Introduction
Chronic rhinosinusitis with nasal polyps (CRSwNP) is
a multifaceted and heterogeneous inflammatory condition affecting
the nasal mucosa. The formation of nasal polyps is characterized by
notable edema of the nasal mucosa, with histopathological features
revealing basal cell hyperproliferation (1). Moreover, basal cells in the nasal
epithelium demonstrate imbalanced differentiation, with goblet cell
hyperplasia and decreased levels of ciliated cells, accompanied by
the infiltration of inflammatory cells (2). CRS is further classified into
eosinophilic CRSwNP (ECRSwNP) and non-ECRSwNP (nECRSwNP), based on
the histological quantification of eosinophilia (3). The recommended treatment approach, as
per European Position Paper on Rhinosinusitis and Nasal Polyps
guidelines (EPOS2020) (3),
involves a comprehensive strategy comprising appropriate medical
therapy and functional endoscopic sinus surgery. However, patients
with CRSwNP often face challenges with poor compliance to long-term
medication, and relapse is common following surgical intervention,
with rates ranging from 35% at 6 months to >40% at 18 months
post-endoscopic sinus surgery (4).
Notably, a subset of patients with CRSwNP experience insufficient
control of the condition despite extended and standardized
treatment (5). Therefore,
understanding of the fundamental mechanisms underlying CRSwNP is
key for exploring alternative therapeutic strategies in managing
this intricate and diverse inflammatory disorder.
Emerging data have indicated an association between
pyroptosis and CRSwNP, as evidenced by positive staining of the
NLRP3, a primary signal to induce pyroptosis, in patient samples
(6,7). Pyroptosis is a form of gasdermin D
(GSDMD)-mediated programmed cell death, primarily involving
Caspase-1 activation. When NLRP3 inflammatory bodies are activated
by pathogens, they activate and cleave pro-Caspase-1 to active
Caspase-1. Caspase-1 cleaves GSDMD, leading to the activation of
N-terminal and C-terminal fragments. N-terminal fragments assemble
to create pores in the cell membrane, leading to cell enlargement,
lysis and death. Simultaneously, inflammatory cytokines are
released from the cell, attracting additional inflammatory cells
and intensifying the inflammatory response (8,9).
Previous studies have demonstrated NLRP3 activation and pyroptosis
markers in CRSwNP samples (6,7,10–12),
however, the role of pyroptosis in epithelial dysfunction,
including basal cell hyperproliferation and goblet cell
differentiation, remains poorly understood.
Previous studies (10,13–18)
have highlighted the involvement of cytokines IL-5 and IL-17A in
CRSwNP pathogenesis. IL-5 is a key regulator of eosinophil
differentiation, migration, activation and survival. Elevated IL-5
levels and increased eosinophil counts are associated with severe
NPs and higher postoperative recurrence rates, positioning IL-5 as
a potential biomarker for disease diagnosis, severity assessment
and recurrence prediction in CRSwNP (13,14).
By contrast, IL-17A serves a key role in neutrophilic inflammation
(15). It drives neutrophil
recruitment, activation and infiltration by inducing chemokines
such as CXCL1, CXCL2 and CXCL8 (16) via the PI3K/Akt/hypoxia inducible
factor (HIF)-1α signaling pathway (17). Elevated IL-17A levels in nasal
tissue are positively associated with increased NP burden, tissue
remodeling and disease severity (18). Furthermore, heightened IL-17A
expression is associated with enhanced neutrophil migration and
pyroptosis-like inflammatory responses (10,16).
The present study aimed to elucidate the
contribution of pyroptosis to the pathophysiology of CRSwNP and to
examine how differential cytokine environments may influence
epithelial cell behavior through this inflammasome-mediated
pathways.
Materials and methods
Patients
The present study was approved by the Ethical
Committee of the Affiliated Hospital of Qingdao University,
Qingdao, China (approval no. QYFY WZLL28477). The present study
included 103 patients (68 males, 35 females; aged 27–73 years)
diagnosed with CRSwNP who underwent surgical treatment at the
Department of Otolaryngology-Head and Neck Surgery, Affiliated
Hospital of Qingdao University, Qingdao, China between January 2019
and June 2020. For the control group, the middle turbinate mucosal
tissue was collected during procedures such as septoplasty, middle
turbinate reduction, optic nerve decompression, orbital wall
fracture repair or cerebrospinal fluid leak repair. The inclusion
criteria required patients to meet the EPOS2020 guidelines
(3) and have postoperative
histopathological confirmation of NP tissue. Patients with the
following conditions were excluded: Use of antibiotics or oral
corticosteroids within 3 months prior to surgery and coexisting
parasitic infection, cystic fibrosis, aspirin-exacerbated
respiratory disease, hematological disorder or psychiatric
conditions. All patients were followed up for a duration of 26–41
months to assess clinical outcomes and disease progression.
Follow-up included in-person hospital visits once monthly for the
first 3 months, every 2 months from months 3 to 9, every 3 months
from months 9 to 12 and annually thereafter via telephone or
in-person visits when disease progression was suspected.
All patients were examined by paranasal sinus
computed tomography (PNCT) using Discovery CT750 HD (GE
Healthcare), endoscopic nasal examination and symptoms such as
nasal obstruction, purulent nasal discharge, headache and olfactory
dysfunction by physical examination. For endoscopic nasal
examination, Lund-Kennedy was used as the endoscopic scoring system
(19). For PNCT examination, the
Lund-Mackay scoring system was applied (20,21).
To quantify the symptoms of patients, the visual analogue scale
(VAS) was applied (22).
Human specimen collection
Control and CRSwNP samples were removed during
endoscopic sinus surgery and fixed in 10% formalin at room
temperature for 24 h for embedding, sectioned at 5 µm and stained
with hematoxylin (10 min) and eosin (3 min) at room temperature. An
additional segment from each sample was stored at −80°C for reverse
transcription-quantitative (RT-q)PCR and western blotting
analyses.
Reagents
Antibodies against IL-1β (ab283818) were purchased
from Abcam. Antibodies against GAPDH (60004-1-1g), NLRP3
(19771-1-AP) and GSDMD (20770-1-AP) were purchased from Proteintech
Group, Inc. Antibodies against Caspase-1 (cat. no. 2225T) and
cleaved Caspase-1 (4199T) were purchased from Cell Signaling
Technology, Inc. The secondary antibodies Donkey anti-Rabbit IgG
(H+L) Cross-Adsorbed Secondary Antibody, DyLight™ 488 (SA5-10038),
Donkey anti-Mouse IgG (H+L) Cross-Adsorbed Secondary Antibody,
DyLight™ 650 (SA5-10169) and DAPI (D1306) were purchased from
Invitrogen. The secondary antibodies Goat Anti-Rabbit IgG(H+L)
(peroxidase/HRP conjugated) (E-AB-1003) and Goat Anti-Mouse
IgG(H+L)(peroxidase/HRP conjugated) (E-AB-1001) were purchased from
Elabscience. Recombinant human IL-5 protein (90106ES10) was
purchased from Shanghai Yeasen Biotechnology Co., Ltd. Recombinant
human IL-17A (200-17) protein was purchased from PeproTech, Inc.
ECL substrate kit (MA0186-2) was purchased from Dalian Meilun
Biology Technology Co., Ltd.
Cell culture
Primary human nasal epithelial cells (HNEpCs) were
derived as previously described (23). In brief, nasal tissue samples were
cut into small pieces, washed with PBS, centrifuged at 300 g at
room temperature for 5 mins, incubated with dispase II (2.4 U/ml,
Stem Cell Technology) overnight at 4°C, centrifuged, incubated with
trypsin (Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C for 15
min, and passed through a 100-µm filter. To recapitulate airway
epithelial architecture, HNEpCs were seeded in the upper chamber of
Transwell inserts (Corning) pre-coated with collagen I (Sigma). The
upper chamber contained DMEM supplemented with 10% fetal bovine
serum (FBS; Invitrogen), while the lower chamber was filled with a
1:1 mixture of bronchial epithelial growth medium and DMEM/F12
(Sigma). Cells were allowed to differentiate under this air-liquid
interface conditions.
Basal RPMI-2650 cells were obtained from the Cell
Bank of the Chinese Academy of Sciences, and maintained in
RPMI-1640, 10% FBS (both Gibco; Thermo Fisher Scientific, Inc.) and
penicillin-streptomycin at 37°C and 5% CO2. Cells were
passaged every 3–4 days by adding 2.5% trypsin (Gibco; Thermo
Fisher Scientific, Inc.) for 3–5 min at 37°C and re-plating at a
1:4 ratio.
For stimulation assays, confluent HNEpCs or
RPMI-2650 cells were treated with either human serum collected
preoperatively from whole blood (5 ml) obtained from patients
clinically diagnosed with ECRSwNP or non-ECRSwNP, or IL-5, 100
ng/ml; PeproTech) or interleukin-17A (IL-17A, 100 ng/mL; PeproTech)
for 72 h at 37°C. Control cells were exposed to the same cytokine
concentrations for 72 h or co-treated with dimethyl fumarate (DMF,
50 µM; Sigma).
