Introduction
Giant cell tumors (GCTs) are benign tumors commonly
occurring at the ends of the long bones, representing approximately
5% of bone tumors (1). GCTs rarely
occur in the spine, with 2–5% of tumors found in the spine above
the sacrum (2,3). When occurring in the spine, these
tumors most commonly present with localized pain and swelling of
the affected side, and may also result in neurological deficit
(3,4).
In this study, the authors present an extremely rare
case of GCT occurring in the axial skeleton, involving the sacrum,
thoracic spine and parieto-occipital skull in more than 15 years of
follow up. The study was approved by the Institutional Review Board
(IRB) for Human Subjects Research and Ethics Committees of Hanyang
University Guri Hospital, Korea.
Case report
In March 1993, a 24-year-old male without a
significant past medical history presented with a several-month
history of localized pain in the right buttock area. There was
induration on the buttock and a firm, palpable mass.
Neurological examination revealed mild weakness of
the flexor hallucis longus and flexor digitorum longus with grade
4/5 power. The patient experienced diminished sensation in the
perineal region, and sphincter tone was slightly diminished.
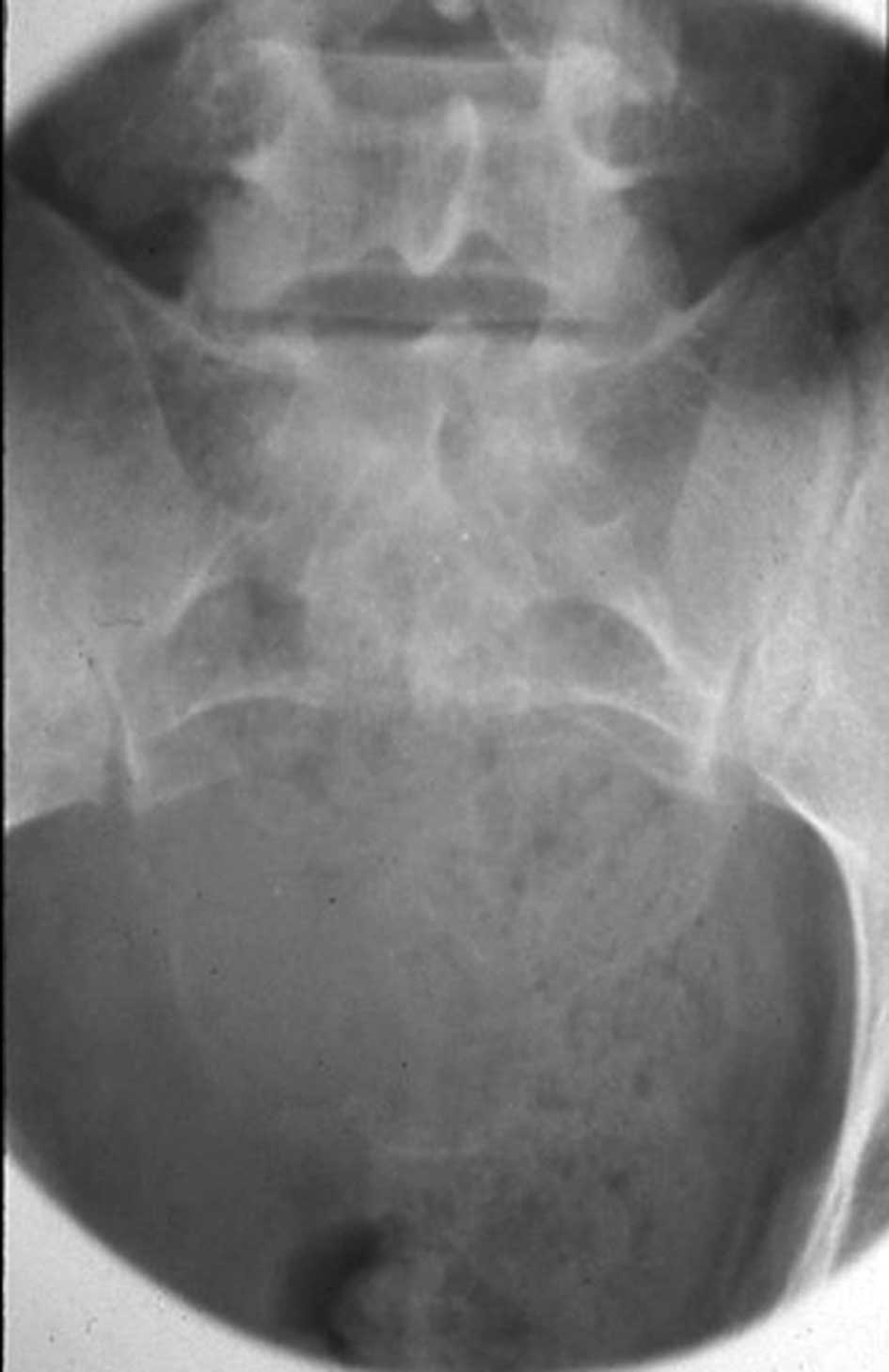
Radiographs revealed a large, osteolytic lesion involving almost
the entire sacrum (Fig. 1).
