Introduction
Sepsis, a life-threatening organ dysfunction caused
by a dysregulated host response to infection (1), remains a significant global health
burden despite the implementation of evidence-based clinical
guidelines and notable advances in its treatment. The high
mortality rate, prolonged hospital stay and substantial medical
costs associated with sepsis (2,3)
highlight the need for a deeper understanding of its complex and
multifaceted pathogenesis. Furthermore, the mechanisms underlying
its pathogenesis include immune dysregulation, complement system
inactivation, mitochondrial damage, endoplasmic reticulum stress
(ERS), autophagy, cell death and endothelial barrier disruption
(4).
Silent information regulator 1 (SIRT1), a member of
the sirtuins protein family, is a class III histone deacetylase
that is dependent on nicotinamide adenine dinucleotide
(NAD+). SIRT1 has been extensively studied for its role
in sepsis and found to be involved in modulating gene expression
and cellular processes (5).
Moreover, the SIRT1-mediated deacetylation of both histone and
non-histone proteins influences a wide range of cellular functions.
Recent studies have shed light on the intricate regulatory
mechanisms and signaling pathways modulated by SIRT1, offering new
insights into the pathophysiological processes of sepsis (6,7).
Research has demonstrated that SIRT1 plays a crucial role in the
inflammatory response, oxidative damage, apoptosis and metabolic
dysregulation associated with sepsis (8).
The present review focused on elucidating the role
of SIRT1 in sepsis pathogenesis based on the recent advances in the
understanding of the potential regulatory mechanisms and associated
signaling pathways of SIRT1 in sepsis. Additionally, it aimed to
provide new insights into the pathophysiological processes of
sepsis and explore potential novel therapeutic strategies.
SIRT1 and sepsis-induced inflammation
SIRT1 and inflammatory cells
Inflammatory cells, including macrophages (MQs),
dendritic cells (DCs) and neutrophils (NEUs), play a pivotal role
in the inflammatory response. As a deacetylase, SIRT1 modulates the
secretion of inflammatory cells, influencing their differentiation,
activation and maturation (5).
SIRT1 can inhibit the activation of nuclear transcription factor-κB
(NF-κB) by promoting the deacetylation of Akt, a serine/threonine
kinase, thereby decreasing the production of pro-inflammatory
cytokines and alleviating macrophage inflammation. Conversely, the
absence of SIRT1 leads to excessive acetylation of Akt,
exacerbating inflammatory cytokine production by MQs and promoting
sepsis progression (9). SIRT1 is
also involved in the inflammatory signaling of DCs, regulating the
balance between type 1 T helper cells and regulatory T cells
(10). DCs with SIRT1 knockout
inhibit regulatory T cell generation and promote type 1 T helper
cell development, leading to an enhanced T cell-mediated
inflammatory response against pathogens (11). A study found that SIRT1-deficient
murine model has reduced neutrophil infiltration at the infection
site, an immature phenotype shift in NEUs and a decrease in the
number of myeloperoxidase-positive NEUs, which have been
hypothesized to lead to impaired neutrophil function and pathogen
clearance (12).
SIRT1 and inflammatory mediators
Inflammatory mediators are pivotal in the
development of sepsis owing to their role in pathogen clearance;
however, their overactivation may lead to severe pathological
outcomes (13). Research indicates
that SIRT1 plays a significant role in regulating inflammatory
mediators, with its activation or enhanced expression effectively
mitigating inflammation and exerting anti-inflammatory effects
(14,15). Tumor necrosis factor α (TNF-α), a
pleiotropic pro-inflammatory cytokine produced by MQs and
monocytes, generates a critical cytokine storm in sepsis and its
levels are highly elevated (~10-fold compared with healthy
individuals) in sepsis patients (16). SIRT1 reduces TNF-α secretion by
deacetylating the NF-κB p65 subunit, thereby inhibiting
TNF-α-induced NF-κB transcriptional activation. Furthermore, SIRT1
overexpression markedly decreases the levels of the
pro-inflammatory cytokines, such as interleukin (IL)-1β, IL-6,
TNF-α and monocyte chemoattractant protein-1, alleviating
lipopolysaccharide (LPS)-induced inflammation and organ damage
(17).
SIRT1 and inflammatory signaling
pathways
SIRT1 negatively regulates inflammatory response via
various signaling pathways, particularly the NF-κB pathway. NF-κB,
a heterodimer consisting of p50 and p65 subunits, exists in an
inactive form in the cytoplasm and typically binds to the IκB
subunit (18). IκB kinase (IKK)
catalyzes the phosphorylation and subsequent degradation of the IκB
subunit, under the influence of various pro-inflammatory factors,
including IL-1β, IL-6 and TNF-α. This allows the NF-κB complex to
be released and rapidly translocated into the nucleus, where it
regulates the expression of inflammation-related genes (19). Studies have shown that SIRT1
inhibits the transcriptional activity of NF-κB by deacetylating
lysine (Lys)-310, a key site of the NF-κB p65 subunit, thereby
playing an anti-inflammatory role (20,21).
SIRT1 can also inhibit IKK activity, subsequently inhibiting IκB
degradation, thereby alleviating LPS-induced inflammation (22). In addition to confining the NF-κB
complex within the cytoplasm, SIRT1 impairs its interaction with
coactivators and RNA polymerase II, thus inhibiting gene
transcription. Moreover, SIRT1 indirectly inhibits NF-κB signaling
by modulating the expression of mediator proteins, including high
mobility group box 1 (HMGB1), adenosine monophosphate-activated
protein kinase (AMPK) and peroxisome proliferator-activated
receptors (23,24). A decrease or absence of SIRT1
activity increases NF-κB activity, further promoting inflammation
(25). These findings highlight
the intricate interplay between SIRT1 and the NF-κB signaling
pathway in modulating inflammatory response.
In sepsis, pathogenic microorganisms or endogenous
molecules activate NF-κB, promoting NOD-like receptor protein 3
(NLRP3) inflammasome formation and pro-IL-1β expression (26). Activation of NLRP3 inflammasome
further activates caspase-1, promoting the secretion of mature
pro-inflammatory cytokines, such as IL-1β, IL-18, TNF-α and
transforming growth factor-β. In addition to enhancing the
inflammatory response, these factors recruit immune cells to the
infection site and modulate the activity of adaptive immune cells
(27). However, excessive
activation of the NLRP3 inflammasome can lead to an uncontrolled
inflammatory response (28). Guo
et al (29) found that
upregulating SIRT1 expression and activity can induce NF-κB
deacetylation, inhibiting NLRP3 inflammasome-associated
transcription factors and decreasing inflammasome assembly and
activation in a sepsis murine model. This reduction in inflammasome
activity decreases the maturation and secretion of IL-1β and IL-18,
improving myocardial inflammatory status and decreasing myocardial
cell apoptosis and damage.
