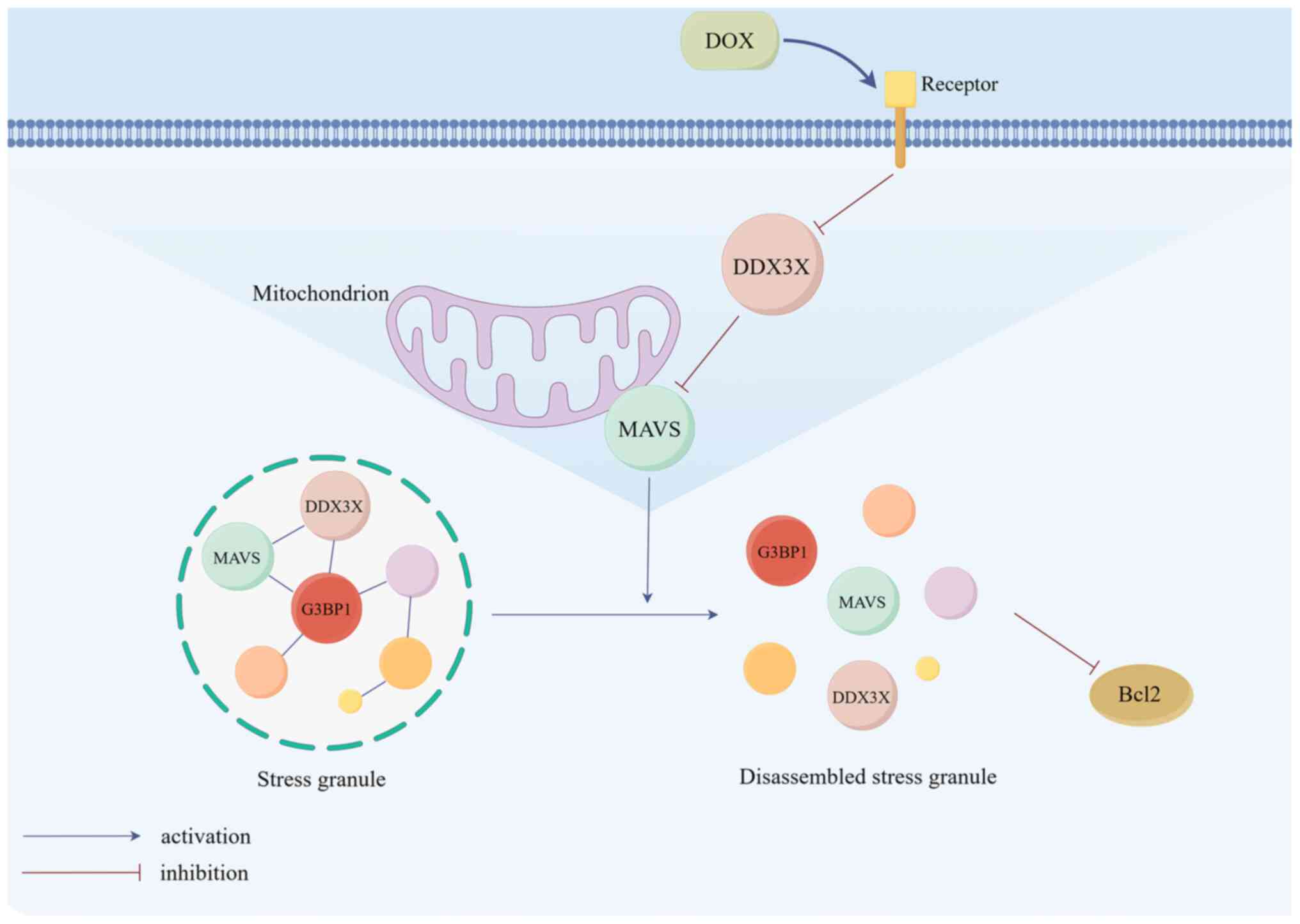
|
1
|
Momparler RL, Karon M, Siegel SE and Avila
F: Effect of adriamycin on DNA, RNA, and protein synthesis in
cell-free systems and intact cells. Cancer Res. 36:2891–2895.
