Introduction
The camptothecin derivative irinotecan hydrochloride
(CPT-11) is an anticancer agent and is now regarded as the most
active drug for the treatment of colorectal cancer patients
(1). A small amount of CPT-11 is
converted into its active metabolite 7-ethyl-10-hydroxycamptothecin
(SN-38) by carboxyesterase in the liver and other tissues, and then
into a water-soluble inactive metabolite SN-38 glucuronide (SN-38G)
by UDP-glucuronosyltransferases (UGTs). Since SN-38 is 100- to
1000-fold more cytotoxic than CPT-11, plasma levels of SN-38,
clearance of SN-38 and/or polymorphism of UGT1A1 are clinically
important influencing factors in CPT-11-induced side-effects,
including neutropenia and diarrhea (1–4).
However, in clinical practice, the adjustment of the
optimal dose of CPT-11 for an individual patient remains unclear,
since pharmacokinetic parameters of CPT-11, as well as the
incidence of CPT-11-related side effects, are markedly varied among
patients (1,3,5,6). The
clinical significance of inherited genetic polymorphisms of UGTs
involved in SN-38 glucuronidation is well recognized in association
with CPT-11-induced side effects (2,7–11).
UGTs are classified into 2 families (UGT1 and UGT2) and 3
subfamilies (UGT1A, UGT2A and UGT2B), and UGT1A1 is thought to be
the most predominant catalyst in the metabolism of SN-38 among
several UGT1A isoforms (1,12). However, it is still considered that
the optimal dose of CPT-11 cannot be adjusted solely from the
information of genetic polymorphisms of UGT1A1, and we frequently
encounter the cases of dose reduction of CPT-11, even in patients
with the wild-type UGT1A1 gene.
In the present study, 17 Japanese patients with
colorectal cancer and the wild-type UGT1A1 gene were enrolled, and
treated with CPT-11 (150 mg/m2) as a part of the CPT-11
plus infusional 5-fluorouracil/leucovorin (FOLFIRI regimen) in
Osaka Rosai Hospital (Japan) (13).
To establish pharmacokinetic-based dose adjustment of CPT-11, the
clinical significance of plasma SN-38G/SN-38 ratios in predicting
CPT-11-induced neutropenia was examined. The concentration ratios
of SN-38G against SN-38 in the plasma were determined exactly 15
min following a 2-h continuous infusion of CPT-11. Neutropenia was
evaluated by counting the number of neutrophils prior to and
following CPT-11 infusion. Plasma concentrations of SN-38, SN-38G
and CPT-11 were simultaneously determined by a previously reported
high-performance liquid chromatography (HPLC) method with a small
modification (14).
Materials and methods
Materials
CPT-11, SN-38 and SN-38G were supplied by Yakult
Honsha Co., Ltd. (Tokyo, Japan) and used without further
purification. Camptothecin, potassium dihydrogen phosphate
(KH2PO4), zinc sulfate and ethylene glycol
were purchased from Wako Pure Chemical Industries, Ltd. (Osaka,
Japan). Phosphoric acid (1 N), acetonitrile for HPLC and methanol
for HPLC were purchased from Kanto Chemical Co., Inc. (Tokyo,
Japan). Sodium 1-decanesulfonate was obtained from Sigma-Aldrich
Corporation (St. Louis, MO, USA).
CPT-11 treatment in patients
Genotyping of wild-type UGT1A1 was performed by the
Invader UGT1A1 Molecular Assay in the same manner as reported
previously (2,10,11).
Colorectal cancer patients (11 males and 6 females) with wild-type
UGT1A1 gene were treated with CPT-11 as part of the FOLFIRI regimen
(Table I) (13). The FOLFIRI regimen consisted of a
2-h continuous infusion of CPT-11 (150 mg/m2) and
leucovorin (200 mg/m2), immediately followed by bolus
injection of 5-FU (400 mg/m2), and followed by a 46-h
continuous infusion of 5-FU (2,400 mg/m2). Patients
received this FOLFIRI regimen every 2 weeks after counting the
number of neutrophils prior to and following CPT-11 infusion. The
number of neutrophils was counted by a Beckman coulter LH 780
analyzer (Beckman Coulter, Inc. Tokyo, Japan). Informed consent was
obtained from all patients. This study was approved by the
Institutional Review Board of Osaka Rosai Hospital and conducted in
accordance with the Declaration of Helsinki.
