Introduction
Early gastric cancer (EGC) is defined as
adenocarcinoma localized to the mucosa or the submucosa,
irrespective of lymph node metastasis (1). The incidence of EGC has increased due
to mass screening and advances in diagnostic technology. In Korea,
EGC increased from 29% in 1995 to 33% in 1999. This trend was more
prominent in Japan, where EGC represents more than 50% of all
gastric cancers (2–4). While the prognosis for surgically
treated EGC is generally excellent, with five-year survival greater
than 90%, patients with lymph node metastasis have lower survival
rates than those without metastasis (4–8).
Accurate prediction of lymph node involvement is of crucial
significance for appropriate curative treatment planning. Lymph
node-negative EGC patients may be curatively treated with minimally
invasive endoscopic mucosal resection or endoscopic submucosal
dissection, whereas lymph node-positive patients should undergo
gastrectomy with lymph node dissection (9–11).
Gastrectomy is associated with a high morbidity and mortality, and
postoperative quality of life may be impaired by weight loss, loss
of appetite and other metabolic and nutritional changes. This
aggressive surgical approach should be reserved only for EGC
patients at high risk of lymph node metastasis (9). Therefore, clarification of the
biological features of EGC with lymph node metastasis may aid in
the identification of a high-risk group of patients, and assist in
planning a strategy for their treatment.
Stromal cell-derived factor (SDF)-1α, also known as
CXCL12, is a small, cytokine-like protein that regulates leukocyte
trafficking to appropriate organs and maintains normal immune
system function. However, in addition to its role in the immune
system, it is now clear that SDF-1α is expressed in a number of
distinct types of normal and cancerous tissues, and that SDF-1α has
significant functions in development, proliferation, angiogenesis
and motility (12,13). It has also been reported that this
chemokine and its receptor, CXCR4, are involved in tumor
progression and metastasis (13–16).
In the case of gastric cancer, SDF-1α expression in primary cancers
was reported to be an independent prognostic factor among patients
with cancers at various stages, suggesting a role as a biological
marker (17,18). Thus, it is possible that there may
be a correlation between SDF-1α expression and lymph node
metastasis in patients with EGC.
Patients and methods
Patients and tumor samples
This study used tissue samples from 138 consecutive
patients with EGC undergoing surgical resection at Chungnam
National University Hospital, Daejeon, Korea, between 2001 and
2003. The patients had histologically confirmed adenocarcinoma of
the stomach. All patients signed informed consent for therapy as
well as for subsequent tissue studies, which had received prior
approval by an institutional review board. Clinicopathological
characteristics at the time of surgery were assessed using the
general rules established by the Japanese Gastric Cancer
Association (19).
Immunohistochemical staining of
SDF-1α
Immunohisto-chemical staining was performed using a
monoclonal anti-SDF-1α antibody (MAB350; R&D Systems,
Minneapolis, MN, USA) and the EnVision-HRP detection system
(DakoCytomation, Carpinteria, CA, USA) according to the
manufacturer’s instructions. Sections (3 μm) were cut from gastric
cancer tissue microarray blocks, mounted on slides treated with
3-aminopropyltriethoxysilane (APES, Sigma Chemical, St. Louis, MO,
USA) and dried for 2 h at 56°C prior to staining. The sections were
deparaffinized in xylene and rehydrated in a graded alcohol series.
Following antigen retrieval by heating under pressure in citrate
buffer (pH 6.0) for 3 min, tissue sections were treated with 3%
hydrogen peroxide for 10 min to block endogenous peroxidases. The
sections were then incubated for 30 min in a humid chamber at room
temperature with the anti-SDF-1α antibody (1:50) diluted in a
background-reducing diluent (S0809; DakoCytomation). Slides were
then incubated with EnVision reagent for 30 min, followed by
3,3′-diaminobenzidine (DAB) chromogen for 5 min, counterstained
with Mayer’s hematoxylin and mounted. Exclusion of the primary
antibody during immunostaining was used in the negative controls,
while lymphocytes of normal spleen sections served as positive
controls. Immunostaining was evaluated independently by two of the
authors, who were blinded to each patient’s clinicopathological
findings. Tumors were classified into four grades according to
staining intensity (grade 0, no staining; grade 1, weak staining
intensity; grade 2, moderate staining intensity; grade 3, strong
staining intensity). In the case of heterogeneous sample staining,
the higher score was selected if more than 50% of the cells
exhibited a higher intensity of staining.