Immunohistochemical and
immunofluorescent (IF) staining
For immunohistochemistry (IHC), paraffin-embedded
sections (5-µm thick) were deparaffinized in fresh xylene for 10
min, rehydrated through a graded ethanol series (100%, 95% and 70%
ethanol), washed with water, blocked with 5% BSA (Yeasen) in PBS at
room temperature for 1 h, incubated with primary antibodies (all
1:500) against NLRP3, IL-1β, cleaved Caspase 1, cleaved GSDMD, IL-5
and IL-17A overnight at 4°C, followed by a 60 min incubation with
goat anti-rabbit IgG(H+L) secondary antibody (1:1,000). The
sections were stained with 3,3′diaminobenzidine tetrahydrochloride
for 5 mins and counterstained with hematoxylin for 2 mins at room
temperature. Images of the immunostained samples were captured
using a light microscope.
For the IF experiment, NP sections or fixed nasal
epithelial cells treated with serum from human patients were
blocked with 5% BSA (Yeasen) in PBS at room temperature for 1 h,
treated with primary antibodies against Cleaved GSDMD or Cleaved
Caspase-1 overnight at 4°C, followed by a 60 min incubation with
secondary antibodies conjugated to fluorescein. The nuclei were
counterstained with DAPI for 5 min at room temperature.
High-resolution images were obtained using a Nikon ECLIPSE Ti
confocal microscope (Nikon Corporation).
Western blotting
Total protein from tissue or cell samples was
extracted using RIPA (Yeasen) buffer. The protein concentrations
were assessed using a BCA Protein Assay kit (Beyotime Institute of
Biotechnology). A total of 40 µg of total protein were loaded onto
SDS-PAGE gels (4–20%) for separation and transferred to a PVDF
membrane. After blocking in 5% skimmed milk for 1 h at room
temperature, dissolved in Tris-buffered saline with 0.1% Tween-20
(TBST), the PVDF membrane was incubated with the primary antibodies
overnight at 4C° as follows: GAPDH (1:4,000), IL-1β (1:5,000),
NLRP3 (1:1,000), GSDMD (1:2,000), Caspase-1 (1:1,000) and cleaved
Caspase-1 (1:2,000). After washing the PVDF membranes three times
with TBST for 5 min each, they were incubated with secondary
antibodies (1:5,000) at room temperature for 60 min. Signal
detection was performed using the ChemiDoc XRS+ Imaging System
(Bio-Rad Laboratories, Inc). Band density was quantified using
Image Lab Software 6.0 (Bio-Rad Laboratories, Inc.). All expression
values were normalized to GAPDH.
RT-qPCR
Total RNA was extracted from RPMI-2650 cells
(1×106) using Total RNA Extraction Reagent (Vazyme
Biotech Co., Ltd.). A total of 1 µg total RNA was
reverse-transcribed with HiScript III RT SuperMix for qPCR
according to the manufacturer's protocol (Vazyme Biotech Co.,
Ltd.). qPCR was conducted using the Taq Pro Universal SYBR qPCR
Master Mix according to the manufacturer's protocol (Vazyme Biotech
Co., Ltd.). The thermocycling conditions were as follows: initial
denaturation at 95°C for 3 min, followed by 40 cycles of
denaturation at 95°C for 15 sec and annealing/extension at 60°C for
30 sec. Melt curve analysis was performed at the end of
amplification by increasing the temperature from 65°C to 95°C in
0.5°C increments every 5 sec to confirm the specificity of the
amplification products. Standard curves were generated for each
primer set and were used to calculate Cq values with the expression
threshold set to 100 RFU (24).
Expression values were normalized to GAPDH. The forward and reverse
primer sequences (5′→3′) were as follows: GAPDH
(GGCTGAGAACGGGAAGCTTGTCAT; CAGCCTTCTCCATGGTGGTGAAGA); dynein
axonemal Intermediate Chain 2 (DNAI2) (CGATCAGCATGTCGGAACAC;
CGAGCTGCATGATGGCGTTA); Chloride Channel Accessory 1 (CLCA1)
(ACAACAATGGCTATGAAGGCA; GGTCTCAAGTTTTGGTCTCACAT); Mucin 5AC
(MUC5AC) (ACCAATGCTCTGTATCCTTCCC; TGGTGGACGGACAGTCAC T) and
Forkhead Box J1 (FOXJ1) (TCTGAGCCAGGCACCACATA;
CCATGTCTGCGGGGACTCT).
RNA-sequencing (seq)
The quality and integrity of total RNA samples were
analyzed using the Agilent 2100 Bioanalyzer (Agilent Technologies)
according to the manufacturer's instructions, and libraries were
prepared utilizing the VAHTS® Universal V8 RNA-Seq
Library Prep kit (NRM605-02) for MGI and VAHTS® RNA
Adapters Set 8 for MGI (NM208-01) (both Vazyme Biotech Co., Ltd.),
according to the manufacturer's instructions. Final libraries were
quantified using the Qubit 4.0 Fluorometer (Thermo Fisher
Scientific) and their size distribution was confirmed via the
Agilent 2100 Bioanalyzer. The libraries were diluted to a final
loading concentration of 1.5 nM. Sequencing was performed as
paired-end 150 bp reads, with strand-specific sequencing using the
MGI-SEQ 2000 platform at BGI Group. The demultiplexed reads were
mapped to the GRCh38 version of the human genome using HISAT2
(25). To quantify transcripts and
analyze differential expression, the demultiplexed reads were
processed with feature Counts using R package (version 4.4.1)
(26). Differential expression was
evaluated using DESeq2 (27).
Additionally, Gene Ontology (28)
and Kyoto Encyclopedia of Genes and Genomes (KEGG) (29) pathway enrichment analyses were
conducted using Database for Annotation, Visualization and
Integrated Discovery (DAVID; david.ncifcrf.gov/).
Statistical analysis
Statistical analyses were performed using SPSS 19.0
(IBM Corp.) and GraphPad Prism 5.0 (Dotmatics). Comparisons between
three groups were conducted using one-way analysis of variance with
Tukey's HSD (Honest Significant Difference) Test. Fisher's Exact
Test was used to assess associations between two categorical
variables and the data were represented as frequency and
percentages. Independent samples t-test was used to assess the
continuous variables. All assays were repeated at least three times
to ensure reproducibility. Data are presented as the mean ±
standard deviation. A power analysis was carried out using
R-package (pwr) to validate the sample size, with the α level set
at 0.01. For datasets with n≤50, the Shapiro-Wilk test was used to
assess the normality of data distribution. P<0.05 was considered
to indicate a statistically significant difference.
Results
Increased number of pyroptosis signals
are observed in patients with nECRSwNP compared with ECRSwNP
To mitigate the influence of potential confounding
variables, the demographic and clinical data between patients with
ECRSwNP or nECRSwNP groups were compared. The present study
involved 103 patients diagnosed with CRSwNP, of whom 58 met the
inclusion criteria. Of these, 27 were excluded based on the
aforementioned criteria. Among the included 31 patients, 15 were
classified as nECRSwNP and 16 as ECRSwNP. To enhance the robustness
of the findings, a control group comprising 13 individuals without
CRSwNP was included. These controls were matched to the patient
cohort for age and sex, and screened to exclude any nasal pathology
or systemic inflammatory conditions. There were no significant
disparities in key parameters such as age, sex distribution,
prevalence of asthma, allergic rhinitis, history of NP surgery,
smoking status or allergy to food or drugs between the ECRSwNP and
nECRSwNP groups, with marked difference for Total VAS scores,
Endoscopy scores, and CT scores (Table
I), indicating heterogeneous clinical features.
 | Table I.Demographic and clinical
characteristics of participants. |
Table I.
Demographic and clinical
characteristics of participants.
| Characteristic | ECRSwNP (n=16) | nECRSwNP
(n=15) | Controls
(n=13) | P-value |
|---|
| Mean age,
years | 46.06±10.19 | 52.27±12.81 | 47.23±15.40 | 0.145 |
| Male (%) | 13 (81.25) | 9 (60.00) | 9 (69.23) | 0.252 |
| Asthma (%) | 2 (12.50) | 1 (6.67) | 0 (0.00) | >0.999 |
| AR (%) | 5 (31.25) | 2 (13.33) | 0 (0.00) | 0.394 |
| History of NP
surgery (%) | 4 (25.00) | 1 (6.67) | 0 (0.00) | 0.333 |
| Smoking (%) | 6 (37.50) | 7 (46.67) | 2 (15.38) | 0.722 |
| Allergy to food or
drugs (%) | 2 (12.50) | 0 (0.00) | 0 (0.00) | 0.484 |
| Mean total VAS
score | 15.88±3.05 | 11.47±2.92 | N/A | <0.001 |
| Mean endoscopy
score | 10.81±1.22 | 6.60±1.92 | N/A | <0.001 |
| Mean CT score | 20.13±3.20 | 11.93±5.40 | N/A | <0.001 |
Shapiro-Wilk test was conducted to assess normality.
