Introduction
Atherosclerosis (AS) is one of the most common
causes of cardiovascular disease (1), responsible for ~62% of global
cardiovascular disease-associated mortality (23.6 million deaths
annually), as reported in the 2025 American Heart Association Heart
Disease and Stroke Statistics (2).
AS is a multifactorial chronic inflammatory disease in which
lipids, inflammatory and smooth muscle cells and necrotic cell
debris accumulate in the arterial intima and low-density
lipoprotein (LDL) accumulates under the endothelium, forming
atheromatous lipid-containing necrotic foci that promote the AS
process (3). Its occurrence is
associated with dyslipidemia, lipid metabolism dysfunction, smoking
and obesity (4). Elevated LDL
cholesterol levels caused by lipid metabolism disorders are a key
risk factor for AS, as concentrations >0.5–1.0 mmol/l (20–40
mg/dl) increase the likelihood of LDL retention in the intima in a
dose-dependent manner, promoting the initiation and progression of
AS plaques and markedly raising the risk of cardiovascular disease
(5). LDL oxidative modification is
a key promoter of AS (6). Studies
have shown that oxidized LDL promotes reactive oxygen species (ROS)
production in endothelial and smooth muscle cells and macrophages,
inhibits endothelial nitric oxide synthase activity in endothelial
cells and enhances platelet activity (7,8).
Therefore, the decrease of ROS levels and enhancing antioxidant
capacity, particularly by mitigating the oxidative modification of
LDL, serve a key role in the prevention and treatment of AS.
Xylitol is a sweetener that is often used as a
substitute for glucose and is naturally present in fruits and
vegetables. Studies have revealed that xylitol can markedly prevent
dental caries and decrease gum inflammation (9). Additionally, xylitol has been found
to decrease postprandial hyperglycemia, which can help manage
diabetes, obesity and metabolic syndrome (10). Chukwuma and Islam (11) demonstrated that xylitol exerts
antioxidant effects by increasing expression of antioxidant enzymes
in normal rats and in rats with type 2 diabetes, suggesting the
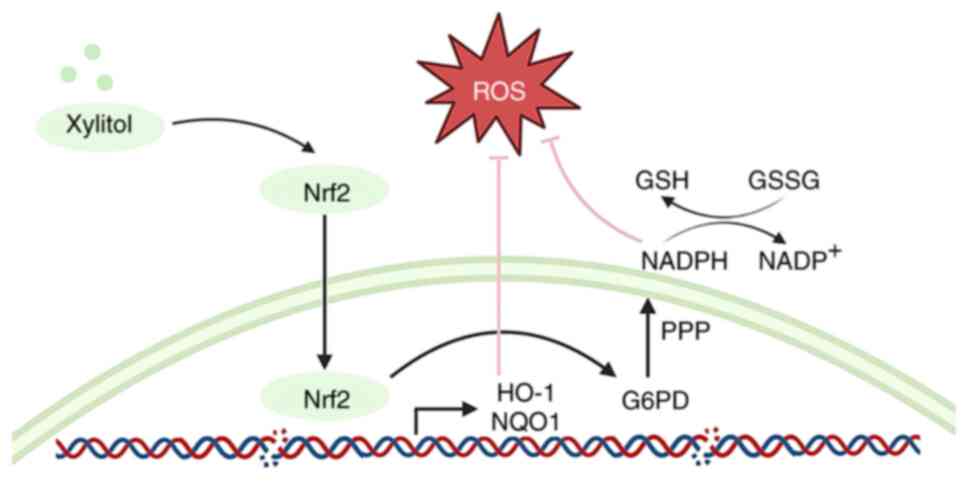
antioxidant potential of xylitol. The pentose phosphate pathway
(PPP) is a glucose-oxidizing pathway that produces ribose
5-phosphate and NADPH (Fig. 1)
(12). Xylitol is also involved in
PPP metabolism through the polyol pathway, and PPP serves a key
role in suppressing oxidative stress through NADPH (13). Oxidative stress is a key factor in
the development of AS. By decreasing the levels of oxidative stress
(14), xylitol may help attenuate
the progression of AS. The present study aimed to assess whether
xylitol mitigates oxidative stress in AS cell models and influences
LDL oxidation and to elucidate the underlying molecular
mechanisms.
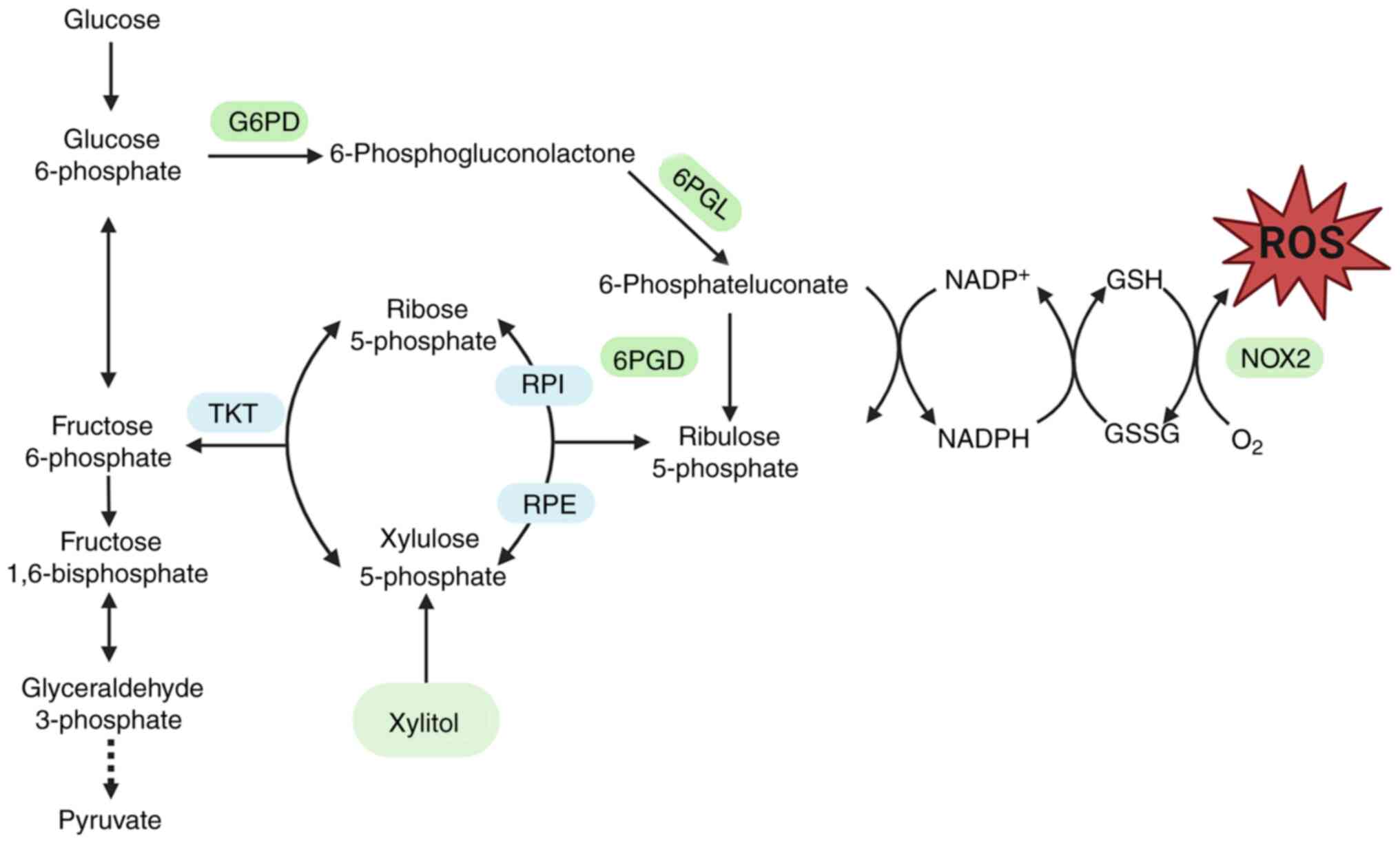 | Figure 1.Metabolites and enzymes in glycolysis
and oxidative PPP. Xylitol activates the PPP by upregulating the
expression of key enzymes, thereby influencing oxidative stress.
ROS, reactive oxygen species; G6PD, glucose-6-phosphate
dehydrogenase; TKT, transketolase; 6PGL, 6-phosphogluconolactonase;
RPI, ribose 5-phosphate isomerase; RPE, ribulose 5-phosphate
epimerase; GSH, glutathione; GSSG, glutathione disulfide; PPP,
pentose phosphate pathway. |
Materials and methods
Cells, culture and treatment
Tohoku Hospital Pediatrics-1 (THP-1) cells, a human
monocyte cell line, were supplied by Procell Life Science &
Technology Co., Ltd., and verified by STR analysis. THP-1 cells
between passages 4 and 10 post-thawing were used. THP-1 cells were
maintained as suspension cultures and subcultured at 90% confluence
(approximately 1×106 cells/ml) by replacing half of the
spent medium with fresh complete medium. THP-1 cells at a density
of 70% were divided into three groups: Untreated control, model
(100 µg/ml LDL) and experimental (100 µg/ml LDL + 100 mM xylitol;
molecular weight, 152.146 g/mol, Cat#B20885; Shanghai Yuanye
Biotechnology Co., Ltd.). THP-1 specialized medium was purchased
from Procell Life Science & Technology Co., Ltd.
