Introduction
Lung cancer is the leading cause of cancer deaths
worldwide (1). Despite the
improvements in diagnosis and treatment, the prognosis of patients
with advanced-stage non-small cell lung cancer (NSCLC) remains a
challenge, with a 5-year survival of less than 15%. Thus, novel
treatment strategies are urgently required.
Studies investigating the mechanism of oncogenesis
showed that NSCLC is a disease with multigenetic abnormalities.
Among these abnormalities, the epidermal growth factor receptor
(EGFR) mutation has received considerable attention for its marked
response to small molecule tyrosine kinase inhibitor (TKI)
treatment. EGFR, together with HER2/neu (erbB2), HER3 (erbB3) and
HER4 (erbB4), belongs to the erbB family of receptor tyrosine
kinase proteins. It contains an extracellular ligand-binding
domain, a transmembrane lipophilic domain and an intracellular
tyrosine kinase domain, and is critical for cell proliferation,
differentiation, motility and metastasis.
Somatic mutations in the EGFR kinase domain have
mainly been identified in a subset of NSCLC patients. The subset
included females, never smokers, patients with adenocarcinoma
histology and patients of an East Asian origin (2,3). The
presence of these mutations in NSCLC correlates with the
sensitivity to TKI treatment, including gefitinib and erlotinib. A
number of distinct mutations have been identified, most of which
cluster around the tyrosine kinase domain. The in-frame deletion in
exon 19 (del-19) accounts for 45% of the mutations, while the point
mutation L858R in exon 21 accounts for 40–45% of the EGFR mutations
in lung cancer (4–6). Patients with these mutations have a
marked response to EGFR TKIs. Thus, these mutations are usually
termed classical mutations (7). Due
to the optimal response in this subtype of NSCLC patients, EGFR
mutation screening for the selection of TKI therapy has been
recommended in the clinical care of NSCLC patients. In addition to
classical mutations, other rare EGFR mutations in exons 18–21 have
also been reported, and patients with these non-classical mutations
have variable responses to EGFR TKIs (8,9). Among
these rare mutations is the EGFR-P848L mutation, which was first
reported by Sequist et al (10) in a patient of Western origin.
However, such studies do not refer to the function of this
mutant.
Therefore, in the present study, all of the EGFR
exons were sequenced to evaluate the frequencies of EGFR
gene mutations and to identify the rare or novel mutations of EGFR
in 55 Chinese NSCLC patients. When a patient with a EGFR-P848L
mutation was identified, the biological properties of the tumor
were further investigated.
Materials and methods
Tumor samples
Tumor samples were obtained from patients suffering
from NSCLC at the First Affiliated Hospital of Bengbu Medical
College (Bengbu, China) and Ruijin Hospital (Shanghai, China).
Clinical data for the NSCLC patients were available, including
gender, age at diagnosis, tumor histology type, clinical staging,
smoking status and response to treatment. All 55 patients provided
written informed consent, and the study was approved by the
institutional review board.
Genomic DNA extraction and direct
sequencing
Genomic DNA from frozen tumor specimens was
extracted using the phenol-chloroform method. Polymerase chain
reaction (PCR) was used to amplify the exon 1–28 fragments of EGFR.
The PCR products were purified by shrimp alkaline
phosphatase/exonuclease I and then directly sequenced on ABI PRISM
3700 (Applied Biosystems, Carlsbad, CA, USA). Mutations were
confirmed by at least two independent PCR amplifications and
repeated sequencing reactions.
Site-directed mutagenesis of the
epidermal growth factor receptor
PCDNA3.1-EGFR-WT and EGFR with a mutation of L858R
or delE746-A750 were kindly provided by Dr William Pao of the
Memorial Sloan-Kettering Cancer Center (New York, NY, USA). The
P848L mutant EGFR construct was generated by introducing a point
mutation into the wild-type EGFR vector using a site-directed
mutagenesis kit (QuikChange XL, Stratagene). The primers were F:
GTACTGGTGAAAACACTGCAGCATGTCAAGATC, R:
GATCTTGACATGCTGCAGTGTTTTCACCAGTAC. The T790M mutation was then
introduced into pCDNA3.1- EGFR-P848L using the same technique. The
primers were F: ACCGTGCAGCTCATCATGCAGCTCATGCCCTTC, R:
GAAGGGCATGAGCTGCATGATGAGCTGCACGGT. The mutated nucleotides are
underlined.