Age, total VAS, and CT scores in ECRSwNP and nECRSwNP groups, as
well as endoscopy scores in the nECRSwNP group were normally
distributed. While endoscopy scores in the ECRSwNP group showed
slight deviation, absolute skewness (<3) and kurtosis (<10)
values indicated acceptable approximation to normality, justifying
the use of parametric tests (Table
II). The total VAS was 15.88±3.05 for patients with ECRSwNP and
11.47±2.92 for patients with nECRSwNP. However, notable
distinctions were observed in the endoscopy and CT scores between
the ECRSwNP and nECRSwNP cohorts. The mean endoscopy score was
10.81±1.22 for patients with ECRSwNP and 6.60±1.92 for patients
with nECRSwNP, while the mean CT score was 20.13±3.20 for ECRSwNP
and 11.93±5.40 for nECRSwNP. These discrepancies in endoscopy and
CT score reflect variations in disease severity between the two
CRSwNP phenotypes as the power analysis demonstrated statistical
power values of 0.9019, 0.9999 and 0.9891 for total VAS, endoscopy
and CT scores, respectively.
 | Table II.Shapiro-Wilk test for continuous
variables. |
Table II.
Shapiro-Wilk test for continuous
variables.
| Characteristic | Group | n | Mean | SD | Skewness | Kurtosis | W-value | P-value |
|---|
| Age | ECRSwNP | 16 | 46.063 | 10.188 | −0.724 | 0.008 | 0.938 | 0.330 |
|
| nECRSwNP | 15 | 52.267 | 12.814 | −0.276 | −0.665 | 0.938 | 0.364 |
| Total VAS
score | ECRSwNP | 16 | 15.875 | 3.052 | −0.379 | −0.862 | 0.943 | 0.389 |
|
| nECRSwNP | 15 | 11.467 | 2.924 | 0.751 | −0.692 | 0.887 | 0.060 |
| Endoscopy
score | ECRSwNP | 16 | 10.813 | 1.223 | −0.345 | −1.570 | 0.808 | 0.003 |
|
| nECRSwNP | 15 | 6.600 | 1.920 | −0.036 | −1.059 | 0.881 | 0.050 |
| CT score | ECRSwNP | 16 | 20.125 | 3.202 | −0.937 | 1.281 | 0.902 | 0.087 |
|
| nECRSwNP | 15 | 11.933 | 5.405 | 0.274 | −1.394 | 0.915 | 0.164 |
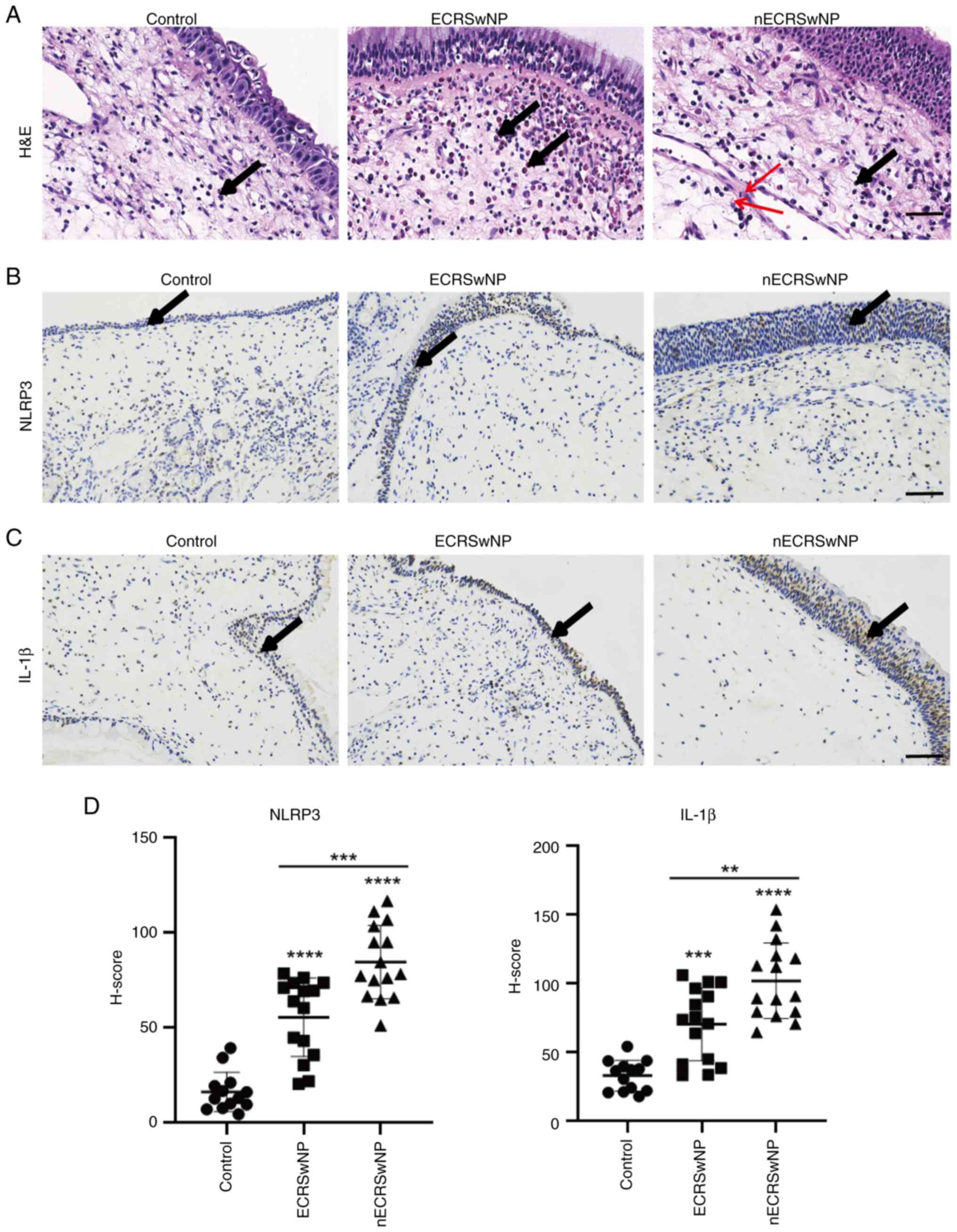
To assess pyroptosis in patients with nECRSwNP and
ECRSwNP, the expression of NLRP3 and IL-1β was analyzed. H&E
staining was used to evaluate morphological changes and the
infiltration of inflammatory cells in NP samples from patients with
CRSwNP (Fig. 1A). Consistent with
clinical observations, the control group showed no notable
infiltration of inflammatory cells. By contrast, the ECRSwNP group
exhibited a substantial infiltration of eosinophils. Conversely,
the nECRSwNP group displayed few eosinophils and basement membrane
thickening and infiltration of inflammatory cells, including
neutrophils, plasma cells and lymphocytes. Immunohistochemical
staining demonstrated a significant increase in the number of
NLRP3- and IL-1β-positive cells in both nECRSwNP and ECRSwNP groups
compared with the control (Fig.
