Introduction
Collecting duct carcinoma (CDC) or Bellini duct
carcinoma, which accounts for 1–3% of all renal neoplasms (1), is a rare variant of renal cell
carcinoma (RCC) originating in the collecting duct epithelium and
occurring more frequently in relatively young adults (2). Patients with CDC often require a more
aggressive clinical course, have a poor long-term survival rate
and, currently no standard treatment regimens exist (3). CDC and clear cell RCC typically
present with a renal mass, macroscopic hematuria and associated
flank pain. However, the majority of patients with CDC show
evidence of metastatic disease at the time of presentation
(3). Pleural metastasis of CDC is
resistant to chemotherapy and radiotherapy, while immunotherapy,
especially adoptive immune cell treatment, becomes a promising
treatment for pleural metastasis. The present study evaluated a
patient with pleural metastasis of CDC who achieved partial success
following intrapleural infusion with modified cytokine-induced
killer (mCIK) cells.
Case report
A 33-year-old male with no significant past medical
history was referred for urology review after a mass was found
during a regular examination. A left radical nephrectomy was
performed in December 2006 in the First Affiliated Hospital,
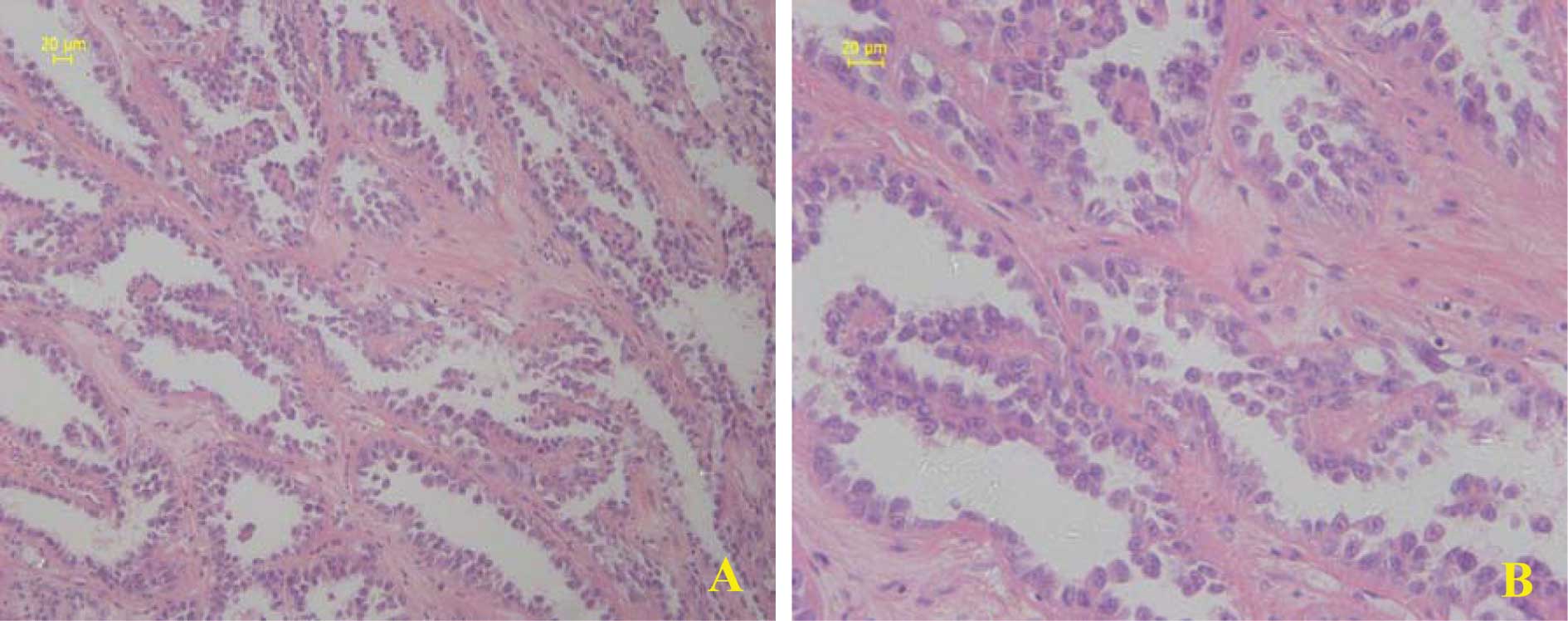
Kunming Medical College, China. The pathology revealed CDC
(Fig. 1) in the left kidney with no
lymph node metastasis. After 1 year, the patient suffered from
constant coughing, dyspnea, chest distress and thoracalgia. A chest
X-Ray revealed a left pleural effusion. The pleural effusion
recurred 1 month after B-ultrasound-guided therapeutic
thoracentesis. For further treatment, the patient was referred to
our institution on May 5th, 2008.
