally, a drop of sealer was added to the slide,
and the coverslip was covered with the cell side down and fixed
with sealing adhesive. The operation was carried out with attention
to aseptic conditions, cell density control and avoiding air
bubbles when sealing the slides. The sections were then baked in an
oven at 65°C for 1 h. The sections were deparaffinized by soaking
them in xylene twice (10 min each time). The sections were then
soaked twice in anhydrous ethanol (5 min each) and were incubated
in different ethanol concentrations (95, 90, 85, 80 and 75%
ethanol; 2 min each), before being rinsed under running water, and
washed and soaked in 1X PBS. Subsequently, the sections were
completely soaked in citric acid antigen repair solution containing
EDTA, boiled at 200°C for 150 sec, cooled at room temperature, and
then removed and soaked in distilled water for 3 min. The sections
were then soaked in 3% hydrogen peroxide solution for 10 min to
fully block endogenous peroxidase and washed in distilled water
(room temperature). The sections were incubated with diluted
anti-CD68 primary antibody (Abcam; cat. no. ab955; 1:50) overnight
at 4°C in a wet box. The following day, the incubated sections were
washed three times with PBS (5 min each), were shaken dry, and a
FITC-conjugated secondary antibody (Abcam; cat. no. ab150113;
1:200) was added dropwise, before being incubated for 50 min at
room temperature. The sections were then washed three times with
PBS (5 min each), were air-dried at room temperature, and the DAPI
staining solution was added dropwise, before being incubated for 10
min at room temperature. Subsequently, the sections were dried, and
then sealed with an anti-fluorescent quenching sealer. The sections
were observed under a fluorescence microscope and the images were
collected.
Transwell assay
Macrophages were seeded into the upper chamber of a
Transwell system (6-well plate, 24 mm) at a density of
1×105 cells/well, and DMEM containing 10% FBS was added
to the lower chamber. After 24 h, the cells in the lower chamber
were removed, fixed with 4% methanol (room temperature, 15 min) and
stained with 0.5% crystal violet (room temperature, 10 min). Cell
migration was recorded using a light microscope ×10 objective, and
migration numbers were assessed using ImageJ software (National
Institutes of Health, 1.8.0).
Statistical analysis
GraphPad Prism 9 (Dotmatics) was used for the
statistics and the experiments were repeated three times. Data were
presented as the mean ± standard error of the mean. One-way ANOVA
was used for comparisons between groups, followed by Tukey's HSD
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
DEG expression analysis between
allergic rhinitis and healthy controls in the GSE44037 dataset
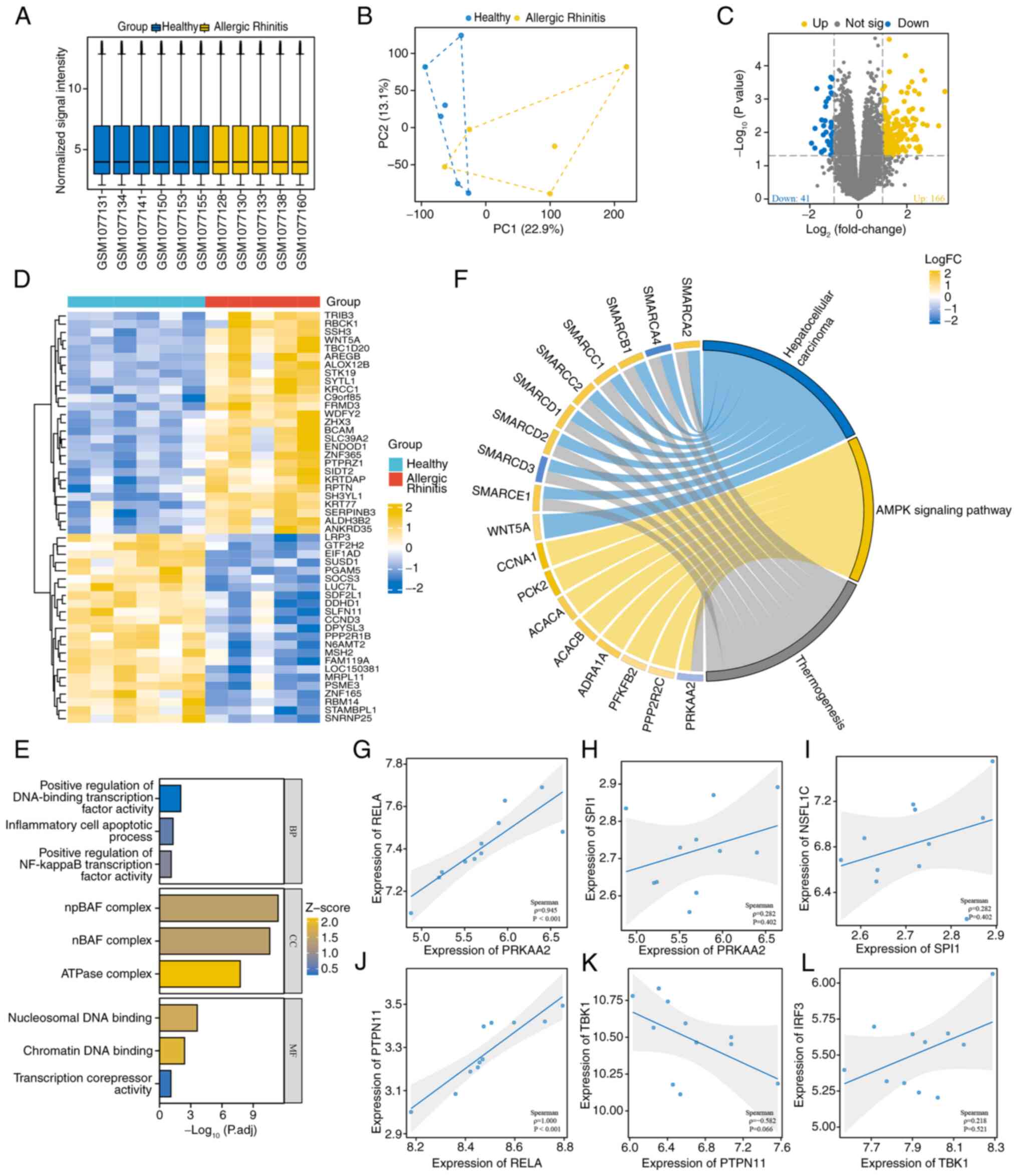
During the data preprocessing stage, the consistency
of the distribution of normalized expression levels across samples
was first verified using box plots (Fig. 1A). Next, principal component
analysis (PCA) was used to visually assess batch effects and it
showed a significant improvement in sample clustering after Combat
correction (Fig. 1B). For the
GSE44037 dataset, differential expression analysis was conducted
between the allergic rhinitis group and the healthy controls using
the limma package. Strict criteria of |log2FC|>1 and FDR
<0.05 was applied, which led to the identification of 207
significantly DEGs from the volcano plot. Among these, 166 genes
were upregulated and 41 genes were downregulated (Fig. 1C). The differential gene expression
patterns were further visualized using a hierarchical clustering
heatmap (Fig. 1D). The GO analysis
indicated that the DEGs were enriched in the CC terms ‘npBAF
complex’, ‘nBAF complex’ and ‘ATPase complex’; in the MF terms
‘nucleosomal DNA binding’, ‘chromatin DNA binding’ and
‘transcription corepressor activity’; and in the BP terms ‘positive
regulation of DNA-binding transcription factor activity’,
‘inflammatory cell apoptotic process’ and ‘positive regulation of
NF-kappaB transcription factor activity’ (Fig. 1E). KEGG pathway analysis further
revealed significant enrichment of DEGs in the ‘AMPK signaling
pathway’ (Fig. 1F). The dataset
GSE44037 identified positive correlations among several
differential genes: AMPK (PRKAA2) and NF-κB (RELA) (Fig. 1G), AMPK (PRKAA2) and PU.1 (SPI1)
(Fig. 1H), PU.1 (SPI1) and SHP1
(NSFL1C) (Fig. 1I), and NF-κB
(RELA) and SHP2 (PTPN11) (Fig.