PubMed/NCBI
|
|
2
|
Fornari FA, Randolph JK, Yalowich JC,
Ritke MK and Gewirtz DA: Interference by doxorubicin with DNA
unwinding in MCF-7 breast tumor cells. Mol Pharmacol. 45:649–656.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pommier Y, Capranico G, Orr A and Kohn KW:
Local base sequence preferences for DNA cleavage by mammalian
topoisomerase II in the presence of amsacrine or teniposide.
Nucleic Acids Res. 19:5973–5980. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tewey KM, Rowe TC, Yang L, Halligan BD and
Liu LF: Adriamycin-induced DNA damage mediated by mammalian DNA
topoisomerase II. Science. 226:466–468. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zamorano JL, Lancellotti P, Rodriguez
Muñoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ,
Lip GYH, Lyon AR, et al: 2016 ESC Position Paper on cancer
treatments and cardiovascular toxicity developed under the auspices
of the ESC Committee for Practice Guidelines: The Task Force for
cancer treatments and cardiovascular toxicity of the European
Society of Cardiology (ESC). Eur Heart J. 37:2768–2801. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cui L, Huang J, Zhan Y, Qiu N, Jin H, Li
J, Huang H and Li H: Association between the genetic polymorphisms
of the pharmacokinetics of anthracycline drug and myelosuppression
in a patient with breast cancer with Anthracycline-based
chemotherapy. Life Sci. 276:1193922021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ramalingayya GV, Cheruku SP, Nayak PG,
Kishore A, Shenoy R, Rao CM and Krishnadas N: Rutin protects
against neuronal damage in vitro and ameliorates
doxorubicin-induced memory deficits in vivo in Wistar rats. Drug
Des Devel Ther. 11:1011–1026. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dean JC, Salmon SE and Griffith KS:
Prevention of doxorubicin-induced hair loss with scalp hypothermia.
N Engl J Med. 301:1427–1429. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Swain SM, Whaley FS and Ewer MS:
Congestive heart failure in patients treated with doxorubicin: A
retrospective analysis of three trials. Cancer. 97:2869–2879. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fornaro A, Olivotto I, Rigacci L,
Ciaccheri M, Tomberli B, Ferrantini C, Coppini R, Girolami F,
Mazzarotto F and Chiostri M: Comparison of long-term outcome in
anthracycline-related versus idiopathic dilated cardiomyopathy: A
single centre experience. Eur J Heart Fail. 20:898–906. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cardinale D, Colombo A, Bacchiani G,
Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N,
Curigliano G, et al: Early detection of anthracycline
cardiotoxicity and improvement with heart failure therapy.
Circulation. 131:1981–1988. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Berthiaume JM and Wallace KB:
Adriamycin-induced oxidative mitochondrial cardiotoxicity. Cell
Biology Toxicol. 23:15–25. 2006. View Article : Google Scholar
|
|
13
|
Goormaghtigh E, Huart P, Praet M, Brasseur
R and Ruysschaert JM: Structure of the adriamycin-cardiolipin
complex. Role in mitochondrial toxicity. Biophys Chem. 35:247–257.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hoye AT, Davoren JE, Wipf P, Fink MP and
Kagan VE: Targeting mitochondria. Acc Chem Res. 41:87–97. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Y, Jungsuwadee P, Vore M, Butterfield
DA and St Clair DK: Collateral damage in cancer chemotherapy:
Oxidative stress in nontargeted tissues. Mol Interv. 7:147–156.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deng S, Kruger A, Kleschyov AL, Kalinowski
L, Daiber A and Wojnowski L: Gp91phox-containing NAD(P)H oxidase
increases superoxide formation by doxorubicin and NADPH. Free Radic
Biol Med. 42:466–473. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Doroshow JH, Esworthy RS and Chu FF:
Control of doxorubicin-induced, reactive oxygen-related apoptosis
by glutathione peroxidase 1 in cardiac fibroblasts. Biochem Biophys
Rep. 21:1007092020.PubMed/NCBI
|
|
18
|
Kong CY, Guo Z, Song P, Zhang X, Yuan YP,
Teng T, Yan L and Tang QZ: Underlying the mechanisms of
doxorubicin-induced acute cardiotoxicity: Oxidative stress and cell
death. Int J Biol Sci. 18:760–770. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xiong Y, Liu X, Lee CP, Chua BHL and Ho
YS: Attenuation of doxorubicin-induced contractile and
mitochondrial dysfunction in mouse heart by cellular glutathione
peroxidase. Free Radic Biol Med. 41:46–55. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sharma D and Jankowsky E: The Ded1/DDX3
subfamily of DEAD-box RNA helicases. Crit Rev Biochem Mol Biol.