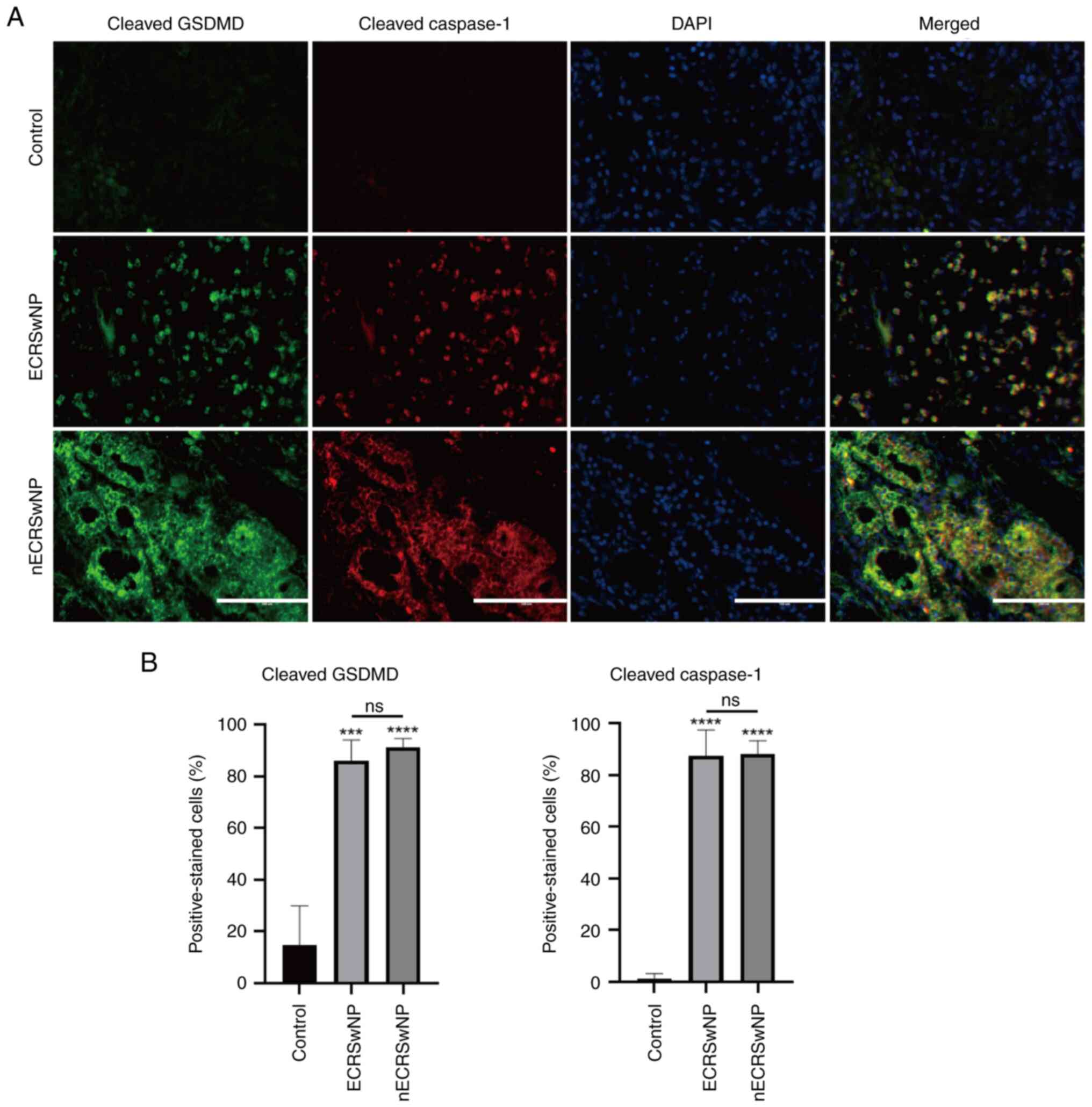
1B-D). To validate these findings, cleaved GSDMD and Caspase-1
expression was assessed using IF staining. Consistently, >80% of
the cells were positive for both cleaved GSDMD and Caspase-1,
indicating the activation of the pyroptosis signaling pathway. The
intensity of cleaved GSDMD and cleaved caspase-1 signals was
stronger in the nECRSwNP group compared with the ECRSwNP group
(Fig. 2). These results suggest an
association between pyroptosis and the development of CRSwNP,
particularly in patients with nECRSwNP.
Peripheral venous serum from patients
with CRSwNP induces pyroptosis of basal cells
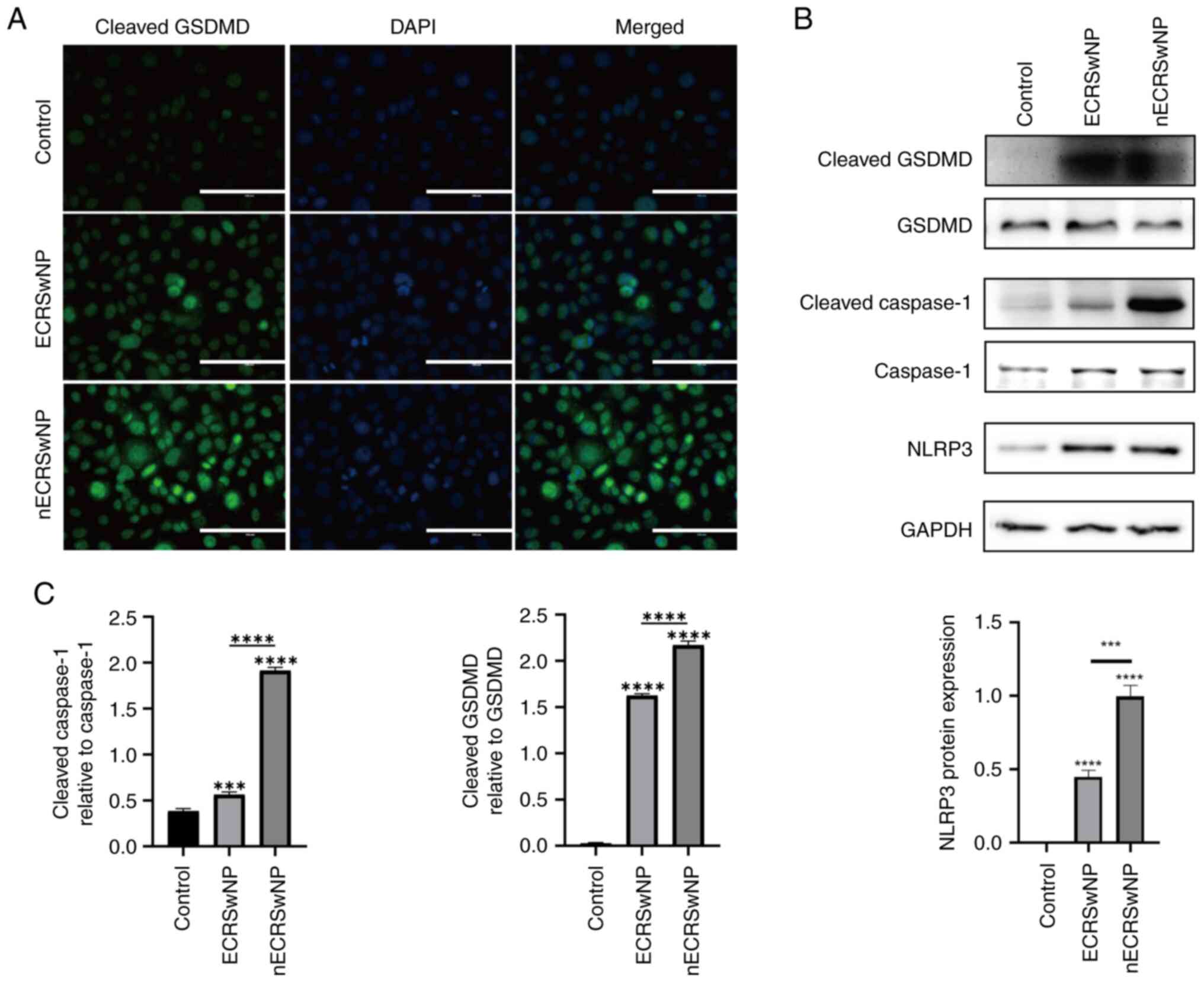
To assess the potential of serum from patients with
CRSwNP to induce pyroptosis in basal cells, RPMI-2650 cells were
treated with diluted serum from patients with either nECRSwNP or
CRSwNP for 72 h. Immunocytochemistry revealed a notable increase in
the expression of cleaved GSDMD in both groups compared with the
control group, with a pronounced increase observed in the nECRSwNP
group (Fig. 3A). The expression of
total GSDMD, Caspase-1, cleaved GSDMD, cleaved Caspase-1 and NLRP3
was further investigated by western blotting. Consistent with the
immunocytochemistry results, cleaved GSDMD and Caspase-1 and NLRP3
showed a notable increase in expression in cells treated with serum
from patients with nECRSwNP and ECRSwNP compared with the control
group, despite no substantial changes in the total protein levels
of GSDMD and Caspase-1. The levels of these proteins were
significantly higher in the nECRSwNP compared with the ECRSwNP
group (Fig. 3B and C). These
findings implicated that components present in the serum from
patients with CRSwNP may induce pyroptosis, with a more pronounced
effect in nECRSwNP, contributing to the manifestation of
CRSwNP.
IL-5 and IL-17A induce the ECRSwNP and
nECRSwNP phenotype, respectively
Previous studies indicate that IL-5 and IL-17A are
the dominant immune cytokines in the peripheral venous serum of
patients with ECRSwN (30) and
nECRSwNP (31), respectively. To
investigate whether these trigger pyroptosis in CRSwNP, their
expression in nECRSwNP and ECRSwNP samples was investigated. IHC
revealed increased expression of IL-5 in patients with ECRSwNP and
IL-17A in patients with nECRSwNP (Fig.
4A). RPMI-2650 cells were treated with IL-5 or IL-17A for 24 h
to assess the activation of pyroptosis. No differences were
observed in cell morphology and number between groups (Fig. 4B). However, western blot analysis
revealed the induction of cleaved GSDMD and cleaved Caspase-1
stimulated by IL-5 and IL-17A compared with the control group,
despite no substantial changes for total GSDMD and Caspase-1.
Conversely, the addition of pyroptosis inhibitor DMF) resulted in
significantly reduced levels of cleaved GSDMD and cleaved Caspase-1
compared with the IL5- or IL17-alone groups (Fig. 4C).