1J). Furthermore, there was a negative correlation between SHP2
(PTPN11) and TBK1 (Fig. 1K), along
with a positive correlation between TBK1 and IRF3 (Fig. 1L).
HPE alleviates AR-induced inflammatory
damage and is associated with AMPK
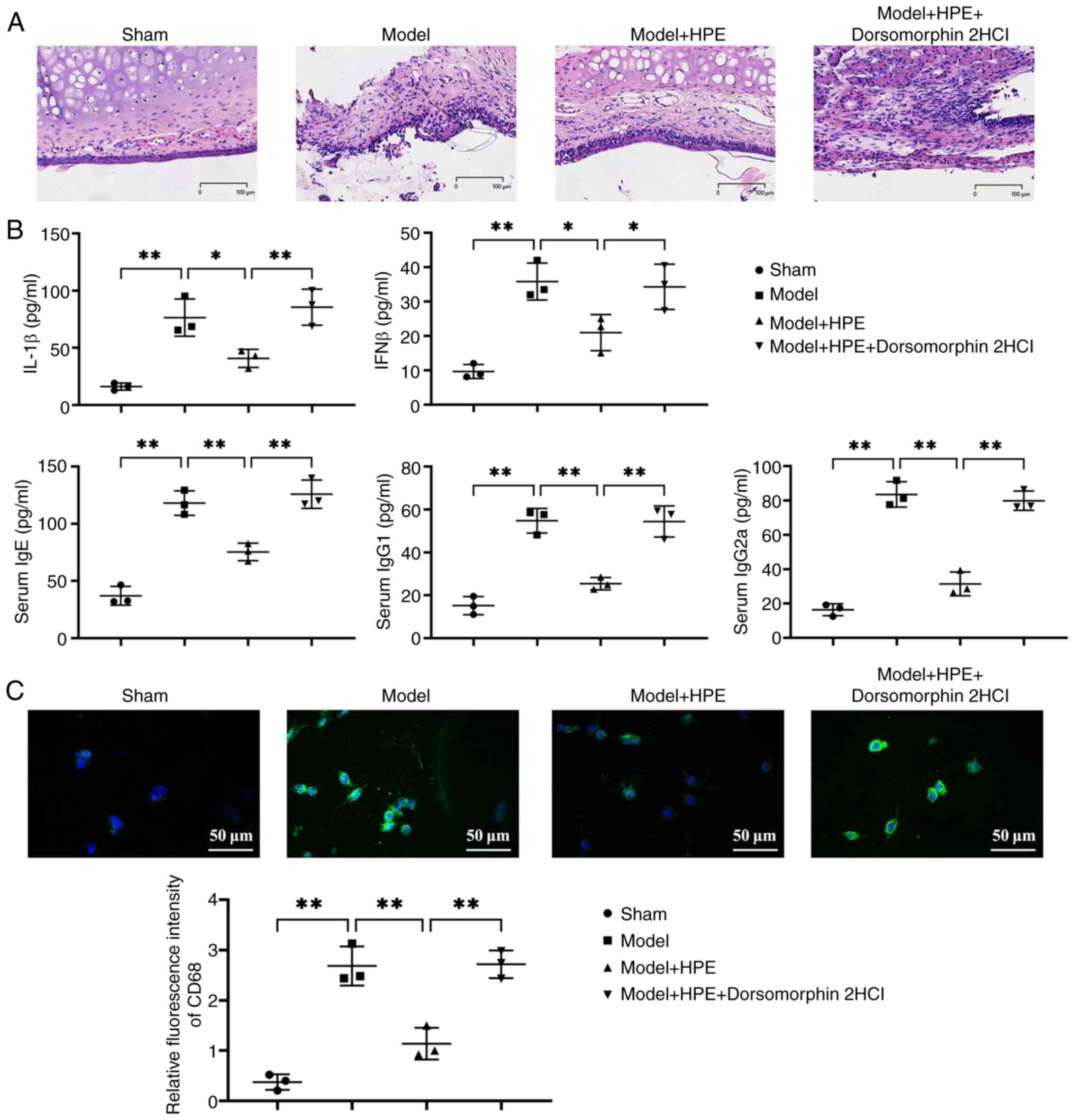
Histological examination via H&E staining showed
that the sham group exhibited intact tissue structure with no
notable pathological changes (Fig.
2A). The model group exhibited pathological phenomena,
including extensive inflammatory cell infiltration, tissue
detachment and edema. In the model + HPE group, tissue structure
gradually recovered, with a reduction in edema and inflammatory
infiltration. Compared with in the model + HPE group, the model +
HPE + AMPK inhibitor group exhibited marked pathological damage.
Furthermore, serum levels of IgE, IgG1, IgG2a, IL-1β and IFNβ were
significantly elevated in the model group compared with those in
the sham group, whereas these levels were significantly decreased
in the model + HPE group compared with those in the model group.
However, in the model + HPE + AMPK inhibitor group, the serum
levels of IgE, IgG1, IgG2a, IL-1β and IFNβ were significantly
increased compared with those in the model + HPE group, with no
significant differences observed between the model and model + HPE
+ AMPK inhibitor groups (Fig. 2B).
The expression levels of CD68 were further detected by
immunofluorescence, and it was revealed that the expression levels
of CD68 were elevated in the model group compared with those in the
sham group, and were decreased in the model + HPE group compared
with in the model group, whereas the addition of an AMPK inhibitor
increased the expression levels of CD68 (Fig. 2C).
HPE acts on AMPK, promoting SHP1/SHP2
protein expression and suppressing STING/TBK1 expression in nasal
mucosal tissue
Compared with in the sham group, the model group
exhibited decreased expression of phosphorylated (p)-AMPK/total
(t)-AMPK, p-SHP1/t-SHP1 and p-SHP2/t-SHP2, whereas p-STING/t-STING
and p-TBK1/t-TBK1 expression levels were elevated. In the model +
HPE group, the levels of p-AMPK/t-AMPK, p-SHP1/t-SHP1 and
p-SHP2/t-SHP2 were increased, whereas those of p-STING/t-STING and
p-TBK1/t-TBK1 were decreased compared with those in the model
group. In the model + HPE + AMPK inhibitor group, p-AMPK/t-AMPK,
p-SHP1/t-SHP1 and p-SHP2/t-SHP2 levels were decreased, whereas
p-STING/t-STING and p-TBK1/t-TBK1 were increased compared with
those in the model + HPE group (Fig.