49:343–360. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Högbom M, Collins R, van den Berg S,
Jenvert RM, Karlberg T, Kotenyova T, Flores A, Karlsson Hedestam GB
and Schiavone LH: Crystal structure of conserved domains 1 and 2 of
the human DEAD-box helicase DDX3X in complex with the
mononucleotide AMP. J Mol Biol. 372:150–159. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tantravedi S, Vesuna F, Winnard PT Jr, Van
Voss MRH, Van Diest PJ and Raman V: Role of DDX3 in the
pathogenesis of inflammatory bowel disease. Oncotarget.
8:115280–115289. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schröder M: Viruses and the human DEAD-box
helicase DDX3: Inhibition or exploitation? Biochem Soc Trans.
39:679–683. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Linder P and Jankowsky E: From unwinding
to clamping-the DEAD box RNA helicase family. Nat Rev Mol Cell
Biol. 12:505–516. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee CS, Dias AP, Jedrychowski M, Patel AH,
Hsu JL and Reed R: Human DDX3 functions in translation and
interacts with the translation initiation factor eIF3. Nucleic
Acids Res. 36:4708–4718. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kellaris G, Khan K, Baig SM, Tsai IC,
Zamora FM, Ruggieri P, Natowicz MR and Katsanis N: A hypomorphic
inherited pathogenic variant in DDX3X causes male intellectual
disability with additional neurodevelopmental and neurodegenerative
features. Hum Genomics. 12:112018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jankowsky A, Guenther UP and Jankowsky E:
The RNA helicase database. Nucleic Acids Res. 39:D338–D341. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lai MC, Lee YH and Tarn WY: The DEAD-box
RNA helicase DDX3 associates with export messenger
ribonucleoproteins as well as tip-associated protein and
participates in translational control. Mol Biol Cell. 19:3847–3858.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brennan R, Haap-Hoff A, Gu L, Gautier V,
Long A and Schröder M: Investigating nucleo-cytoplasmic shuttling
of the human DEAD-box helicase DDX3. Eur J Cell Biol. 97:501–511.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen HH, Yu HI, Cho WC and Tarn WY: DDX3
modulates cell adhesion and motility and cancer cell metastasis via
Rac1-mediated signaling pathway. Oncogene. 34:2790–2800. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Valentin-Vega YA, Wang YD, Parker M,
Patmore DM, Kanagaraj A, Moore J, Rusch M, Finkelstein D, Ellison
DW, Gilbertson RJ, et al: Cancer-associated DDX3X mutations drive
stress granule assembly and impair global translation. Sci Rep.
6:259962016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Soulat D, Bürckstümmer T, Westermayer S,
Goncalves A, Bauch A, Stefanovic A, Hantschel O, Bennett KL, Decker
T and Superti-Furga G: The DEAD-box helicase DDX3X is a critical
component of the TANK-binding kinase 1-dependent innate immune
response. EMBO J. 27:2135–2146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kienes I, Bauer S, Gottschild C, Mirza N,
Pfannstiel J, Schröder M and Kufer TA: DDX3X links NLRP11 to the
regulation of type I interferon responses and NLRP3 inflammasome
activation. Front Immunol. 12:6538832021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gu L, Fullam A, Brennan R and Schröder M:
Human DEAD box helicase 3 couples IκB kinase ε to interferon
regulatory factor 3 activation. Mol Cell Biol. 33:2004–2015. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Samir P, Kesavardhana S, Patmore DM,
Gingras S, Malireddi RKS, Karki R, Guy CS, Briard B, Place DE,
Bhattacharya A, et al: DDX3X acts as a live-or-die checkpoint in
stressed cells by regulating NLRP3 inflammasome. Nature.