Bioinformatics analysis
Raw gene expression profile of dataset GSE54666 was
downloaded from the Gene Expression Omnibus (GEO;
ncbi.nlm.nih.gov/geo/). The GSE54666 dataset is a transcriptomic
dataset collected using Illumina gene microarrays after treating
primary human monocyte-derived macrophages with oxidized LDL for 48
h. This dataset was used to screen for differentially expressed
genes associated with AS to explore potential molecular
mechanisms.
To extract key information from the gene expression
microarray, the AnnoProbe package in R software (R-project.org;
version 4.2.3) was used to convert probe IDs into gene symbols. If
a gene symbol corresponded to multiple probe IDs, the average
expression of the probes was calculated as the representative
expression of the gene. Group analysis was performed using Python
software (version 3.8.5; python.org/downloads/release/python-385/)
and the limma package (v3.46.0) in R, with screening thresholds set
at fold change ≥1.5 and P<0.05. Subsequently, the
clusterProfiler package was used for Gene Ontology (GO;
geneontology.org/) and Kyoto Encyclopedia of Genes and Genomes
(KEGG; enome.jp/kegg/) enrichment analyses, as well as gene set
enrichment analysis (GSEA; gsea-msigdb.org/gsea/index.jsp).
Finally, the ggplot2 package (version 3.3.5; ggplot2.tidyverse.org)
was used for visualization.
Hoechst 33342/PI staining
Cells were fixed with 4% paraformaldehyde (PFA)
(Sigma-Aldrich) at room temperature for 15 min. A total of 1 ml
working solution per sample was prepared, consisting of 5 µl each
PI and Hoechst 33342 staining solution (Beijing Solarbio Science
& Technology Co., Ltd.) and 990 µl RPMI-1640 medium (Gibco).
Cells were centrifuged at 8,000 × g for 2 min at 4°C, the medium
was discarded and the cells were resuspended in working solution
then incubated in a 37°C cell culture incubator for 20 min. The
cells were washed twice with PBS and resuspended in 100 µl PBS. A
10 µl aliquot of the resuspended cell solution was carefully placed
on a glass slide and examined under a fluorescence microscope
(Olympus IX73) at 200× magnification.
Cell Counting Kit-8 (CCK-8) assay
Cells were plated in 96-well plates at a density of
~5,000 cells/well and cultured at 37°C in a 5% CO2
humidified incubator. The cells were then treated with xylitol at
concentrations of 25, 50, 100, 200 and 400 mM. After 24 h, 10 µl
CCK-8 reagent (Beijing Solarbio Science & Technology Co., Ltd.)
was added for 2.5 h. The absorbance (A) at 450 nm was detected
using an EON microplate reader (BioTek Instruments, Inc.) Cell
viability was calculated as follows: Cell viability=(A
test-A blank)/(A
control-Ablank).
Western blot analysis
THP-1 cells were washed with PBS three times and
lysed to extract total protein using RIPA lysis buffer (Beyotime
Institute of Biotechnology; containing 1% protease and phosphatase
inhibitor cocktail and 1 mmol/l PMSF). Following protein
quantification using the BCA method, an equal volume of 2X loading
buffer was added, followed by mixing boiling at 100°C and
denaturation for 10 min, immediately cooled on ice, and stored at
−20°C until further use. A total of 30 µg protein lysate per lane
was separated using 8–15% gels by SDS-PAGE, transferred to a PVDF
membrane, blocked with 5% skimmed milk in TBST (0.1% Tween-20) for
2 h at 25°C, the membranes were incubated with primary antibodies
(Table I; β-actin, 1:2,000; all
others 1:800) overnight at 4°C. The membrane was washed four times
with 1X TBST for 10 min each time, and horseradish peroxidase
(HRP)-conjugated secondary antibody (1:10,000) was added at room
temperature for 2 h. After washing four times with 1X TBST, ECL
(Beyotime Institute of Biotechnology, Cat#P0018S) was used for
advanced development. β-actin was used as the internal reference
protein and the gray values of protein bands were analyzed using
ImageJ software 1.53 (National Institutes of Health).
 | Table I.Primary antibodies. |
Table I.
Primary antibodies.
| Antibody | Supplier | Catalog No. |
|---|
| Nrf2 | Zenbioscience | R380773 |
| p-Nrf2 | ABclonal | AP1133 |
| G6PD | ABclonal | A1537 |
| PGD | ABclonal | A0563 |
| TKT | ABclonal | A6314 |
| HO-1 | ABclonal | A1346 |
| HRP-conjugated goat
anti-Rabbit IgG | ABclonal | AS014 |
| HRP-conjugated Goat
anti-Mouse IgG | ABclonal | AS003 |
| β-actin | Sanjian |
|
|
| Biotechnology | KM9006T |
Malondialdehyde (MDA), glutathione
(GSH) and ROS assay
MDA content was quantified using a commercial assay
kit (Beyotime Institute of Biotechnology, Cat# S0131S) based on the
chromogenic reaction between MDA) and thiobarbituric acid followed
by colorimetric analysis, according to the manufacturer's protocol.
The absorbance was measured at 532 nm using the EON microplate
reader (Sartorius) and a standard curve was plotted to calculate
the MDA content.
GSH content was measured using a GSH assay kit
(Beijing Solarbio Science & Technology Co., Ltd.; cat. no.
BC1175) according to the manufacturer's instructions (Beijing
Solarbio Science & Technology Co., Ltd.). The absorbance was
measured at 412 nm using the EON microplate reader and a standard
curve was plotted to calculate the GSH content of the samples.
Dichlorodihydrofluorescein diacetate (DCFH-DA) was
diluted in serum-free RPMI-1640 at a ratio of 1:2,000. Cells were
resuspended in the diluted DCFH-DA solution at a concentration of
~5×106 cells/ml, followed by incubation at 37°C for 20
min. During incubation, the cells were gently mixed every 3–5 min.
Cells were washed with PBS three times and the fluorescence
intensity was measured using a CytoFLEX flow cytometry (Beckman
Coulter, Model: CytoFLEX S; Software: CytExpert 2.4;
beckmancoulter.com/products/flow-cytometry/cytoflex/software) to
detect ROS levels.
Measurement of superoxide dismutase
(SOD) and glucose-6-phosphate dehydrogenase (G6PD) activity
SOD activity was assessed using a commercially
available kit (Nanjing Jiancheng Bioengineering Institute,
Cat#A001-3-1) based on the autooxidation of hydroxylamine,
according to the manufacturer's instructions. The absorbance was
measured at 450 nm using the EON microplate reader (Sartorius) G6PD
activity was assessed using a commercially available kit (Shanghai
Enzyme-linked Biotechnology Co. Ltd., Cat#MC8C5L). A total of 10
sample and 190 µl working solution were added to a 96-well plate,
mixed and the absorbance at 340 nm (A1) was read using the EON
microplate reader. Subsequently, the absorbance at 340 nm (A2) was
read following incubation for 6 min at 37°C. G6PD activity was
calculated as follows: G6PD enzyme activity=1,286 ×
(A2-A1)/Cpr.
NADPH and NADP+ content
assay
NADPH and NADP+ contents were measured
using a coenzyme II (NADPH, NADP+) content test kit
(Nanjing Jiancheng Bioengineering Institute; cat#A115-1-1)
according to the manufacturer's instructions (Nanjing Jiancheng
Bioengineering Institute). Following centrifugation at 300 × g for
5 min at 4°C in a 1.5 ml microcentrifuge tube, ~5×106
cells were collected, followed by the addition of 0.5 ml alkaline
extraction buffer. The cells were sonicated on ice for 90 sec (2
sec on, 1 sec off) and boiled at 100°C for 5 min. The cells were
cooled on ice and centrifuged at 10,000 × g for 10 min at 4°C. A
total of 250 µl supernatant was neutralized with an equal volume of
acidic extraction buffer and centrifuged again at 12,000 × g for 5
min at 4°C. The protein concentration was measured using the BCA
method. Absorbance was measured at 570 nm using a
spectrophotometer, with double-distilled water as the blank
control. The NADPH content was calculated as follows: NADPH content
(nmol/mg protein)=0.8 × (ΔA-0.0259)/protein concentration.
For the measurement of the intracellular
NADP+ content, following centrifugation at 300 × g for 5
min at 4°C, ~5×106 cells were collected into an EP tube
followed by the addition of 0.5 ml acidic extraction buffer. The
cells were sonicated and centrifuged as aforementioned. A total of
250 µl supernatant was transferred to a new EP tube, neutralized
with an equal volume of alkaline extraction buffer and centrifuged
again at 12,000 × g for 5 min at 4°C. The protein concentration was
measured using the BCA method. The reaction and detection were
carried out as aforementioned. The NADP+ content was
calculated as follows: NADP+ content (nmol/mg
protein)=5.1 × (ΔA-0.0144)/protein concentration.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from approximately
5×106 THP-1 cells using TRIzol reagent (Thermo Fisher
Scientific, Inc.) and reverse-transcribed using a PrimeScript RT
reagent kit (Takara Biotechnology Co., Ltd.) according to the
manufacturer's instructions. qPCR was conducted on a Bio-Rad CFX96
system using the SYBR qPCR master mix (Toyobo Co., Ltd.) according
to the manufacturer's protocol. Thermocycling conditions were as
follows: Initial denaturation at 95°C for 5 min; followed by 40
cycles of denaturation at 95°C for 15 sec, annealing at 60°C for 30
sec and extension at 72°C for 30 sec. Analysis of relative gene
expression data was performed via the 2-ΔΔCq method with β-actin as
the endogenous control (15). RNA
purity was assessed by measuring the A260/A280 ratio (1.8–2.0)
using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific)
to confirm the absence of protein or solvent contamination. To
ensure experimental reproducibility in downstream procedures, RNA
concentrations were adjusted to 1 µg/µl using nuclease-free water
(Thermo Fisher Scientific, Cat# AM9937). Primer-BLAST (v2.12.0;
ncbi.nlm.nih.gov/tools/primer-blast/) was used to design the
primers (Table II).
 | Table II.Reverse transcription-quantitative
PCR primers. |
Table II.