3).
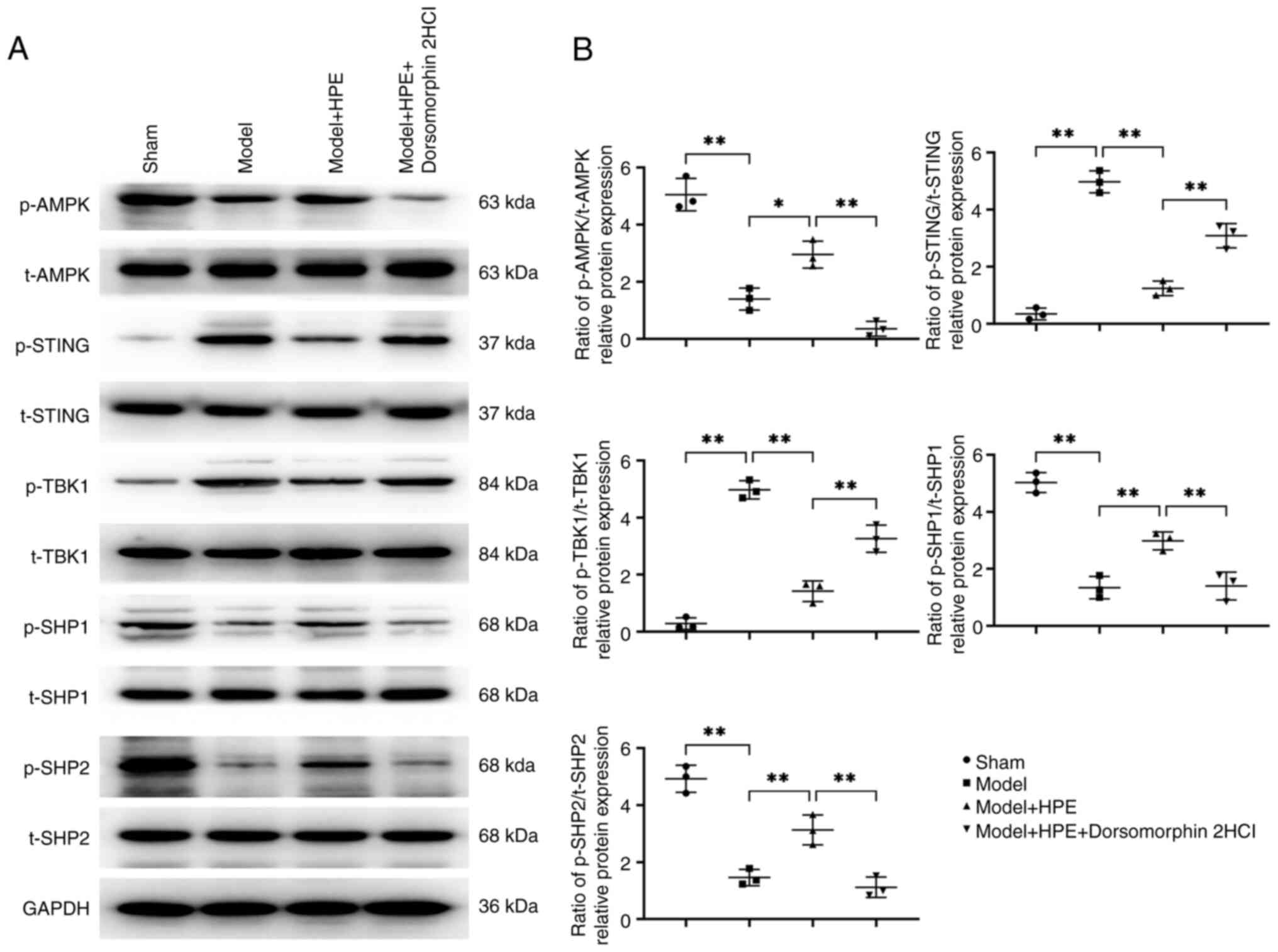 | Figure 3.HPE acts on AMPK and promotes
SHP1/SHP2 protein expression while inhibiting STING/TBK1
expression. (A) Protein banding maps of p-AMPK, t-AMPK, p-STING,
t-STING, p-TBK1, t-TBK1, p-SHP1, t-SHP1, and p-SHP2, t-SHP2 were
detected in nasal mucosal tissues by Western blot analysis. (B)
Western blot analysis was performed to detect the protein
expression levels of p-AMPK/t-AMPK, p-STING/t-STING, p-TBK1/t-TBK1,
p-SHP1/t-SHP1 and p-SHP2/t-SHP2 in nasal mucosal tissues.
**P<0.01, *P<0.05. HPE, human placental extracts; p-,
phosphorylated; SHP, Src homology 2-containing phosphatase; t-t,
total. |
HPE alleviates inflammatory responses,
and reduces cell apoptosis and migration in rat macrophages
Research has shown that the mechanism by which
macrophages act in AR significantly affects the inflammatory
response of the nasal mucosa and the levels of immune factors
through the inflammatory mediators they produce. This includes
promoting nasal mucosal inflammation by secreting Th2-type
cytokines (such as IL-4, IL-5 and IL-13) and regulating the levels
of immune factors in serum, further supporting the key role of
macrophages in AR (21,22). Macrophages are the primary
regulators in initiating immune responses, whereas other cells
participate in local and systemic inflammatory responses through
synergistic or auxiliary mechanisms. The results of
immunofluorescence staining of CD68 confirmed the success of
alveolar macrophage extraction (Fig.
4A). In this study, alveolar macrophages from normal rats were
isolated and subjected to in vitro experiments. Western blot
analysis (Fig. 4B and C) revealed
that the LPS + IFN-γ group showed significantly elevated levels of
NOX4, p-STING/t-STING, p-TBK1/t-TBK1, NLRP3, and IFNβ, but reduced
p-AMPK/t-AMPK, p-SHP1/t-SHP1, and p-SHP2/t-SHP2 compared with the
NC group. The LPS + IFN-γ + HPE group exhibited decreased levels of
NOX4, p-STING/t-STING, p-TBK1/t-TBK1, NLRP3, and IFNβ, alongside
increased p-AMPK/t-AMPK, p-SHP1/t-SHP1, and p-SHP2/t-SHP2.
Conversely, the LPS + IFN-γ + HPE + AMPK inhibitor group displayed
elevated NOX4, p-STING/t-STING, p-TBK1/t-TBK1, NLRP3, and IFNβ,
with reduced p-AMPK/t-AMPK, p-SHP1/t-SHP1, and p-SHP2/t-SHP2. The
LPS + IFN-γ + HPE + DMXAA group had higher NOX4, p-STING/t-STING,
p-TBK1/t-TBK1, NLRP3, and IFNβ levels compared with the LPS + IFN-γ
group, but lower p-AMPK/t-AMPK, p-SHP1/t-SHP1, and p-SHP2/t-SHP2.