Cell culture and Western blotting
293T cells were cultured in DMEM supplemented with
10% FBS, 100 pg penicillin/streptomycin and 2 mM glutamine.
According to the manufacturer's instructions, wild-type EGFR and
EGFR with different mutations were transiently transfected into
cells with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA).
Cells were then serum-starved for 16 h prior to treatment with
various concentrations of gefitinib (Tocris, UK). Following
incubation (3 h) with the inhibitors, cells were stimulated with
EGF at 100 ng/ml for 30 min. Cells were then collected and the
protein was extracted with RIPA lysis buffer and PMSF. Protein was
electrophoresed in 10% sodium dodecyl sulphate polyacrylamide gel
electrophoresis (SDS-PAGE) gels, and then transferred to the
nitrocellulose membrane. Anti-phospho-EGFR (pY1068) (Cell Signaling
Technology, Danvers, MA, USA) was used for the detection of
autophosphorylation of EGFR, anti-EGFR (Cell Signaling Technology)
for total EGFR expression and anti-β-actin (abcom) as a loading
control. A Western blotting luminal reagent (Thermo, Newington, NH,
USA) was used to detect the signals. Results were repeated at least
twice.
Computer modeling of EGFR
In order to better understand how this mutation
affects the binding of EGFR with gefitinib, we constructed a
three-dimensional computer model with RasMol software (version 2.6;
http://www.umass.edu/microbio/rasmol/). Using the
crystal structure of the wild-type EGFR (PDB code 2JITB) as a
template, 321 residues (E697-L1017) of EGFR-P848L were modeled by
homology using SWISS-MODEL (http://swissmodel.expasy.org/) (11) at a resolution of 3.10 Å.
Results
Clinical characteristics and EGFR
mutations of lung adeno-carcinoma patients
In total, 55 tumor tissues were collected from
patients with NSCLC. Of these, 25 were squamous, 24 were
adenocarcinomas and 6 were large cell lung cancers (Table I). The exons of the EGFR gene
were sequenced in the 55 NSCLC patients using the ABI PRISM 3700
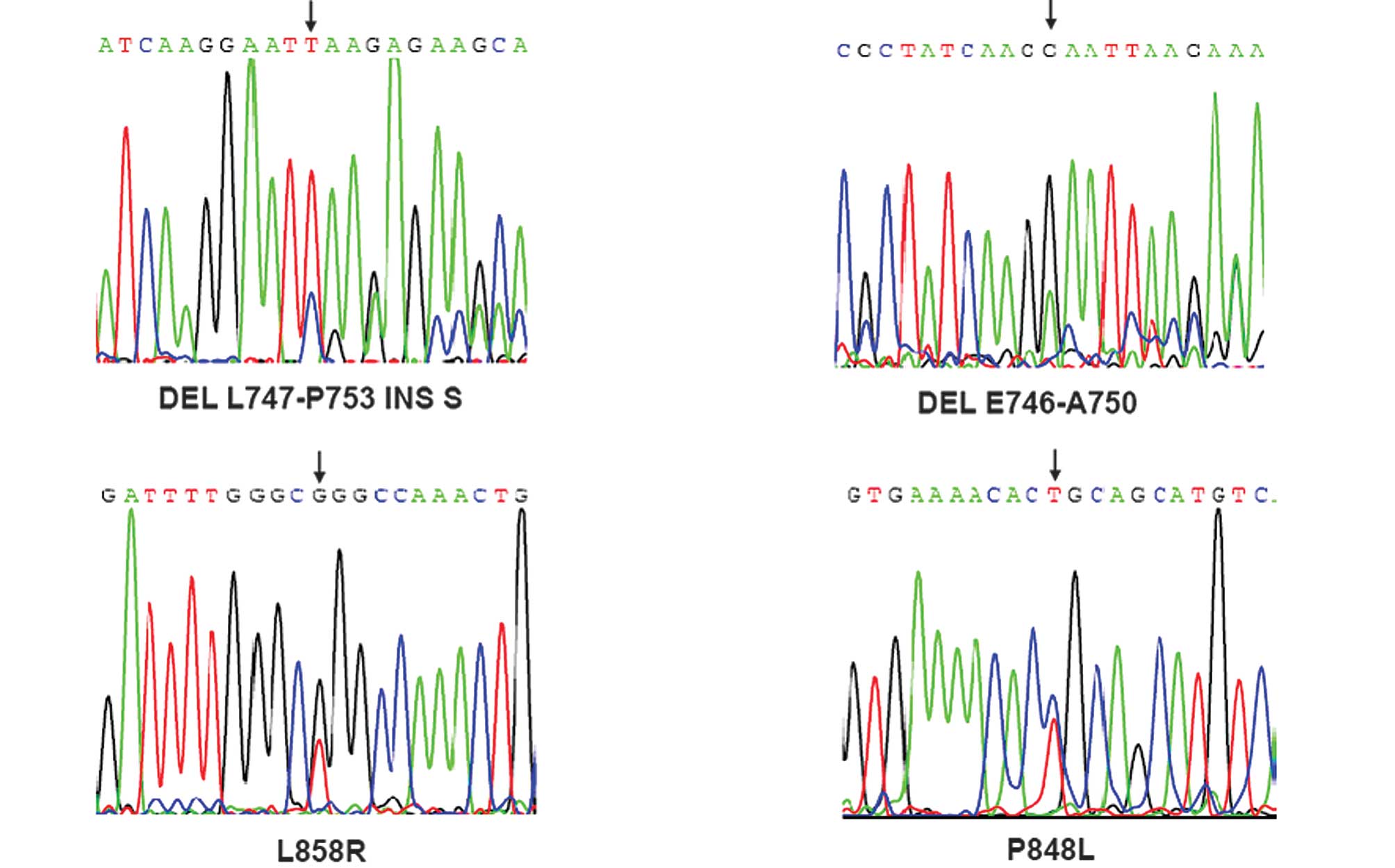
DNA sequencer. Eight (14.5%) patients harboring EGFR mutations were
detected, including substitutions in exon 21 (5 patients with L858R