 | Table ISerological diagnosis of Japanese
colorectal cancer patients with wild-type UGT1A1 gene. |
Table I
Serological diagnosis of Japanese
colorectal cancer patients with wild-type UGT1A1 gene.
| No. | Age (years) | Gender | Body surface area
(m2) | Cr (mg/dl) | BUN (mg/dl) | T-BIL (mg/dl) | AST (U/l) | ALT (U/l) | Neutrophils
(μl-1) |
|---|
|
|---|
| Before | After |
|---|
| 1 | 77 | M | 1.77 | 0.7 | 16 | 0.5 | 24 | 22 | 1547 | 1232 |
| 2 | 60 | M | 1.79 | 0.7 | 11 | 0.3 | 16 | 9 | 3705 | 1806 |
| 3 | 62 | F | 1.72 | 0.8 | 12 | 0.4 | 48 | 62 | 3886 | 2434 |
| 4 | 60 | F | 1.51 | 0.7 | 7 | 0.6 | 26 | 35 | 1300 | 530 |
| 5 | 67 | M | 1.54 | 0.7 | 13 | 0.6 | 16 | 10 | 3154 | 2751 |
| 6 | 72 | M | 1.56 | 1.2 | 27 | 0.3 | 48 | 26 | 2796 | 1716 |
| 7 | 49 | M | 1.68 | 1.0 | 14 | 0.4 | 26 | 20 | 4537 | 2300 |
| 8 | 79 | M | 1.66 | 0.6 | 12 | 1.1 | 40 | 31 | 2639 | 1141 |
| 9 | 63 | M | 1.82 | 0.7 | 12 | 0.2 | 22 | 21 | 3813 | 1005 |
| 10 | 59 | M | 1.78 | 0.9 | 22 | 0.7 | 22 | 14 | 2646 | 2305 |
| 11 | 74 | M | 1.56 | 0.6 | 22 | 0.2 | 13 | 8 | 4219 | 2862 |
| 12 | 61 | F | 1.50 | 0.4 | 11 | 0.4 | 18 | 14 | 3426 | 2268 |
| 13 | 72 | F | 1.36 | 1.0 | 16 | 0.5 | 19 | 21 | 6313 | 4312 |
| 14 | 73 | F | 1.42 | 0.5 | 14 | 0.4 | 28 | 21 | 2780 | 1916 |
| 15 | 76 | M | 1.40 | 0.9 | 18 | 0.9 | 22 | 16 | 3788 | 489 |
| 16 | 65 | F | 1.49 | 0.5 | 11 | 0.3 | 19 | 13 | 6029 | 3396 |
| 17 | 68 | M | 1.62 | 0.7 | 8 | 0.5 | 19 | 22 | 4906 | 3552 |
Blood (5 ml each) was collected into an
ethylenediaminetetraacetic acid (EDTA)-containing blood collection
tube to determine plasma concentrations of SN-38, SN-38G and CPT-11
15 min following the completion of a 2-h continuous infusion by
measuring the time with a stopwatch. Blood samples were centrifuged
for 10 min at 3,000 × g to obtain plasma samples, and the plasma
samples were stored at −30°C until further analysis. Another part
of the blood sample was separately subjected to analysis of the
concentrations of creatinine (Cr), blood urea nitrogen (BUN), total
bilirubin (T-BIL), aspartate aminotransferase (AST), alanine
aminotransferase (ALT) and the number of neutrophils in plasma
(Table I).
Analytical method of CPT-11-related
compounds by HPLC
Stock solutions of CPT-11, SN-38, SN-38G and
camptothecin [an internal standard (IS)] were prepared by
dissolving stock in dimethylsulfoxide (DMSO) and storing at −30°C.
For the calibration curve, DMSO solution (50 μl) of CPT-11, SN-38
and SN-38G was diluted with plasma (450 μl) at a concentration
range of 500–7,500, 10–100 and 25–200 ng/ml, respectively. A total
of 50 μl of DMSO solution of camptothecin was then added to each
sample (400 μl) in a test tube, and the mixture solution was
deproteinized with 150 μl of deproteinizing agent [a mixture of 1 M
zinc sulfate solution, methanol and ethylene glycol (1:1:1)].