Computed tomography (CT) also revealed a destructive sacral mass
involving almost the entire sacrum below the S1 vertebral body.
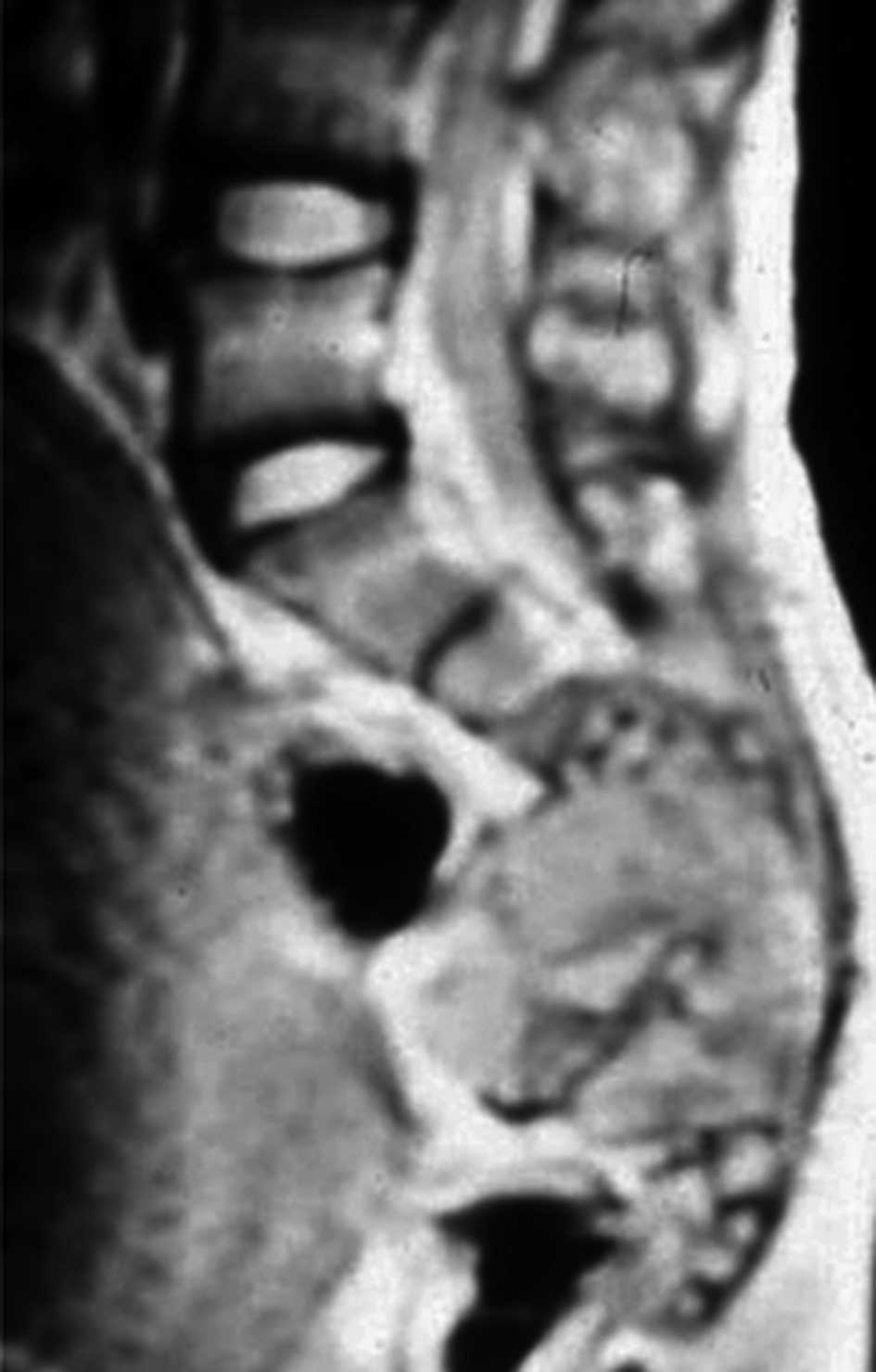
T2-weighted magnetic resonance imaging (MRI) showed an expansile
soft tissue mass with a heterogeneous signal density. The tumor
infiltrated into the spinal and sacral canal, and into the
presacral area above S2 (Fig. 2).