Statistical analysis
The association of SDF-1α expression with
clinicopathological characteristics was assessed using the
Pearson’s χ2 test or linear-by-linear associations. For
analysis of the factors responsible for lymph node metastasis, a
logistic regression analysis was used. P<0.05 was considered to
be statistically significant. Statistical analyses were conducted
using SPSS 13.0 (SPSS, Chicago, IL, USA).
Results
SDF-1α immunostaining in tumor
tissues
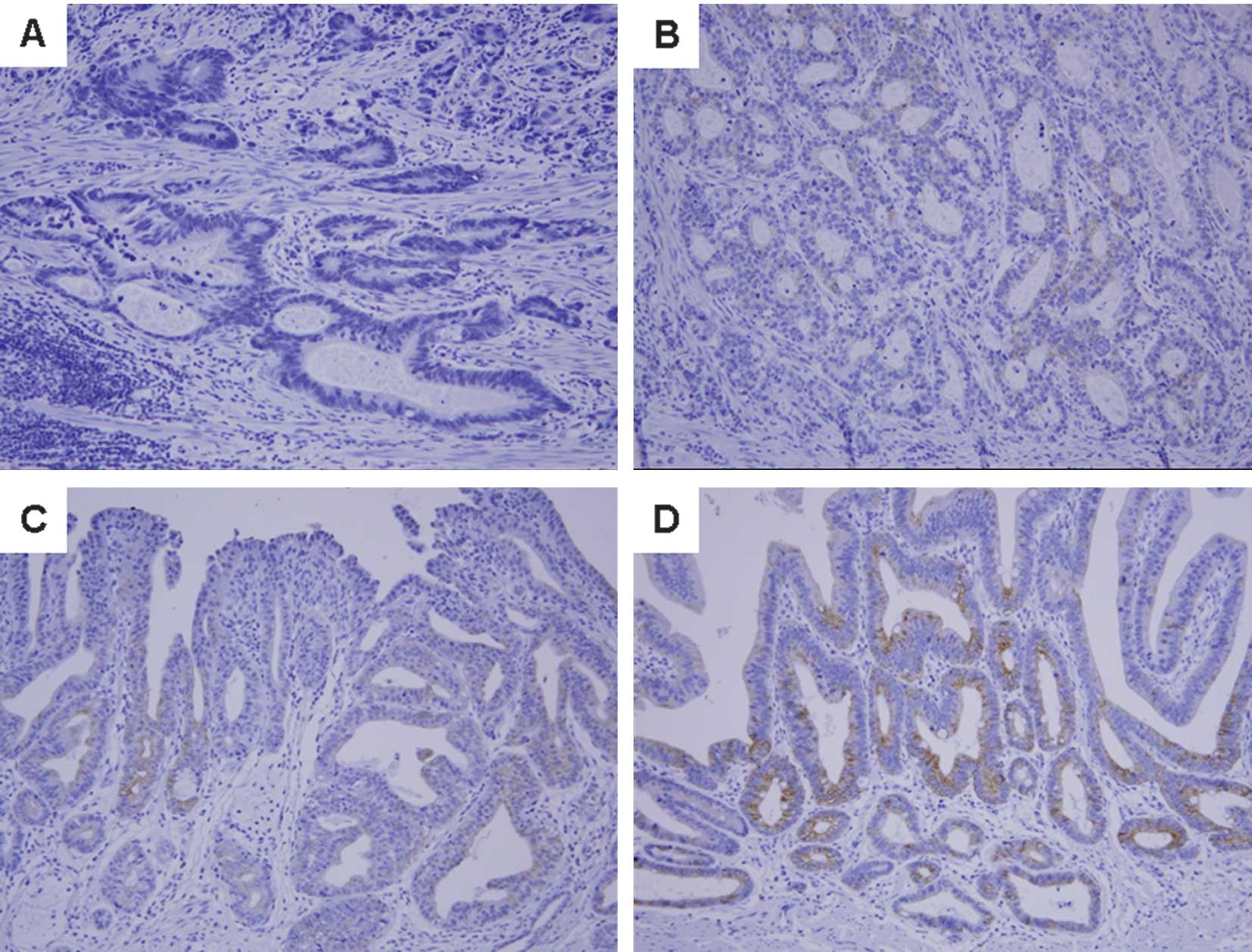
SDF-1α was detected in the cytoplasm and cellular
membrane of gastric cancer cells. SDF-1α expression was variable
[no staining, 79 patients (57.2%); weak staining, 30 (21.7%);
moderate staining, 17 (12.3%); and strong staining, 12 patients
(8.7%); Fig. 1]. The patients were
divided into two groups according to SDF-1α expression; the
SDF-1α-positive group (n=59) was defined as patients with weak to
strong SDF-1α expression, and the SDF-1α-negative group (n=79) was
defined as patients with no SDF-1α expression.
Association between SDF-1α expression and
clinicopathological factors
The correlation of SDF-1α expression and
clinicopathological characteristics is shown in Table I. No significant differences existed
with respect to age, gender, tumor location, proportion of tumors
>20 mm in size, macroscopic type, depth of invasion or histology
between SDF-1α-positive and -negative groups. However, the
SDF-1α-positive group was significantly correlated with
lymphovascular invasion (P=0.042) and with lymph node metastasis
(P=0.018).
 | Table IClinicopathological characteristics
according to SDF-1α expression. |
Table I
Clinicopathological characteristics
according to SDF-1α expression.
| Variable | Total n=138 | SDF-1α | P-value |
|---|
|
|---|
| Positive n=59
(%) | Negative n=79
(%) |
|---|
| Age (years) | | | | 0.342a |
| ≤ 65 | 96 | 38 (64.4) | 58 (73.4) | |
| > 65 | 42 | 21 (35.6) | 21 (26.6) | |
| Gender | | | | 0.544a |
| Male | 98 | 44 (74.6) | 54 (68.4) | |
| Female | 40 | 15 (25.4) | 25 (31.6) | |
| Tumor location | | | | 0.672b |
| Upper | 4 | 1 (1.7) | 3 (3.8) | |
| Middle | 77 | 33 (55.9) | 44 (55.7) | |