Activator protein-1 (AP-1) is a key transcription
factor (TF) in the inflammatory response and is composed of c-Jun
and c-Fos proteins. SIRT1 can deacetylate specific Lys residues
(such as Lys271) of the c-Jun protein, decreasing AP-1 activity and
subsequently inhibiting the expression of inflammatory genes.
Additionally, SIRT1 may indirectly regulate AP-1 activity by
affecting other signaling pathways, including the mitogen-activated
protein kinase pathway, further affecting the inflammatory response
(23).
SIRT1 and non-coding RNAs in sepsis
SIRT1 has been found to interact with various
non-coding RNAs, including long non-coding RNAs, microRNAs
(miRNAs/miR) and circular RNAs, thereby affecting sepsis
development (6). Growth
arrest-specific 5 (GAS5), which acts as a miR-155-5p sponge,
promotes the expression of SIRT1 in patients and murine models with
sepsis. Additionally, GAS5 alleviates cellular inflammatory
responses by inhibiting excessive acetylation and release of HMGB1
(7). Zou et al (30) found that under sepsis conditions,
increased connexin 43 expression (Cx43) leads to enhanced
intercellular miR-181b transfer, affecting the SIRT1/forkhead box
O3a (FOXO3a)-signaling pathway and subsequently causing cell and
tissue damage. Cx43 inhibitors can inhibit the SIRT1/FOXO3a
signaling pathway by regulating the intercellular transfer of
miR-181b, thereby mitigating organ damage during sepsis (30). Circular RNA vesicle-associated
membrane protein-associated protein A targets miR-212-3p to
negatively regulate the expression of SIRT1 and cell
pyroptosis-related factors, including nuclear erythroid 2-related
factor 2 (Nrf2) and NLRP3 and inhibit LPS-induced cell pyroptosis
and type 17 T helper cell-related inflammatory responses, which are
involved in alleviating inflammatory damage in sepsis-induced acute
lung injury (31). Additionally,
SIRT1 has shown potential for inhibiting inflammation and the
expression of cyclooxygenase-2 and inducible nitric oxide synthase
by targeting the p53/miR-22 axis (32).
Role of SIRT1 in metabolism during
sepsis
Metabolism is a fundamental physiological process
that sustains growth, reproduction and normal functions in a living
organism. Patients with sepsis frequently exhibit a series of acute
responses, including tachycardia, fever, tachypnea, as well as
activation of the immune, coagulation and complement systems,
accompanied by a significant imbalance of energy metabolism
(33). Glycolysis, glycogenolysis
and lipolysis, along with accelerated fatty acid oxidation, lead to
the breakdown of muscle tissue proteins, promoting a cachectic
state (34). As immune cells
compete with pathogens for glucose during their function execution,
the disruption of glycolysis can impair the phagocytic and
bactericidal capabilities of immune cells (35).
SIRT1 regulates glucose metabolism, lipid metabolism
and mitochondrial quality control by forming a complex sensing
network with various sensing proteins, such as AMPK, forkhead box
protein O1 (FOXO1) and peroxisome proliferator-activated receptor
gamma coactivator-1 alpha (PGC-1α) (36). During sepsis, SIRT1 promotes a
transition from a high-inflammatory stage characterized by
glycolysis to a low-inflammatory stage characterized by fatty acid
oxidation, by regulating metabolic pathways to meet the energy
demands of immune cells and maintaining the balance between immune
function and metabolism (37).
However, persistent SIRT1 overexpression may suppress immune
function and hinder infection foci clearance, ultimately resulting
in energy exhaustion and organ dysfunction (38). Stark et al (39) found that SIRT1 can directly act on
key rate-limiting glycolytic enzymes, including hexokinase 2,
platelet-type phosphofructokinase and M2-type pyruvate kinase, to
regulate the glycolytic process in endothelial cells, which may
affect host's immunity against pathogens during sepsis. However,
research on the effect of SIRT1 on energy metabolism and its
potential regulatory mechanisms during sepsis remains limited,
necessitating further in-depth exploration.
Role of SIRT1 in oxidative stress (OS)
during sepsis
OS results from an imbalance between reactive oxygen
species (ROS) production and the antioxidant defense system
(40) and is a key factor in
cellular damage. In the pathophysiology of sepsis, OS and
inflammation reciprocally amplify each other, driving a
self-perpetuating cycle that exacerbates tissue injury and systemic
immune dysfunction. ROS, encompassing superoxide anions
(O2−), hydrogen peroxide
(H2O2) and hydroxyl radicals (•OH), serve
dual roles as both initiators and enhancers of inflammatory
cascades. Mechanistically, IKK undergoes oxidation by ROS,
triggering sequential phosphorylation and proteasomal degradation
of IκB (41). This molecular event
facilitates nuclear translocation of NF-κB, subsequently
upregulating transcription of pro-inflammatory mediators that
orchestrate systemic inflammatory responses. ROS-mediated oxidation
of mitochondrial DNA (mtDNA) or induction of mitochondrial
permeability transition pore opening enables cytosolic mtDNA
release, thereby activating the NLRP3 inflammasome (42,43).
Activated NLRP3 promotes caspase-1-mediated cleavage of pro-IL-1β
and pro-IL-18 into their bioactive forms, perpetuating inflammatory
cascades. Additionally, ROS activate the p38 mitogen-activated
protein kinase and c-Jun N-terminal kinase pathways, enhancing AP-1
transcriptional activity, which synergizes with NF-κB to potentiate
pro-inflammatory gene expression (41). Conversely, inflammatory cytokines
exacerbate OS through multiple mechanisms. For instance, TNF-α and
IL-1β enhance NADPH oxidase activity while suppressing antioxidant
defense systems and disrupting mitochondrial electron transport
chain integrity, collectively resulting in pathological ROS
overproduction (44).