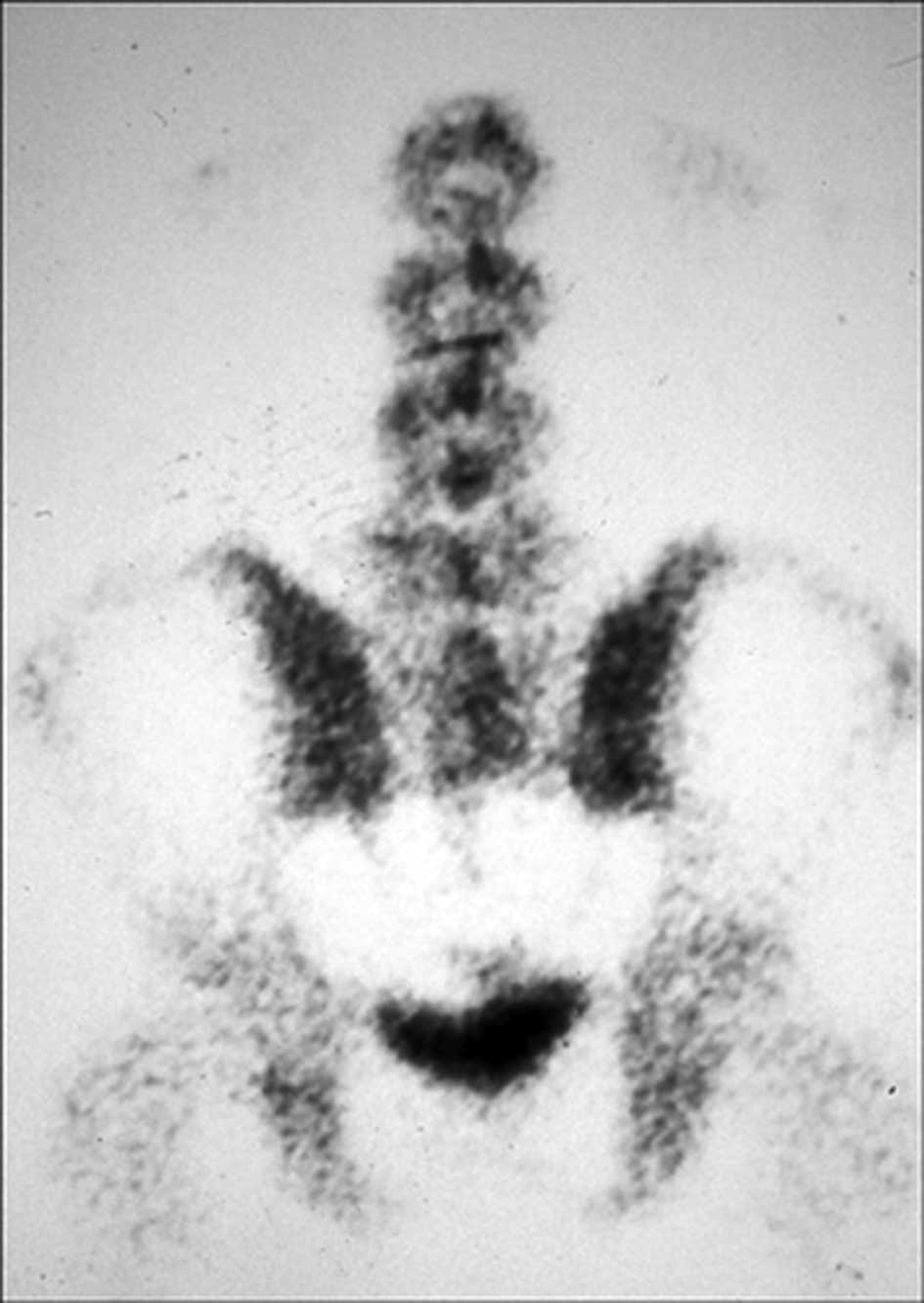
The technetium-99m methylene diphosphonate whole-body bone scan
revealed an abnormally high uptake in the sacral region (Fig. 3). Metastases were not found. To
prevent an improper diagnosis or a delay in treatment occurring,