A physical examination revealed a dull sound on
percussion and the breath sounds disappeared below the sixth left
rib. A computed tomography (CT) scan of the chest, abdomen and
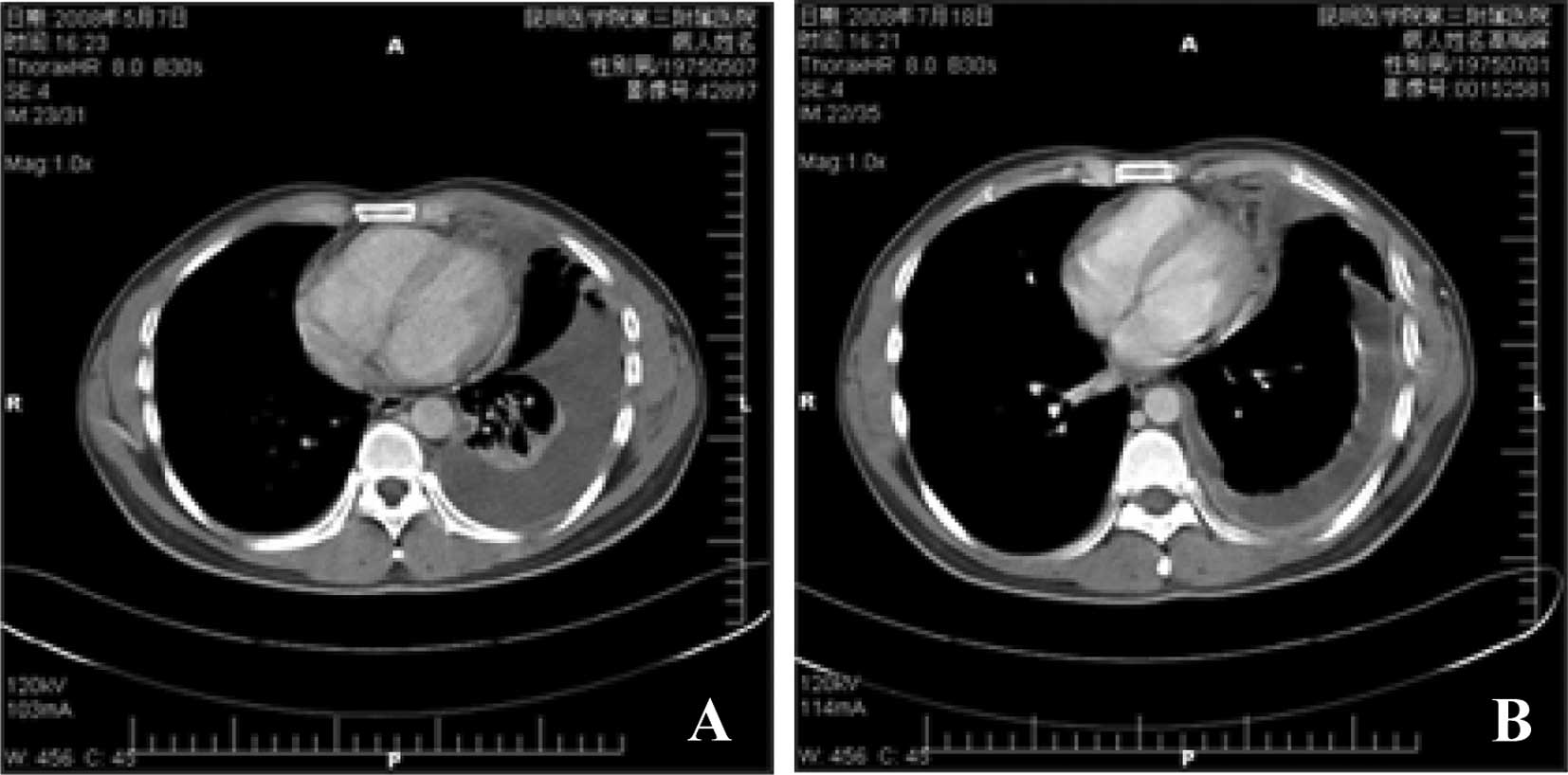
pelvis revealed no local recurrence and no lymph node metastasis,
although severe pleural metastasis was noted. The examination
results revealed that the left pleural had thickened, the pleural
effusion was partly encapsulated and there was left pulmonary
atelectasis (Fig. 3A). An
ultrasound-guided aspiration revealed haematodes fluid. The results
of the laboratory examinations were insignificant. Haematology
showed a haemoglobin level of 171 g/l (120–160), a white cell count
of 11.72×109/l (4–10) and
a platelet count of 236×109/l (100–300).
To alleviate pleural effusion and dyspnea, the
patient was treated with a mCIK cell intrapleural infusion.
Mononuclear cells were obtained aseptically from blood and infused
back to the patient through intrapleural infusion, following in
vitro culture of the mCIK cells.
The mCIK cells were cascade-primed immune cells
(CAPRI) generated using a procedure to enhance cytokine-induced
killer cells (4), with
modification. Briefly, using a blood cell separator (Spectra,
Gambro BCT, USA), leukapheresis was conducted to obtain peripheral
blood mononuclear cells (PBMCs). Half of the mononuclear cells were
resuspended in culture flasks coated with anti-CD3 antibody (BD
Pharmingen, NJ, USA), and incubated at 37°C in a humidified
atmosphere of 5% CO2. After 3 h, a mixture of 700 U/ml
interleukin (IL)-2, 800 U/ml IL-18 and 2,000 U/ml interferon
(IFN)-γ (all from R&D Systems, MN, USA) were added. After
another 3 h, the remaining mononuclear cells were added. After 24
h, the cells were transferred to a larger culture flask for
expansion. IL-2, IL-18 and IFN-γ were added every 2 days. After
incubation for 7 days, the activated immune cells were harvested
and preserved in liquid nitrogen. The cells were defrosted,
resuspended in phosphate-buffered saline and freshly transfused to
the patient when required.
In every cycle, the patient received mCIK cells at a
dose of 100 million (40 ml) once a day for 5 days, through
intrapleural infusion. Following the initial cycle of treatment,
the coughing disappeared and symptoms including dyspnea, chest
distress and thoracalgia were relieved. In order to receive further
treatment, the patient returned to the department for the second
cycle of therapy on July 18, 2008. An ultrasound-guided aspiration
revealed 600 ml of clear fluid (Fig.