Reverse transcription-quantitative
PCR primers.
| Gene | Forward primer, 5′
to 3′ | Reverse primer (5′
to 3′) |
|---|
| SOD1 |
ACAAAGATGGTGTGGCCGAT |
AACGACTTCCAGCGTTTCCT |
| SOD2 |
GCACTAGCAGCATGTTGAGC |
CCGTTAGGGCTGAGGTTTGT |
| G6PD |
CGACGACGAAGCGCAGA |
TGAAGGTGTTTTCGGGCAGA |
| PGD |
GCTCTTCGGTTCTGCTCTGT |
TCTCGGCACCGTCTCAATTT |
| TKT |
ACTTCGACAAGGCCAGCTAC |
GCCCAGGCGATTGATGTCTA |
| HO-1 |
AAGATTGCCCAGAAAGCCCTGGAC |
AACTGTCGCCACCAGAAAGCTGAG |
| Nrf2 |
CAGTCAGCGACGGAAAGAGTA |
CTGGGAGTAGTTGGCAGATCC |
| NQO1 |
GCTGGTTTGAGCGAGTGTTC |
CTGCCTTCTTACTCCGGAAGG |
| KEAP1 |
CCTCTGGCCGGGTAATAGG |
CCCCTCCCAGGTATCCAAGA |
| Nox1 |
TAAAGGCTCACAGACCCTGC |
AGCCTAACCAAACAACCAGA |
| Nox2 |
TGTGTGAATGCCCGAGTCAA |
GCCCACGTACAATTCGTTCAG |
| Nox4 |
CAGATGTTGGGGCTAGGATTG |
GAGTGTTCGGCACATGGGTA |
| β-actin |
CTTCGCGGGCGACGAT |
CCACATAGGAATCCTTCTGACC |
Nuclear and cytoplasmic protein
extraction
To investigate xylitol-induced nuclear translocation
of Nrf2 by western blotting, nuclear and cytoplasmic protein
fractions were isolated using a Nuclear and Cytoplasmic Protein
Extraction kit (Solarbio, Cat# EX1470). A total of
~5×106 treated THP-1 cells were washed with PBS and
centrifuged at 500 × g for 2–3 min at 4°C. Subsequently, the
supernatant was aspirated, followed by the addition of 100 µl
plasma protein extraction reagent (Solarbio, Cat# EX1470), mixing
by blowing with a pipette and vortexing at 14 × g for 30 sec at 4°C
to obtain a single cell suspension. Following 10 min in an ice
bath, the suspension was vortexed at 14 × g for 30 sec at 4°C and
centrifuged at 16,000 × g for 10 min at 4°C. The supernatant
containing cytoplasmic protein was stored at −80°C for subsequent
analysis. To isolate nuclear proteins, the remaining pellet was
resuspended in 50 µl nuclear protein extraction reagent (Solarbio,
Cat# EX1470) by pipette mixing (10× with a 200 µl tip) followed by
vortexing at 14 × g for 30 sec at 4°C. After 10 min incubation on
ice, the suspension was vortexed (14 × g for 10 sec at 4°C) and
centrifuged at 16,000 × g for 10 min at 4°C. The resulting nuclear
protein supernatant was collected for downstream applications. The
nuclear protein supernatant was either immediately processed or
stored at −80°C for future use, while the cytoplasmic fraction was
preserved at −80°C.
Statistical analysis
All data are presented as the mean ± SD of ≥3
experiments performed in parallel. Statistical analyses were
performed using one-way analysis of variance followed by
Bonferroni's multiple comparison test using GraphPad Prism 10
software (Dotmatics, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
PPP is a Key Metabolic Signature in
Atherosclerosis
Gene differential expression analysis was performed
for the control and AS model groups, followed by GO and KEGG
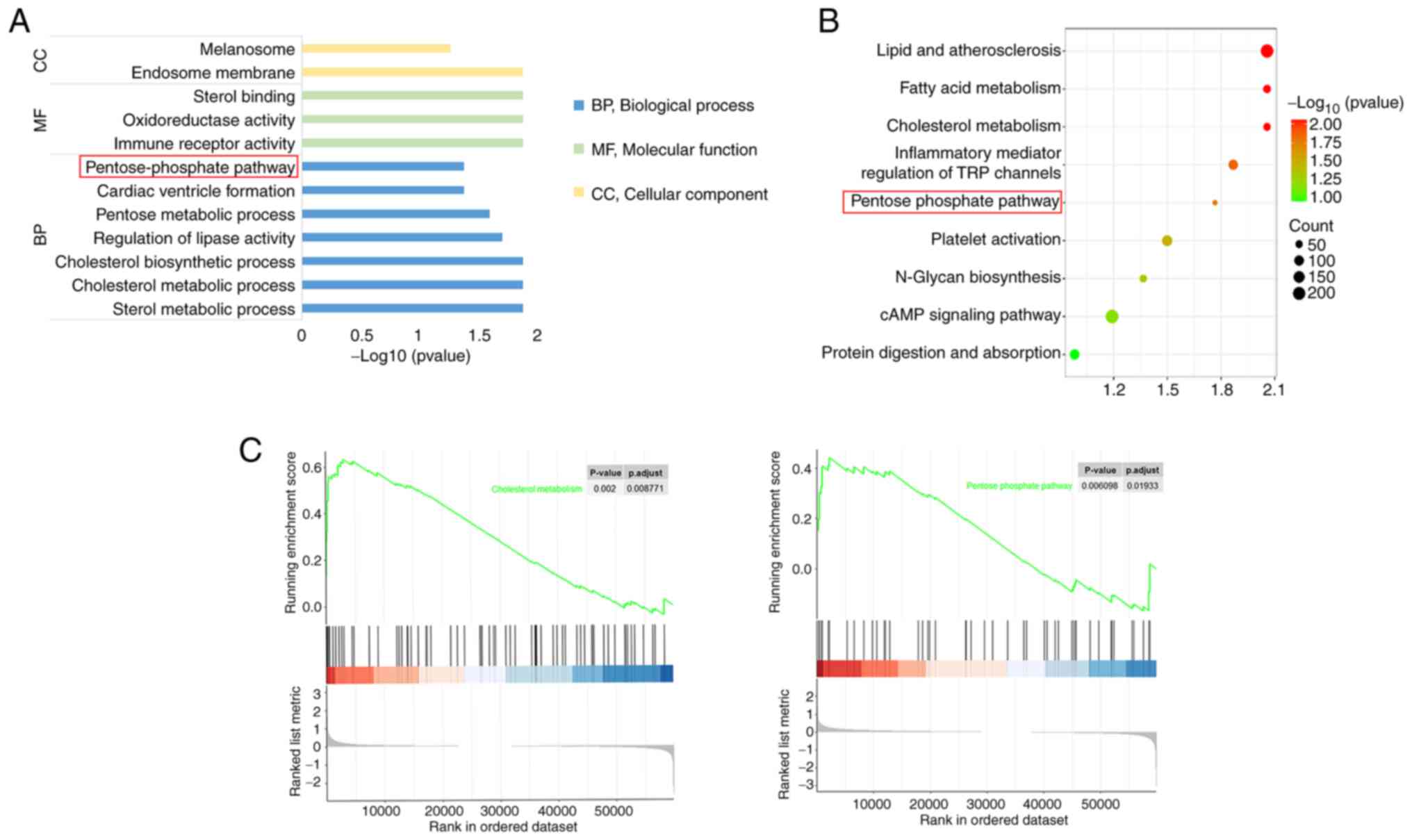
analyses. GO enrichment analysis revealed that the differentially
expressed genes were enriched in ‘endosome membrane’ and
‘melanosome’, molecular functions, such as ‘immune receptor
activity’, ‘oxidoreductase activity’ and ‘sterol binding’
molecules, and ‘pentose-phosphate pathway’ (Fig. 2A). KEGG analysis (Fig. 2B) demonstrated that several
pathways, including ‘pentose-phosphate pathway’, were enriched. To
explore the expression of the PPP in the AS model group, GSEA was
performed out to examine the differences in expression between the
control and AS model groups (Fig.
2C). Analysis revealed that the PPP had enrichment score (ES)
>0.4, indicating significant upregulation in the model group,
suggesting that AS was positively associated with the PPP.