Additionally, the LPS + IFN-γ + HPE + DMXAA group showed increased
NOX4, p-STING/t-STING, p-TBK1/t-TBK1, NLRP3, and IFNβ, and
decreased p-AMPK/t-AMPK, p-SHP1/t-SHP1, and p-SHP2/t-SHP2 compared
with the LPS + IFN-γ + HPE group. Compared with the LPS + IFN-γ +
HPE + AMPK inhibitor and LPS + IFN-γ + HPE + NSC-87877 groups, the
LPS + IFN-γ + HPE + DMXAA group demonstrated elevated NOX4,
p-STING/t-STING, p-TBK1/t-TBK1, NLRP3, and IFNβ, with reduced
p-AMPK/t-AMPK, p-SHP1/t-SHP1, and p-SHP2/t-SHP2.
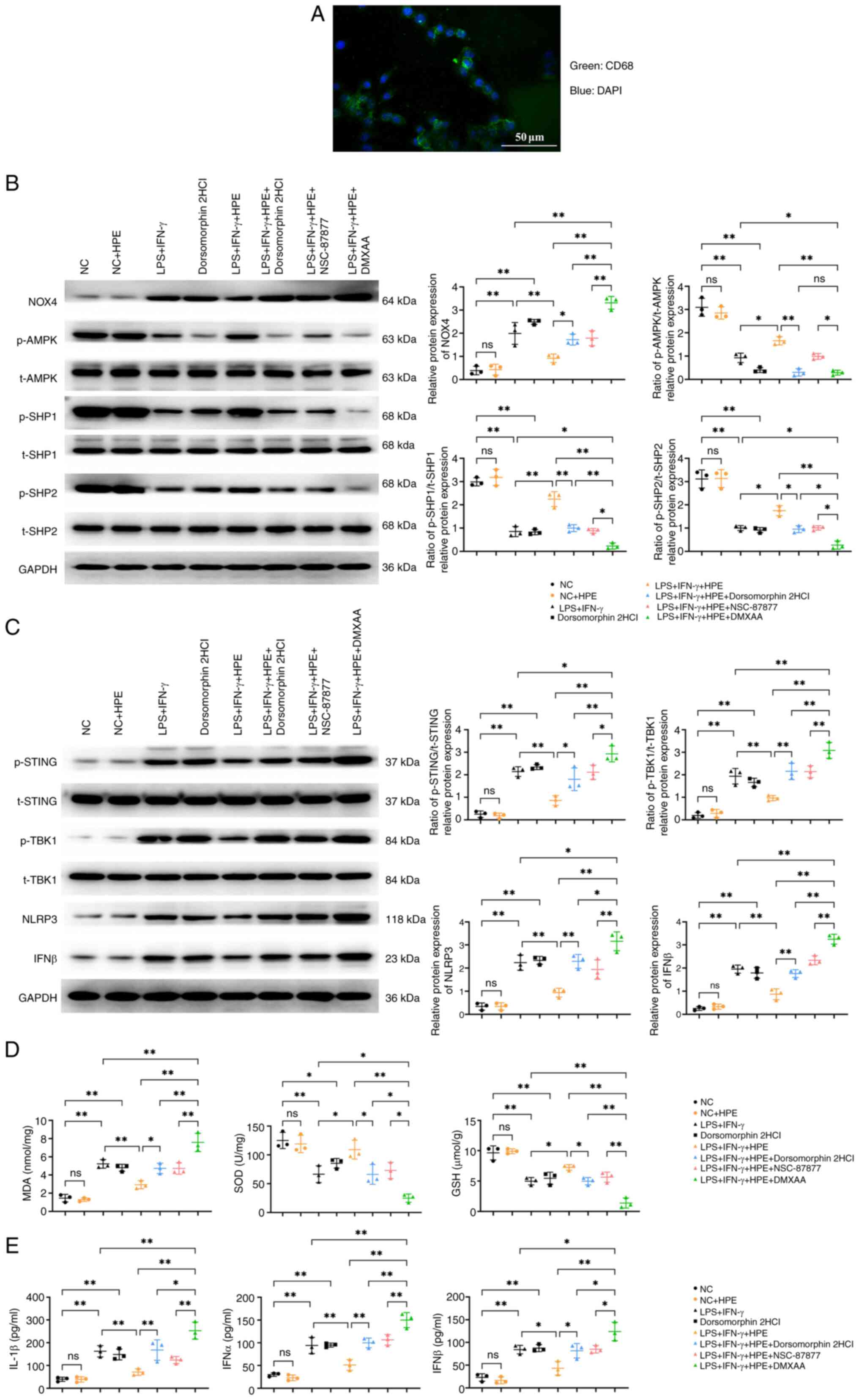 | Figure 4.HPE alleviates the inflammatory
response. (A) Immunofluorescence staining was used to detect
extracted alveolar macrophages. (B) Western blot analysis was
conducted to detect the protein expression levels of NOX4,
p-AMPK/t-AMPK, p-SHP1/t-SHP1 and p-SHP2/t-SHP2 in macrophages. (C)
Western blot analysis was conducted to detect the protein
expression levels of p-STING/t-STING, p-TBK1/t-TBK1, NLRP3 and IFNβ
in macrophages. ELISA was used to measure the levels of (D) MDA,
SOD and GSH, and (E) IL-1β, IFNβ and IFNα in macrophage
supernatants. **P<0.01, *P<0.05, nsP>0.05. GSH,
glutathione; HPE, human placental extracts; IFN, interferon; LPS,
lipopolysaccharide; MDA, malondialdehyde; NC, negative control;
NOX4, NADPH oxidase 4; p-, phosphorylated; SHP, Src homology
2-containing phosphatase; SOD, superoxide dismutase; t-t,
total. |
ELISA results (Fig. 4D
and E) showed that the LPS + IFN-γ group had elevated levels of
IL-1β, IFNβ, IFNα, and MDA, but reduced SOD and GSH compared with
the NC group. The LPS + IFN-γ + HPE group exhibited decreased
IL-1β, IFNβ, IFNα, and MDA, and increased SOD and GSH. In contrast,
the LPS + IFN-γ + HPE + AMPK inhibitor group displayed higher
IL-1β, IFNβ, IFNα, and MDA, and lower SOD and GSH. The LPS + IFN-γ
+ HPE + DMXAA group showed elevated IL-1β, IFNβ, IFNα, and MDA, and
reduced SOD and GSH compared with both the LPS + IFN-γ group and
the LPS + IFN-γ + HPE group.. Compared with the LPS + IFN-γ + HPE +
AMPK inhibitor group, IL-1β, IFNβ, IFNα and MDA levels were
elevated and SOD and GSH levels were decreased in the LPS + IFN-γ +
HPE + DMXAA group. These results suggested that HPE attenuated the
inflammatory response in rat macrophages by mediating the reactive
oxygen species (ROS)/AMPK/SHP1/SHP2/STING signaling pathway.