2B). A CT scan (Fig. 3B)
revealed that the left pulmonary had re-expanded and the level of
pleural fluid had decreased. After the third cycle of therapy, the
dyspnea was evidently relieved and the chest distress and
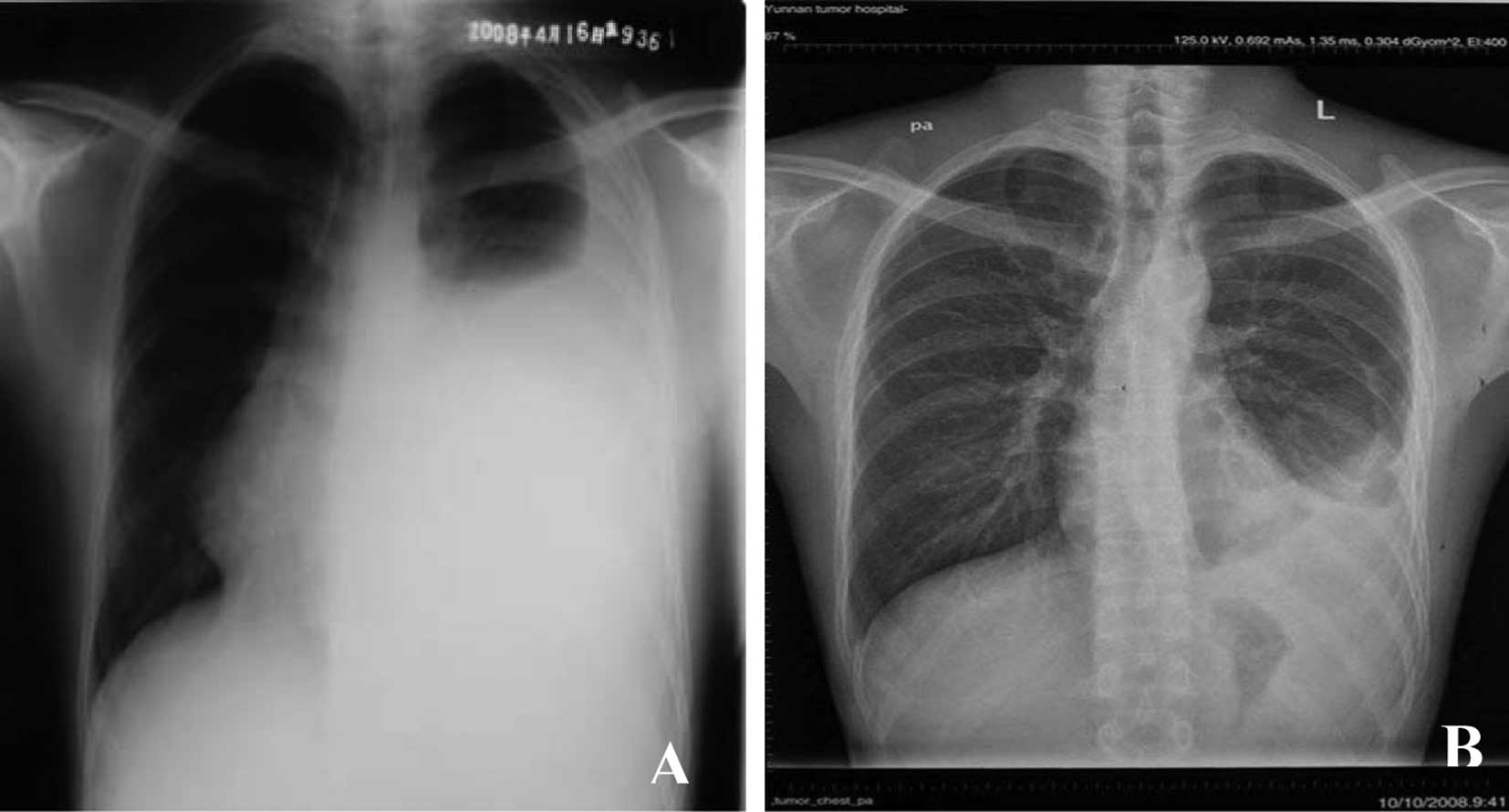
thoracalgia were relieved completely. The chest X-Ray (Fig. 4A) revealed a great reduction in the
level of pleural fluid compared to the initial examination
(Fig. 4B).
The patient had a fever (~38°C) after intrapleural
infusion, but recovered 2 days later. No other adverse effects were
noted during therapy.
The evaluation of efficacy was based on the relief
of symptoms and absence or reduction of pleural fluid (5,6).