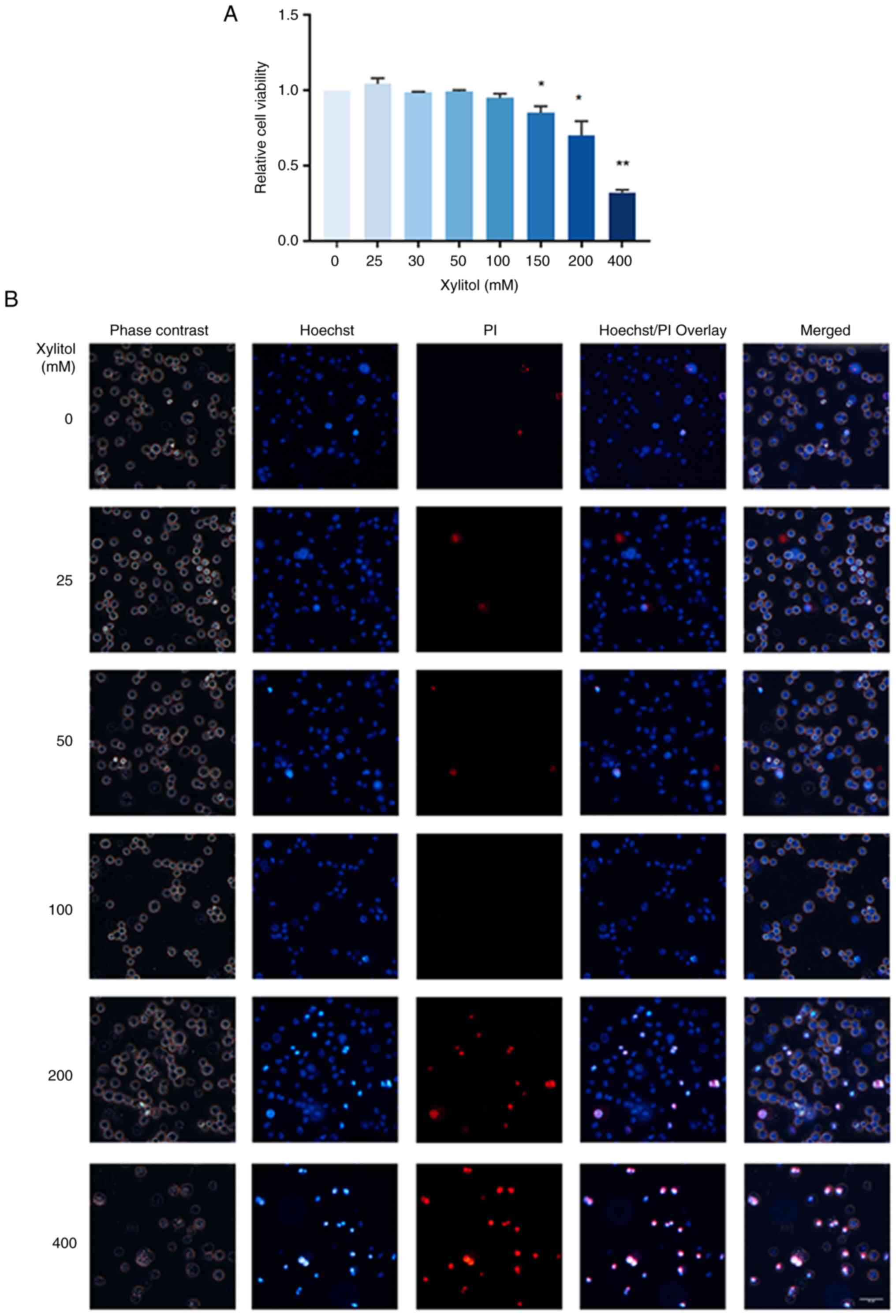
Optimal concentration of xylitol
Based on the CCK-8 assay, the optimal concentration
of xylitol was determined. Xylitol significantly affected THP-1
cell viability in a concentration-dependent manner (Fig. 3A) at ≥150 mM. Cell viability was
not notably affected by xylitol treatment at low concentrations
(0–100 mM; Fig. 3B). However, at
concentrations ≥200 mM, the viability of the cells decreased and
apoptosis and necrosis increased. Therefore, 100 mM xylitol was
used as the highest concentration in subsequent experiments.
Xylitol inhibits oxidative stress in
THP-1 cells induced by high levels of LDL
Levels of oxidative stress were assessed by
detecting the MDA content. MDA levels were unaltered across
experimental groups exposed to xylitol concentrations ranging from
100 µM to 10 mM, demonstrating no substantial assay interference
from the polyol across the tested concentration spectrum (Fig. S1). Compared with the control, the
MDA levels were significantly increased in the LDL-treated group.
Conversely, co-incubation with LDL and xylitol led to a significant
decrease in MDA levels compared with the LDL group (Fig. 4A). DCFH-DA probe to detect levels
of ROS demonstrated increased relative fluorescence quantification
in the native LDL group compared with the control, suggesting
elevated levels of ROS (Fig. 4B and
C). However, following the addition of xylitol, the relative
fluorescence quantification decreased, accompanied by a leftward
peak shift relative to the LDL group, indicating decreased levels
of ROS in the THP-1 cells. GSH levels, a key antioxidant for
cellular regulation (16), were
increased in the LDL + xylitol compared with the LDL-only group
(Fig. 4D). These findings
suggested that xylitol mitigated oxidative stress induced by high
LDL levels in THP-1 cells.
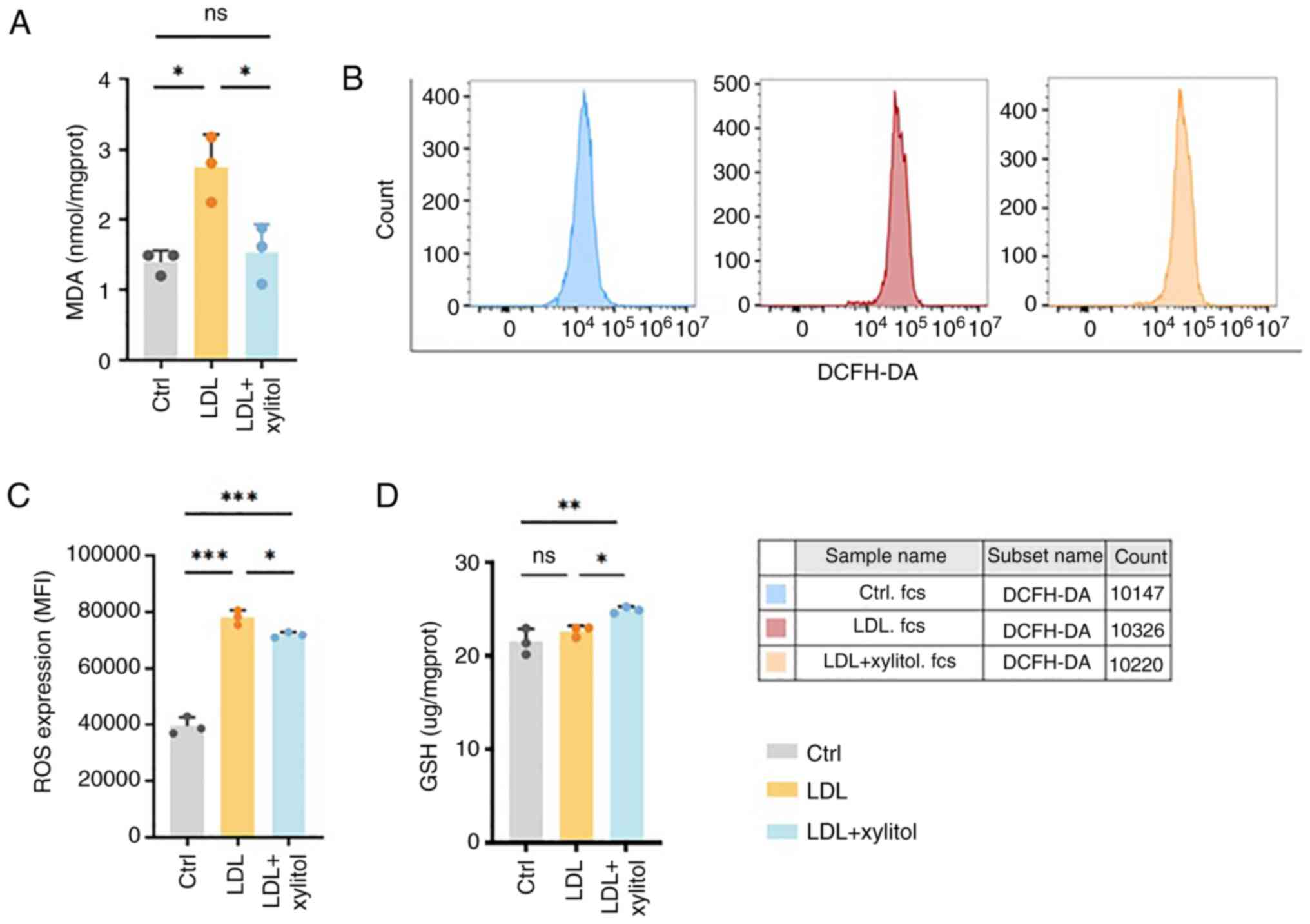 | Figure 4.Effect of xylitol on LDL oxidation in
THP-1 cells. (A) MDA content. (B) Flow cytometry. (C) Quantified
relative fluorescence. (D) GSH levels. *P<0.05, **P<0.01,
***P<0.001. LDL, low-density lipoprotein; MDA, malondialdehyde;
GSH, glutathione; ROS, reactive oxygen species; DCFH-DA,
dichlorodihydrofluorescein diacetate; MFI, mean fluorescence
intensity; ns, not significant; ctrl, control; prot, protein. |
Xylitol decreases intracellular LDL
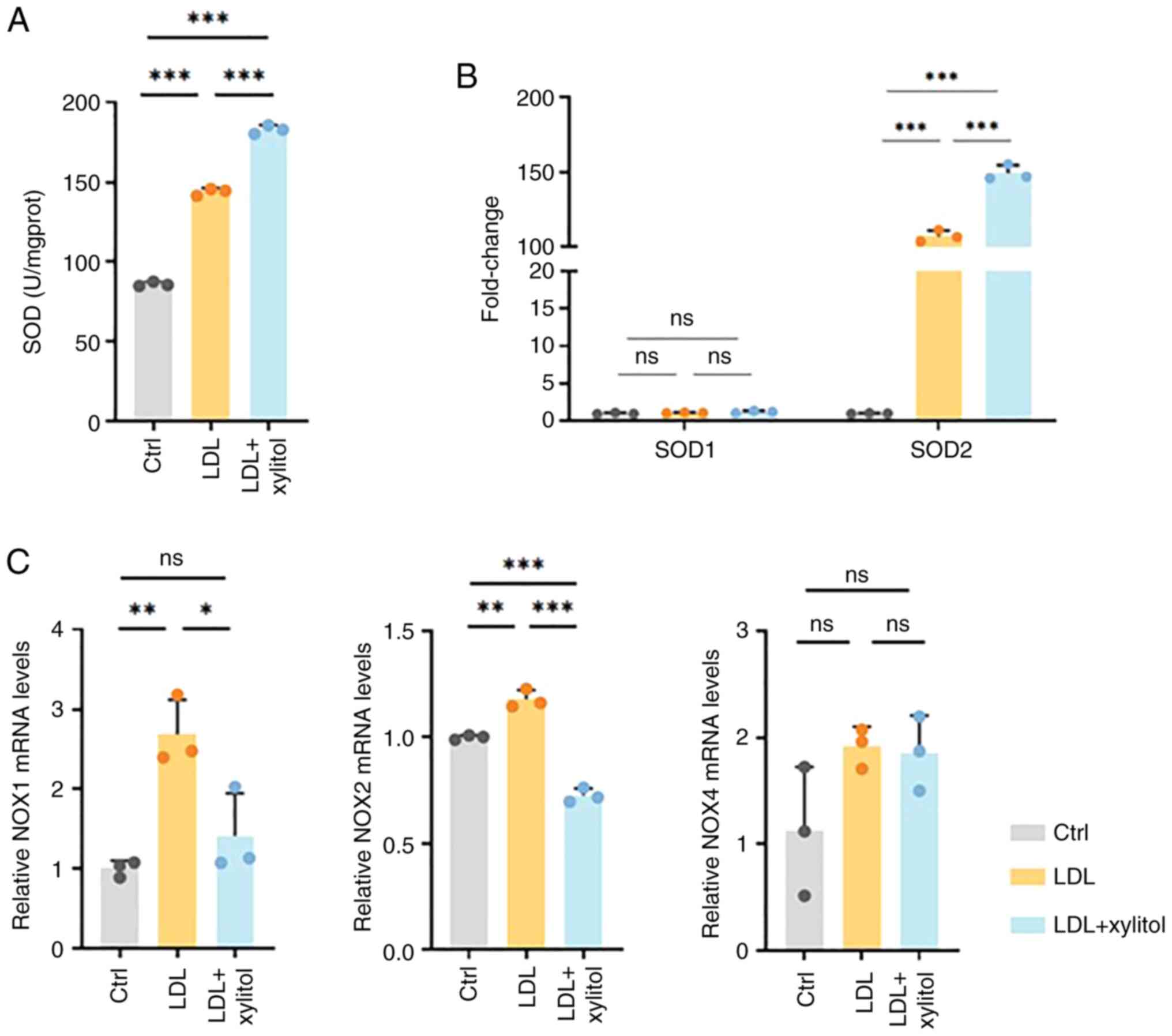
oxidation in THP-1 cells through the NADPH and SOD systems
Intracellular SOD activity increased in the LDL-only
compared with the control group, and this elevation was further
amplified by xylitol co-treatment (Fig. 5A). There was no significant
alteration in SOD1 mRNA expression in the LDL + xylitol
co-incubation group compared with that in the LDL group. However,