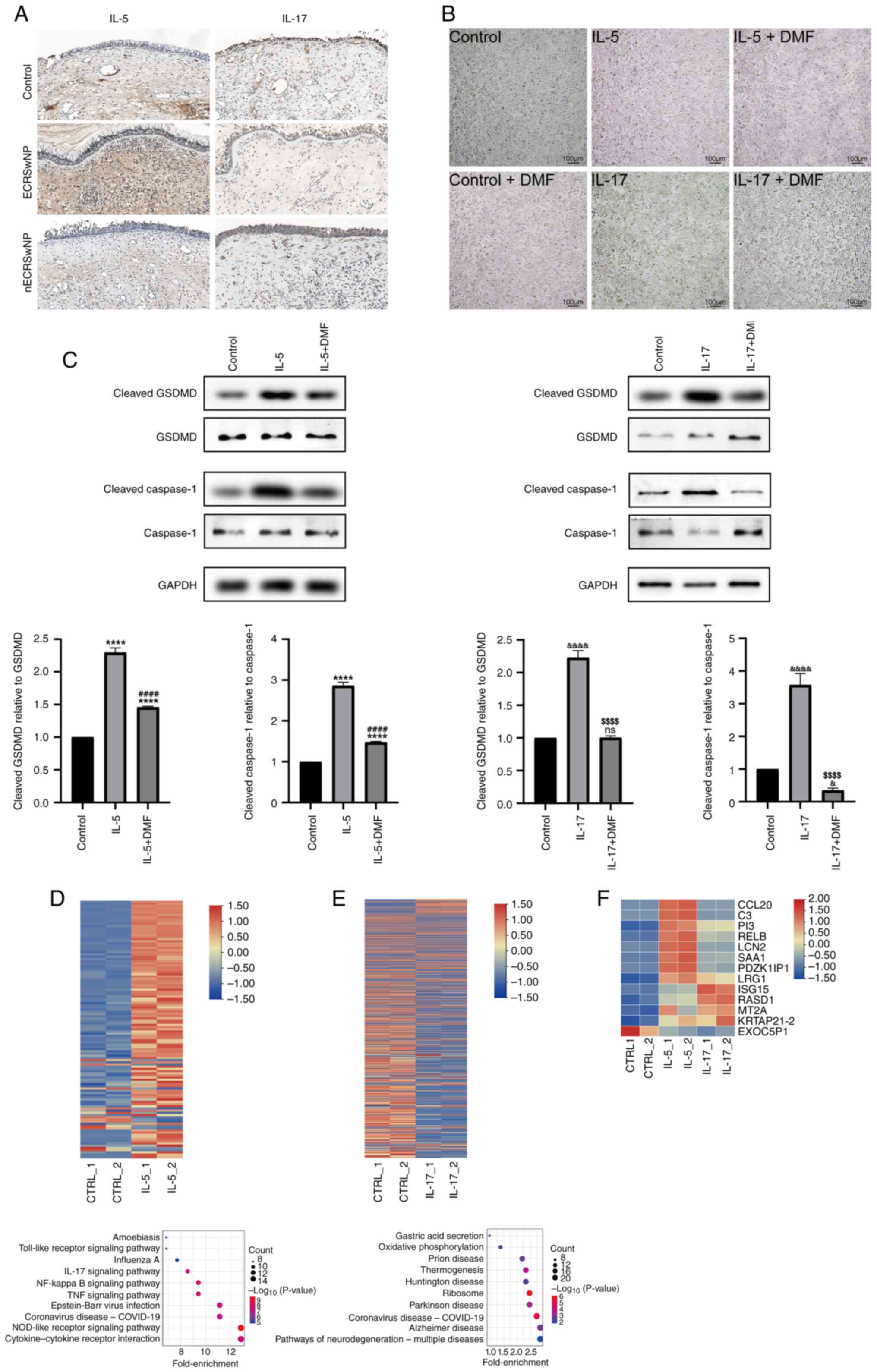 | Figure 4.IL-5 and IL-17A induce the ECRSwNP
and nECRSwNP phenotype, respectively. (A) Immunohistochemical
staining of IL-5 and IL-17A protein. (B) Morphology and quantity of
basal HNEpCs cells stimulated with IL-5, IL-17A and their inhibitor
DMF for 24 h. (C) GSDMD, cleaved GSDMD, Caspase-1 and cleaved
Caspase-1 were analyzed by western blotting in HNEpCs stimulated
with IL-5 (****P<0.0001 IL-5 group, IL-5+DMF group vs. Control
group; ####P<0.0001 IL-5+DMF group vs. IL-5 group) or
IL-17A (&&&&P<0.0001 IL-17 group vs.
Control group; &P<0.05 IL-17+DMF group vs.
Control group; $$$$P<0.0001 IL-17+DMF group vs. IL-17
group). RNA-sequencing analysis of basal cells treated with (D)
IL-5 or (E) IL-17. Heatmap shows differential gene expression. Red,
upregulation; blue, downregulation. Kyoto Encyclopedia of Genes and
Genomes pathway analysis was conducted to display the pathway of
differentially expressed genes. (F) Common up- and downregulated
genes of basal cells treated with IL-5 and IL-17. DMF, dimethyl
fumarate; CTRL, control; nECRSwNP, non-eosinophilic chronic
rhinosinusitis with nasal polyps; GSDMD, Gasdermin D; HNEpC, human
nasal epithelial cells; ns, not significant. |
To gain molecular insight into the effects of IL-5
and IL-17A on RPMI-2650 cells, RNA-seq analysis was performed on
control cells and cells stimulated with either IL-5 or IL-17 to
analyze associated genes and signaling pathways involved. Relative
to the control cells, 105 genes were up- and 12 genes were
downregulated in the IL-5 group, while 245 genes were up- and 484
genes were downregulated in the IL-17A group. Among these genes, 12
were up- and one was downregulated in the IL-5 vs. IL-17A overlap.
KEGG analysis revealed that inflammatory signaling pathways such as
‘cytokine-cytokine receptor interaction’, ‘NOD-like receptor
signaling pathway’, ‘TNF signaling’, ‘NF-κβ signaling pathway’,
‘toll-like receptor signaling pathway’ and ‘IL-17 signaling
pathway’ were associated with IL-5 stimulation. Conversely,
‘coronavirus disease-COVlD-19’ and ‘ribosome’ were ranked as top in
the IL-17A-stimulated group (Fig. 4D
and E).
Inhibition of pyroptosis rescues the
differentiation imbalance of basal cells
To understand the role of pyroptosis in the
development of CRSwNP, its impact on basal cell differentiation
capacity was examined. RT-qPCR revealed that the expression of
CLCA1, the key marker of goblet cells, was upregulated in RPMI-2650
cells treated with either IL-5 or IL-17A, indicating a shift
towards goblet cell differentiation. Conversely, the expression of
DNAI2, the key regulator of ciliated cells, was downregulated,
suggesting a decrease in ciliated cell differentiation (Fig. 5A). DMF, a pyroptosis inhibitor,
rescued this differentiation imbalance, implicating the activation
of pyroptosis as a key trigger of the differentiation imbalance in
basal cells (Fig. 5A and B). These
results were validated in HNEpCs, demonstrating that pyroptosis
inhibition partially rescued the differentiation imbalance,
particularly in IL-17A-treated cells (Fig. 4C).
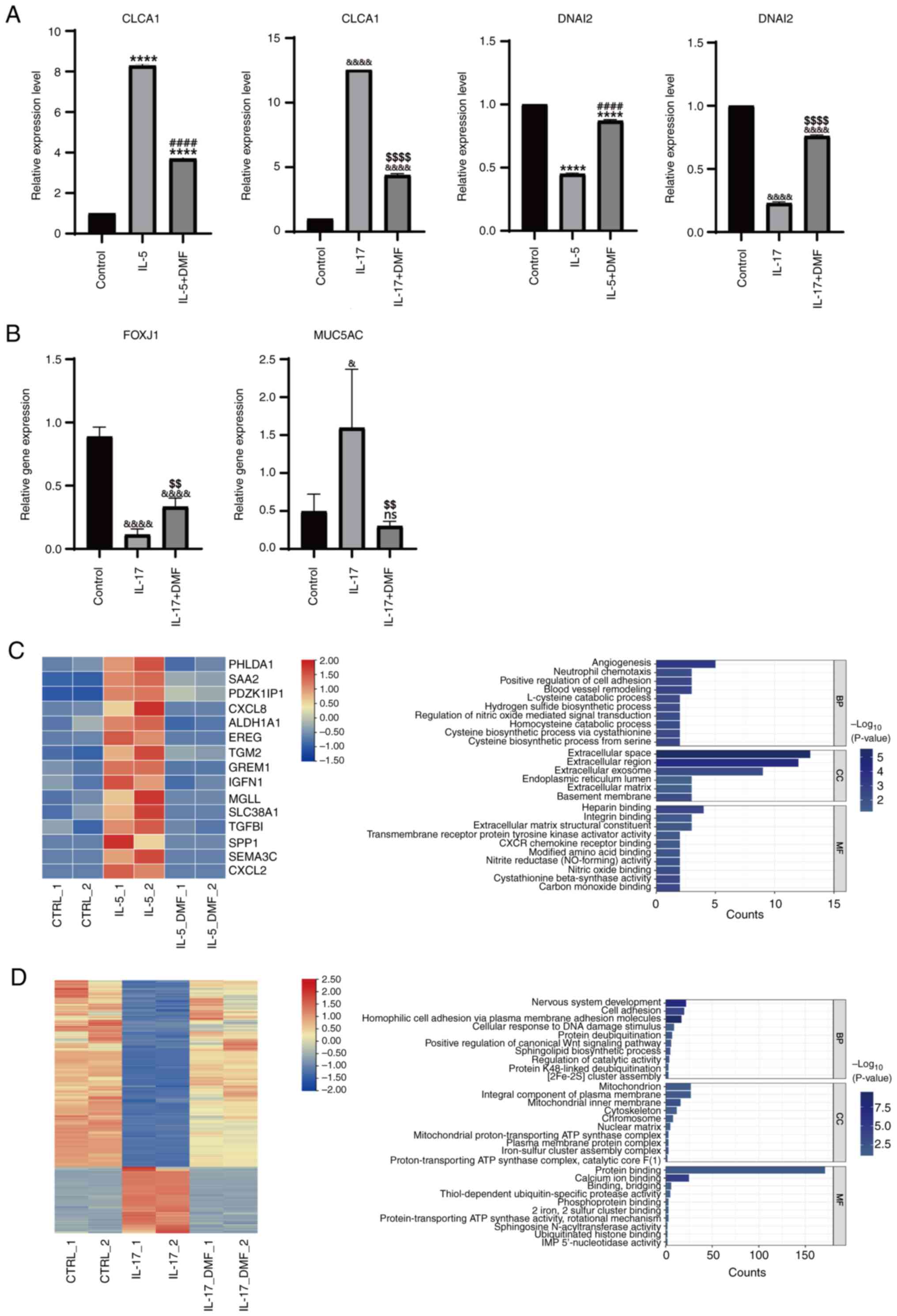 | Figure 5.Inhibition of pyroptosis mitigates
the differentiation imbalance of basal cells. mRNA expression of
(A) CLCA1 and DNAI2(****P<0.0001 IL-5 group, IL-5+DMF group vs.