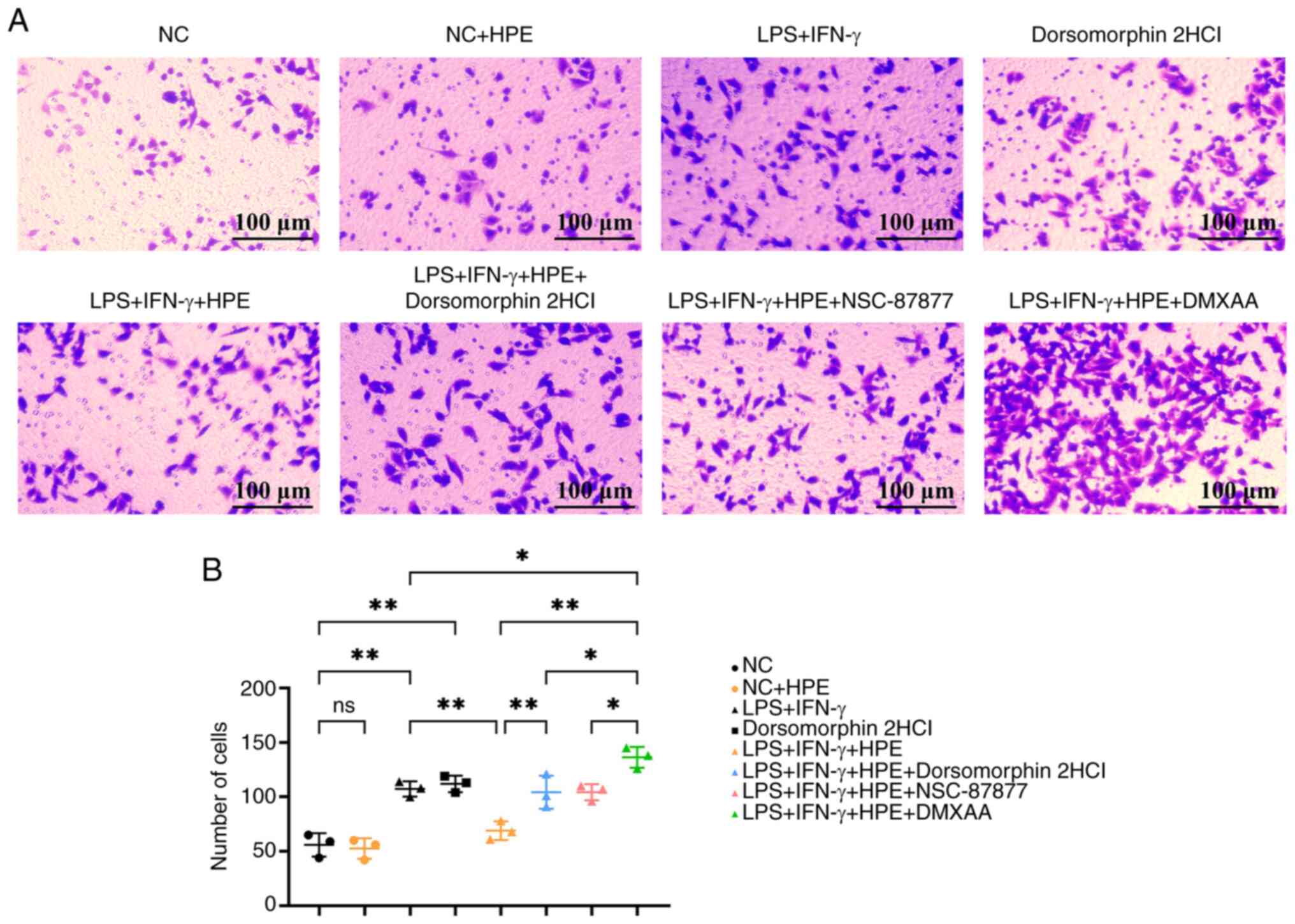
The important role of macrophage migration in nasal
mucosal remodeling was assessed in the present study, as
macrophages migrating to the site of inflammation can influence the
expression of signaling pathway proteins (23). Additionally, the signaling factors
released by apoptotic macrophages can be recognized by other immune
cells, thus affecting the levels of immune factors in both the
nasal mucosa and serum. Therefore, Transwell migration experiments
were conducted on macrophages from different groups. In the
Transwell experiment (Fig. 5A and
B), compared with in the NC group, cell migration was increased
in the LPS + IFN-γ group; however, when compared with the LPS +
IFN-γ group, cell migration was decreased in the LPS + IFN-γ + HPE
group. In addition, when compared with the LPS + IFN-γ + HPE group,
cell migration was increased in the LPS + IFN-γ + HPE + AMPK
inhibitor group, and when compared with the LPS + IFN-γ group, cell
migration was increased in the LPS + IFN-γ + HPE + DMXAA group.
Furthermore, when compared with the LPS + IFN-γ + HPE, LPS + IFN-γ
+ HPE + AMPK inhibitor and LPS + IFN-γ + HPE + NSC-87877 groups,
cell migration was significantly increased in the LPS + IFN-γ + HPE
+ DMXAA group. HPE promotes the NF-κB signaling pathway through
activation of AMPK, and NF-κB directly activates SHP2 on the one
hand, and SHP1 on the other hand via C/EBPα/PU.1. SHP1 inhibits
STING, and SHP2 inhibits TBK1, thereby inhibiting the STING/TBK1
signaling pathway, which mediates inflammatory responses and
promotes mitochondrial via IRF3 Oxidative Stress. Mitochondrial
oxidative stress upregulates the expression of cGAMP and P53: cGAMP
binds to STING and stabilizes its dimers and oligomers; P53
inhibits SHP1 and SHP2, and promotes the expression of both SYK and
EGFR. STING interacts with EGFR, leading to the autophosphorylation
of EGFR and activation of SYK, which then phosphorylates STING and
EGFR at Y240 and Y245 sites, respectively. SYK and EGFR
phosphorylate STING at the Y240 and Y245 sites, respectively,
promoting its translocation to ERGIC for signaling. In conclusion,
HPE may inhibit AR by regulating the AMPK/SHP1/SHP2/STING signaling
pathway (Fig. 6).