Numerous studies have indicated that SIRT1 plays a
pivotal role in modulating OS processes during sepsis by regulating
OS-related signaling pathways and gene expression. The multifaceted
mechanisms underlying the SIRT1/AMPK signaling pathway are involved
in alleviating OS, maintaining mitochondrial function and enhancing
cellular resistance to OS. A study found that quercetin (a SIRT1
agonist) can bind to SIRT1 to elevate intracellular NAD+
levels and activate antioxidant enzymes, such as superoxide
dismutase (SOD), glutathione peroxidase (GPX) and catalase (CAT),
thus facilitating in reducing intracellular ROS levels and
alleviating OS (45). SIRT1 can
also directly improve mitochondrial function by deacetylating and
activating AMPK, decreasing ROS production and promoting
mitochondrial biogenesis, thus maintaining mitochondrial integrity
and function. Furthermore, activated AMPK enhances fatty acid
oxidation and increases intracellular adenosine triphosphate
production, thus improving cellular resistance to OS. SIRT1
regulates members of the FOX family, including FOXO1, thereby
promoting the expression of antioxidant enzymes, like SOD and CAT,
consequently decreasing OS-induced cellular damage (46). Zhu et al (47) found that SIRT1 reduces
malondialdehyde concentrations and increases SOD and CAT activities
through the SIRT1/FOXO1 pathway, thus alleviating OS and protecting
against sepsis-related brain cell damage. Similarly, SIRT1-mediated
regulation of FOXO3 and FOXO4 helps mitigate OS response (48,49).
Moreover, SIRT1 activation can inhibit NF-κB activity, alleviating
OS injury in sepsis hepatocytes (50). One study showed that the
SIRT1/NF-κB signaling pathway is involved in alleviating
LPS-induced OS and subsequent renal damage in mice (51). SIRT1 activates p53-encoded
antioxidant enzyme-related genes, such as SOD and GPX, to
neutralize ROS, protecting cells from OS injury. Simultaneously,
SIRT1 inhibits p53 activity by deacetylation, thereby decreasing
the expression of oxidative factors and enhancing cell resistance
to OS (52). Nrf2 is a leucine
zipper TF that directly modulates the expression of antioxidant
genes. Deacetylation of Nrf2 by SIRT1 promotes its binding to
antioxidant response elements, enhancing the expression of
downstream antioxidant proteins, such as quinone oxidoreductase and
heme oxygenase-1, effectively clearing excess ROS and alleviating
OS-induced damage (53). In sepsis
models, SIRT1 increases PGC-1α acetylation to enhance its activity,
thereby strengthening mitochondrial function, decreasing OS and
protecting neurons from OS-induced damage (54).
SIRT1 and ERS in sepsis
SIRT1 plays a significant role in ERS, which is a
critical component of sepsis pathophysiology. ERS is closely
related to several important processes, including inflammatory
responses, immune cell dysfunction and apoptosis (55). A study has shown that increased
SIRT1 expression can markedly suppress ERS response in lung tissues
and MQs through the protein kinase R-like ER kinase/eukaryotic
initiation factor 2 α/activating TF 4/C/EBP homologous protein
signaling pathway, thereby alleviating sepsis-related lung injury
and pulmonary inflammation (56).
Moreover, quercetin can inhibit OS-mediated ERS by activating the
SIRT1/AMPK signaling pathway, thus decreasing sepsis-induced acute
lung injury (45).
SIRT1 and programmed cell death in
sepsis
SIRT1 mediates programmed cell death processes
across various tissues and organs in sepsis via multiple
mechanisms. SIRT1-mediated cell death includes autophagy,
apoptosis, pyroptosis and ferroptosis. An understanding of the
different types of cell death is essential for developing targeted
therapeutic strategies that can effectively mitigate organ-specific
damage in sepsis.
SIRT1 and autophagy
In autophagy, intracellular materials or pathogens
are engulfed by autophagosomes and degraded upon fusion with
lysosomes (57). Autophagy plays a
protective role by clearing pathogens, neutralizing microbial
toxins, regulating cytokine release, decreasing target cell
apoptosis and promoting antigen presentation (58). SIRT1 exerts multifaceted regulation
of autophagy in sepsis. In LPS-induced sepsis, recombinant human
erythropoietin alters the expression of autophagy-related proteins,
including microtubule-associated protein 1A/1B-light chain 3 (LC3)
I/LC3-II and P62, through the SIRT1/AMPK pathway, activating
autophagy to prevent hepatic cell apoptosis (59). Sun et al (60) demonstrated that SIRT1 enhances
autophagy in renal tubular epithelial cells through p53
deacetylation, thereby mitigating acute kidney injury in a murine
model of LPS-induced sepsis. Furthermore, the authors observed that
acetylated p53 is more prone to bind with Beclin1, accelerating its
ubiquitination-mediated degradation and inhibiting autophagy
(60). Deng et al (61) found that SIRT1 promotes autophagy
and mitigates septic kidney damage by deacetylating Beclin-1 at
Lys430 and Lys437. SIRT1 activation has been shown to promote
Beclin-1 deacetylation and enhance autophagy, thereby alleviating
sepsis-induced myocardial damage (62,63).
Wang et al (64)
demonstrated that aquaporin 3 promotes LPS-induced autophagy in
Caco-2 cells by modulating the SIRT1/p62 signaling pathway, thus
alleviating sepsis-induced intestinal epithelial cell damage.
Furthermore, the authors found that EX 527, a SIRT1 inhibitor,
abrogated the effects of aquaporin 3 overexpression. A recent study
showed that SIRT1 signaling plays a critical role in limiting the
hyperactivation of the stimulator of interferon genes and NLRP3
inflammasome through endosomal-mediated mitophagy during
sepsis-induced acute lung injury (65). These findings suggest that SIRT1
activators can stimulate autophagy and mitophagy, highlighting
their potential use in sepsis treatment.
SIRT1 and apoptosis
Apoptosis, a critical component in sepsis
pathogenesis, is regulated by multiple signaling pathways and
exerts its effects in various organs. SIRT1 prevents apoptosis by
mediating the deacetylation of downstream targets, including p53,
NF-κB and FOXO1. p53 potently induces apoptosis and was identified
as the first non-histone deacetylation substrate of SIRT1 (46). SIRT1 catalyzes the
NAD+-dependent deacetylation of p53 at its C-terminal
lysine 382 residue by cleaving the nicotinamide-ribose bond and
transferring the acetyl group to co-substrates, thereby inhibiting
its trans-activating capacity and suppressing
transcription-dependent apoptosis mediated by p53 (66). SIRT1-mediated p53 deacetylation
plays a significant role in reducing apoptosis in septic
conditions, thereby contributing to the preservation of organ
function (67). Lin et al
(68) demonstrated that SIRT1
overexpression attenuates apoptosis in sepsis-induced
cardiomyopathy models by modulating p53. In a murine model of
sepsis-induced lung injury, Yang et al (69) demonstrated that matrine treatment
elicited comparable therapeutic outcomes, which were
mechanistically associated with SIRT1/p53 signaling pathway
activation and subsequent inhibition of sepsis-associated cellular
apoptosis. Notably, while SIRT1 has been shown to suppress
transcription-dependent apoptosis mediated by p53, its potential
role in suppressing or promoting p53-mediated
transcription-independent apoptosis remains unknown. Yang et
al (69) also revealed the
anti-apoptotic role of the SIRT1/NF-κB pathway, wherein SIRT1
modulates the NF-κB pathway to regulate inflammatory cytokine
secretion, thereby inhibiting apoptosis. Furthermore,
SIRT1-mediated regulation of FOXO1 alleviates OS-induced apoptosis
and mitochondrial dysfunction (70).