SOD2 mRNA expression was significantly upregulated (Fig. 5B). Compared to the control group,
LDL treatment significantly elevated oxidative stress markers NOX1
and NOX2, while NOX4 showed no significant alteration (Fig. 5C). These increased levels indicated
an increase in oxidative stress. Following the addition of xylitol,
the mRNA levels of NOX1 and NOX2 decreased significantly, whereas
the mRNA expression of NOX4 was not significantly altered.
Xylitol decreases intracellular LDL
oxidation in THP-1 cells by activating the PPP
Compared with the LDL group, xylitol resulted in the
upregulation of G6PD protein and mRNA (Fig. 6A-C). G6PD/TKT expression after LDL
treatment was not significantly altered compared with control.
Furthermore, G6PD enzyme activity significantly increased (Fig. 6D). 6-phosphogluconate dehydrogenase
(PGD) is the second enzyme in the oxidative phase of the PPP
(17). Protein and RNA levels of
PGD significantly increased, indicating that xylitol significantly
promoted the upregulation of molecules associated with the
oxidative phase of PPP (Fig.
6A-C). Transketolase (TKT) is a thiamine diphosphate-dependent
enzyme that catalyzes reversible reactions in the non-oxidative
branch of PPP and serves as a bridge between PPP and glycolysis
(18). TKT catalyzes the
conversion of xylulose-5-phosphate (X5P) and ribulose-5-phosphate,
which are key enzymes for the participation of the xylitol
metabolite X5P in the PPP (19). A
significant increase in both the protein and mRNA levels of TKT was
observed following the addition of xylitol compared with the model
group, suggesting that xylitol promoted expression of molecules
associated with the non-oxidative phase of the PPP, but TKT
expression showed no significant difference between the LDL-treated
and control groups (Fig. 6A-C).
NADPH, a key product of the PPP, and its derivative glutathione
disulfide, which is reduced to glutathione (GSH) by glutathione
reductase, serve key roles in cellular antioxidant defense
(13). Xylitol increased NADPH and
NADP+ levels compared with the LDL model group (Fig. 6E). As shown in Fig. 6E, the NADPH/NADP+ ratio
was significantly decreased in the LDL-treated group compared with
the control, whereas xylitol supplementation restored this ratio to
levels exceeding those of the LDL-only group. These results
suggested that the addition of xylitol promoted the upregulation of
the PPP and the expression of key molecules in both the oxidative
and non-oxidative phases at both the protein and mRNA levels, which
promoted the NADPH/NADP+ ratio and attenuated the
oxidation of LDL in THP-1 cells.
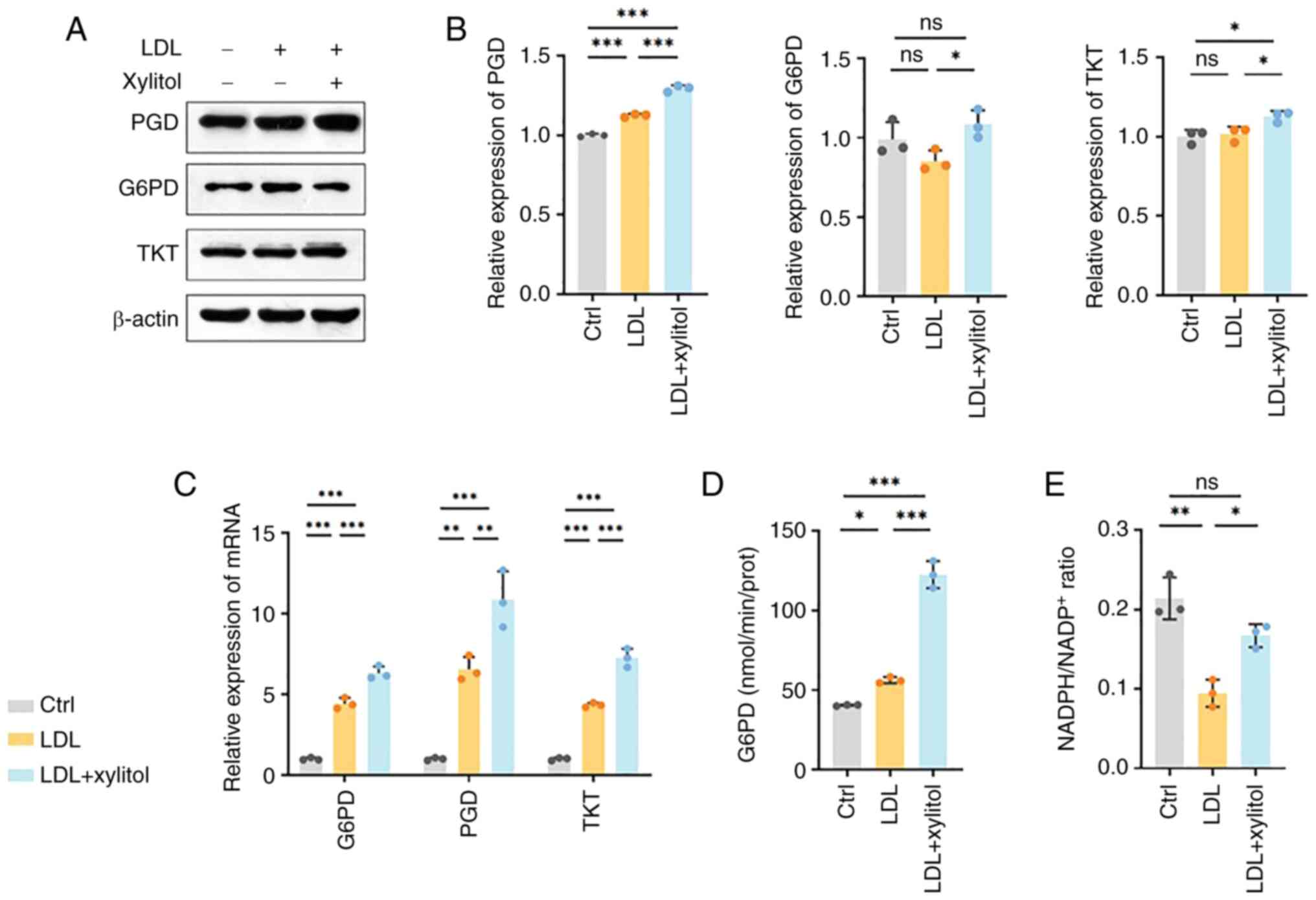 | Figure 6.Xylitol activates the pentose
phosphate pathway. (A) Representative western blot bands of (B)
PGD, G6PD and TKT. (C) mRNA expression levels of G6PD, PGD and TKT.