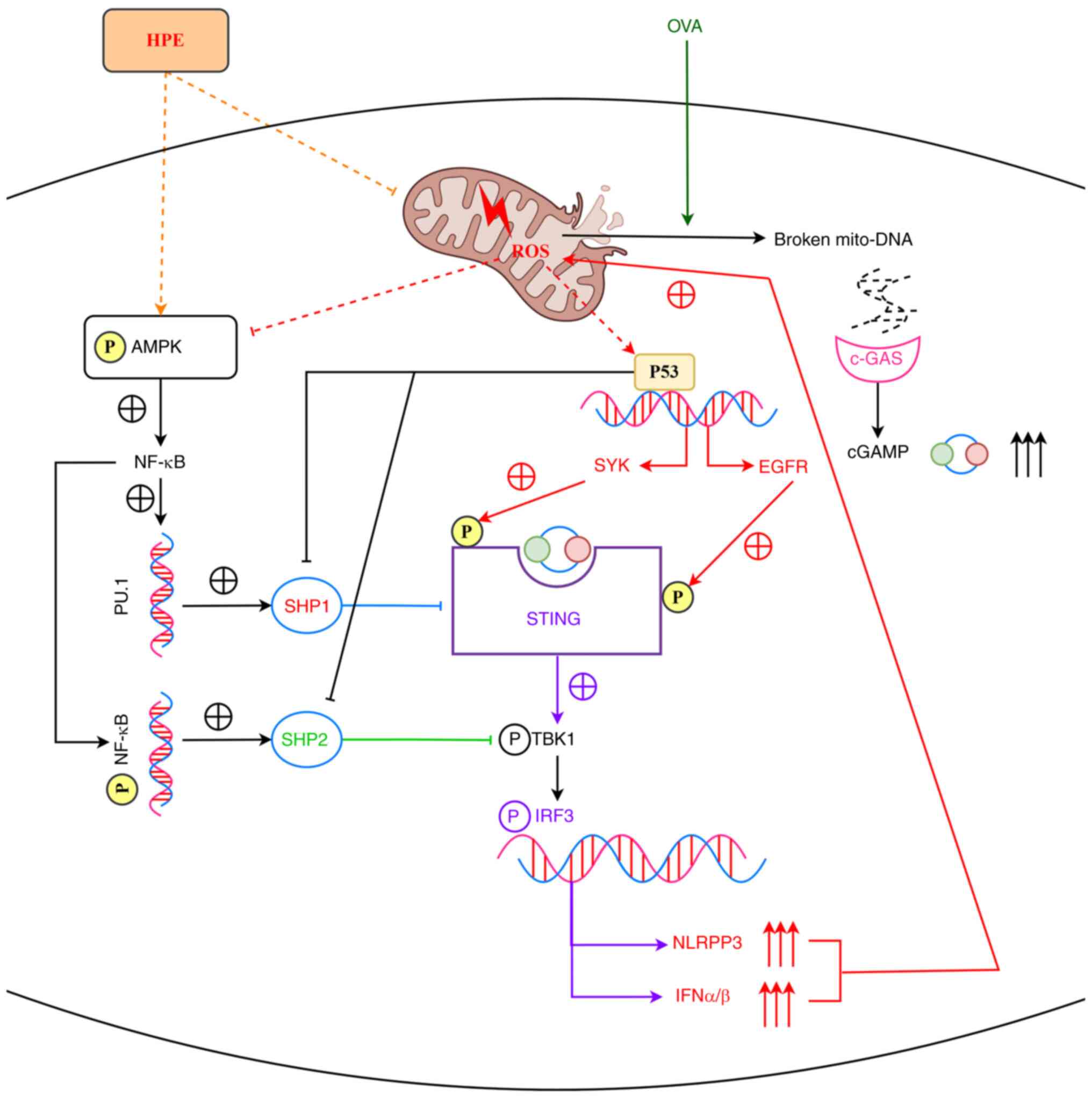 | Figure 6.HPE inhibits allergic rhinitis by
modulating the AMPK/SHP1/SHP2/STING signaling pathway. HPE promotes
the NF-κB signaling pathway through activation of AMPK, and NF-κB
directly activates SHP2 on the one hand, and SHP1 on the other hand
via C/EBPα/PU.1. SHP1 inhibits STING, and SHP2 inhibits TBK1,
thereby inhibiting the STING/TBK1 signaling pathway, which mediates
inflammatory responses and promotes mitochondrial via IRF3
Oxidative Stress. Mitochondrial oxidative stress upregulates the
expression of cGAMP and P53: cGAMP binds to STING and stabilizes
its dimers and oligomers; P53 inhibits SHP1 and SHP2, and promotes
the expression of both SYK and EGFR. STING interacts with EGFR,
leading to the autophosphorylation of EGFR and activation of SYK,
which then phosphorylates STING and EGFR at Y240 and Y245 sites,
respectively. SYK and EGFR phosphorylate STING at the Y240 and Y245
sites, respectively, promoting its translocation to ERGIC for
signaling. cGAMP, cyclic GMP-AMP; EGFR, epidermal growth factor;
HPE, human placental extracts; IFN, interferon; IRF3, IFN
regulatory factor 3; OVA, ovalbumin; SHP, Src homology 2-containing
phosphatase; SYK, spleen tyrosine kinase. |
Discussion
AR is a chronic inflammatory disease of the nasal
mucosa in sensitized individuals caused by a type I
hypersensitivity reaction upon exposure to common allergens
(24). The cascade of allergic
inflammation serves a crucial role in the pathogenesis of AR.
Inhaled allergens enter the nasal mucosa epithelial cells,
triggering the release of a series of chemokines and the
recruitment of immature dendritic cells, upregulating histone
deacetylase activity, damaging tight junction proteins and
compromising the integrity of the nasal mucosal epithelial barrier,
thereby increasing the likelihood of allergen invasion (the process
by which an allergen breaks through the epithelial barrier of the
nasal mucosa, enters the body, and triggers an immune response) and
exacerbating the allergic inflammatory response (25).
The results of the present study can be summarized
as follows: i) OVA promotes the production of mitochondrial ROS
(the present study examined the expression of MDA and NOX4), which
in turn promotes the action of P53 (26). Mitochondrial damage leads to the
production of cyclic GMP-AMP (cGAMP) (27), which binds to STING and mediates
the inflammatory response. ii) HPE inhibits ROS activity (the
present study examined the expression of MDA and NOX4) and promotes
AMPK phosphorylation, which further acts on NF-κB to promote
SHP1/SHP2 expression, thereby inhibiting the inflammatory response
(9).
After OVA induces AR, it causes cells to produce
large amounts of ROS, leading to oxidative stress (28). The main source of ROS is the
mitochondrial respiratory chain, and when mitochondria are
stimulated by external factors, the cellular ROS level increases.
Excessive ROS, in turn, causes mitochondrial damage, which leads to
more severe oxidative stress (29). When mitochondrial damage occurs,
mitochondrial DNA (mtDNA) may be released from the mitochondria
into the cytoplasm. In the cytoplasm, cGAS can recognize and bind
to the free mtDNA; when cGAS binds to mtDNA, it undergoes a
conformational change, which activates cGAS. Activated cGAS can
then catalyze the synthesis of cGAMP from ATP and GTP (30). Meanwhile, high levels of ROS can
cause DNA damage, which activates P53, and P53 can influence ROS
levels by regulating a series of reactions. Upon activation, P53
directs the cell to repair DNA damage or induce apoptosis to
prevent damaged cells from continuing to proliferate; however, in
certain situations, such as under stress conditions, activation of
P53 can lead to further increases in ROS levels, forming a positive
feedback loop that enhances P53 activation and the apoptotic
process. Spleen tyrosine kinase (SYK) is a non-receptor tyrosine
kinase involved in signal transduction and regulation of cellular
functions. It has previously been shown that P53 can directly or
indirectly influence SYK expression through transcriptional
regulatory mechanisms (31).