SIRT1 and pyroptosis
Pyroptosis is a form of programmed cell death that
is primarily induced by inflammasome-mediated activation of caspase
family proteins, which cleave gasdermin proteins, such as gasdermin
D (71). Pyroptosis leads to the
release of cellular contents and subsequent cell death by releasing
the N-terminal active fragments of gasdermin proteins that form
pores on the cell membrane (72).
SIRT1 has been found to regulate pyroptosis by modulating NLRP3
inflammasome activity. A study found that quercetin upregulated
SIRT1 expression and reduced NLRP3 inflammasome activation, thereby
inhibiting pyroptosis in the target organs of a septic murine model
(73). Jiao et al (74) showed that exosomal miR-30d-5p
increased p65 acetylation and activated NF-κB by targeting and
suppressing SIRT1 expression in MQs, leading to MQ pyroptosis,
which is associated with sepsis-related pneumonia. SIRT1 can also
activate PGC-1α through deacetylation, which in turn activates
Nrf2, allowing it to enter the nucleus and promote the expression
of antioxidant genes, thereby decreasing OS and pyroptosis
(75).
SIRT1 and ferroptosis
Ferroptosis is a recently discovered form of cell
death that is induced by iron-dependent lipid peroxidation
(76) and its pathogenesis is
closely related to that of sepsis (77). A study demonstrated that quercetin
exerts an anti-ferroptotic effect by activating the
SIRT1/p53/solute carrier family 7 member 11 signaling pathway to
alleviate sepsis-induced myocardial injury both in vivo and
in vitro (68).
Additionally, quercetin inhibits ferroptosis by activating the
SIRT1/Nrf2/GPX4 signaling pathway, providing significant protection
against LPS-induced lung injury (78). Another study found that irisin
suppresses ferroptosis in a cecal ligation and puncture murine
model through the SIRT1/Nrf2 signaling pathway, reducing the extent
of renal damage (79). Ferroptosis
inhibition involves a decrease in malondialdehyde levels, increase
in glutathione levels to inhibit lipid peroxidation, decrease in
hepatic iron content, increase in GPX4 expression and decrease in
acetyl-CoA synthetase 4 expression (79).
Endothelial protective mechanism of SIRT1 in
sepsis
Damage to the endothelial glycocalyx is an essential
component of sepsis pathology. It exacerbates inflammatory response
and promotes the activation of the coagulation system, leading to
vascular dysregulation and inducing endothelial cell apoptosis
(80). An increase in
NAD+ (a SIRT1 substrate) levels can lead to the
activation of the deacetylation function of SIRT1. A recent study
found that combination therapy with interferon-β and nicotinamide
riboside (as a SIRT1) effectively alleviated sepsis-induced
vascular endothelial injury in an LPS-induced sepsis model
(81). However, this protective
mechanism was markedly weakened in a model with SIRT1 knockout
endothelial cells, indicating the essential role of SIRT1 in
maintaining vascular endothelial integrity. This study also
revealed that SIRT1 can regulate the SIRT1/heparanase 1 pathway,
contributing to the repair of damaged endothelial glycocalyx.
Conclusion and future perspectives
As illustrated in Fig.
1, SIRT1 modulates various signaling pathways and mechanisms
and regulates inflammation, immune function, cellular metabolism,
autophagy, apoptosis, pyroptosis and ferroptosis in sepsis.
Moreover, SIRT1 exerts anti-inflammatory, anti-apoptotic and
cytoprotective effects, making it a promising therapeutic target
for sepsis.
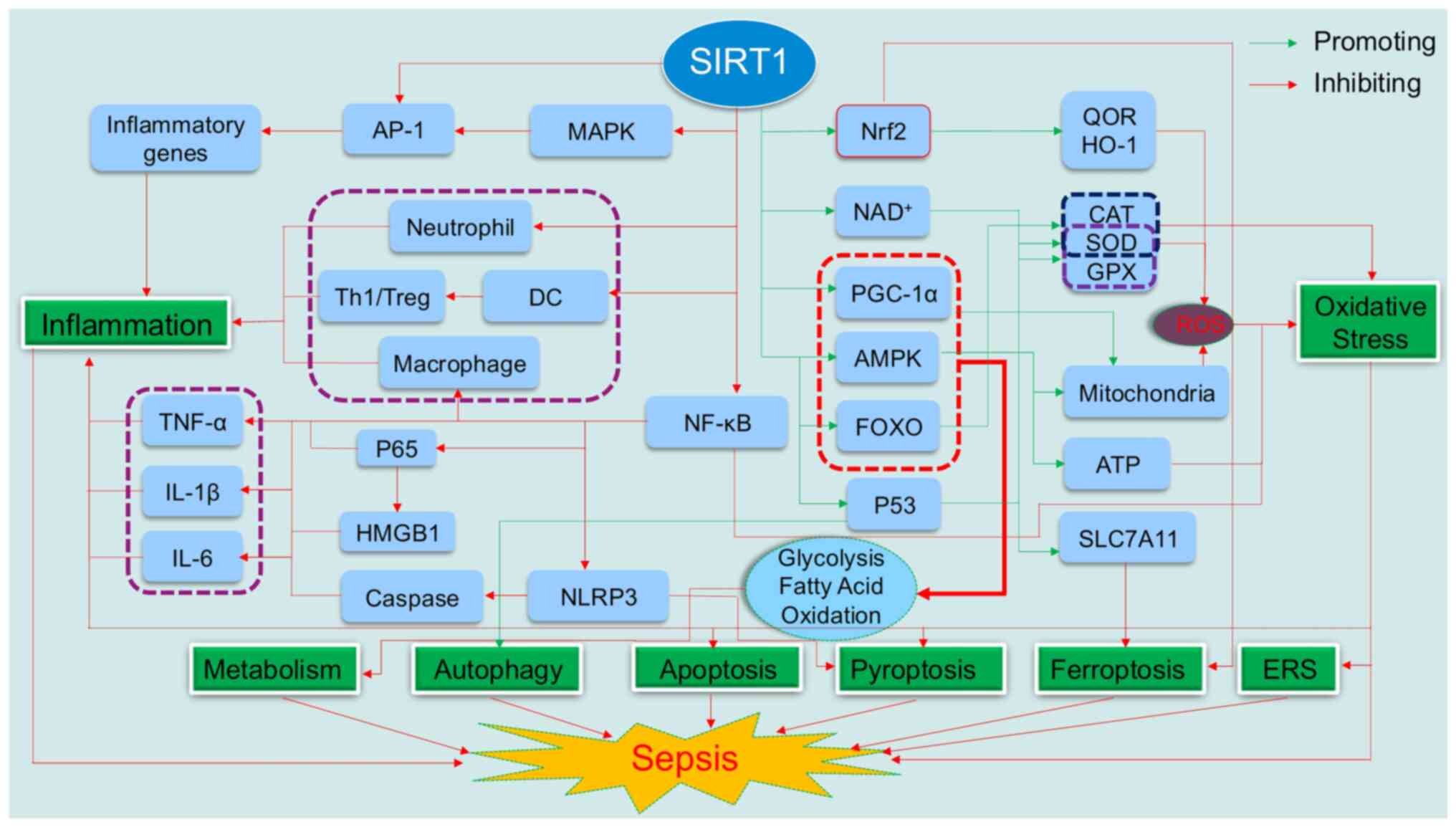 | Figure 1.The central regulatory role of SIRT1
in sepsis pathophysiology. SIRT1, a NAD+-dependent
deacetylase, plays a pivotal role in modulating the
pathophysiological processes in sepsis. SIRT1 initiates its
anti-inflammatory action by deacetylating key proteins, such as Akt
and NF-κB p65 subunit, which in turn suppress the activation of
inflammatory signaling cascades and diminish the secretion of
pro-inflammatory cytokines, leading to a regulated inflammatory
response. Furthermore, SIRT1 attenuates oxidative stress by
upregulating antioxidant enzymes and improving mitochondrial
function, thereby reducing oxidative damage to cellular components.