Control group; ####P<0.0001 IL-5+DMF group vs. IL-5
group; &&&&P<0.0001 IL-17, IL-17+DMF
group vs. Control group; $$$$P<0.0001 IL-17+DMF group
vs. IL-17 group) and (B) FOXJ1
(&&&&P<0.0001 IL-17, IL-17+DMF group
vs. Control group; $$P<0.01 IL-17+DMF group vs. IL-17
group) and MUC5AC (&P<0.05 IL-17 group vs.
Control group; $$P<0.01 IL-17+DMF group vs. IL-17
group). RNA-sequencing analysis of basal cells treated with (C)
IL-5 and (D) IL-17, with and without DMF. The bar chart was
generated using DAVID, http://david.ncifcrf.gov/). DMF, dimethyl fumarate,
BP, biological process; CC, cellular component; MF, molecular
function; CTRL, control; CLCA1, chloride Channel Accessory 1;
DNAI2, Dynein Axonemal Intermediate Chain 2; MUC5AC, Mucin 5AC. |
RNA-seq analysis revealed a significant reversal of
gene expression patterns after DMF administration, particularly in
the IL-17A-treated group. Gene Ontology function analysis of
differentially expressed genes in the IL-5-treated group revealed
enrichment in ‘angiogenesis’, ‘cell adhesion’, ‘regulation of
nitric oxide-mediated signal transduction’, ‘neutrophil chemotaxis’
and ‘extracellular space’, as well as ‘hydrogen sulfide
biosynthetic process’, ‘heparin binding’ and ‘carbon monoxide
binding’. By contrast, in the IL-17A-treated group, targeting
pyroptosis by DMF resulted in enrichment of ‘cell adhesion’,
‘positive regulation of canonical Wnt signaling pathway’,
‘mitochondrion’, ‘cytoskeleton’ and ‘plasma membrane protein
complex’, as well as ‘nervous system development’, ‘sphingolipid
biosynthetic process’ and ‘iron-sulfur cluster assembly complex’
(Fig. 5C and D). These findings
suggest that pyroptosis may contribute to the impairment of basal
cell differentiation in the nasal epithelium of patients with
CRSwNP.
Discussion
Despite the identification of the NLRP3 inflammasome
signal pathway being implicated in the pathogenesis of CRSwNP,
notable gaps persist in the understanding of whether this phenotype
influences the development of CRSwNP. The present study revealed
that the activation of pyroptosis mediates the imbalanced
differentiation of basal cells in the nasal epithelium in CRSwNP.
This discovery suggests that targeting pyroptosis may represent a
novel therapeutic approach for managing CRSwNP.
A notable increase in the expression of
pyroptosis-associated markers was observed, including NLRP3,
Caspase-1, GSDMD and IL-1β, in NP, with increased levels detected
in nECRSwNP compared with ECRSwNP samples. These findings were
consistent across cell experiments in which basal cells were
exposed to IL-5 and IL-17A and serum from patients with ECRSwNP or
nECRSwNP. This aligns with previous research, indicating a
potential association between the predominance of neutrophil
infiltration in nECRSwNP and the heightened neutrophilic response
associated with type 3 immune reactions (10,11).
However, the typical features of pyroptosis-induced cell death,
such as cell membrane perforation and cleavage, were not observed.
The low concentrations of IL-5 and IL-17A used in the present study
may not have been sufficient to induce cell death.
RNA-seq suggested that IL-5 and IL-17A participate
in pyroptosis primarily by modulating inflammatory pathways, which
further exacerbate the impairment of nasal epithelial cell
differentiation. Previous studies (32–34)
have reported that inflammasome NLRP3/Caspase-1-mediated pyroptosis
induced by viral infection occurs in both airway epithelial and
immune cells (32). HIF-1α has
been revealed to activate NLRP3 by phosphorylating NLRP3 at serine
295 (12). This activation of the
inflammasome leads to the recruitment of Caspase-1 and
apoptosis-associated speck-like protein containing a CARD,
inhibition of ciliated cell expression and promotion of goblet cell
differentiation and basal cell proliferation (12). For example, during rhinovirus
infection, there is a notable increase in the expression of NLRP3,
IL-1β and MUC5AC in nasal mucosa, leading to goblet cell
proliferation. The NLRP3 inflammasome relies on the DEAH-Box
Helicase 33 (DDX33)- DDX58-NLRP3-Caspase-1-GSDMD-IL-1β signaling
axis to mediate airway epithelial inflammation, pyroptosis and
mucin production (32). Studies on
bronchial epithelium have revealed increased expression of
Caspase-1, NLRP3 and IL-1β in human bronchial epithelium (35,36).
Additionally, asthmatic patients exhibit increased expression of
IL-1β and MUC5AC, with IL-1β upregulating MUC5AC expression via the
NF-κB pathway (37). RT-qPCR data
confirmed the differentiation deficit of basal cells biased toward
goblet cell fate, which is a typical hallmark of CRSwNP. Therefore
it was hypothesized that inflammatory signaling pathway may exhibit
crosstalk with differentiation capacity via pyroptosis.
Previous clinical data have indicated marked
increases in vascularity, pro-angiogenic gene expression (including
platelet and endothelial cell adhesion molecule 1 and
platelet-activating factor receptor), blood vessel morphogenesis
and blood flow in the mucosal tissue of patients with CRSwNP
(38,39). Additionally, intercellular adhesion
molecule genes are significantly elevated in the blood and sinuses
of patients with CRSwNP (40).
Basal cell adhesion molecules, which serve as markers of airway
stem cells, have a key role in epithelial remodeling during Type 2
inflammation (41). Conversely,
the expression of eosinophil adhesion molecule vascular cell
adhesion molecule-1 (VCAM-1) markedly decreases after therapy with
dupilumab, a monoclonal anti-IL-4Rα antibody (42). Furthermore, nitric oxide (NO)
increases ciliary beating, the airway primary physical defense
mechanism. Fractional exhaled NO is a convenient and sensitive
marker for evaluating the efficacy of standard anti-inflammatory
therapy for CRSwNP. Studies have demonstrated that the airway
epithelial layers contribute to exhaled NO in type 2 inflammation,
including eosinophilic chronic rhinosinusitis (43,44).
Of note, the extracellular space may be related to tight junctions
(TJs) between nasal epithelial cells, with TJs serving an essential
role in the development and progression of CRSwNP (45). Rescue data in the IL-5 group of the
present study suggest that DMF alleviated the CRSwNP phenotype,
potentially via modulation of these signaling pathways.
In nECRSwNP, existing literature indicates
activation of the canonical Wnt pathway, which has been associated
with cytokine release and inhibition of multiciliated cell
differentiation in nasal epithelium regeneration (46). Inhibiting the Wnt/β-catenin
signaling pathway mitigates inflammation and the
epithelial-to-mesenchymal transition in CRSwNP, both in vivo
and in vitro (47).
Moreover, the pathogenesis of CRSwNP may involve the production of
mitochondrial reactive oxygen species, disrupted mitochondrial
function and structural changes in nasal epithelial cells (48). The downregulation of
tryptophan-aspartic acid repeat-containing planar cell polarity
effector in nasal epithelium may impact mitochondria through the
MAPK/ERK pathway, potentially contributing to ciliary dysfunction
in CRSwNP (49). Additionally, the
cytoskeleton, which is involved in cell morphology and integrity,
may undergo alterations associated with changes in cell morphology
during pyroptosis. Future investigations should explore how basal
and neural cells use inflammation-induced pyroptosis, providing
valuable insights into the pathogenesis of CRSwNP.