Epidermal growth factor receptor (EGFR) is an important receptor
tyrosine kinase involved in the regulation of cell proliferation,
differentiation and survival. In certain cases, P53 and the EGFR
signaling pathway may coordinate to regulate cellular stress
responses and inflammatory responses (32). In the present study, P53 promoted
SYK transcription, which acted on the STING phosphorylation site,
and P53 also acted on the EGFR promoter, thereby affecting the
STING phosphorylation site, as shown in Figs. 3 and 4). As a second messenger, cGAMP binds to
and activates STING on the endoplasmic reticulum membrane.
Activated STING translocates to the Golgi apparatus, and recruits
and activates multiple downstream effectors, including TBK1 and
IKKε (33). These effectors
further phosphorylate and activate IFN regulatory factor 3 (IRF3),
and phosphorylated IRF3 acts on the promoters of inflammatory
factors, such as NLRP3 and IFNα/β, leading to their upregulation,
further promoting ROS expression and forming a vicious cycle
(34–36).
HPE is rich in active substances such as peptides,
cytokines and amino acids. Peptides promote cell signaling, enhance
cell proliferation and differentiation, and thus contribute to
tissue repair and regeneration. Amino acids are the basic building
blocks of proteins, and serve key roles in wound healing,
anti-inflammation and antioxidant processes. Growth factors,
including EGF and insulin-like growth factor, accelerate wound
healing and tissue regeneration by activating receptors on the cell
surface and promoting matrix protein synthesis (37). Therefore, the antioxidant effect of
HPE may be due to the action of amino acids (38). HPE acts on cells through
endocytosis to treat AR. The western blot and ELISA assays also
showed that HPE inhibited ROS activity, and bioinformatics analysis
revealed that HPE promotes AMPK phosphorylation, and p-AMPK
promotes NF-κB activity (Figs. 3
and 4). In a non-inflammatory
state, activated AMPK promotes the expression of antioxidant
factors through NF-κB and inhibits STING expression via SHP1,
thereby reducing the inflammatory response (39). In an inflammatory state, STING
activates NF-κB, exacerbating the inflammatory response. AMPK
inhibits TBK1 activity by promoting SHP2 expression, indirectly
inhibiting the STING signaling pathway. ROS inhibits AMPK
phosphorylation, reducing the AMPK-induced promotion of NF-κB,
thereby indirectly affecting the inflammatory response; P53
inhibits SHP1 and SHP2, relieving inhibition of STING and TBK1,
thereby enhancing the inflammatory response, so the two pathways,
SHP2/SHP1 and STING/TBK1, mutually inhibit each other (39,40).
In conclusion, HPE may inhibit AR by modulating
AMPK/SHP1/SHP2/STING signaling. This provides a therapeutic target
for the treatment of AR; however, relevant clinical studies are
needed to provide a more robust basis for this conclusion. This
study illustrates that HPEs have potential clinical significance,
such as providing new therapeutic strategies for prostate cancer or
allergic diseases, and may be suitable for long-term use and
personalized therapy due to their multi-target modulation and
natural compound advantages. However, relevant clinical trials are
needed to provide a stronger basis for the conclusions of this
study.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
BW contributed to the conception and design of the
study, organized the AR dataset GSE44037 and wrote the first draft
of the manuscript. SL conducted statistical analysis of the data.
ZL and XL carried out experiments. All authors participated in the
revision of the manuscript and read and approved the final version
of the manuscript. BW and SL confirm the authenticity of all the
raw data.
Ethics approval and consent to
participate
The present study was approved by the Animal
Protection and Use Committee of Hebei Medical University (approval
no. IACUC-Hebmu-P-2024024).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siddiqui ZA, Walker A, Pirwani MM, Tahiri
M and Syed I: Allergic rhinitis: Diagnosis and management. Br J
Hosp Med (Lond). 83:1–9. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schuler Iv CF and Montejo JM: Allergic
rhinitis in children and adolescents. Pediatr Clin North Am.
66:981–993. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pyun BJ, Lee JY, Kim YJ, Ji KY, Jung DH,
Park KS, Jo K, Choi S, Jung MA, Kim YH and Kim T: Gardenia
jasminoides Attenuates Allergic Rhinitis-induced inflammation by
inhibiting periostin production. Pharmaceuticals (Basel).
14:9862021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bjermer L, Westman M, Holmström M and
Wickman MC: The complex pathophysiology of allergic rhinitis:
Scientific rationale for the development of an alternative
treatment option. Allergy Asthma Clin Immunol. 15:242019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
De D, Chakraborty PD and Bhattacharyya D:
Regulation of trypsin activity by peptide fraction of an aqueous
extract of human placenta used as wound healer. J Cell Physiol.
226:2033–2040. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee JO, Jang Y, Park AY, Lee JM, Jeong K,
Jeon SH, Jin H, Im M, Kim JW and Kim BJ: Human Placenta Extract
(HPH) suppresses inflammatory responses in TNF-α/IFN-γ-Stimulated
HaCaT cells and a DNCB atopic dermatitis (AD)-like mouse model. J
Microbiol Biotechnol. 34:1969–1980. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hackethal J, Weihs AM, Karner L, Metzger
M, Dungel P, Hennerbichler S, Redl H and Teuschl-Woller AH: Novel
human Placenta-based extract for vascularization strategies in
tissue engineering. Tissue Eng Part C Methods. 27:616–632. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gwam C, Ohanele C, Hamby J, Chughtai N,
Mufti Z and Ma X: Human placental extract: A potential therapeutic
in treating osteoarthritis. Ann Transl Med. 11:3222023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Samiei F, Jamshidzadeh A, Noorafshan A and
Ghaderi A: Human placental extract ameliorates structural lung
changes iinduced by amiodarone in rats. Iran J Pharm Res. 15 (Suppl
1):S75–S82. 2016.PubMed/NCBI
|
|
10
|
Lee YK, Chung HH and Kang SB: Efficacy and
safety of human placenta extract in alleviating climacteric
symptoms: Prospective, randomized, double-blind, placebo-controlled
trial. J Obstet Gynaecol Res. 35:1096–1101. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang W, Lu J, Wang Y, Sun P, Gao T, Xu N,
Zhang Y and Xie W: Canagliflozin attenuates lipotoxicity in
cardiomyocytes by inhibiting inflammation and ferroptosis through
activating AMPK pathway. Int J Mol Sci. 24:8582023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang J, Sun Y, Tang K, Xu H, Xiao J and
Li Y: RGC32 promotes the progression of ccRCC by activating the
NF-κB/SHP2/EGFR signaling pathway. Aging (Albany NY). May
27–2024.(Epub ahead of print).
|
|
13
|
Wang D, Paz-Priel I and Friedman AD:
NF-kappa B p50 regulates C/EBP alpha expression and inflammatory
cytokine-induced neutrophil production. J Immunol. 182:5757–5762.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bi L, Yu Z, Wu J, Yu K, Hong G, Lu Z and
Gao S: Honokiol inhibits constitutive and inducible STAT3 signaling
via PU.1-Induced SHP1 expression in acute myeloid leukemia cells.