Following thorough mixing, the solution was centrifuged at 3,000 ×
g for 10 min, 100 μl of the supernatant was obtained and 20 μl of
0.5 M KH2PO4 was added. After mixing again,
the solution was transferred to a HPLC tube. The volume of each
sample loaded to the HPLC column was 20 μl. As for the calibration
curve, plasma samples obtained from each patient treated with
CPT-11 were similarly treated and concentrations of SN-38, SN-38G
and CPT-11 in the plasma were determined by the modified HPLC
method.
The HPLC system used was composed of a system
controller (SCL-10 AVP), an autosampler (SIL-20AC), degasser
(DGU-20A3), pump (LC-20ADXL) and a fluorescence detector (RF-10AXL)
(Shimadzu, Kyoto, Japan). The column used was Luna® 5 μm
C18 100 Å (250×4.60 mm), a reverse-phase column (Shimadzu). The
mobile phase was a mixture of solution A [50 mM
KH2PO4, 4 mM sodium 1-decanesulfonate and 20%
acetonitrile (CH3CN)] and solution B (50 mM
KH2PO4, 4 mM sodium 1-decanesulfonate and 30%
CH3CN). Solutions A and B were adjusted to pH 3.5 using
phosphoric acid. Gradient elution was performed using solutions A
and B, with the concentration of solution B being set at 0, 100 and