Metabolically, SIRT1 interacts with AMPK and PGC-1α, orchestrating
a metabolic reprogramming that aligns with the energy requirements
of immune cells, thus preserving immune-metabolic homeostasis.
SIRT1 also safeguards cells from ERS-induced damage and modulates
autophagy, a critical process for cellular homeostasis.
Additionally, SIRT1 exerts its influence through p53/SLC7A11
pathways to inhibit both apoptosis and pyroptosis, which are
significant contributors to tissue damage in sepsis. SIRT1, silent
information regulator 1; NF-κB, nuclear transcription factor-κB;
AMPK, adenosine monophosphate -activated protein kinase; PGC-1α,
peroxisome proliferator-activated receptor gamma coactivator-1α;
AP-1, activator protein-1; CAT, catalase; DC, dendritic cell; ERS,
endoplasmic reticulum stress; FOXO, forkhead box O; GPX,
glutathione peroxidase; HMGB1, high mobility group box 1; HO-1,
heme oxygenase-1; IL-1β, interleukin-1β; IL-6, interleukin-6; MAPK,
mitogen-activated protein kinase; NAD+, nicotinamide
adenine dinucleotide; NLRP3, NOD-like receptor protein 3; Nrf2,
nuclear erythroid 2-related factor 2; QOR, quinone oxidoreductase;
ROS, reactive oxygen species; SLC7A11, solute carrier family 7
member 11; SOD, superoxide dismutase; Th1, type 1 T helper cells;
Treg, regulatory T cells; TNF-α, tumor necrosis factor α. |
Researchers have discovered and developed a range of
SIRT1 agonists and antagonists, providing valuable insights into
potential avenues for future research (82). Several strategies can be employed
to ensure effective delivery of SIRT1-based drugs: i) Employing
mononuclear-MQ cells as carriers for SIRT1 drugs; ii) employing
target-specific nanotechnology-driven delivery systems; iii)
administering SIRT1 modulators, such as resveratrol and quercetin;
iv) activating signaling pathways, such as the AMPK signal pathway,
to modulate autophagy and mitochondrial autophagy; and v) employing
miRNA sponge technology to boost SIRT1 expression in targeted cells
or organs. These approaches establish a foundation for the targeted
administration of SIRT1-based therapies to address sepsis-induced
organ dysfunction.
Despite significant progress in in vitro and
in vivo experiments, our understanding of the regulatory
mechanisms of SIRT1 in sepsis remains unclear. Most studies are
focused on the role of SIRT1 in a single target organ and clinical
trial data for SIRT1 modulators are extremely scarce, which limits
the comprehensive understanding of the role of SIRT1 in sepsis
(83,84). To address these limitations, future
research should delve deeper into the regulatory mechanisms of
SIRT1 in sepsis using a multidisciplinary approach combining
advanced techniques, such as genomics, proteomics and metabolomics,
to fully elucidate these mechanisms (85). Using the findings of these studies,
more specific and effective SIRT1 modulators can be developed,
providing new strategies for the prevention and treatment of
sepsis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Traditional Chinese
Medicine research project of Chongqing Health Commission (grant no.
2022ZY7501), Chongqing Science and Technology Commission (grant no.
CSTC2020JCYJ-MSXMX1069) and the Chongqing Medical Scientific
Research Project (Joint project of Chongqing Health Commission and
Science and Technology Bureau; grant no. 2024QNXM054).
Availability of data and materials
Not applicable.
Authors' contributions
QZ and WG were responsible for writing and editing
the original draft. WZ was responsible for writing and reviewing
the original draft and funding acquisition. ST was responsible for
writing the original draft. HF was responsible for editing the
manuscript. PP was responsible for writing, reviewing and editing
the manuscript and supervision and funding acquisition. Data
authentication is not applicable. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Dr Pengfei Pan: https://orcid.org/0000-0002-7024-3863
References
|
1
|
Singer M, Deutschman CS, Seymour CW,
Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche
JD, Coopersmith CM, et al: The third international consensus
definitions for sepsis and septic shock (Sepsis-3). JAMA.
315:801–810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rudd KE, Johnson SC, Agesa KM, Shackelford
KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer
S, et al: Global, regional, and national sepsis incidence and
mortality, 1990–2017: Analysis for the global burden of disease
study. Lancet. 395:200–211. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weng L, Xu Y, Yin P, Wang Y, Chen Y, Liu
W, Li S, Peng JM, Dong R, Hu XY, et al: National incidence and
mortality of hospitalized sepsis in China. Crit Care. 27:842023.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Font MD, Thyagarajan B and Khanna AK:
Sepsis and septic shock-basics of diagnosis, pathophysiology and
clinical decision making. Med Clin North Am. 104:573–585. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim JK, Silwal P and Jo EK: Sirtuin 1 in
host defense during infection. Cells. 11:29212022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ghafouri-Fard S, Shoorei H, Hussen BM,
Poornajaf Y, Taheri M and Sharifi G: Interaction between SIRT1 and
non-coding RNAs in different disorders. Front Genet.