(D) Enzyme activity of G6PD. (E) NADPH/NADP+ ratio.
*P<0.05, **P<0.01, ***P<0.001. PGD, phosphogluconate
dehydrogenase; G6PD, glucose-6-phosphate dehydrogenase; TKT,
transketolase; LDL, low-density lipoprotein; ctrl, control; ns, not
significant; prot, protein. |
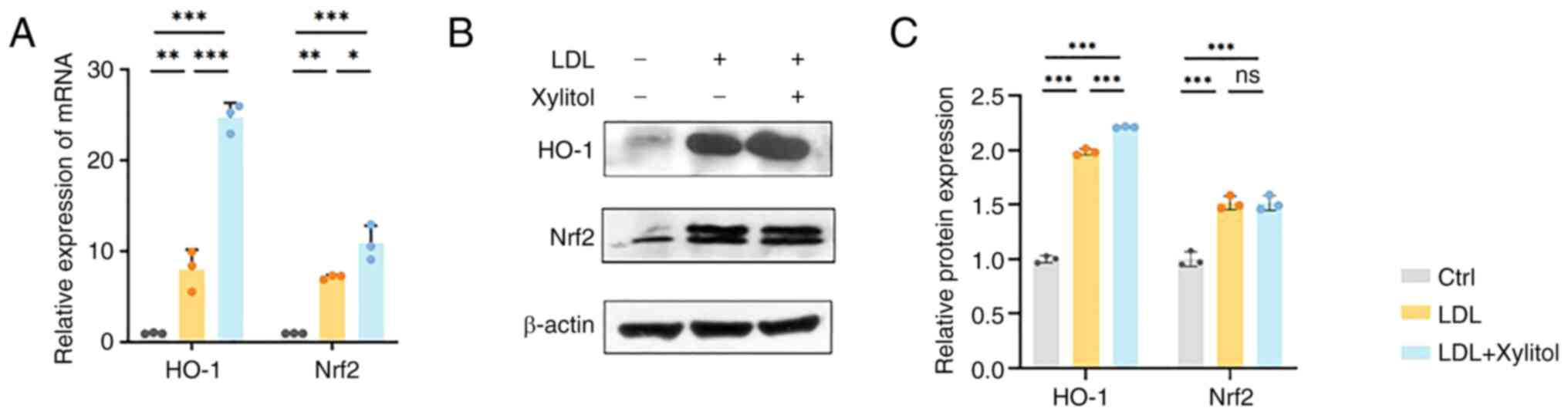
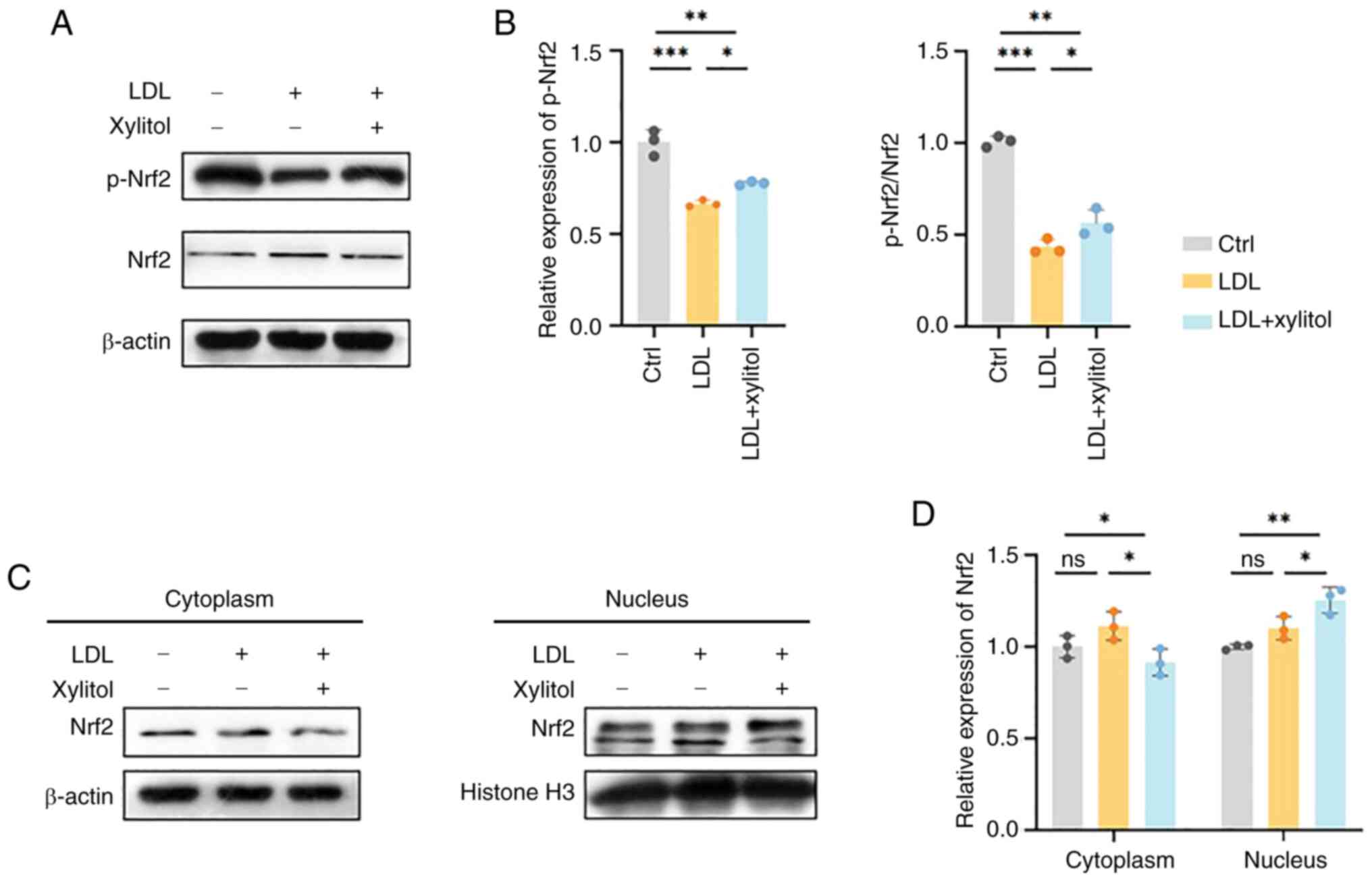
Xylitol promotes Nrf2/heme oxygenase-1
(HO-1) signaling to reduce LDL oxidation in THP-1 cells
As Nrf2 is a transcription factor that regulates the
PPP and redox homeostasis in cells and G6PD is a target gene of
Nrf2 (20), the present study
investigated whether Nrf2 regulates LDL oxidation in THP-1 cells.
Xylitol significantly increased the mRNA expression of Nrf2
compared with that in the LDL group. The mRNA levels of HO-1 (the
target gene of Nrf2) exhibited a similar trend (Fig. 7A). Xylitol significantly increased
the HO-1 protein levels compared with the LDL group (Fig. 7B). Although LDL and xylitol
co-treatment elevated Nrf2 mRNA expression compared with LDL alone,
total Nrf2 protein levels showed no significant difference between
the two groups (Fig. 7C).
Xylitol decreases oxidative stress by
promoting phosphorylation and nuclear isomerization of Nrf2
The phosphorylation of Nrf2 affects the charge
properties that modulate DNA-binding activity (21). As there was no difference in the
expression of Nrf2 protein in the LDL and the LDL and xylitol
co-incubation group, but the Nrf2 mRNA levels increased and there
were changes in expression of target genes of Nrf2, the protein
levels of phosphorylated (p-)Nrf2 was examined. Xylitol
significantly increased the p-Nrf2 protein levels compared with the
LDL group (Fig. 8A and B). Nrf2
increases the transcription of antioxidant proteins by binding
antioxidant response elements, which are translocated to the
nucleus to exert their effects (22). Therefore, the present study
investigated whether xylitol promoted the cytosolic translocation
of Nrf2. However, compared with the LDL group, the expression level
of Nrf2 was increased in the cytoplasm following the addition of
xylitol and expression of Nrf2 was decreased in THP-1 cells,
suggesting that xylitol altered nucleocytoplasmic shuttling rather
than canonical activation of Nrf2 (Fig. 8C and D).
Discussion
Xylitol attenuates LDL oxidative modification in
THP-1 macrophages via Nrf2-dependent transcriptional regulation of
the pentose phosphate pathway, resulting in increased
NADPH/NADP+ ratio, activation of the Nrf2/HO-1
antioxidant axis, and consequent reduction of intracellular ROS
(Fig. 9).