573:590–594. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Oshiumi H, Sakai K, Matsumoto M and Seya
T: DEAD/H BOX 3 (DDX3) helicase binds the RIG-I adaptor IPS-1 to
up-regulate IFN-beta-inducing potential. Eur J Immunol. 40:940–948.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang P, Li Y, Xia J, He J, Pu J, Xie J,
Wu S, Feng L, Huang X and Zhang P: IPS-1 plays an essential role in
dsRNA-induced stress granule formation by interacting with PKR and
promoting its activation. J Cell Sci. 127:2471–2482.
2014.PubMed/NCBI
|
|
38
|
Aoyama-Ishiwatari S, Okazaki T, Iemura SI,
Natsume T, Okada Y and Gotoh Y: NUDT21 links mitochondrial IPS-1 to
RLR-containing stress granules and activates host antiviral
defense. J Immunol. 206:154–163. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lin HB, Naito K, Oh Y, Farber G, Kanaan G,
Valaperti A, Dawood F, Zhang L, Li GH, Smyth D, et al: Innate
Immune Nod1/RIP2 signaling is essential for cardiac hypertrophy but
requires mitochondrial antiviral signaling protein for signal
transductions and energy balance. Circulation. 142:2240–2258. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li WY, Yang F, Li X, Wang LW and Wang Y:
Stress granules inhibit endoplasmic reticulum stress-mediated
apoptosis during hypoxia-induced injury in acute liver failure.
World J Gastroenterol. 29:1315–1329. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Anderson P and Kedersha N: RNA granules:
Post-transcriptional and epigenetic modulators of gene expression.
Nat Rev Mol Cell Biol. 10:430–436. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kedersha N, Stoecklin G, Ayodele M, Yacono
P, Lykke-Andersen J, Fritzler MJ, Scheuner D, Kaufman RJ, Golan DE
and Anderson P: Stress granules and processing bodies are
dynamically linked sites of mRNP remodeling. J Cell Biol.
169:871–884. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Protter DSW and Parker R: Principles and
properties of stress granules. Trends Cell Biol. 26:668–679. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Molliex A, Temirov J, Lee J, Coughlin M,
Kanagaraj AP, Kim HJ, Mittag T and Taylor JP: Phase separation by
low complexity domains promotes stress granule assembly and drives
pathological fibrillization. Cell. 163:123–133. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Saito M, Hess D, Eglinger J, Fritsch AW,
Kreysing M, Weinert BT, Choudhary C and Matthias P: Acetylation of
intrinsically disordered regions regulates phase separation. Nat
Chem Biol. 15:51–61. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wheeler JR, Matheny T, Jain S, Abrisch R
and Parker R: Distinct stages in stress granule assembly and
disassembly. Elife. 5:e184132016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jain S, Wheeler JR, Walters RW, Agrawal A,
Barsic A and Parker R: ATPase-modulated stress granules contain a
diverse proteome and substructure. Cell. 164:487–498. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhao J, Fu X, Chen H, Min L, Sun J, Yin J,
Guo J, Li H, Tang Z, Ruan Y, et al: G3BP1 interacts with YWHAZ to
regulate chemoresistance and predict adjuvant chemotherapy benefit
in gastric cancer. Br J Cancer. 124:425–436. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang X, Chen T, Li C, Li W, Zhou X, Li Y,
Luo D, Zhang N, Chen B, Wang L, et al: CircRNA-CREIT inhibits
stress granule assembly and overcomes doxorubicin resistance in
TNBC by destabilizing PKR. J Hematol Oncol. 15:1222022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Oh SW, Onomoto K, Wakimoto M, Onoguchi K,
Ishidate F, Fujiwara T, Yoneyama M, Kato H and Fujita T:
Leader-containing uncapped viral transcript activates RIG-I in
antiviral stress granules. PLoS Pathog. 12:e10054442016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li YR, King OD, Shorter J and Gitler AD:
Stress granules as crucibles of ALS pathogenesis. J Cell Biol.