14:11219822023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zeng Z, Lan Y, Chen Y, Zuo F, Gong Y, Luo
G, Peng Y and Yuan Z: LncRNA GAS5 suppresses inflammatory responses
by inhibiting HMGB1 release via miR-155-5p/SIRT1 axis in sepsis.
Eur J Pharmacol. 942:1755202023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu QJ, Zhang TN, Chen HH, Yu XF, Lv JL,
Liu YY, Liu YS, Zheng G, Zhao JQ, Wei YF, et al: The sirtuin family
in health and disease. Signal Transduct Target Ther. 7:4022022.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jia Y, Shen K, Liu J, Li Y, Bai X, Yang Y,
He T, Zhang Y, Tong L, Gao X, et al: The deacetylation of Akt by
SIRT1 inhibits inflammation in macrophages and protects against
sepsis. Exp Biol Med (Maywood). 248:922–935. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rasha F, Mims BM, Castro-Piedras I, Barnes
BJ, Grisham MB, Rahman RL and Pruitt K: The versatility of
sirtuin-1 in endocrinology and immunology. Front Cell Dev Biol.
8:5890162020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu G, Bi Y, Xue L, Zhang Y, Yang H, Chen
X, Lu Y, Zhang Z, Liu H, Wang X, et al: Dendritic cell SIRT1-HIF1α
axis programs the differentiation of CD4+ T cells through IL-12 and
TGF-β1. Proc Natl Acad Sci USA. 112:E957–E965. 2015.PubMed/NCBI
|
|
12
|
Labiner HE, Sas KM, Hoying J, Sepeda JA,
Wolf N, Perez EC, Sas AR and Sims CA: SIRT1 downregulation in
pneumonia is associated with an immature neutrophil response and
increased disease severity. J Trauma Acute Care Surg. 96:557–565.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Doganyigit Z, Eroglu E and Akyuz E:
Inflammatory mediators of cytokines and chemokines in sepsis: From
bench to bedside. Hum Exp Toxicol. 41:96032712210788712022.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Singh V and Ubaid S: Role of silent
information regulator 1 (SIRT1) in regulating oxidative stress and
inflammation. Inflammation. 43:1589–1598. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Labiner HE, Sas KM, Baur JA and Sims CA:
Sirtuin 1 deletion increases inflammation and mortality in sepsis.
J Trauma Acute Care Surg. 93:672–678. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gharamti AA, Samara O, Monzon A,
Montalbano G, Scherger S, DeSanto K, Chastain DB, Sillau S, Montoya
JG, Franco-Paredes C, et al: Proinflammatory cytokines levels in
sepsis and healthy volunteers, and tumor necrosis factor-alpha
associated sepsis mortality: A systematic review and meta-analysis.
Cytokine. 158:1560062022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Han S, Li Z, Han F, Jia Y, Qi L, Wu G, Cai
W, Xu Y, Li C, Zhang W and Hu D: ROR alpha protects against
LPS-induced inflammation by down-regulating SIRT1/NF-kappa B
pathway. Arch Biochem Biophys. 668:1–8. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li L, Liu M, Cao M, He T and Bai X:
Research progress on SIRT1 and sepsis. Histol Histopathol.
34:1205–1215. 2019.PubMed/NCBI
|
|
19
|
Liu T, Zhang L, Joo D and Sun SC: NF-κB
signaling in inflammation. Signal Transduct Target Ther.
2:17023–2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li G, Xia Z, Liu Y, Meng F, Wu X, Fang Y,
Zhang C and Liu D: SIRT1 inhibits rheumatoid arthritis
fibroblast-like synoviocyte aggressiveness and inflammatory
response via suppressing NF-κB pathway. Biosci Rep.
38:BSR201805412018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Quan M, Lv Y, Dai Y, Qi B, Fu L, Chen X
and Qian Y: Tanshinone IIA protects against
lipopolysaccharide-induced lung injury through targeting Sirt1. J
Pharm Pharmacol. 71:1142–1151. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoshizaki T, Schenk S, Imamura T,
Babendure JL, Sonoda N, Bae EJ, Oh DY, Lu M, Milne JC, Westphal C,
et al: SIRT1 inhibits inflammatory pathways in macrophages and
modulates insulin sensitivity. Am J Physiol Endocrinol Metab.
298:E419–E428. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang Y, Liu Y, Wang Y, Chao Y, Zhang J,
Jia Y, Tie J and Hu D: Regulation of SIRT1 and its roles in
inflammation. Front Immunol. 13:8311682022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu FJ, Gu TJ and Wei DY: Emodin
alleviates sepsis-mediated lung injury via inhibition and reduction
of NF-kB and HMGB1 pathways mediated by SIRT1. Kaohsiung J Med Sci.
38:253–260. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang Y, Liu Y, He X, Yang F, Han S, Qin A,
Wu G, Liu M, Li Z, Wang, et al: ING4 alleviated
lipopolysaccharide-induced inflammation by regulating the NF-κB
pathway via a direct interaction with SIRT1. Immunol Cell Biol.
98:127–137. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang X, Ning W, Gao G, Zhou Y, Duan XB,
Li X, Li D and Guo R: Bazedoxifene attenuates intestinal injury in
sepsis by suppressing the NF-κB/NLRP3 signaling pathways. Eur J
Pharmacol. 947:1756812023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McKee CM and Coll RC: NLRP3 inflammasome
priming: A riddle wrapped in a mystery inside an enigma. J Leukoc
Biol. 108:937–952. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kelley N, Jeltema D, Duan Y and He Y: The
NLRP3 inflammasome: An overview of mechanisms of activation and
regulation. Int J Mol Sci. 20:33282019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guo T, Jiang ZB, Tong ZY, Zhou Y, Chai XP
and Xiao XZ: Shikonin ameliorates LPS-induced cardiac dysfunction
by SIRT1-dependent inhibition of NLRP3 inflammasome. Front Physiol.
11:5704412020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zou Z and Yu J, Huang R and Yu J:
Cx43-delivered miR-181b negatively regulates sirt1/FOXO3a
signalling pathway-mediated apoptosis on intestinal injury in
sepsis. Digestion. 104:370–380. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang Y, Lin J, Wu Z and Li Y: Circular
RNA circVAPA modulates macrophage pyroptosis in sepsis-induced
acute lung injury through targeting miR-212-3p/Sirt1/Nrf2/NLRP3
axis. Int J Exp Pathol. 105:21–32. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lu H and Wang B: SIRT1 exerts
neuroprotective effects by attenuating cerebral
ischemia/reperfusion-induced injury via targeting p53/microRNA-22.