| Lower | 57 | 25 (42.4) | 32 (40.5) | |
| Tumor size (mm) | | | | 0.688a |
| ≤ 20 | 50 | 23 (39.0) | 27 (34.2) | |
| > 20 | 88 | 36 (61.0) | 52 (65.8) | |
| Macroscopic
types | | | | 0.280b |
| Elevated | 18 | 11 (18.6) | 7 (8.9) | |
| Flat | 12 | 5 (8.5) | 7 (8.9) | |
| Depressed | 95 | 36 (61.0) | 59 (74.7) | |
| Mixed | 13 | 7 (11.9) | 6 (7.6) | |
| Depth of
invasion | | | | 0.497a |
| Mucosal | 9 | 5 (8.5) | 4 (5.1) | |
| Submucosal | 129 | 54 (91.5) | 75 (94.9) | |
| Lymphovascular
invasion | | | | 0.042a |
| Negative | 52 | 16 (27.1) | 36 (45.6) | |
| Positive | 86 | 43 (72.9) | 43 (54.4) | |
| Histology | | | | 0.761a |
|
Differentiated | 81 | 36 (61.0) | 45 (57.0) | |
|
Undifferentiated | 57 | 23 (39.0) | 34 (43.0) | |
| Lymph node
metastasis | | | | 0.018a |
| Negative | 110 | 41 (69.5) | 69 (87.3) | |
| Positive | 28 | 18 (30.5) | 10 (12.7) | |
SDF-1α expression and lymph node
metastasis
To estimate the clinical significance of various
clinicopathological factors that may affect lymph node metastasis
in EGC, univariate analyses were performed. As shown in Table II, lymphovascular invasion [hazard
ratio (HR), 10.833; 95% confidence interval (CI), 2.450–47.895;
P=0.002], undifferentiated histology (HR, 3.277; 95% CI,
1.378–7.791; P=0.007) and SDF-1α positivity (HR, 3.029; 95% CI,
1.276–7.189; P=0.012) were statistically significant risk factors
affecting lymph node metastasis in patients with EGC. To determine
the independent prognostic effects of these variables, multivariate
analyses were performed using logistic regression analysis. The
results again demonstrated that lymphovascular invasion (HR, 8.595;
95% CI, 1.694–43.595; P=0.009), undifferentiated histology (HR,
2.965; 95% CI, 1.037–8.471; P=0.043) and SDF-1α positivity (HR,
2.108; 95% CI, 1.316–10.135; P=0.013) were independent risk factors
predicting lymph node metastasis in EGC patients (Table III).
 | Table IIUnivariate analysis of risk factors
for lymph node metastasis in patients with early gastric
cancer. |
Table II
Univariate analysis of risk factors
for lymph node metastasis in patients with early gastric
cancer.
| Variables | Hazard ratio | 95% Confidence