open biopsy was performed from the posterior aspect of the sacrum,
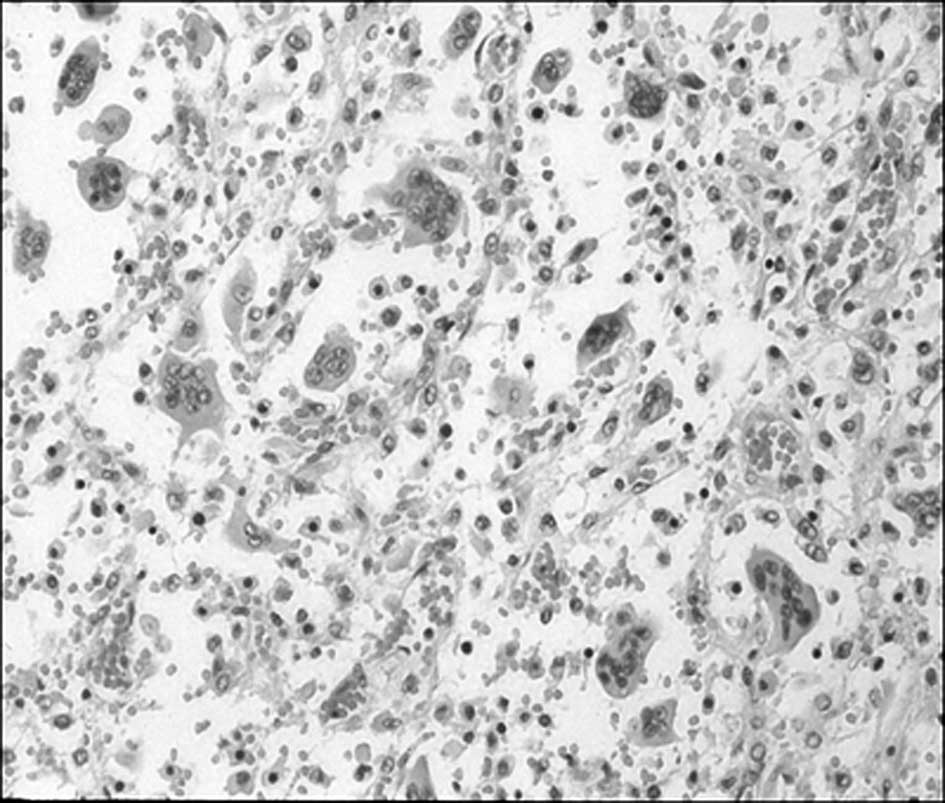
and histopathological examination revealed numerous multinucleated
giant cells within a background of scant stroma consistent with GCT
(Fig. 4).