Given the role of pyroptosis in driving inflammation
and basal cell dysfunction in CRSwNP, targeting this pathway offers
a promising approach to mitigate disease progression and improve
patient outcomes (12). Pyroptosis
inhibition could not only decrease epithelial damage and immune
cell infiltration but also restore normal epithelial
differentiation, potentially breaking the cycle of chronic
inflammation (50). However,
challenges must be addressed before translating these findings into
clinical practice. Effective drug delivery strategies are key,
particularly for targeting the nasal epithelium while minimizing
systemic exposure. Additionally, off-target effects pose a concern,
as systemic pyroptosis inhibition could interfere with essential
immune responses. Lastly, variability in patient responses, driven
by distinct phenotypes of CRSwNP (eosinophilic vs. neutrophilic)
and genetic differences, highlights the need for personalized
therapeutic strategies. Future research should develop localized
delivery systems, identify specific inhibitors with minimal side
effects and stratify patients to maximize therapeutic efficacy.
While the present study offered insight into the
role of pyroptosis in chronic rhinosinusitis with CRSwNP and the
therapeutic potential of targeting pyroptosis, several limitations
should be acknowledged. The present study primarily relied on in
vitro experiments and patient-derived samples to demonstrate
the role of pyroptosis in CRSwNP. Although the data suggested that
inhibiting pyroptosis restores basal cell differentiation balance,
the lack of in vivo models limits the ability to validate
the therapeutic potential and physiological relevance of pyroptosis
inhibition. Future studies using animal models of CRSwNP would
provide more comprehensive insights into the systemic effects of
pyroptosis modulation and its impact on disease progression. The
present study investigated both ECRSwNP and nECRSwNP subtypes of
CRSwNP, but the sample size was relatively small, particularly for
subgroup comparisons. Larger, multicenter cohorts are needed to
validate the differential pyroptosis mechanisms between ECRSwNP and
nECRSwNP and assess the influence of demographic and clinical
variables. Although the present study identified IL-5 and IL-17A as
upstream triggers of pyroptosis, the precise signaling mechanisms
linking these cytokines to pyroptosis activation remain
incompletely defined. More detailed mechanistic studies are
necessary to elucidate the precise molecular interactions. The
present study used DMF to inhibit pyroptosis, but DMF also exerts
anti-inflammatory and immunomodulatory effects through other
pathways, such as NF-κB and oxidative stress modulation (51). Genetic approaches (such as
clustered Regularly Interspaced Short Palindromic Repeat-mediated
gene editing) are necessary to confirm the specificity of the
present findings.
In conclusion, the present study suggested that
pyroptosis, mediated by IL-5 and IL-17A, contributes to the
impaired differentiation of nasal epithelial cells, particularly
promoting the goblet cell phenotype seen in CRSwNP.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from National Natural
Science Foundation of China (grant no. 32070859), Natural Science
Foundation of Shandong Province (grant nos. ZR2020MC083 and
ZR2021MH350) and Taishan Scholars Program of Shandong Province
(grant no. TS20190931).
Availability of data and materials
The data generated in the present study may be found
in the Sequence Read Archive database under accession number
PRJNA1224023 or at the following URL:
ncbi.nlm.nih.gov/sra/?term=PRJNA1224023.
Authors' contributions
GZ, SY and ZW designed the study and wrote the
manuscript. GZ, SJ, JL, LL and XX performed human specimen
collection, cell culture, immunohistochemical and immunofluorescent
staining, western blotting, RT-qPCR and data analysis. YD and ZL
constructed the figures, revised the manuscript, supplemented and
analyzed the data. LL and XX confirm the authenticity of all the
raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study received approval from the Ethics
Committee of the Affiliated Hospital of Qingdao University
(Qingdao, China; Ethical approval no. QYFY WZLL28477). Participants
provided written informed consent to take part in the present
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stevens WW, Lee RJ, Schleimer RP and Cohen
NA: Chronic rhinosinusitis pathogenesis. J Allergy Clin Immunol.
136:1442–1453. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kato A, Schleimer RP and Bleier BS:
Mechanisms and pathogenesis of chronic rhinosinusitis. J Allergy
Clin Immunol. 149:1491–1503. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fokkens WJ, Lund VJ, Hopkins C, Hellings
PW, Kern R, Reitsma S, Toppila-Salmi S, Bernal-Sprekelsen M, Mullol
J, Alobid I, et al: European position paper on rhinosinusitis and
nasal polyps 2020. Rhinology. 58 (Suppl S29):1–464. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
DeConde AS, Mace JC, Levy JM, Rudmik L,
Alt JA and Smith TL: Prevalence of polyp recurrence after
endoscopic sinus surgery for chronic rhinosinusitis with nasal
polyposis. Laryngoscope. 127:550–555. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Riva G, Tavassoli M, Cravero E, Moresco M,
Albera A, Canale A and Pecorari G: Long-term evaluation of nasal
polyposis recurrence: A focus on multiple relapses and nasal
cytology. Am J Otolaryngol. 43:1033252022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chang L, Wu H, Huang W, Li Y, Chen Y, Li
X, Yao Z, Chen X, Lai X, Zheng R, et al: IL-21 induces pyroptosis
of Treg cells via Akt-mTOR-NLRP3-caspase 1 axis in eosinophilic
chronic rhinosinusitis. J Allergy Clin Immunol. 152:641–655.e14.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang Y, Chen H, Zhong J, Shen L and Zheng
X: Role of NLRP3 Inflammasome on different phenotypes of chronic
rhinosinusitis. Am J Rhinol Allergy. 36:607–614. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kayagaki N, Stowe IB, Lee BL, O'Rourke K,
Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT,
et al: Caspase-11 cleaves gasdermin D for non-canonical
inflammasome signalling. Nature. 526:666–671. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi J, Zhao Y, Wang K, Shi X, Wang Y,
Huang H, Zhuang Y, Cai T, Wang F and Shao F: Cleavage of GSDMD by
inflammatory caspases determines pyroptotic cell death. Nature.
526:660–665. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wei Y, Zhang J, Wu X, Sun W, Wei F, Liu W,
Lu T, Ji W, Li H and Wen W: Activated pyrin domain containing 3
(NLRP3) inflammasome in neutrophilic chronic rhinosinusitis with
nasal polyps (CRSwNP). J Allergy Clin Immunol. 145:1002–1005.e16.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Y, Chang LH, Huang WQ, Bao HW, Li X,
Chen XH, Wu HT, Yao ZZ, Huang ZZ, Weinberg SE, et al: IL-17A
mediates pyroptosis via the ERK pathway and contributes to steroid
resistance in CRSwNP. J Allergy Clin Immunol. 150:337–351. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhong B, Sun S, Tan KS, Ong HH, Du J, Liu
F, Liu Y, Liu S, Ba L, Li J, et al: Hypoxia-inducible factor 1α
activates the NLRP3 inflammasome to regulate epithelial
differentiation in chronic rhinosinusitis. J Allergy Clin Immunol.
152:1444–1459.e14. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gevaert P, Han JK, Smith SG, Sousa AR,
Howarth PH, Yancey SW, Chan R and Bachert C: The roles of
eosinophils and interleukin-5 in the pathophysiology of chronic
rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol.
12:1413–1423. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nagase H, Ueki S and Fujieda S: The roles
of IL-5 and anti-IL-5 treatment in eosinophilic diseases: Asthma,
eosinophilic granulomatosis with polyangiitis, and eosinophilic
chronic rhinosinusitis. Allergol Int. 69:178–186. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen XH, Chang LH, Huang JC, Li X, Lai XP,
Wu XF, Huang ZZ, Wang ZY, Bao HW and Zhang GH: [Expression and
cellular provenance of interleukin 17A in non-eosinophilic chronic
rhinosinusitis with nasal polyps]. Zhonghua Er Bi Yan Hou Tou Jing
Wai Ke Za Zhi. 55:604–610. 2020.(In Chinese). PubMed/NCBI
|
|
16
|
Liu Y, Zeng M and Liu Z: Th17 response and
its regulation in inflammatory upper airway diseases. Clin Exp
Allergy. 45:602–612. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng KJ, Zhou ML, Liu YC and Zhou SH:
Roles played by the PI3K/Akt/HIF-1α pathway and IL-17A in the
Chinese subtype of chronic sinusitis with nasal polyps. Mediators
Inflamm. 2022:86095902022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ye X, Li Y, Fang B, Yuan Y, Feng D, Chen
H, Li J, Meng Q, Xiong S, Ye D, et al: Type 17 mucosal-associated
invariant T cells contribute to neutrophilic inflammation in
patients with nasal polyps. J Allergy Clin Immunol.