Int J Mol Med. 39:208–216. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vandewalle J and Libert C: Sepsis: A
failing starvation response. Trends Endocrinol Metab. 33:292–304.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Luiking YC, Poeze M and Deutz NE: A
randomized-controlled trial of arginine infusion in severe sepsis
on microcirculation and metabolism. Clin Nutr. 39:1764–1773. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cheng SC, Scicluna BP, Arts RJ, Gresnigt
MS, Lachmandas E, Giamarellos-Bourboulis EJ, Kox M, Manjeri GR,
Wagenaars JA, Cremer OL, et al: Broad defects in the energy
metabolism of leukocytes underlie immunoparalysis in sepsis. Nat
Immunol. 17:406–413. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lu C, Zhao H, Liu Y, Yang Z, Yao H, Liu T,
Gou T, Wang L, Zhang J, Tian Y, et al: Novel role of the SIRT1 in
endocrine and metabolic diseases. Int J Biol Sci. 19:484–501. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vachharajani VT, Liu T, Wang X, Hoth JJ,
Yoza BK and McCall CE: Sirtuins link inflammation and metabolism. J
Immunol Res. 2016:81672732016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang X, Buechler NL, Woodruff AG, Long DL,
Zabalawi M, Yoza BK, McCall CE and Vachharajani V: Sirtuins and
immuno-metabolism of sepsis. Int J Mol Sci. 19:27382018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Stark RJ, Koch SR, Stothers CL, Pourquoi
A, Lamb CK, Miller MR and Choi H: Loss of Sirtuin 1 (SIRT1)
potentiates endothelial dysfunction via impaired glycolysis during
infectious challenge. Clin Transl Med. 12:e10542022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jomova K, Raptova R, Alomar SY, Alwasel
SH, Nepovimova E, Kuca K and Valko M: Reactive oxygen species,
toxicity, oxidative stress, and antioxidants: chronic diseases and
aging. Arch Toxicol. 97:2499–2574. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Averill-Bates D: Reactive oxygen species
and cell signaling. Review. Biochim Biophys Acta Mol Cell Res.
1871:1195732024. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kent AC, El Baradie KBY and Hamrick MW:
Targeting the mitochondrial permeability transition pore to prevent
age-associated cell damage and neurodegeneration. Oxid Med Cell
Longev. 2021:66264842021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Poli G, Fabi C, Sugoni C, Bellet MM,
Costantini C, Luca G and Brancorsini S: The role of NLRP3
inflammasome activation and oxidative stress in varicocele-mediated
male hypofertility. Int J Mol Sci. 23:52332022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang CC and Yang CM: Chinese herbs and
repurposing old drugs as therapeutic agents in the regulation of
oxidative stress and inflammation in pulmonary diseases. J Inflamm
Res. 14:657–687. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sang A, Wang Y, Wang S, Wang Q, Wang X, Li
X and Song X: Quercetin attenuates sepsis-induced acute lung injury
via suppressing oxidative stress-mediated ER stress through
activation of SIRT1/AMPK pathways. Cell Signal. 96:1103632022.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mai C, Qiu L, Zeng Y and Tan X:
Lactobacillus casei strain Shirota enhances the ability of
geniposide to activate SIRT1 and decrease inflammation and
oxidative stress in septic mice. Front Physiol. 12:6788382021.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhu Y, Wang K, Ma Z, Liu D, Yang Y, Sun M,
Wen A, Hao Y, Ma S, Ren F, et al: SIRT1 activation by butein
attenuates sepsis-induced brain injury in mice subjected to cecal
ligation and puncture via alleviating inflammatory and oxidative
stress. Toxicol Appl Pharmacol. 363:34–46. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mahlooji MA, Heshmati A, Kheiripour N,
Ghasemi H, Asl SS, Solgi G, Ranjbar A and Hosseini A: Evaluation of
protective effects of curcumin and nanocurcumin on aluminium
phosphide-induced subacute lung injury in rats: modulation of
oxidative stress through SIRT1/FOXO3 signalling pathway. Drug Res
(Stuttg). 72:100–108. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li H, Shen L, Lv T, Wang R, Zhang N, Peng
H and Diao W: Salidroside attenuates dextran sulfate sodium-induced
colitis in mice via SIRT1/FoxOs signaling pathway. Eur J Pharmacol.
861:1725912019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Üstündağ H, Kalindemirtaş FD, Doğanay S,
Demir Ö, Kurt N, Huyut MT, Özgeriş B and Kariper İA: Enhanced
efficacy of resveratrol-loaded silver nanoparticle in attenuating
sepsis-induced acute liver injury: Modulation of inflammation,
oxidative stress, and SIRT1 activation. Shock. 60:688–697.
2023.PubMed/NCBI
|
|
51
|
Hu Y, Xiang C and Zhang D, Zhou F and
Zhang D: Nephroprotective effect of Ginsenoside Rg1 in
lipopolysaccharide-induced sepsis in mice through the SIRT1/NF-κB
signaling. Folia Histochem Cytobiol. 62:13–24. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liu X, Fan L, Lu C, Yin S and Hu H:
Functional role of p53 in the regulation of chemical-induced
oxidative stress. Oxid Med Cell Longev. 2020:60397692020.PubMed/NCBI
|
|
53
|
Xie W, Deng L, Lin M, Huang X, Qian R,
Xiong D, Liu W and Tang S: Sirtuin1 mediates the protective effects
of echinacoside against sepsis-induced acute lung injury via
regulating the NOX4-Nrf2 axis. Antioxidants (Basel). 12:19252023.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu Y, Yang H, Luo N, Fu Y, Qiu F, Pan Z,
Li X, Jian W, Yang X, Xue Q, et al: An Fgr kinase inhibitor
attenuates sepsis-associated encephalopathy by ameliorating
mitochondrial dysfunction, oxidative stress, and neuroinflammation
via the SIRT1/PGC-1α signaling pathway. J Transl Med. 21:4862023.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Khan MM, Yang WL and Wang P: Endoplasmic
reticulum stress in sepsis. Shock. 44:294–304. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang F, Ma J, Wang J, Chen M, Xia H, Yao S
and Zhang D: SIRT1 ameliorated septic associated-lung injury and
macrophages apoptosis via inhibiting endoplasmic reticulum stress.
Cell Signal. 97:1103982022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liu S, Yao S, Yang H, Liu S and Wang Y:
Autophagy: Regulator of cell death. Cell Death Dis. 14:6482023.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Qiu P, Liu Y and Zhang J: Review: The role
and mechanisms of macrophage autophagy in sepsis. Inflammation.