Tohoku J Exp Med. 237:163–172. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li BL, Zhao DY, Du PL, Wang XT, Yang Q and
Cai YR: Luteolin alleviates ulcerative colitis through SHP-1/STAT3
pathway. Inflamm Res. 70:705–717. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun Z, Liu Q, Lv Z, Li J, Xu X, Sun H,
Wang M, Sun K, Shi T, Liu Z, et al: Targeting macrophagic SHP2 for
ameliorating osteoarthritis via TLR signaling. Acta Pharm Sin B.
12:3073–3084. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang B, Zeng M, Zhang Q, Wang R, Jia J,
Cao B, Liu M, Guo P, Zhang Y, Zheng X and Feng W: Ephedrae Herba
polysaccharides inhibit the inflammation of ovalbumin induced
asthma by regulating Th1/Th2 and Th17/Treg cell immune imbalance.
Mol Immunol. 152:14–26. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nur Husna SM, Md Shukri N, Tuan Sharif SE,
Tan HTT, Mohd Ashari NS and Wong KK: IL-4/IL-13 axis in allergic
rhinitis: Elevated serum cytokines levels and inverse association
with tight junction molecules expression. Front Mol Biosci.
9:8197722022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shao YY, Zhou YM, Hu M, Li JZ, Chen CJ,
Wang YJ, Shi XY, Wang WJ and Zhang TT: The Anti-allergic rhinitis
effect of traditional Chinese medicine of Shenqi by regulating mast
cell degranulation and Th1/Th2 cytokine balance. Molecules.
22:5042017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Murray PJ and Wynn TA: Protective and
pathogenic functions of macrophage subsets. Nat Rev Immunol.
11:723–737. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hirano M, Ogita-Nakanishi H, Miyachi W,
Hannya N, Yamamoto-Kimoto Y, Sakurai K, Miyoshi-Higashino M,
Tashiro-Yamaji J, Kato R, Ijiri Y, et al: Essential role of
macrophages in the initiation of allergic rhinitis in mice
sensitized intranasally once with cedar pollen: Regulation of class
switching of immunoglobulin in B cells by controlling interleukin-4
production in T cells of submandibular lymph nodes. Microbiol
Immunol. 56:392–405. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saradna A, Do DC, Kumar S, Fu QL and Gao
P: Macrophage polarization and allergic asthma. Transl Res.
191:1–14. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qu J, Sun Y, Liang N, Li C, Huang Q, Wang
M, Wang D and Zhou B: Histopathological characteristics and
inflammatory cell infiltration in sinonasal inverted papilloma. Am
J Rhinol Allergy. 39:21–31. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang H, Ren Y, Liang H and Liu X, Nan J,
Zhao H and Liu X: Mechanism of TCONS_00147848 regulating apoptosis
of nasal mucosa cells and alleviating allergic rhinitis through
FOSL2-mediated JAK/STAT3 signaling pathway. Sci Rep. 11:159912021.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Geng B, Dilley M and Anterasian C:
Biologic therapies for allergic rhinitis and nasal polyposis. Curr
Allergy Asthma Rep. 21:362021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou Y, Que KT, Zhang Z, Yi ZJ, Zhao PX,
You Y, Gong JP and Liu ZJ: Iron overloaded polarizes macrophage to
proinflammation phenotype through ROS/acetyl-p53 pathway. Cancer
Med. 7:4012–4022. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang C, Sharma N, Veleeparambil M, Kessler
PM, Willard B and Sen GC: STING-Mediated interferon induction by
herpes simplex Virus 1 requires the protein tyrosine kinase syk.
mBio. 12:e03228212021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Meng L, Hao D, Liu Y, Yu P, Luo J, Li C,
Jiang T, Yu J, Zhang Q, Liu S and Shi L: LRRC8A drives NADPH
oxidase-mediated mitochondrial dysfunction and inflammation in
allergic rhinitis. J Transl Med. 22:10342024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Drazdauskaitė G, Layhadi JA and Shamji MH:
Mechanisms of allergen immunotherapy in allergic rhinitis. Curr
Allergy Asthma Rep. 21:22020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wen X, Tang L, Zhong R, Liu L, Chen L and
Zhang H: Role of mitophagy in regulating intestinal oxidative
damage. Antioxidants (Basel). 12:4802023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiménez-Loygorri JI, Villarejo-Zori B,
Viedma-Poyatos Á, Zapata-Muñoz J, Benítez-Fernández R, Frutos-Lisón
MD, Tomás-Barberán FA, Espín JC, Area-Gómez E, Gomez-Duran A and
Boya P: Mitophagy curtails cytosolic mtDNA-dependent activation of
cGAS/STING inflammation during aging. Nat Commun. 15:8302024.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ho TLF, Lee MY, Goh HC, Ng GYN, Lee JJH,
Kannan S, Lim YT, Zhao T, Lim EKH, Phua CZJ, et al: Domain-specific
p53 mutants activate EGFR by distinct mechanisms exposing
tissue-independent therapeutic vulnerabilities. Nat Commun.
14:17262023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zajkowicz A, Gdowicz-Kłosok A, Krześniak
M, Janus P, Łasut B and Rusin M: The Alzheimer's disease-associated
TREM2 gene is regulated by p53 tumor suppressor protein. Neuroscie
Lett. 681:62–67. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yum S, Li M, Fang Y and Chen ZJ: TBK1
recruitment to STING activates both IRF3 and NF-κB that mediate
immune defense against tumors and viral infections. Proc Natl Acad
Sci USA. 118:e21002251182021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tanaka Y and Chen ZJ: STING specifies IRF3
phosphorylation by TBK1 in the cytosolic DNA signaling pathway. Sci
Signal. 5:ra202012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Duan N, Zhang Y, Tan S, Sun J, Ye M, Gao
H, Pu K, Wu M, Wang Q and Zhai Q: Therapeutic targeting of
STING-TBK1-IRF3 signalling ameliorates chronic stress induced
depression-like behaviours by modulating neuroinflammation and
microglia phagocytosis. Neurobiol Dis. 169:1057392022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu T, He J, Yan S, Li J, Chen K, Zhang D,
Cheng M, Xiang Z and Fang Y: Human placental extract suppresses
mast cell activation and induces mast cell apoptosis. Allergy
Asthma Clin Immunol. 19:982023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jang SY, Park JW, Bu Y, Kang JO and Kim J:
Protective effects of hominis placenta hydrolysates on radiation
enteropathy in mice. Nat Prod Res. 25:1988–1992. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu C, Wang X, Qin W, Tu J, Li C, Zhao W,
Ma L, Liu B, Qiu H and Yuan X: Combining radiation and the ATR
inhibitor berzosertib activates STING signaling and enhances
immunotherapy via inhibiting SHP1 function in colorectal cancer.
Cancer Commun (Lond). 43:435–454. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ding H and Wu R: The Role of SHP2 in
advancing COPD: Insights into oxidative stress, endoplasmic
reticulum stress, and pyroptosis. Altern Ther Health Med. Apr
18–2024.(Epub ahead of print).
|