interval | P-value |
|---|
| Age (> 65
years) | 1.354 | 0.564–3.251 | 0.497 |
| Females | 1.207 | 0.493–2.955 | 0.680 |
| Tumor location
(lower) | 1.083 | 0.468–2.508 | 0.852 |
| Tumor size (>20
mm) | 2.444 | 0.917–6.513 | 0.074 |
| Macroscopic type
(Depressed or mixed) | 1.857 | 0.590–5.843 | 0.290 |
| Depth of invasion
(Submucosal) | 2.118 | 0.254–17.672 | 0.488 |
| Lymphovascular
invasion (Positive) | 10.833 | 2.450–47.895 | 0.002 |
| Histology
(Undifferentiated) | 3.277 | 1.378–7.791 | 0.007 |
| SDF-1α expression
(Positive) | 3.029 | 1.276–7.189 | 0.012 |
 | Table IIIMultivariate analysis of risk factors
for lymph node metastasis in patients with early gastric
cancer. |
Table III
Multivariate analysis of risk factors
for lymph node metastasis in patients with early gastric
cancer.
| Variables | Hazard ratio | 95% Confidence
interval | P-value |
|---|
| Age (> 65
years) | 1.524 | 0.550–4.225 | 0.418 |
| Females | 1.520 | 0.540–4.277 | 0.428 |
| Tumor location
(lower) | 1.366 | 0.504–3.703 | 1.366 |
| Tumor size (>20
mm) | 2.314 | 0.742–7.216 | 2.314 |
| Macroscopic type
(Depressed or mixed) | 2.108 | 0.566–7.857 | 0.266 |
| Depth of invasion
(Submucosal) | 1.163 | 0.062–21.939 | 0.920 |
| Lymphovascular
invasion (Positive) | 8.595 | 1.694–43.595 | 0.009 |
| Histology
(Undifferentiated) | 2.965 | 1.037–8.471 | 0.043 |
| SDF-1α expression
(Positive) | 2.108 | 1.316–10.135 | 0.013 |
Discussion
The prognosis for EGC is favorable following radical
surgery. Lymph node metastasis is considered to be a significant
prognostic factor for EGC as the 5-year survival rate of patients
without lymph node metastasis is approximately 95%, whereas that of
patients with metastasis is approximately 83% (7,20).
Therefore, a number of studies have been conducted to identify
predictive parameters, particularly biological markers, of lymph
node metastasis in EGC. We report for the first time that SDF-1α
expression in tumor cells is an independent risk factor for lymph
node metastasis in EGC. However, there remains no real consensus on
which patient and/or tumor characteristics are associated with
lymph node metastasis (9,21–23).