and 1 patient with P848L) and deletions in exon 19 (1 patient with
del E746-A750 and one patient with del L747-P753 INS S) (Fig. 1, Table
II). Notably, all 8 patients (5 males and 3 females) with EGFR
mutations had adenocarcinoma histopathology (Table II), among whom 6 were non-smokers
and 2 were ever-smokers. Although only 9 female patients were
enrolled in this series, 6 had adenocarcinoma and the EGFR mutation
rate in female patients with lung adenocarcinoma was 50% (3/6). It
was noted that no mutation in the EGFR gene was detected in
patients with lung squamous carcinoma.
 | Table IClincal data of NSCLC patients. |
Table I
Clincal data of NSCLC patients.
| Histotype | Gender (M/F) | Smoking (E/N) | Total |
|---|
| SCC | 22/3 | 19/6 | 25 |
| ACC | 19/5 | 13/11 | 24 |
| Other types | 5/1 | 4/2 | 6 |
 | Table IISummary of the patients harboring EGFR
mutations. |
Table II
Summary of the patients harboring EGFR
mutations.
| Patient | Gender | Age | Histology | Mutation type | Smoking | Stage |
|---|
| 1 | Female | 33 | AD | L858R | Never | T1N3M0 |
| 2 | Male | 60 | AD | L858R | Never | T2N1M0 |
| 3 | Male | 64 | AD | P848L | Never | T2N0M0 |
| 4 | Male | 49 | AD | L858R | Never | T2N2M0 |
| 5 | Male | 68 | AD | Del747-753INSS | 20 pack years | T3N2M0 |
| 6 | Female | 54 | AD | L858R | Never | T2N2M0 |
| 7 | Male | 61 | AD | Del E746-A750 | 15 pack years | T4N2M1
left humerus metatasis |
| 8 | Female | 54 | AD | L858R | Never | T4N2M1
bone metastasis |
Response to tyrosine kinase inhibitor. The data
revealed that most patients received platinum-based doublet
chemotherapy. After failing this chemotherapy, 2 patients received
gefitinib at a dosage of 250 mg orally once a day. The median
survival following gefitinib therapy was 10.0 months. patient no. 6
is a never smoking, 54-year-old female lung adenocarcinoma patient
with a L858R mutation in the EGFR gene and was in the T2N2M0
stage when she was diagnosed. She was treated with vinorelbine (36
mg on day 1 and day 8) plus cisplatin (110 mg on day 1) for 4
cycles during the initial treatment. When distant metastasis in the
tempus sinistrum meningeal was detected, radiotherapy was applied.