Xylitol is metabolized to D-xylulose 5-phosphate
(C5H11O8P), an essential component
of the PPP (23). Xylitol
undergoes polyol metabolism, and its products are involved in the
PPP. Increased NADPH levels in the PPP decrease oxidative stress.
According to Chukwuma and Islam (11), xylitol exerts antioxidant effects
and ameliorates oxidative stress in a rat model of diabetes.
Therefore, the present study investigated whether xylitol has
similar effects in an in vitro AS model and whether it
influences cellular oxidation levels via the PPP.
Bioinformatics analysis identified changes in the
PPP in AS. Park et al (24)
treated THP-1 cells with xylitol for 24 or 48 h and found that
concentrations ≥197 mmol/l caused a decrease in cell viability
(24), which was consistent with
the results of the present study. Dose-optimization experiments
demonstrated that 100 mM xylitol (the highest concentration tested)
achieved maximal PPP modulation without cytotoxicity. This
concentration was therefore selected for all subsequent
experiments.
Oxidative stress is implicated in the pathogenesis
of AS and there is increasing evidence to indicate that it serves a
key role in its development (25,26).
MDA is a primary end product of membrane lipid peroxidation during
cellular oxidative stress (27).
The present study revealed that MDA levels in THP-1 cells increased
following LDL stimulation, while co-incubation with xylitol
significantly decreased MDA levels, indicating that xylitol
alleviated LDL-induced cellular oxidative damage. Xylitol is a
five-carbon sugar alcohol with relatively weak reducing activity.
Although xylitol exhibits reducing properties, its chemical
structure results in low reactivity with thiobarbituric acid, the
reagent used in the MDA assay, leading to minimal interference
(10). The intracellular GSH
levels in THP-1 cells increased in the LDL + xylitol group compared
with the LDL model group. In a study by Abulizi et al
(28) on serum from a rat model of
AS, the GSH levels decreased in the AS model group compared with
the control. By contrast, in the present study, in the LDL model
group, intracellular GSH levels did not decrease, potentially as it
was in the early compensatory state of regulating oxidation levels.
ROS levels in THP-1 cells were measured. Following LDL stimulation,
the peak of the fluorescence signal appeared to shift to the right
and the oxidation level increased. Following the addition of
xylitol, the peaks shifted to the left compared with those of the
LDL group, indicating that the addition of xylitol reduced ROS
levels and oxidation of LDL in THP-1 cells. Future studies should
use electron spin resonance to enable real-time detection of ROS to
determine the underlying molecular mechanisms.
NOXs are specialized enzymes responsible for
producing ROS, with their activation resulting in the formation of
superoxide (29). To date, seven
NOX isoforms (NOX1, NOX2, NOX3, NOX4, NOX5, Duox1 and Duox2) have
been identified. These enzymes participate in cellular processes
such as signal transduction in cellular stress responses, with
isoform expression being cell-specific and each NOX exhibiting
unique physiological and pathological roles (30). Studies have shown that the
expression of NOX1 in human aortic smooth muscle cells increases
following stimulation with oxidized LDL, which increases the
intracellular oxidation and participates in the occurrence of AS
(31,32). Moreover, NOX2 is a primary source
of ROS and its increased expression is associated with plaque
development (33). NOX2-mediated
oxidative stress homeostasis is key in AS (34). Therefore, the present study
examined the mRNA expression of NOX1, NOX2 and NOX4 in the NADPH
oxidase system and found that xylitol decreased the mRNA levels of
NOX1 and NOX2. SOD, a key endogenous antioxidant enzyme, has a key
role in cardiovascular diseases. SOD2 is associated with
development of AS, and SOD2 deficiency under hyperlipidemic
conditions leads to increased mitochondrial oxidative stress in
mice, which induces AS plaque destabilization (35). In the present study, co-treatment
of xylitol with LDL resulted in a significant increase in SOD
enzyme activity and an increase in SOD2 mRNA levels compared with
the LDL group. The present study demonstrated that xylitol reduced
LDL oxidation in THP-1 cells by increasing the activity and subunit
mRNA levels of the antioxidant SOD system and decreased the subunit
mRNA levels of NOX system.
NADPH is a key intracellular antioxidant required
for the maintenance of redox homeostasis by the GSH system and
other ROS scavengers, and the primary source of cytoplasmic NADPH
is the PPP (36). The present
study indicated that xylitol upregulated the protein and mRNA
levels of key regulatory enzymes, G6PD and PGD, in the PPP.
Furthermore, an increase in content of G6PD was observed. G6PD, the
first rate-limiting enzyme in the oxidative branch of the PPP,
serves a key role in generating NADPH and regenerating GSH, thereby
decreasing ROS levels (37).
Reduced NADPH levels in patients with G6PD deficiency leads to
impaired GSH regeneration and oxidative damage in red blood cells
(12). The elevation of G6PD and
PGD levels suggests enhanced oxidative pathways in the PPP.
Additionally, NADPH/NADP+ ratio increased significantly.
Subsequently, the present study investigated the protein and mRNA
levels of TKT, a non-oxidative pathway enzyme in PPP, which
exhibited elevated levels. Collectively, these findings indicated
that xylitol activates the PPP by increasing the expression levels
of key enzymes, thereby increasing the NADPH/NADP+ ratio
and ameliorating high LDL-induced oxidative stress in THP-1
cells.
Nrf2 is a key transcription factor that regulates
oxidative stress (38). Under
physiological conditions, Nrf2 undergoes ubiquitination and
degradation, mediated by its specific inhibitor, kelch-like
ECH-associated protein 1 (KEAP1). However, under stress, Nrf2
dissociates from KEAP1, translocates from the cytoplasm to the
nucleus and binds antioxidant response elements, thereby activating
the transcription of antioxidant defense genes such as HO-1,
leading to the rapid clearance of ROS and the alleviation of
oxidative damage (39).
Furthermore, Nrf2 regulates GSH synthesis (40). Nrf2 directly activates G6PD by
binding antioxidant response elements in the G6PD promoter
(41). HO-1 serves as an Nrf2
regulatory gene; the Nrf2/HO-1 system has a key role in AS and is a
notable defense mechanism against cardiovascular disease (42). In a previous study, increased
Nrf2/HO-1 signaling was observed in monocyte-derived macrophages
within patients with cardiovascular disease when compared with
healthy individuals (43). The
present study demonstrated that following xylitol supplementation,
the mRNA levels of Nrf2 and its target gene, HO-1, increased.
However, while the expression of HO-1 increased at the protein
level, there was no change in the total Nrf2 protein levels.
Therefore, the present study examined the expression of p-Nrf2 and
observed an increase at the protein level. Further investigation
revealed an increase in nuclear Nrf2 and a decrease in cytoplasmic
Nrf2 expression following xylitol supplementation compared with the
LDL model group, with no change in the total Nrf2 expression. These
results suggested that xylitol decreased LDL-induced oxidative
stress in THP-1 cells by promoting the activation of Nrf2/HO-1
signaling and facilitating Nrf2 phosphorylation and nuclear
translocation.
In conclusion, the present study demonstrated that
xylitol significantly decreased oxidative stress induced by high
levels of LDL in THP-1 cells, as well as ROS and MDA levels and NOX
family enzyme expression, and increased antioxidant enzyme SOD
activity and expression and GSH levels. This was achieved by
regulating the PPP via the Nrf2 transcription factor to increase
the NADPH/NADP+ ratio and activate the Nrf2/HO-1 axis to
decrease oxidative modification of LDL in THP-1 cells.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Natural Science Foundation of
Hubei Province, China (grant no. 2024AFC016).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JW and YL designed the study. ZH, AL, MS, RH, RY, WW
and ZH performed experiments and analyzed data. ZH wrote the
manuscript. All authors have read and approved the final
manuscript. ZH and AL confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AS
|
Atherosclerosis
|
|
THP-1
|
Tohoku Hospital Pediatrics-1
|
|
GO
|
Gene Ontology
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
GSEA
|
Gene Set Enrichment Analysis
|
|
GEO
|
Gene Expression Omnibus
|
|
DCFH-DA
|
dichlorodihydrofluorescein
diacetate
|
|
G6PD
|
glucose-6-phosphate dehydrogenase
|
|
HO-1
|
heme oxygenase 1
|
|
LDL
|
low-density lipoprotein
|
|
PGD
|
phosphogluconate dehydrogenase
|
|
PPP
|
pentose phosphate pathway
|
|
TKT
|
transketolase
|
|
ROS
|
reactive oxygen species
|
|
CCK-8
|
Cell Counting Kit-8
|
|
MDA
|
malondialdehyde
|
|
GSH
|
glutathione
|
|
SOD
|
superoxide dismutase
|
|
RT-q
|
reverse transcription-quantitative
|
|
ES
|
enrichment score
|
|
KEAP1
|
kelch-like ECH-associated protein
1
|
References
|
1
|
Wang B, Tang X, Yao L, Wang Y, Chen Z, Li
M, Wu N, Wu D, Dai X, Jiang H and Ai D: Disruption of USP9X in
macrophages promotes foam cell formation and atherosclerosis. J
Clin Invest. 132:e1542172022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Martin SS, Aday AW, Allen NB, Almarzooq
ZI, Anderson CAM, Arora P, Avery CL, Baker-Smith CM, Bansal N,
Beaton AZ, et al: 2025 Heart disease and stroke statistics: A
report of US and global data from the American heart association.