Recently, certain reports have demonstrated that
SDF-1α expression is associated with the progression and metastasis
of a number of types of cancer, including malignant glioma,
esophageal carcinoma, non-small cell lung cancer and colorectal
cancer (24–29). In gastric cancer, Ishigami et
al reported that SDF-1α expression was significantly associated
with lymph node metastasis, depth of invasion, lymphatic invasion,
tumor diameter and higher stage. In addition, the SDF-1α-positive
group showed significantly poorer surgical outcomes than the
SDF-1α-negative group, suggesting SDF-1α to be an independent
prognostic factor in gastric cancer (17). Iwasa et al also showed that
SDF-1α expression was significantly correlated with lymphovascular
invasion and lymph node and liver metastasis in patients with
intestinal-type gastric cancer (18). These findings led to speculation
that SDF-1α is a predictive marker of lymph node metastasis in
EGC.
In this study, it was found that SDF-1α expression
in EGC was significantly associated with lymphovascular invasion
and lymph node metastasis, whereas SDF-1α expression was not
associated with age, gender, tumor location, tumor size,
macroscopic type, depth of invasion or histology (Table I). Results of the univariate
analyses of risk factors for lymph node metastasis showed that
lymphovascular invasion, undifferentiated histology and SDF-1α
expression are risk factors in patients with EGC (Table II). Furthermore, multivariate
analyses clearly indicated that SDF-1α expression is as much an
independent risk factor for lymph node metastasis as is
lymphovascular invasion or undifferentiated histology. These data
suggest that SDF-1α expression in tumor cells is a useful marker
for the prediction of lymph node metastasis in patients with
EGC.
The mechanism by which SDF-1α contributes to gastric
cancer progression events, including lymph node metastasis, remains
unclear. One potential explanation is that SDF-1α is involved in
tumor progression in an autocrine and/or paracrine manner. The
concomitant expression of SDF-1α and its receptor, CXCR4, in the
same brain tumor cells has been characterized as an autocrine
and/or paracrine mechanism of cancer cell stimulation, resulting in
more aggressive behavior (30,31).
Subsequently, the autocrine/paracrine mitogenic activity of SDF-1α
was reported in cell lines and primary cell cultures of human
glioblastoma multiforme (32,33).
Barbieri et al also reported that the overexpression of
SDF-1α promoted autocrine/paracrine cell proliferation in human
pituitary tumor cells (30).
Another possible mechanism is that SDF-1α may promote tumor
angiogenesis by attracting endothelial cells to the tumor
microenvironment. Pathologically induced SDF-1α secretion by brain
tumor cells increases the recruitment of circulating endothelial
progenitors (34). Furthermore,
inhibition of the SDF-1α/CXCR4 receptor pathway reduced short-term
homing and long-term engraftment of vascular progenitors, and
decreased the growth of gastrointestinal tumors through the
suppression of angiogenesis (35,36).
In conclusion, this study has demonstrated that the
tumor expression of SDF-1α is an independent risk factor for lymph
node metastasis in patients with EGC, suggesting that SDF-1α is a
useful predictive marker. Further studies are required to elucidate
the mechanisms linking SDF-1α secreted by tumor cells to gastric
cancer.
Acknowledgements
This study was supported in part by the Basic
Science Research Program through the National Research Foundation
of Korea (NRF) funded by the Ministry of Education, Science and
Technology (NRF-2009-0076540) and the National Research Foundation
of Korea grant funded by the Korean government (MEST) (No.
2011-0006229).