201:361–372. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Moraes KC, Monteiro CJ and Pacheco-Soares
C: A novel function for CUGBP2 in controlling the pro-inflammatory
stimulus in H9c2 cells: Subcellular trafficking of messenger
molecules. Cell Biol Int. 37:1129–1138. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Alikunju S, Niranjan N, Mohsin M, Sayed N
and Sayed D: G3bp1-microRNA-1 axis regulates cardiomyocyte
hypertrophy. Cell Signal. 91:1102452022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Dong G, Liang F, Sun B, Wang C, Liu Y,
Guan X, Yang B, Xiu C, Yang N, Liu F, et al: Presence and function
of stress granules in atrial fibrillation. PLoS One.
14:e02137692019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang Y, Liu R, Wu K, Yang G, Wang Y, Wang
H and Rui T: Stress granule activation attenuates
lipopolysaccharide-induced cardiomyocyte dysfunction. BMC
Cardiovasc Disord. 23:2772023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Guo Y, Hinchman MM, Lewandrowski M, Cross
ST, Sutherland DM, Welsh OL, Dermody TS and Parker JSL: The
multi-functional reovirus σ3 protein is a virulence factor that
suppresses stress granule formation and is associated with
myocardial injury. PLoS Pathog. 17:e10094942021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using Real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Jiang Y, Liu Y, Xiao W, Zhang D, Liu X,
Xiao H, You S and Yuan L: Xinmailong Attenuates Doxorubicin-induced
lysosomal dysfunction and oxidative stress in H9c2 cells via HO-1.
Oxid Med Cell Longev. 2021:58969312021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhang H, Pan J, Huang S, Chen X, Chang
ACY, Wang C, Zhang J and Zhang H: Hydrogen sulfide protects
cardiomyocytes from doxorubicin-induced ferroptosis through the
SLC7A11/GSH/GPx4 pathway by Keap1 S-sulfhydration and Nrf2
activation. Redox Biology. 70:1030662024. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hu C, Zhang X, Song P, Yuan YP, Kong C-Y,
Wu HM, Xu SC, Ma ZG and Tang QZ: Meteorin-like protein attenuates
doxorubicin-induced cardiotoxicity via activating cAMP/PKA/SIRT1
pathway. Redox Biology. 37:1017472020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Feng D, Li J, Guo L, Liu J, Wang S, Ma X,
Song Y, Liu J and Hao E: DDX3X alleviates doxorubicin-induced
cardiotoxicity by regulating Wnt/β-catenin signaling pathway in an
in vitro model. J Biochem Mol Toxicol. 36:e230772022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Rawat PS, Jaiswal A, Khurana A, Bhatti JS
and Navik U: Doxorubicin-induced cardiotoxicity: An update on the
molecular mechanism and novel therapeutic strategies for effective
management. Biomed Pharmacother. 139:1117082021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lim CC, Zuppinger C, Guo X, Kuster GM,
Helmes M, Eppenberger HM, Suter TM, Liao R and Sawyer DB:
Anthracyclines induce calpain-dependent titin proteolysis and
necrosis in cardiomyocytes. J Biol Chem. 279:8290–8299. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ahmadiasl N, Rostami A, Mohammadi NM and
Rajabi F: Effects of noradrenaline and KCl on peripheral vessels in
doxorubicin induced model of heart failure. Pathophysiology.