With the goal of pain relief, tumor resection and
prevention of further neurological deterioration, the patient
underwent high sacral amputation using a staged anterior and
posterior approach. In the first stage, the anterior procedure with
a transperitoneal approach was performed. The anterior aspect of
the tumor was exposed, and internal iliac and middle sacral vessels
were ligated. The anterior osteotomy was performed through the
lower border of the S1 vertebral body at the level just below the
S1 sacral foramina. In the second stage, the posterior approach was
performed to transect the dural contents, eventually
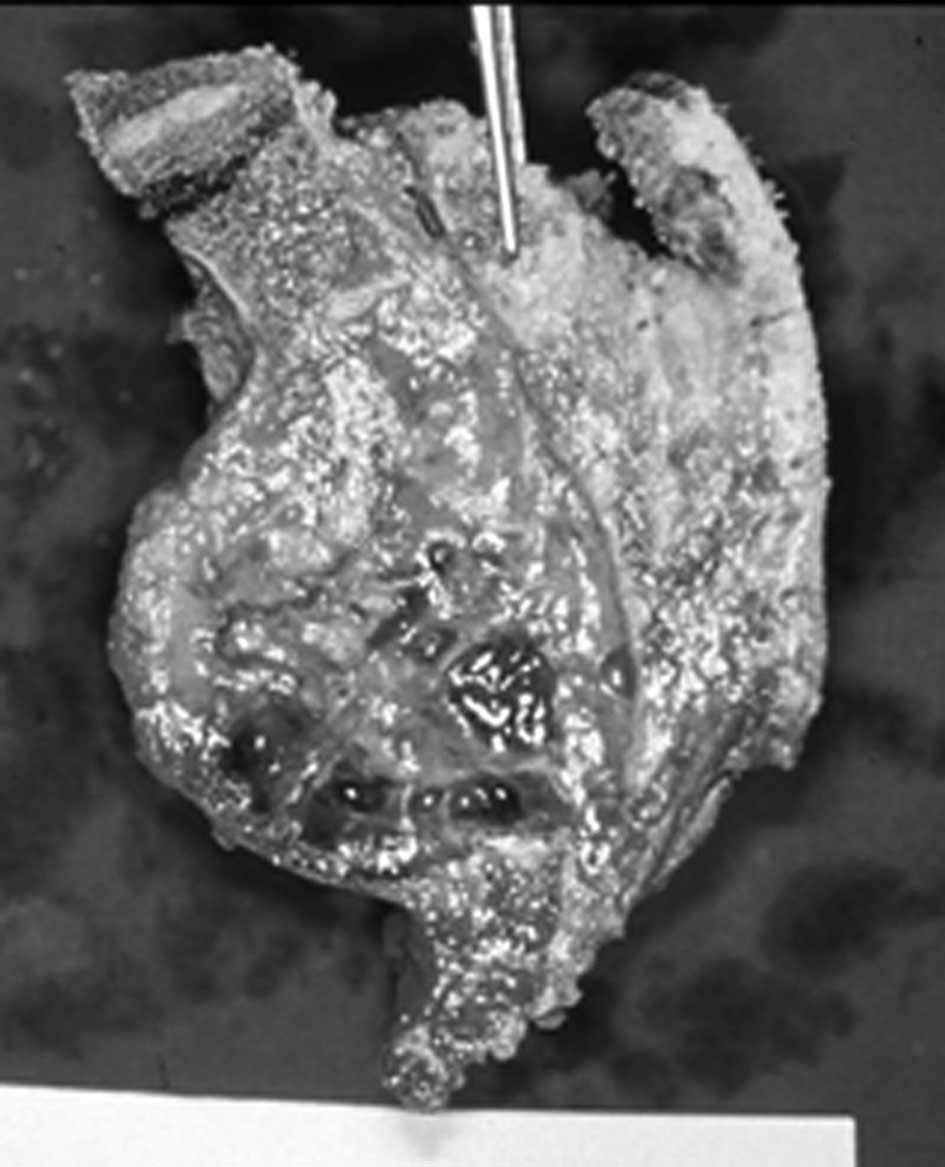
interconnecting the anterior and posterior osteotomies (Fig. 5). The tumor was resected with a
small margin and the biopsy tract was included within the resection
margins. The S1 roots were preserved. For adjuvant therapy, the
patient underwent radiotherapy consisting of a total dose of 5000
rad in 5 weeks.
Postoperatively, the patient was able to ambulate
without the use of external supports by 6 months. A certain amount
of perineal numbness remained, but the patient did not consider the
lower extremity numbness to be particularly disabling. The patient
suffered difficulties with urinary retention, requiring
intermittent urinary self-catheterization. Self-urination and
defecation became possible by a voluntary increase of the abdominal
muscle pressure.
After 5 years, there was no evidence of local
recurrence on serial imaging. The patient had returned to work
without significant complaints and was satisfied with the degree of
mobility.