42:6–19. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li K, Liu TX, Li JF, Ma YR, Liu ML, Wang
YQ, Wu R, Li B, Shi LZ and Chen C: rhEPO inhibited cell apoptosis
to alleviate acute kidney injury in sepsis by AMPK/SIRT1 activated
autophagy. Biochem Biophys Res Commun. 517:557–565. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sun M, Li J, Mao L, Wu J, Deng Z, He M, An
S, Zeng Z, Huang Q and Chen Z: p53 deacetylation alleviates
sepsis-induced acute kidney injury by promoting autophagy. Front
Immunol. 12:6855232021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Deng Z, Sun M, Wu J, Fang H, Cai S, An S,
Huang Q, Chen Z, Wu C, Zhou Z, et al: SIRT1 attenuates
sepsis-induced acute kidney injury via Beclin1
deacetylation-mediated autophagy activation. Cell Death Dis.
12:2172021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhang WX, He BM, Wu Y, Qiao JF and Peng
ZY: Melatonin protects against sepsis-induced cardiac dysfunction
by regulating apoptosis and autophagy via activation of SIRT1 in
mice. Life Sci. 217:8–15. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Pi QZ, Wang XW, Jian ZL, Chen D, Zhang C
and Wu QC: Melatonin alleviates cardiac dysfunction via increasing
Sirt1-mediated Beclin-1 deacetylation and autophagy during sepsis.
Inflammation. 44:1184–1193. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wang C, Hu Y, Song Y and Hu X: AQP3
mediates autophagy through SIRT1/p62 signal to alleviate intestinal
epithelial cell damage caused by sepsis. Int J Colorectal Dis.
39:2052024. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Jiang T, Liu E, Li Z, Yan C, Zhang X, Guan
J, Zhan Y, Zhao B and Ding W: SIRT1-Rab7 axis attenuates NLRP3 and
STING activation through late endosomal-dependent mitophagy during
sepsis-induced acute lung injury. Int J Surg. 110:2649–2668.
2024.PubMed/NCBI
|
|
66
|
Yin JY, Lu XT, Hou ML, Cao T and Tian Z:
Sirtuin1-p53: A potential axis for cancer therapy. Biochem
Pharmacol. 212:1155432023. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ong ALC and Ramasamy TS: Role of
Sirtuin1-p53 regulatory axis in aging, cancer and cellular
reprogramming. Ageing Res Rev. 43:64–80. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Lin X, Zhao X, Chen Q, Wang X, Wu Y and
Zhao H: Quercetin ameliorates ferroptosis of rat cardiomyocytes via
activation of the SIRT1/p53/SLC7A11 signaling pathway to alleviate
sepsis-induced cardiomyopathy. Int J Mol Med. 52:1162023.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yang L, Zhang YM, Guo MN, Zhang H, Zhu XY,
Xu C and Liu YJ: Matrine attenuates lung injury by modulating
macrophage polarization and suppressing apoptosis. J Surg Res.
281:264–274. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Mo X, Wang X, Ge Q and Bian F: The effects
of SIRT1/FoxO1 on LPS induced INS-1 cells dysfunction. Stress.
22:70–82. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Dai Z, Liu WC, Chen XY, Wang X, Li JL and
Zhang X: Gasdermin D-mediated pyroptosis: Mechanisms, diseases, and
inhibitors. Front Immunol. 14:11786622023. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wen R, Liu YP, Tong XX, Zhang TN and Yang
N: Molecular mechanisms and functions of pyroptosis in sepsis and
sepsis-associated organ dysfunction. Front Cell Infect Microbiol.
12:9621392022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chen LL, Song C, Zhang Y, Li Y, Zhao YH,
Lin FY, Han DD, Dai MH, Li W and Pan PH: Quercetin protects against
LPS-induced lung injury in mice via SIRT1-mediated suppression of
PKM2 nuclear accumulation. Eur J Pharmacol. 936:1753522022.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Jiao Y, Zhang T, Zhang C, Ji H, Tong X,
Xia R, Wang W, Ma Z and Shi X: Exosomal miR-30d-5p of neutrophils
induces M1 macrophage polarization and primes macrophage pyroptosis
in sepsis-related acute lung injury. Crit Care. 25:3562021.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ling H, Li Q, Duan ZP, Wang YJ, Hu BQ and
Dai XG: LncRNA GAS5 inhibits miR-579-3p to activate
SIRT1/PGC-1α/Nrf2 signaling pathway to reduce cell pyroptosis in
sepsis-associated renal injury. Am J Physiol Cell Physiol.
321:C117–C133. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Stockwell BR: Ferroptosis turns 10:
Emerging mechanisms, physiological functions, and therapeutic
applications. Cell. 185:2401–2421. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Yang J, Yan C, Chen S, Li M, Miao Y, Ma X,
Zeng J and Xie P: The possible mechanisms of ferroptosis in
sepsis-associated acquired weakness. Front Physiol. 15:13809922024.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Deng S, Li J, Li L, Lin S, Yang Y, Liu T,
Zhang T, Xie G, Wu D and Xu Y: Quercetin alleviates
lipopolysaccharide-induced acute lung injury by inhibiting
ferroptosis via the Sirt1/Nrf2/Gpx4 pathway. Int J Mol Med.
52:1182023. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Qiongyue Z, Xin Y, Meng P, Sulin M, Yanlin
W, Xinyi L and Xuemin S: Post-treatment with irisin attenuates
acute kidney injury in sepsis mice through anti-ferroptosis via the
SIRT1/Nrf2 pathway. Front Pharmacol. 13:8570672022. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
McMullan RR, McAuley DF, O'Kane CM and
Silversides JA: Vascular leak in sepsis: physiological basis and
potential therapeutic advances. Crit Care. 28:972024. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Duan S, Kim SG, Lim HJ, Song HR and Han
MK: Interferon-β alleviates sepsis by SIRT1-mediated blockage of
endothelial glycocalyx shedding. BMB Rep. 56:314–319. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Chen M, Tan J, Jin Z, Jiang T, Wu J and Yu
X: Research progress on Sirtuins (SIRTs) family modulators. Biomed
Pharmacother. 174:1164812024. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Yang XR, Wen R, Yang N and Zhang TN: Role
of sirtuins in sepsis and sepsis-induced organ dysfunction: A
review. Int J Biol Macromol. 278:1348532024. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Bursch KL, Goetz CJ and Smith BC: Current
trends in sirtuin activator and inhibitor development. Molecules.
29:11852024. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
You J, Li Y and Chong W: The role and
therapeutic potential of SIRTs in sepsis. Front Immunol.
15:13949252024. View Article : Google Scholar : PubMed/NCBI
|