8:259–262. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lin RW, Ho CJ, Chen HW, Pao YH, Chen LE,
Yang MC, Huang SB, Wang S, Chen CH and Wang C: P53 enhances
apoptosis induced by doxorubicin only under conditions of severe
DNA damage. Cell Cycle. 17:2175–2186. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Cui N, Wu F, Lu WJ, Bai R, Ke B, Liu T, Li
L, Lan F and Cui M: Doxorubicin-induced cardiotoxicity is
maturation dependent due to the shift from topoisomerase IIα to IIβ
in human stem cell derived cardiomyocytes. J Cell Mol Med.
23:4627–4639. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhang X, Wang F, Hu Y, Chen R, Meng D, Guo
L, Lv H, Guan J and Jia Y: In vivo stress granule misprocessing
evidenced in a FUS knock-in ALS mouse model. Brain. 143:1350–1367.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wolozin B and Ivanov P: Stress granules
and neurodegeneration. Nat Rev Neurosci. 20:649–666. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Cui Q, Bi H, Lv Z, Wu Q, Hua J, Gu B, Huo
C, Tang M, Chen Y, Chen C, et al: Diverse CMT2 neuropathies are
linked to aberrant G3BP interactions in stress granules. Cell.
186:803–820.e25. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Asadi MR, Sadat Moslehian M, Sabaie H,
Jalaiei A, Ghafouri-Fard S, Taheri M and Rezazadeh M: Stress
granules and neurodegenerative disorders: A scoping review. Front
Aging Neurosci. 13:6507402021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Arimoto K, Fukuda H, Imajoh-Ohmi S, Saito
H and Takekawa M: Formation of stress granules inhibits apoptosis
by suppressing stress-responsive MAPK pathways. Nat Cell Biol.
10:1324–1332. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Si W, Ye S, Ren Z, Liu X, Wu Z, Li Y, Zhou
J, Zhang S, Li Y, Deng R and Chen D: miR-335 promotes stress
granule formation to inhibit apoptosis by targeting ROCK2 in acute
ischemic stroke. Int J Mol Med. 43:1452–1466. 2019.PubMed/NCBI
|
|
73
|
Kedersha N, Ivanov P and Anderson P:
Stress granules and cell signaling: More than just a passing phase?
Trends Biochem Sci. 38:494–506. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Thedieck K, Holzwarth B, Prentzell MT,
Boehlke C, Kläsener K, Ruf S, Sonntag AG, Maerz L, Grellscheid SN,
Kremmer E, et al: Inhibition of mTORC1 by astrin and stress
granules prevents apoptosis in cancer cells. Cell. 154:859–874.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Doroshow JH, Locker GY and Myers CE:
Enzymatic defenses of the mouse heart against reactive oxygen
metabolites: Alterations produced by doxorubicin. J Clin Invest.
65:128–1235. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
do Nascimento TC, Cazarin CBB, Maróstica
MR Jr, Mercadante AZ, Jacob-Lopes E and Zepka LQ: Microalgae
carotenoids intake: Influence on cholesterol levels, lipid
peroxidation and antioxidant enzymes. Food Res Int. 128:1087702020.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Mihm MJ, Yu F, Weinstein DM, Reiser PJ and
Bauer JA: Intracellular distribution of peroxynitrite during
doxorubicin cardiomyopathy: Evidence for selective impairment of
myofibrillar creatine kinase. Br J Pharmacol. 135:581–588. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Deavall DG, Martin EA, Horner JM and
Roberts R: Drug-induced oxidative stress and toxicity. J Toxicol.
2012:6454602012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Takahashi M, Higuchi M, Matsuki H, Yoshita
M, Ohsawa T, Oie M and Fujii M: Stress granules inhibit apoptosis
by reducing reactive oxygen species production. Mol Cell Biol.
33:815–829. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Kim YS, Lee SG, Park SH and Song K: Gene
structure of the human DDX3 and chromosome mapping of its related
sequences. Mol Cells. 12:209–214. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Perfetto M, Xu X, Lu C, Shi Y, Yousaf N,
Li J, Yien YY and Wei S: The RNA helicase DDX3 induces neural crest
by promoting AKT activity. Development.