Gefitinib was then administered at a dosage of 250 mg per day, and
4 months later the patient achieved a complete response. Gefitinib
treatment continued and there was a complete response status for 20
months without tempus sinistrum meningeal metatasis progression.
Another patient without an EGFR mutation who received gefitinib had
coronale metastasis upon diagnosis. The conventional strategy of
chemotherapy for 2 cycles did not provide relief, and follow-up
evidence revealed the disease had progressed. Gefitinib was
administered for 3 months, and the patient had no relapse.
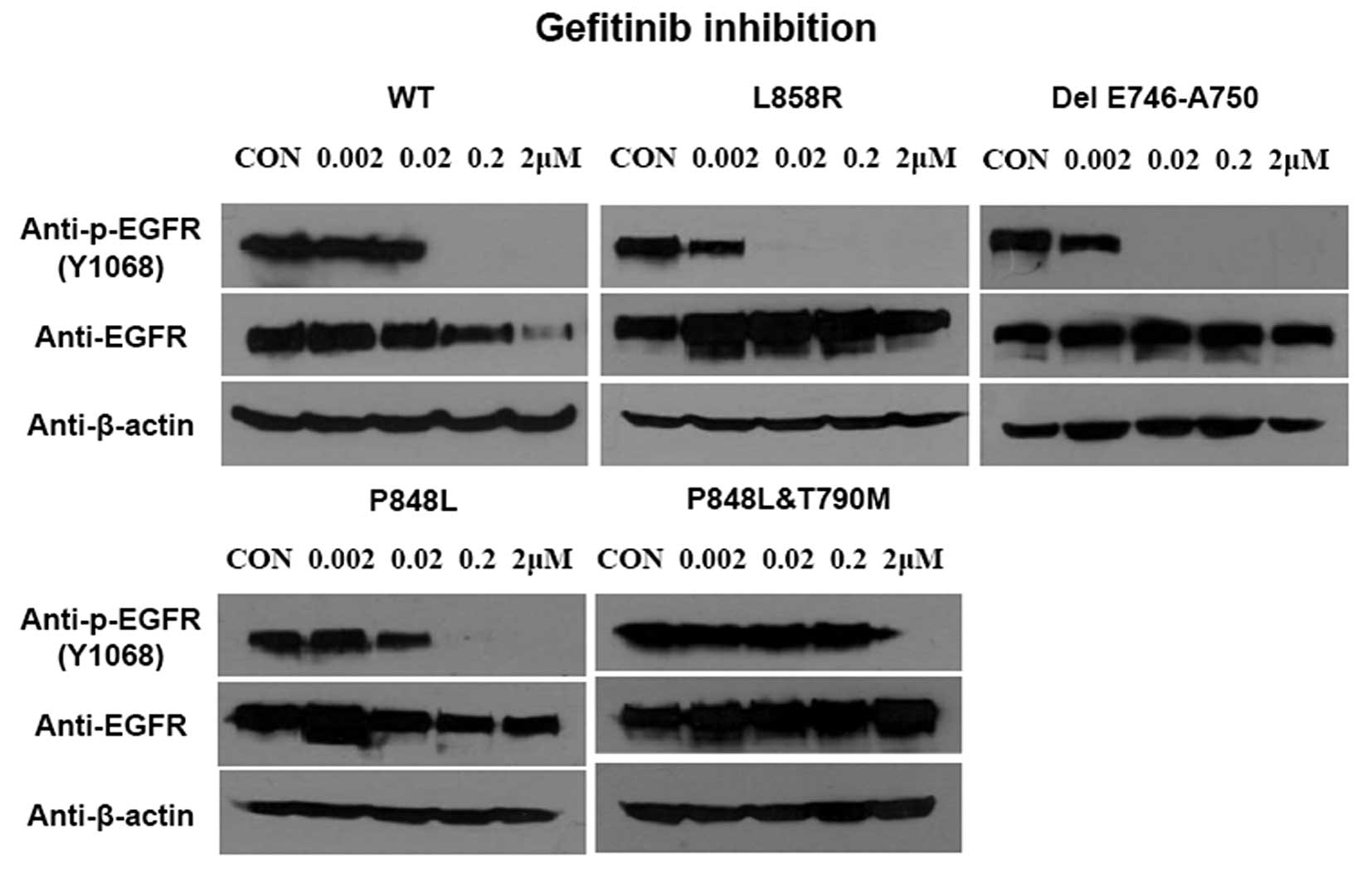
Sensitivity of the wild-type and the
P848L mutant EGFR to TKI
To compare the sensitivity of EGFR-P848L mutation to
TKIs with the most common classical mutations, the EGFR with L858R
and delE746-A750 mutation were transfected into 293T cells. In
addition, the EGFR with P848L mutation or P848L and T790M double
mutations were also transfected into cells to assess the change of
sensitivity to TKI after T790M mutation. The EGFR-P848L mutant had
a similar sensitivity to TKIs, as did the wild-type one. When the
TKI concentration increased to 0.02 μM, the autophosphorylation of
all three mutants was inhibited completely. Introducing T790M to
P848L mutated EGFR markedly decreased the sensitivity to TKIs
(Fig. 2).