References
|
1
|
Okamura T, Tsujitani S, Korenaga D,
Haraguchi M, Baba H, Hiramoto Y and Sugimachi K: Lymphadenectomy
for cure in patients with early gastric cancer and lymph node
metastasis. Am J Surg. 155:476–480. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Park YD, Chung YJ, Chung HY, et al:
Factors related to lymph node metastasis and the feasibility of
endoscopic mucosal resection for treating poorly differentiated
adenocarcinoma of the stomach. Endoscopy. 40:7–10. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Isozaki H, Tanaka N and Okajima K: General
and specific prognostic factors of early gastric carcinoma treated
with curative surgery. Hepatogastroenterology. 46:1800–1808.
1999.PubMed/NCBI
|
|
4
|
Maehara Y, Orita H, Okuyama T, Moriguchi
S, Tsujitani S, Korenaga D and Sugimachi K: Predictors of lymph
node metastasis in early gastric cancer. Br J Surg. 79:245–247.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Folli S, Dente M, Dell’Amore D, Gaudio M,
Nanni O, Saragoni L and Vio A: Early gastric cancer: prognostic
factors in 223 patients. Br J Surg. 82:952–956. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kitamura K, Yamaguchi T, Taniguchi H,
Hagiwara A, Sawai K and Takahashi T: Analysis of lymph node
metastasis in early gastric cancer: rationale of limited surgery. J
Surg Oncol. 64:42–47. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Habu H, Takeshita K, Sunagawa M and Endo
M: Lymph node metastasis in early gastric cancer. Int Surg.
71:244–247. 1986.PubMed/NCBI
|
|
8
|
Folli S, Morgagni P, Roviello F, et al;
Italian Research Group for Gastric Cancer (IRGGC). Risk factors for
lymph node metastases and their prognostic significance in early
gastric cancer (EGC) for the Italian Research Group for Gastric
Cancer (IRGGC). Jpn J Clin Oncol. 31:495–499. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kwee RM and Kwee TC: Predicting lymph node
status in early gastric cancer. Gastric Cancer. 11:134–148. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brennan MF: Current status of surgery for
gastric cancer: a review. Gastric Cancer. 8:64–70. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakajima T: Gastric cancer treatment
guidelines in Japan. Gastric Cancer. 5:1–5. 2002. View Article : Google Scholar
|
|
12
|
Krop IE: Chemokine signaling in gliomas:
prognostic factor, therapeutic target or both? Cancer Biol Ther.
5:1039–1041. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Burger JA and Kipps TJ: CXCR4: a key
receptor in the crosstalk between tumor cells and their
microenvironment. Blood. 107:1761–1767. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Arya M, Patel HR and Williamson M:
Chemokines: key players in cancer. Curr Med Res Opin. 19:557–564.
2003. View Article : Google Scholar
|
|
15
|
Scotton CJ, Wilson JL, Scott K, et al:
Multiple actions of the chemokine CXCL12 on epithelial tumor cells
in human ovarian cancer. Cancer Res. 62:5930–5938. 2002.PubMed/NCBI
|
|
16
|
Koshiba T, Hosotani R, Miyamoto Y, et al:
Expression of stromal cell-derived factor 1 and CXCR4 ligand
receptor system in pancreatic cancer: a possible role for tumor
progression. Clin Cancer Res. 6:3530–3535. 2000.PubMed/NCBI
|
|
17
|
Ishigami S, Natsugoe S, Okumura H, et al:
Clinical implication of CXCL12 expression in gastric cancer. Ann
Surg Oncol. 14:3154–3158. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Iwasa S, Yanagawa T, Fan J and Katoh R:
Expression of CXCR4 and its ligand SDF-1 in intestinal-type gastric
cancer is associated with lymph node and liver metastasis.
Anticancer Res. 29:4751–4758. 2009.PubMed/NCBI
|
|
19
|
Japanese Gastric Cancer Association.
Japanese classification of gastric carcinoma. 2nd English edition.
Gastric Cancer. 1. pp. 10–24. 1998, View Article : Google Scholar
|
|
20
|
Li C, Kim S, Lai JF, et al: Risk factors
for lymph node metastasis in undifferentiated early gastric cancer.