148:dev1843412021.PubMed/NCBI
|
|
82
|
Snijders Blok L, Madsen E, Juusola J,
Gilissen C, Baralle D, Reijnders MR, Venselaar H, Helsmoortel C,
Cho MT, Hoischen A, et al: Mutations in DDX3X Are a common cause of
unexplained intellectual disability with Gender-specific effects on
wnt signaling. Am J Hum Genet. 97:343–352. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Molina-Navarro MM, Triviño JC,
Martínez-Dolz L, Lago F, González-Juanatey JR, Portolés M and
Rivera M: Functional networks of nucleocytoplasmic
transport-related genes differentiate ischemic and dilated
cardiomyopathies. A new therapeutic opportunity. PLoS One.
9:e1047092014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Vesuna F, Akhrymuk I, Smith A, Winnard PT
Jr, Lin SC, Panny L, Scharpf R, Kehn-Hall K and Raman V: RK-33, a
small molecule inhibitor of host RNA helicase DDX3, suppresses
multiple variants of SARS-CoV-2. Front Microbiol. 13:9595772022.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Pène V, Li Q, Sodroski C, Hsu CS and Liang
TJ: Dynamic interaction of stress granules, DDX3X, and IKK-α
mediates multiple functions in Hepatitis C virus infection. J
Virol. 89:5462–5477. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
He S, Gou H, Zhou Y, Wu C, Ren X, Wu X,
Guan G, Jin B, Huang J, Jin Z and Zhao T: The SARS-CoV-2
nucleocapsid protein suppresses innate immunity by remodeling
stress granules to atypical foci. FASEB J. 37:e232692023.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ciccosanti F, Di Rienzo M, Romagnoli A,
Colavita F, Refolo G, Castilletti C, Agrati C, Brai A, Manetti F,
Botta L, et al: Proteomic analysis identifies the RNA helicase
DDX3X as a host target against SARS-CoV-2 infection. Antiviral Res.
190:1050642021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Yang S, Zhou L, Zhao T, Zhu H, Luo T,
Jiang K, Shi X, Chen C, Zhang H, Zhao S, et al: Protective and
adverse roles of DDX3X in different cell types in nonalcoholic
steatohepatitis progression. Research (Wash D C).
6:02752023.PubMed/NCBI
|
|
89
|
Chen H, Li B, Zhao X, Yang C, Zhou S and
Ma W: Cell-free analysis reveals the role of RG/RGG motifs in DDX3X
phase separation and their potential link to cancer pathogenesis.
Int J Biol Macromol. 279:1352512024. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Gu L, Fullam A, McCormack N, Höhn Y and
Schröder M: DDX3 directly regulates TRAF3 ubiquitination and acts
as a scaffold to co-ordinate assembly of signalling complexes
downstream from MAVS. Biochem J. 474:571–587. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ventura-Clapier R, Garnier A and Veksler
V: Energy metabolism in heart failure. J Physiol. 555:1–13. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Oliveira PJ and Wallace KB: Depletion of
adenine nucleotide translocator protein in heart mitochondria from
doxorubicin-treated rats-relevance for mitochondrial dysfunction.
Toxicology. 220:160–168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wang Q, Sun Z, Cao S, Lin X, Wu M, Li Y,
Yin J, Zhou W, Huang S, Zhang A, et al: Reduced immunity regulator
MAVS contributes to Non-hypertrophic cardiac dysfunction by
disturbing energy metabolism and mitochondrial homeostasis. Front
Immunol. 13:9190382022. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Carvalho RA, Sousa RP, Cadete VJ,
Lopaschuk GD, Palmeira CM, Bjork JA and Wallace KB: Metabolic
remodeling associated with subchronic doxorubicin cardiomyopathy.
Toxicology. 270:92–98. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Fu J, Hu F, Ma T, Zhao WJ, Tian H, Zhang
Y, Hu M, Zhou J, Zhang Y, Jian C, et al: A conventional immune
regulator mitochondrial antiviral signaling protein blocks hepatic
steatosis by maintaining mitochondrial homeostasis. Hepatology.
75:403–418. 2022. View Article : Google Scholar : PubMed/NCBI
|