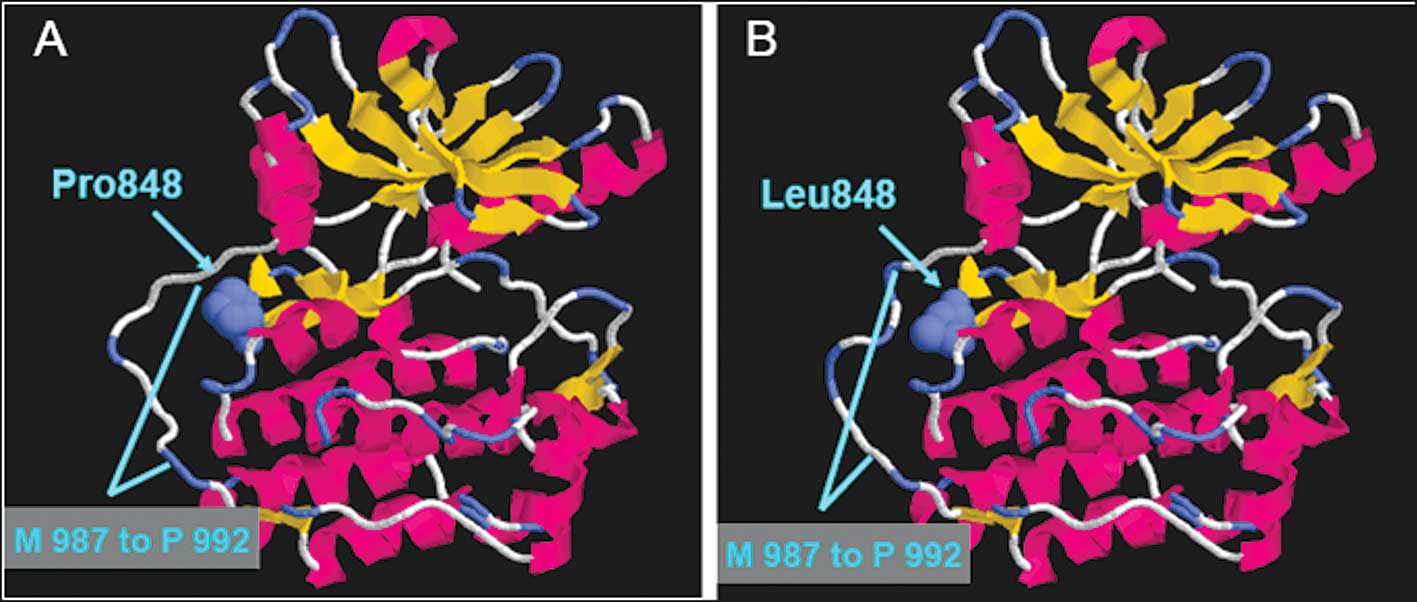
Computer modeling
To further determine the change after the P848L
mutation of EGFR, we constructed a three-dimensional computer model
using the crystal structure of wild-type EGFR as the template.
Fig. 3A and B reveal the crystal
structure of wild-type and P848L EGFR, respectively. Although the
structure of the ATP-binding pocket in the EGFR protein does not
have any marked alteration, the structure of the region M987 to
P992 in the EGFR gene was changed after the EGFR-P848L
mutation.