In March 2008, the patient reported increasing pain
in the back and mid-buttock area. Physical examination of the
buttock area revealed a draining sinus on the previous surgery scar
with pus-like discharge. MRI of the lumbar and thoracic spine was
performed. T2-weighted MRI of the lumbar spine revealed high signal
intensity pus draining with a sinus tract into the buttock area
(Fig. 6A). MRI studies of the
thoracic spine showed diffuse enhancement on the T11 vertebral body
with an associated compression deformity suggestive of a bone
tumor. There was no cord compression, nor a signal change within
the cord (Fig. 6B). A
technetium-99m methylene diphosphonate whole-body bone scan was
also performed and showed abnormal uptake only at the T11 vertebral
body.
Considering the patient’s clinical history and
findings on MRI, a diagnosis of metastatic GCT was considered,
involving anterior zones of the vertebral body according to the
Weinstein-Boriani-Biagini (WBB) classification system (5,6). The
patient subsequently underwent a wide en block resection of T11 and
anterior interbody fusion with autogenous iliac strut bone graft. A
complete resection of the vertebral body was achieved and specimens
were sent for histopathological analysis. The spinal reconstruction
and stabilization were performed by posterolateral fusion with
autogenous iliac bone graft and pedicle screw fixation.
Additionally, the patient had open irrigation and debridement for
the infection on the buttock.
The histopathological examination revealed numerous
multinucleated giant cells within a background of scant stroma,
consistent with GCT. For adjunctive therapy, the patient again
underwent radiotherapy consisting of a total dose of 5000 rads in 5
weeks.
In October 2008, the patient reported newly
developed localized swelling on the right occipital area. A diffuse
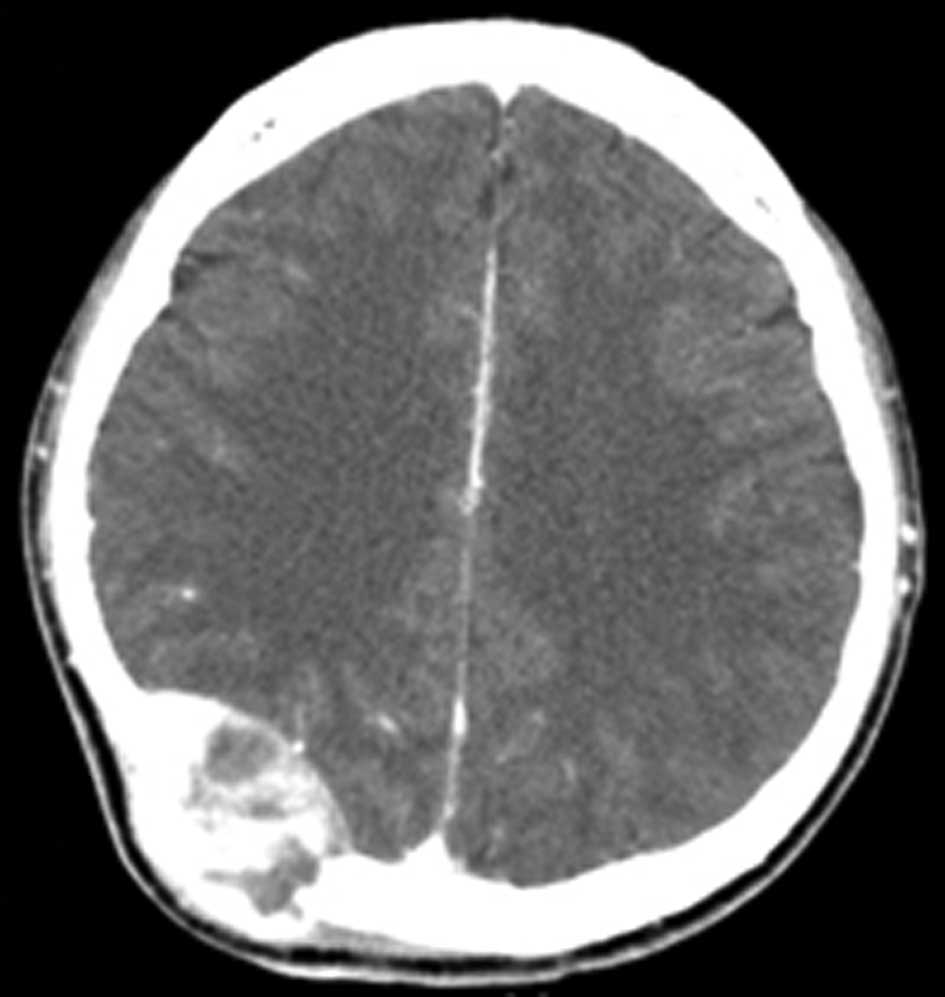
swelling was noted in the right parieto-occipital area. CT scan of
the brain revealed a large well-defined hyperdense
contrast-enhancing lesion originating from the right
parieto-occipital bone (Fig. 7).