Patients with symptomatic improvement and no detectable fluid on
the roentgenogram prior to discharge are deemed a success. Those
with symptomatic improvement, but residual fluid in the
costophrenic angle, with no tendency for increase, are considered
to be a partial success. Patients whose pleural effusion recurred
or who had no symptomatic improvement, are deemed unsuccessful.
Based on these criteria, the patient received mCIK cells and
achieved partial success.
Discussion
The rare renal carcinoma type CDC, first reported by
Mancilla-Jimenz (7), exhibits
highly aggressive behavior (8).
Although radical surgery is the basic type of therapy and different
protocols have been applied, the challenge involves the
identification of novel therapeutic modalities (9). No standard protocols have been
established for the treatment of CDC. Numerous agents have been
reported over the last few decades as being effective by
intrapleural injection in the suppression of recurrent malignant
pleural effusion (10).
Intrapleural injection by mCIKs is a novel therapeutic strategy for
the treatment of malignant pleural effusions. We evaluated this
case due to the efficacy of this novel immunotherapy method in the
supression of pleural metastasis of CDC.
mCIK cells are a cluster of cascade-primed immune
cells, including mainly CD3+CD56+ and
CD3+CD8+ T cells. PBMCs were stimulated in
vitro with CD3-activated T lymphocytes in order to increase the
expression of HLA molecules and costimulatory molecules. In
particular, the antigen-presenting cells in the molecules express
tumor peptides after stimulation. Freshly added PBMCs were primed
by the stimulated antigen-presenting cells and immune cytokines
(IL-18, IFN-γ and IL-2) and matured to helper T and cytotoxic T
cells. IL-18 induces enhanced CD8+ T-cell proliferation
(11). Yamada et al found
that IL-18 immunotherapy decreased the number of macroscopic
pulmonary metastases (12). IFN-γ
is the hallmark cytokine of Th1 cells and stimulates anti-tumor
immunity (13). IL-2 stimulates the
growth, differentiation and survival of antigen-selected cytotoxic
T cells and enhances anti-tumor immunity (14). The mCIK cells proliferated rapidly
and showed anti-tumor effects on various types of cancer.
We treated pleural metastasis of the patient with
this novel immunotherapy method. Following the initial cycle of
treatment, the symptoms including coughing, chest distress and
thoracalgia were relieved. After the second cycle of treatment, we
found that pleural fluid previously exhibiting haematodes had
become clear. Although the pleural effusion remained, pulmonary
re-expansion was evident (Fig. 3B).
After the third cycle of treatment was completed, the levels of
pleural fluid had decreased significantly (Fig. 4B). According to the previous
criteria, the patient achieved partial success.
Various agents have been instilled into the pleural
space to treat malignant pleural effusions and agents, such as
talc, tetracycline and bleomycin, creating a chemical pleuritis
(10,15). However, when we treated the patient
with mCIK cells, overt pleuritis was not observed, only a
transitory fever, suggesting the safety of this method for the
treatment of pleural metastasis.
The intrapleural infusion of activated immune cells
causes the accumulation of effector cells and significantly
decreases the number of tumor cells. The precise mechanisms of
eradication of tumor cells by the adoptive transfer of activated
killer cells have yet to be elucidated, but this transfer may be
mediated mainly by the immune responses of cytotoxic killer T
cells. Intrapleural infusion is an innovative method aimed at
curing pleural metastasis with activated immune cells. Thus, the
efficacy and mechanisms of this exploratory treatment require
further study.
In conclusion, to control malignant pleural fluid,
drainage and intrapleural chemotherapy are applied in the majority
of cases of pleural metastasis. However, successful supression of
pleural fluid is difficult and always recurs in advanced cases.
This study suggested that mCIK cell intrapleural infusion is a
candidate for the therapy of pleural metastasis with refractory
pleural fluid.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (Grant No. 30671904, 30760014
and 81060185), the New Century Excellent Talent Supporting Project
of Ministry of Education of China (Grant No. NCET-08-0923), the
Cultivation Program for Reserve Talent of Middle-Young Aged
Academic and Technology Leader of Yunnan Province (Grant No.