Ann Surg Oncol. 15:1464–1469. 2008.
|
|
21
|
Kanai T, Konno H, Maruyama K, et al: p53
overexpression and proliferative activity do not correlate with
lymph node metastasis in early gastric cancer. Eur Surg Res.
29:35–41. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kabashima A, Maehara Y, Kakeji Y, Baba H,
Koga T and Sugimachi K: Clinicopathological features and
overexpression of matrix metalloproteinases in intramucosal gastric
carcinoma with lymph node metastasis. Clin Cancer Res. 6:3581–3584.
2000.PubMed/NCBI
|
|
23
|
Yonemura Y, Ninomiya I, Ohoyama S, et al:
Correlation of c-erbB-2 protein expression and lymph node status in
early gastric cancer. Oncology. 49:363–367. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Salmaggi A, Gelati M, Pollo B, et al:
CXCL12 expression is predictive of a shorter time to tumor
progression in low-grade glioma: a single-institution study in 50
patients. J Neurooncol. 74:287–293. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Calatozzolo C, Maderna E, Pollo B, et al:
Prognostic value of CXCL12 expression in 40 low-grade
oligodendrogliomas and oligoastrocytomas. Cancer Biol Ther.
5:827–832. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sasaki K, Natsugoe S, Ishigami S, et al:
Expression of CXCL12 and its receptor CXCR4 correlates with lymph
node metastasis in submucosal esophageal cancer. J Surg Oncol.
97:397–402. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sasaki K, Natsugoe S, Ishigami S, et al:
Expression of CXCL12 and its receptor CXCR4 in esophageal squamous
cell carcinoma. Oncol Rep. 21:65–71. 2009.PubMed/NCBI
|
|
28
|
Wagner PL, Hyjek E, Vazquez MF, et al:
CXCL12 and CXCR4 in adenocarcinoma of the lung: association with
metastasis and survival. J Thorac Cardiovasc Surg. 137:615–621.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yoshitake N, Fukui H, Yamagishi H, et al:
Expression of SDF-1 α and nuclear CXCR4 predicts lymph node
metastasis in colorectal cancer. Br J Cancer. 98:1682–1689.
2008.
|
|
30
|
Barbieri F, Bajetto A, Stumm R, et al:
Overexpression of stromal cell-derived factor 1 and its receptor
CXCR4 induces autocrine/paracrine cell proliferation in human
pituitary adenomas. Clin Cancer Res. 14:5022–5032. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rempel SA, Dudas S, Ge S and Gutiérrez JA:
Identification and localization of the cytokine SDF1 and its
receptor, CXC chemokine receptor 4, to regions of necrosis and
angiogenesis in human glioblastoma. Clin Cancer Res. 6:102–111.
2000.PubMed/NCBI
|
|
32
|
Bajetto A, Barbieri F, Dorcaratto A, et
al: Expression of CXC chemokine receptors 1–5 and their ligands in
human glioma tissues: role of CXCR4 and SDF1 in glioma cell
proliferation and migration. Neurochem Int. 49:423–432. 2006.
|
|
33
|
Barbero S, Bonavia R, Bajetto A, et al:
Stromal cell-derived factor 1α stimulates human glioblastoma cell
growth through the activation of both extracellular
signal-regulated kinases 1/2 and Akt. Cancer Res. 63:1969–1974.
2003.
|
|
34
|
Li M and Ransohoff RM: The roles of
chemokine CXCL12 in embryonic and brain tumor angiogenesis. Semin
Cancer Biol. 19:111–115. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guleng B, Tateishi K, Ohta M, et al:
Blockade of the stromal cell-derived factor-1/CXCR4 axis attenuates
in vivo tumor growth by inhibiting angiogenesis in a
vascular endothelial growth factor-independent manner. Cancer Res.
65:5864–5871. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Orimo A, Gupta PB, Sgroi DC, et al:
Stromal fibroblasts present in invasive human breast carcinomas
promote tumor growth and angiogenesis through elevated SDF-1/CXCL12
secretion. Cell. 121:335–348. 2005. View Article : Google Scholar : PubMed/NCBI
|