152:1153–1166.e12. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Psaltis AJ, Li G, Vaezeafshar R, Cho KS
and Hwang PH: Modification of the Lund-Kennedy endoscopic scoring
system improves its reliability and correlation with
patient-reported outcome measures. Laryngoscope. 124:2216–2223.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kennedy DW: Prognostic factors, outcomes
and staging in ethmoid sinus surgery. Laryngoscope. 102 (Suppl
57):1–18. 1992.PubMed/NCBI
|
|
21
|
Lund VJ and Kennedy DW: Quantification for
staging sinusitis. The staging and therapy group. Ann Otol Rhinol
Laryngol Suppl. 167:17–21. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bachert C, Sousa AR, Lund VJ, Scadding GK,
Gevaert P, Nasser S, Durham SR, Cornet ME, Kariyawasam HH, Gilbert
J, et al: Reduced need for surgery in severe nasal polyposis with
mepolizumab: Randomized trial. J Allergy Clin Immunol.
140:1024–1031.e14. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu C, Wang K, Liu W, Zhang J, Fan Y and
Sun Y: ALOX15+ M2 macrophages contribute to epithelial
remodeling in eosinophilic chronic rhinosinusitis with nasal
polyps. J Allergy Clin Immunol. 154:592–608. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Z, Gearhart MD, Lee YW, Kumar I,
Ramazanov B, Zhang Y, Hernandez C, Lu AY, Neuenkirchen N, Deng J,
et al: A non-canonical BCOR-PRC1.1 complex represses
differentiation programs in human ESCs. Cell Stem Cell.
22:235–251.e9. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pertea M, Kim D, Pertea GM, Leek JT and
Salzberg SL: Transcript-level expression analysis of RNA-seq
experiments with HISAT, StringTie and ballgown. Nat Protoc.
11:1650–1667. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liao Y, Smyth GK and Shi W: featureCounts:
An efficient general purpose program for assigning sequence reads
to genomic features. Bioinformatics. 30:923–930. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Love MI, Huber W and Anders S: Moderated
estimation of fold change and dispersion for RNA-seq data with
DESeq2. Genome Biol. 15:5502014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene ontology: Tool for the unification of biology. The gene
ontology consortium. Nat Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kanehisa M, Furumichi M, Sato Y,
Ishiguro-Watanabe M and Tanabe M: KEGG: Integrating viruses and
cellular organisms. Nucleic Acids Res. 49:D545–D551. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shin SH, Ye MK, Park J and Geum SY:
Immunopathologic role of eosinophils in eosinophilic chronic
rhinosinusitis. Int J Mol Sci. 23:133132022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ryu G, Bae JS, Kim JH, Kim EH, Lyu L,
Chung YJ and Mo JH: Role of IL-17A in chronic rhinosinusitis with
nasal polyp. Allergy Asthma Immunol Res. 12:507–522. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu T, Zhou YT, Wang LQ, Li LY, Bao Q,
Tian S, Chen MX, Chen HX, Cui J and Li CW: NOD-like receptor
family, pyrin domain containing 3 (NLRP3) contributes to
inflammation, pyroptosis, and mucin production in human airway
epithelium on rhinovirus infection. J Allergy Clin Immunol.
144:777–787.e9. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Duan Y, Zhu Y, Zhang L, Wang W, Zhang M,
Tian J, Li Q, Ai J, Wang R and Xie Z: Activation of the NLRP3
inflammasome by human adenovirus type 7 L4 100-kilodalton protein.
Front Immunol. 15:12948982024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hsieh LL, Looney M, Figueroa A, Massaccesi
G, Stavrakis G, Anaya EU, D'Alessio FR, Ordonez AA, Pekosz AS,
DeFilippis VR, et al: Bystander monocytic cells drive
infection-independent NLRP3 inflammasome response to SARS-CoV-2.
mBio. 15:e00810242024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hirota JA, Hirota SA, Warner SM,
Stefanowicz D, Shaheen F, Beck PL, Macdonald JA, Hackett TL, Sin
DD, Van Eeden S and Knight DA: The airway epithelium
nucleotide-binding domain and leucine-rich repeat protein 3
inflammasome is activated by urban particulate matter. J Allergy
Clin Immunol. 129:1116–1125.e6. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dino P, Giuffrè MR, Buscetta M, Di
Vincenzo S, La Mensa A, Cristaldi M, Bucchieri F, Lo Iacono G,
Bertani A, Pace E and Cipollina C: Release of IL-1β and IL-18 in
human primary bronchial epithelial cells exposed to cigarette smoke
is independent of NLRP3. Eur J Immunol. 54:e24510532024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu S, Li H, Yu L, Wang N, Li X and Chen W:
IL-1β upregulates Muc5ac expression via NF-κB-induced HIF-1α in
asthma. Immunol Lett. 192:20–26. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Khurana N, Pulsipher A, Jedrzkiewicz J,
Ashby S, Pollard CE, Ghandehari H and Alt JA: Inflammation-driven
vascular dysregulation in chronic rhinosinusitis. Int Forum Allergy
Rhinol. 11:976–983. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vukadinović T, Vuksanović Božarić A,
Vukomanović Durđević B, Radunović M and Perić A: Angiogenesis and
eosinophilia in the nasal mucosa of patients with different
clinical phenotypes of chronic rhinosinusitis. J Infect Dev Ctries.
17:1480–1488. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Blight BJ, Gill AS, Sumsion JS, Pollard
CE, Ashby S, Oakley GM, Alt JA and Pulsipher A: Cell adhesion
molecules are upregulated and may drive inflammation in chronic
rhinosinusitis with nasal polyposis. J Asthma Allergy. 14:585–593.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang X, Hallen NR, Lee M, Samuchiwal S, Ye
Q, Buchheit KM, Maxfield AZ, Roditi RE, Bergmark RW, Bhattacharyya
N, et al: Type 2 inflammation drives an airway basal stem cell
program through insulin receptor substrate signaling. J Allergy
Clin Immunol. 151:1536–1549. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ryser FS, Yalamanoglu A, Valaperti A,
Brühlmann C, Mauthe T, Traidl S, Soyka MB and Steiner UC:
Dupilumab-induced eosinophilia in patients with diffuse type 2
chronic rhinosinusitis. Allergy. 78:2712–2723. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kawasumi T, Takeno S, Ishikawa C, Takahara
D, Taruya T, Takemoto K, Hamamoto T, Ishino T and Ueda T: The
functional diversity of nitric oxide synthase isoforms in human
nose and paranasal sinuses: Contrasting pathophysiological aspects
in nasal allergy and chronic rhinosinusitis. Int J Mol Sci.
22:75612021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Maniscalco M, Fuschillo S, Mormile I,
Detoraki A, Sarnelli G, Paulis A, Spadaro G, Cantone E and PATH-2
TASK FORCE: Exhaled nitric oxide as biomarker of type 2 diseases.
Cells. 12:25182023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Huang ZQ, Liu J, Sun LY, Ong HH, Ye J, Xu
Y and Wang DY: Updated epithelial barrier dysfunction in chronic
rhinosinusitis: Targeting pathophysiology and treatment response of
tight junctions. Allergy. 79:1146–1165. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Böscke R, Vladar EK, Könnecke M, Hüsing B,
Linke R, Pries R, Reiling N, Axelrod JD, Nayak JV and Wollenberg B:
Wnt signaling in chronic rhinosinusitis with nasal polyps. Am J
Respir Cell Mol Biol. 56:575–584. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Su H and Zhao Y: Eupatilin alleviates
inflammation and epithelial-to-mesenchymal transition in chronic
rhinosinusitis with nasal polyps by upregulating TFF1 and
inhibiting the Wnt/β-catenin signaling pathway. Histol Histopathol.
39:357–365. 2024.PubMed/NCBI
|
|
48
|
Yoon YH, Yeon SH, Choi MR, Jang YS, Kim
JA, Oh HW, Jun X, Park SK, Heo JY, Rha KS and Kim YM: Altered
mitochondrial functions and morphologies in epithelial cells are
associated with pathogenesis of chronic rhinosinusitis with nasal
polyps. Allergy Asthma Immunol Res. 12:653–668. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ma Y, Tian P, Zhong H, Wu F, Zhang Q, Liu
X, Dang H, Chen Q, Zou H and Zheng Y: WDPCP modulates cilia beating
through the MAPK/ERK pathway in chronic rhinosinusitis with nasal
polyps. Front Cell Dev Biol. 8:6303402020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xie X, Wang P, Jin M, Wang Y, Qi L, Wu C,
Guo S, Li C, Zhang X, Yuan Y, et al: IL-1β-induced epithelial cell
and fibroblast transdifferentiation promotes neutrophil recruitment
in chronic rhinosinusitis with nasal polyps. Nat Commun.
15:91012024. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fox RJ, Kita M, Cohan SL, Henson LJ,
Zambrano J, Scannevin RH, O'Gorman J, Novas M, Dawson KT and
Phillips JT: BG-12 (dimethyl fumarate): A review of mechanism of
action, efficacy, and safety. Curr Med Res Opin. 30:251–262. 2014.
View Article : Google Scholar : PubMed/NCBI
|