2006Y01-12), the Key Program of Science and Technology Project of
Kunming (Grant No. 07S060202), the Yunnan Provincial Applied Basic
Research Program (Grant No. 2007C0022R, 2007C0025R and 2007C0023R),
the Science and Technology Program of Yunnan Province (Grant No.
2007C009Z), the Yunnan Provincial Key Program for Applied Basic
Research (Grant No. 2008CC006), the Innovation talent Team Program
for Prevention and Treatment of High Incidence Lung Cancer of
Yunnan Province (Grant No. 20080C014), and the International
Technology Cooperation Project of Scientific and Technological
Innovation Program of Yunnan Province Science and Technology Agency
(Grant No. 2009AC016).
References
|
1
|
Dimopoulos MA, Logothetis CJ, Markowitz A,
et al: Collecting duct carcinoma of the kidney. Br J Urol.
71:388–391. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Méjean A, Rouprêt M, Larousserie F, et al:
Is there a place for radical nephrectomy in the presence of
metastatic collecting duct (Bellini) carcinoma? J Urol.
169:1287–1290. 2003.PubMed/NCBI
|
|
3
|
Milowsky MI, Rosmarin A, Tickoo SK,
Papanicolaou N and Nanus DM: Active chemotherapy for collecting
duct carcinoma of the kidney: a case report and review of the
literature. Cancer. 94:111–116. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shi M, Zhang B, Tang ZR, et al: Autologous
cytokine-induced killer cell therapy in clinical trial phase is
safe in patients with primary hepatocellular carcinoma. World J
Gastroenterol. 10:1146–1151. 2004.PubMed/NCBI
|
|
5
|
Millar JW, Hunter AM and Horne NW:
Intrapleural immunotherapy with Corynebacterium parvum in
recurrent malignant pleural effusions. Thorax. 35:856–858.
1980.PubMed/NCBI
|
|
6
|
Schulze M, Boehle AS, Kurdow R, et al:
Effective treatment of malignant pleural effusion by minimal
invasive thoracic surgery: thoracoscopic talc pleurodesis and
pleuroperitoneal shunts in 101 patients. Ann Thorac Surg.
71:1809–1812. 2001. View Article : Google Scholar
|
|
7
|
Mancilla-Jimenez R, Stanley RJ and Blath
RA: Papillary renal cell carcinoma. A clinical, radiologic, and
pathologic study of 34 cases. Cancer. 38:2469–2480. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Anjum MI, Ting PY, Shrotri N, Randall B
and Mufti GR: An unusual case of Bellini duct carcinoma. Int Urol
Nephrol. 28:695–698. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ansari J, Fatima A, Chaudhri S, et al:
Sorafenib induces therapeutic response in a patient with metastatic
collecting duct carcinoma of kidney. Onkologie. 32:44–46. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Musani AI: Treatment options for malignant
pleural effusion. Curr Opin Pulm Med. 15:380–387. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Balkow S, Loser K, Krummen M, et al:
Dendritic cell activation by combined exposure to anti-CD40 plus
interleukin (IL)-12 and IL-18 efficiently stimulates anti-tumor
immunity. Exp Dermatol. 18:78–87. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamada N, Hata M, Ohyama H, et al:
Immunotherapy with interleukin-18 in combination with preoperative
chemotherapy with ifosfamide effectively inhibits postoperative
progression of pulmonary metastases in a mouse osteosarcoma model.
Tumour Biol. 30:176–184. 2009. View Article : Google Scholar
|
|
13
|
Olioso P, Giancola R, Di Riti M, et al:
Immunotherapy with cytokine induced killer cells in solid and
hematopoietic tumours: a pilot clinical trial. Hematol Oncol.
27:130–139. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
McDermott DF: Immunotherapy of metastatic
renal cell carcinoma. Cancer. 115:2298–2305. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hausheer FH and Yarbro JW: Diagnosis and
treatment of the malignant pleural effusion. Semin Oncol. 12:54–75.
1985.
|