The patient subsequently underwent a right parieto-occipital
craniectomy, followed by cranioplasty. Adjunctive radiotherapy was
repeated. The histopathological examination has confirmed the
diagnosis of GCT of bone.
Currently, the patient continues to be free of
recurrence 26 months following the T11 corpectomy, and 19 months
following the craniectomy.
Discussion
The majority of GCTs occur in the ends of the long
bones, usually the distal femur, proximal tibia and distal radius
(1,2). GCTs rarely occur in the spine, with
2–5% of tumors found in the spine above the sacrum (2–4).
Neoplasia of the skull bones are also extremely rare, accounting
for only 2.4–2.6% of all primary bone tumors, and when they occur,
the sphenoid bone is known to be the most common site, followed by
the temporal bone (5,7). Although GCTs exhibit a propensity for
aggressive local recurrence, there are few reports of metastases or
recurrence from GCT of the spine. Additionally, GCTs are difficult
to determine due to the low incidence of these lesions (2,4).
Multifocal GCTs of bone have been addressed in
previous reports, mostly occurring in the limbs. On only one
occasion has the multifocal GCT of bone been reported in the spine,
occurring primarily in the thoracic spine, and two years later, in
the sacrum (8). Due to multiple
lesions in the axial skeleton in the absence of lung involvement
and an open foramen ovale, it is difficult to confirm whether the
tumors were actually multifocal in their origin or spread through
the vertebral venous complex (9,10). The
Batson’s venous plexus, which connects the deep pelvic veins to the
internal vertebral venous plexuses, may provide a route for the
spread of tumors arising from the pelvis to the vertebral column or
brain (10). The primary tumor and
metastatic lesions usually have the same histological features;
however, histological grades and findings do not have practical
value in the prediction of the outcome or the risk of metastatic
spread (11,12).
Optimal management of GCT of bone is known to be
complete resection of the tumor with wide margins if possible, and
according to Mnaymneh et al (13), there was no recurrence when the
primary tumor excision was performed, whereas recurrent malignant
changes were reported in approximately half or more of groups
undergoing curettage and irradiation (3,14). Due
to the propensity of GCTs for aggressive local recurrence, adjuvant
radiation therapy to the resection site may be adopted. Although it
is widely held that radiation is capable of inducing sarcomatous
transformation and soft tissue damage, it has been suggested that
this risk was possibly associated with the now obsolete
ortho-voltage radiation therapy rather than the currently used
mega-voltage radiation therapy (15–17).
Fujimoto et al (18) report
favorable results in three consecutive cases diagnosed with GCT of
the spine, which were treated with radiotherapy and bisphosphonate
(BP) as a new treatment option.
In the present case, the patient was found to have
multifocal GCTs in extremely unusual locations. The first in the
sacrum, the second, 15 years later, in the thoracic spine, and the
third, 15 years and 6 months later from the onset of the first
tumor in the sacrum, in the parieto-occipital area of the skull.
The patient had neither lung involvement nor a sign of cardiac
septal defect. The tumors in the thoracic spine and skull may be
thought of as metastases of the primary lesion in the sacrum with
late recurrence. As the patient had an open biopsy for the initial
diagnosis, it is possible that the biopsy margins were contaminated
in the first resection and the infection of primary site, which
occured 15 years later may be related to residual active disease
(15,19,20).
The 15-year interval between the primary lesion in the sacrum and
recurrences in the thoracic spine and skull was longer than the
usual interval that has been reported in other studies (19). Thus, we report an extremely rare
case of GCT occurring in the axial skeleton, involving the sacrum,
thoracic spine and parieto-occipital skull in more than 15 years of
follow up.