Discussion
A better mechanistic understanding of NSCLC would
aid in the effort to find an optimal treatment for the disease. The
oncogenesis of NSCLC involves numerous factors, including a large
number of genetic abnormalities. Therapies targeted to these
genetic abnormalities, which are different from the classical
cytotoxic chemotherapy, have emerged in recent years. Culture
studies have provided clear evidence that EGFR over-expression
and/or mutation commonly occur in NSCLC, but only certain types of
EGFR mutations have thus far been revealed to have therapeutic
significance. Mutants of EGFR often show greater
autophosphorylation activity, which increases the sensitivity to
treatment with TKIs. The clinical observations in this study
confirmed the same result as reported in the literature; patients
harboring mutant EGFR show a marked response to TKI. This suggests
that accurate mutation information is crucial, and only those NSCLC
patients harboring an active EGFR mutation respond to the existing
TKI treatment.
In the present study, all of the exons of the EGFR
in 55 lung cancer patients were sequenced. In this cohort of NSCLC
patients, a similar pattern of EGFR mutation was found as that
reported previously. The EGFR mutations occurred in cases of
adenocarcinoma, and more frequently in non-smokers, with a total
gross mutation rate of 14.5%. A rare mutation in exon 21, P848L,
was first identified in a patient of Chinese origin. A further
functional study was carried out to observe the sensitivity of this
rare mutant to gefitinib. The result showed gefitinib to have a
modest inhibition of the P848L mutant EGFR, similar to that of the
wild-type one. This result demonstrated that not all patients with
mutations of EGFR are associated with sensitivity to gefitinib
treatment. However, this does not indicate that the P848L mutant is
resistant to all small molecule TKIs.
Sequist et al (10) first reported P848L and other rare
point mutations in exons 18, 20 and 21 of EGFR, and in that study
none of the patients with these rare point mutations responded to
EGFR TKI agents. In the present study, the patient with the
EGFR-P848L mutation did not adopt TKI treatment; subsequently,
assessment of the clinical response to TKI was not possible. The
in vitro result showed that the EGFR-P848L mutant had a
similar sensitivity to gefitinib as that of the wild-type one,
i.e., lower than that of the classical mutations.
With the aid of advances in computer technology to
help achieve a better understanding of the alteration of this
mutant, we were able to directly explore the spatial conformation
using a homology model of EGFR-P848L. Marked change was not
detected in the ATP-binding pocket in the EGFR-P848L mutant.
However, we found that the structure of the region M987 to P992 was
changed after the EGFR-P848L mutation. Combined with the in
vitro analysis, it indicated that this mutation did not affect
the binding of small molecule TKI with EGFR. Furthermore, we could
also not exclude the possibility that mutations located around the
ATP-binding pocket of the tyrosine kinase domain indirectly affect
the binding affinity of mutant EGFR and gefitinib.
Although the patients with the EGFR mutation showed
sensitivity to TKI at the initial stage of treatment, the majority
of these patients became resistant to TKI. Two reasons could
elucidate the acquired resistance in the patients treated with TKI:
one reason is that a secondary mutation in the EGFR gene,
T790M, occurred during the patient's TKI treatment. Another reason
is that the patient treated with TKI appeared to have amplification
of the MET protooncogene (12,13).
However, T790M is the primary secondary mutation responsible for
acquired resistance in NSCLC patients treated with TKI (14). In the present study, the EGFR
gene with a double mutation, P848L and T790M, revealed a similar
feature in that T790M serves a gate-residue function, even though
it is a non-sensitive mutation. Therefore, it is essential to
monitor in ‘real-time’ the resistant T790M mutation of the EGFR
gene in the lung cancer cells of NSCLC patients. Maheswaran et
al (15) have established a
method to capture circulating tumor cells from the blood of NSCLC
patients by using a microfluidic device containing microposts
coated with antibodies against epithelial cells, leading to the
probable real-time detection of the resistant mutation T790M of the
EGFR gene in the lung cancer cells of NSCLC patients.
In conclusion, we studied the EGFR mutation of 55
Chinese NSCLC patients and further determined the biological
properties of a rare mutation. The results revealed that the
mutational hotspot was located in exons 19 and 21, which
coordinates with patients of Western origin. Future functional
studies are required, particularly for the rare EGFR mutations.
Finally, individualized treatment of patients with different
mutations is also required in our clinical work.
Acknowledgements
This study was supported in part by the National
Natural Science Foundation of China (30530370, 30771017 and
30470816), the Chinese High Tech Program (863) (2006AA02Z175) and
the Commission for Science and Technology of Shanghai (055407029,
06XD14016 and 06JC14054). Pacific Edit reviewed the manuscript
prior to submission.