Circulation. 151:e41–e660. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kong P, Cui ZY, Huang XF, Zhang DD, Guo RJ
and Han M: Inflammation and atherosclerosis: Signaling pathways and
therapeutic intervention. Signal Transduct Target Ther. 7:1312022.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Raitakari O, Pahkala K and Magnussen CG:
Prevention of atherosclerosis from childhood. Nat Rev Cardiol.
19:543–554. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ference BA, Ginsberg HN, Graham I, Ray KK,
Packard CJ, Bruckert E, Hegele RA, Krauss RM, Raal FJ, Schunkert H,
et al: Low-density lipoproteins cause atherosclerotic
cardiovascular disease. 1. Evidence from genetic, epidemiologic,
and clinical studies. A consensus statement from the European
atherosclerosis society consensus panel. Eur Heart J. 38:2459–2472.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kianmehr A, Qujeq D, Bagheri A and Mahrooz
A: Oxidized LDL-regulated microRNAs for evaluating vascular
endothelial function: Molecular mechanisms and potential biomarker
roles in atherosclerosis. Crit Rev Clin Lab Sci. 59:40–53. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yan R, Zhang X, Xu W, Li J, Sun Y, Cui S,
Xu R, Li W, Jiao L and Wang T: ROS-induced endothelial dysfunction
in the pathogenesis of atherosclerosis. Aging Dis. 16:250–268.
2024.(Epub ahead of print). PubMed/NCBI
|
|
8
|
Prasad K and Mishra M: Mechanism of
hypercholesterolemia-induced atherosclerosis. Rev Cardiovasc Med.
23:2122022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gasmi Benahmed A, Gasmi A, Arshad M,
Shanaida M, Lysiuk R, Peana M, Pshyk-Titko I, Adamiv S, Shanaida Y
and Bjørklund G: Health benefits of xylitol. Appl Microbiol
Biotechnol. 104:7225–7237. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Msomi NZ, Erukainure OL, Salau VF,
Olofinsan KA and Islam MS: Xylitol improves antioxidant, purinergic
and cholinergic dysfunction, and lipid metabolic homeostasis in
hepatic injury in type 2 diabetic rats. J Food Biochem.
46:e140402022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chukwuma CI and Islam S: Xylitol improves
anti-oxidative defense system in serum, liver, heart, kidney and
pancreas of normal and type 2 diabetes model of rats. Acta Pol
Pharm. 74:817–826. 2017.PubMed/NCBI
|
|
12
|
TeSlaa T, Ralser M, Fan J and Rabinowitz
JD: The pentose phosphate pathway in health and disease. Nat Metab.
5:1275–1289. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hayes JD, Dinkova-Kostova AT and Tew KD:
Oxidative stress in cancer. Cancer Cell. 38:167–197. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koutsaliaris IK, Moschonas IC, Pechlivani
LM, Tsouka AN and Tselepis AD: Inflammation, oxidative stress,
vascular aging and atherosclerotic ischemic stroke. Curr Med Chem.
29:5496–5509. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y, Liu S, Tomar A, Yen FS, Unlu G,
Ropek N, Weber RA, Wang Y, Khan A, Gad M, et al: Autoregulatory
control of mitochondrial glutathione homeostasis. Science.
382:820–828. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Patra KC and Hay N: The pentose phosphate
pathway and cancer. Trends Biochem Sci. 39:347–354. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stincone A, Prigione A, Cramer T, Wamelink
MM, Campbell K, Cheung E, Olin-Sandoval V, Grüning NM, Krüger A,
Tauqeer Alam M, et al: The return of metabolism: biochemistry and
physiology of the pentose phosphate pathway. Biol Rev Camb Philos
Soc. 90:927–963. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fullam E, Pojer F, Bergfors T, Jones TA
and Cole ST: Structure and function of the transketolase from
Mycobacterium tuberculosis and comparison with the human enzyme.
Open Biol. 2:1100262012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Smith MJ, Yang F, Griffiths A, Morrell A,
Chapple SJ, Siow RCM, Stewart T, Maret W and Mann GE: Redox and
metal profiles in human coronary endothelial and smooth muscle
cells under hyperoxia, physiological normoxia and hypoxia: Effects
of NRF2 signaling on intracellular zinc. Redox Biol. 62:1027122023.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang HC, Nguyen T and Pickett CB:
Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates
antioxidant response element-mediated transcription. J Biol Chem.
277:42769–42774. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang X, Yu M, Wang WK, Zhu LY, Wang X,
Jin HC and Feng LF: The regulation and function of Nrf2 signaling
in ferroptosis-activated cancer therapy. Acta Pharmacol Sin.
45:2229–2240. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vincent MF, Van den Berghe G and Hers HG:
D-xylulose-induced depletion of ATP and Pi in isolated rat
hepatocytes. FASEB J. 3:1855–1861. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park E, Na HS, Kim SM, Wallet S, Cha S and
Chung J: Xylitol, an anticaries agent, exhibits potent inhibition
of inflammatory responses in human THP-1-derived macrophages
infected with Porphyromonas gingivalis. J Periodontol.
85:e212–e223. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Batty M, Bennett MR and Yu E: The role of
oxidative stress in atherosclerosis. Cells. 11:38432022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Violi F, Pignatelli P and Valeriani E:
Oxidative stress and atherosclerosis: Basic and clinical open
issues. Kardiol Pol. 82:689–691. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cordiano R, Di Gioacchino M, Mangifesta R,
Panzera C, Gangemi S and Minciullo PL: Malondialdehyde as a
potential oxidative stress marker for allergy-oriented diseases: An
update. Molecules. 28:59792023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abulizi A, Simayi J, Nuermaimaiti M, Han
M, Hailati S, Talihati Z, Maihemuti N, Nuer M, Khan N, Abudurousuli
K, et al: Quince extract resists atherosclerosis in rats by
down-regulating the EGFR/PI3K/Akt/GSK-3β pathway. Biomed
Pharmacother. 160:1143302023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu Q, Choksi S, Qu J, Jang J, Choe M,
Banfi B, Engelhardt JF and Liu ZG: NADPH oxidases are essential for
macrophage differentiation. J Biol Chem. 291:20030–20041. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang F, Zhang Y and Dusting GJ: NADPH
oxidase-mediated redox signaling: Roles in cellular stress
response, stress tolerance, and tissue repair. Pharmacol Rev.
63:218–242. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yin CC and Huang KT: H2O2 but not
O2-elevated by oxidized LDL enhances human aortic smooth muscle
cell proliferation. J Biomed Sci. 14:245–254. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gimenez M, Schickling BM, Lopes LR and
Miller FJ Jr: Nox1 in cardiovascular diseases: Regulation and
pathophysiology. Clin Sci (Lond). 130:151–165. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vermot A, Petit-Härtlein I, Smith SME and
Fieschi F: NADPH oxidases (NOX): An overview from discovery,
molecular mechanisms to physiology and pathology. Antioxidants
(Basel). 10:8902021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Y, Liu XY, Wang Y, Zhao WX, Li FD,
Guo PR, Fan Q and Wu XF: NOX2 inhibition stabilizes vulnerable
plaques by enhancing macrophage efferocytosis via MertK/PI3K/AKT
pathway. Redox Biol. 64:1027632023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vendrov AE, Stevenson MD, Alahari S, Pan
H, Wickline SA, Madamanchi NR and Runge MS: Attenuated superoxide
dismutase 2 activity induces atherosclerotic plaque instability
during aging in hyperlipidemic mice. J Am Heart Assoc.
6:e0067752017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Masi A, Mach RL and Mach-Aigner AR: The
pentose phosphate pathway in industrially relevant fungi: Crucial
insights for bioprocessing. Appl Microbiol Biotechnol.
105:4017–4031. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen PH, Tjong WY, Yang HC, Liu HY, Stern
A and Chiu DT: Glucose-6-phosphate dehydrogenase, redox homeostasis
and embryogenesis. Int J Mol Sci. 23:20172022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
He F, Ru X and Wen T: NRF2, a
transcription factor for stress response and beyond. Int J Mol Sci.
21:47772020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sun YY, Zhu HJ, Zhao RY, Zhou SY, Wang MQ,
Yang Y and Guo ZN: Remote ischemic conditioning attenuates
oxidative stress and inflammation via the Nrf2/HO-1 pathway in MCAO
mice. Redox Biol. 66:1028522023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu J, Huang C, Liu J, Meng C, Gu Q, Du X,
Yan M, Yu Y, Liu F and Xia C: Nrf2 and its dependent autophagy
activation cooperatively counteract ferroptosis to alleviate acute
liver injury. Pharmacol Res. 187:1065632023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang YY, Chen J, Liu XM, Zhao R and Zhe H:
Nrf2-mediated metabolic reprogramming in cancer. Oxid Med Cell
Longev. 2018:93040912018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Alonso-Piñeiro JA, Gonzalez-Rovira A,
Sánchez-Gomar I, Moreno JA and Durán-Ruiz MC: Nrf2 and heme
oxygenase-1 involvement in atherosclerosis related oxidative
stress. Antioxidants (Basel). 10:14632021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fiorelli S, Porro B, Cosentino N, Di Minno
A, Manega CM, Fabbiocchi F, Niccoli G, Fracassi F, Barbieri MS,
Marenzi G, et al: Activation of Nrf2/HO-1 pathway and human
atherosclerotic plaque vulnerability:An in vitro and in vivo study.
Cells. 8:3562019. View Article : Google Scholar : PubMed/NCBI
|