References
|
1
|
Donthineni R, Boriani L, Ofluoglu O and
Bandiera S: Metastatic behaviour of giant cell tumour of the spine.
Int Orthop. 33:497–501. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goldenberg RR, Campbell CJ and Bonfiglio
M: Giant-cell tumor of bone. An analysis of two hundred and
eighteen cases. J Bone Joint Surg Am. 52:619–664. 1970.PubMed/NCBI
|
|
3
|
Sanjay BK, Sim FH, Unni KK, McLeod RA and
Klassen RA: Giant-cell tumours of the spine. J Bone Joint Surg Br.
75:148–154. 1993.PubMed/NCBI
|
|
4
|
Kumar R, Guinto FC Jr, Madewell JE, David
R and Shirkhoda A: Expansile bone lesions of the vertebra.
Radiographics. 8:749–769. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bitoh S, Takimoto N, Nakagawa H, Namba J,
Sakaki S and Gohma T: Giant cell tumor of the skull. Surg Neurol.
9:185–188. 1978.PubMed/NCBI
|
|
6
|
Boriani S, Weinstein JN and Biagini R:
Primary bone tumors of the spine. Terminology and surgical staging
Spine (Phila Pa 1976). 22:1036–1044. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bertoni F, Unni KK, Beabout JW and
Ebersold MJ: Giant cell tumor of the skull. Cancer. 70:1124–1132.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kos CB, Taconis WK, Fidler MW and ten
Velden JJ: Multifocal giant cell tumors in the spine. A case report
Spine (Phila Pa 1976). 22:821–822. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Onuigbo WI: Batson’s theory of vertebral
venous metastasis: a review. Oncology. 32:145–150. 1975.
|
|
10
|
Batson OV: The function of the vertebral
veins and their role in the spread of metastases. Clin Orthop Relat
Res. 312:4–9. 1995.PubMed/NCBI
|
|
11
|
McGough RL, Rutledge J, Lewis VO, Lin PP
and Yasko AW: Impact severity of local recurrence in giant cell
tumor of bone. Clin Orthop Relat Res. 438:116–122. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lausten GS, Jensen PK, Schiodt T and Lund
B: Local recurrences in giant cell tumour of bone. long-term follow
up of 31 cases. Int Orthop. 20:172–176. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mnaymneh WA, Dudley HR and Mnaymneh LG:
Giant-cell tumor of bone. an analysis and follow-up study of the
forty-one cases observed at the Massachusetts General Hospital
between 1925 and 1960. J Bone Joint Surg Am. 46:63–75.
1964.PubMed/NCBI
|
|
14
|
Shikata J, Yamamuro T, Kotoura Y, Mikawa
Y, Iida H and Maetani S: Total sacrectomy and reconstruction for
primary tumors. report of two cases. J Bone Joint Surg Am.
70:122–125. 1988.PubMed/NCBI
|
|
15
|
Refai D, Dunn GP and Santiago P: Giant
cell tumor of the thoracic spine: case report and review of the
literature. Surg Neurol. 71:228–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Caudell JJ, Ballo MT, Zagars GK, et al:
Radiotherapy in the management of giant cell tumor of bone. Int J
Radiat Oncol Biol Phys. 57:158–165. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nair MK and Jyothirmayi R: Radiation
therapy in the treatment of giant cell tumor of bone. Int J Radiat
Oncol Biol Phys. 43:1065–1069. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fujimoto N, Nakagawa K, Seichi A, et al: A
new bisphosphonate treatment option for giant cell tumors. Oncol
Rep. 8:643–647. 2001.PubMed/NCBI
|
|
19
|
Bergh P, Kindblom LG, Gunterberg B,
Remotti F, Ryd W and Meis-Kindblom JM: Prognostic factors in
chordoma of the sacrum and mobile spine: a study of 39 patients.
Cancer. 88:2122–2134. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fourney DR, Rhines LD, Hentschel SJ, et
al: En bloc resection of primary sacral tumors: classification of
surgical approaches and outcome. J Neurosurg Spine. 3:111–122.
2005. View Article : Google Scholar : PubMed/NCBI
|