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics. CA Cancer J Clin. 59:225–249. 2009.
|
|
2
|
Kris MG, Natale RB, Herbst RS, Lynch TJ
Jr, Prager D, Belani CP, Schiller JH, Kelly K, Spiridonidis H,
Sandler A, Albain KS, Cella D, Wolf MK, Averbuch SD, Ochs JJ and
Kay AC: Efficacy of gefitinib, an inhibitor of the epidermal growth
factor receptor tyrosine kinase, in symptomatic patients with
non-small cell lung cancer: A randomized trial. JAMA.
290:2149–2158. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fukuoka M, Yano S, Giaccone G, Tamura T,
Nakagawa K, Douillard JY, Nishiwaki Y, Vansteenkiste J, Kudoh S,
Rischin D, Eek R, Horai T, Noda K, Takata I, Smit E, Averbuch S,
Macleod A, Feyereislova A, Dong RP and Baselga J:
Multi-institutional randomized phase II trial of gefitinib for
previously treated patients with advanced nonsmall-cell lung cancer
(The IDEAL 1 Trial). J Clin Oncol. 21:2237–2246. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sharma SV, Bell DW, Settleman J and Haber
DA: Epidermal growth factor receptor mutations in lung cancer. Nat
Rev Cancer. 7:169–181. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J and
Haber DA: Activating mutations in the epidermal growth factor
receptor underlying responsiveness of non-small-cell lung cancer to
gefitinib. N Engl J Med. 350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ,
Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE and
Meyerson M: EGFR mutations in lung cancer: Correlation with
clinical response to gefitinib therapy. Science. 304:1497–1500.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yatabe Y and Mitsudomi T: Epidermal growth
factor receptor mutations in lung cancers. Pathol Int. 57:233–244.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gu D, Scaringe WA, Li K, Saldivar JS, Hill
KA, Chen Z, Gonzalez KD and Sommer SS: Database of somatic
mutations in EGFR with analyses revealing indel hotspots but no
smoking-associated signature. Hum Mutat. 28:760–770. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu JY, Wu SG, Yang CH, Gow CH, Chang YL,
Yu CJ, Shih JY and Yang PC: Lung cancer with epidermal growth
factor receptor exon 20 mutations is associated with poor gefitinib
treatment response. Clin Cancer Res. 14:4877–4882. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sequist LV, Joshi VA, Jänne PA, Muzikansky
A, Fidias P, Meyerson M, Haber DA, Kucherlapati R, Johnson BE and
Lynch TJ: Response to Treatment and Survival of patients with
Non-Small Cell Lung Cancer Undergoing Somatic EGFR Mutation
Testing. Oncologist. 12:90–98. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arnold K, Bordoli L, Kopp J and Schwede T:
The SWISS-MODEL Workspace: A web-based environment for protein
structure homology modelling. Bioinformatics. 22:195–201. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Engelman JA and Janne PA: Mechanisms of
acquired resistance to epidermal growth factor receptor tyrosine
kinase inhibitors in non-small cell lung cancer. Clin Cancer Res.
14:2895–2899. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bean J, Brennan C, Shih JY, Riely G, Viale
A, Wang L, Chitale D, Motoi N, Szoke J, Broderick S, Balak M, Chang
WC, Yu CJ, Gazdar A, Pass H, Rusch V, Gerald W, Huang SF, Yang PC,
Miller V, Ladanyi M, Yang CH and Pao W: MET amplification occurs
with or without T790M mutations in EGFR mutant lung tumors with
acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci
USA. 104:20932–20937. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yun CH, Mengwasser KE, Toms AV, Woo MS,
Greulich H, Wong KK, Meyerson M and Eck MJ: The T790M mutation in
EGFR kinase causes drug resistance by increasing the affinity for
ATP. Proc Natl Acad Sci USA. 105:2070–2075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maheswaran S, Sequist LV, Nagrath S, Ulkus
L, Brannigan B, Collura CV, Inserra E, Diederichs S, Iafrate AJ,
Bell DW, Digumarthy S, Muzikansky A, Irimia D, Settleman J,
Tompkins RG, Lynch TJ, Toner M and Haber DA: Detection of mutations
in EGFR in circulating lung-cancer cells. N Engl J Med.
359:366–377. 2008. View Article : Google Scholar : PubMed/NCBI
|