0% during the first 4 min, from 4 to 30 min, and from 30 min to
completion of elution, respectively. The flow rate was 1.5 ml/min,
and detection was made at 373 nm for the excitation wavelength and
540 nm for the emission wavelength, respectively.
Results
HPLC chromatograms
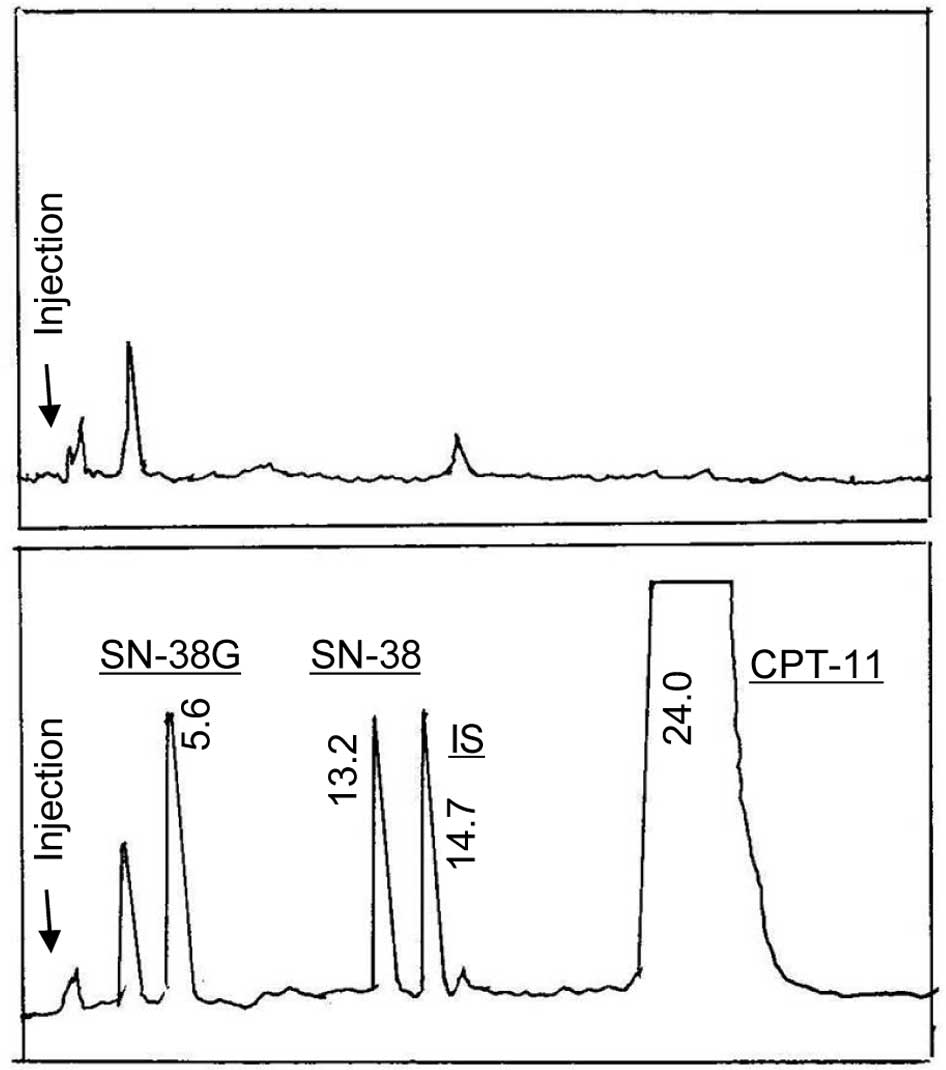
HPLC chromatograms of blank plasma and a plasma
sample containing CPT-11, SN-38, SN-38G and camptothecin (IS) are
shown in Fig. 1A and B,
respectively. The retention times of SN-38G, SN-38, IS and CPT-11
were 5.6, 13.2, 14.7 and 24.0 min, respectively. Endogenous
substances in plasma did not interfere with the peaks of
CPT-11-related compounds at all in the present analytical
conditions. A good regression line was obtained as follows: CPT-11
in a concentration range of 500–7,500 ng/ml, y=0.0155x+0.308,
r2=0.9997; SN-38 in a concentration range of 10–100
ng/ml, y=0.0211x−0.0112, r2=0.9986; SN-38G in a
concentration range of 25–200 ng/ml, y=0.0118x+0.0534,
r2=0.9961, where ‘y’ is the peak area ratio between each
compound and IS and ‘x’ is the concentration (ng/ml) of each
compound in the plasma.
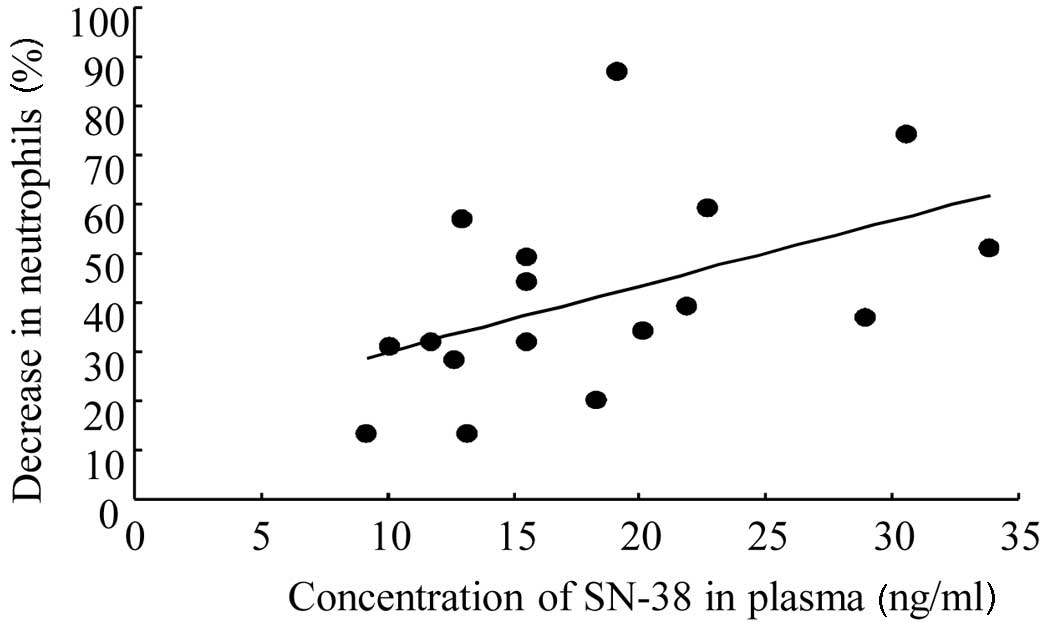
Correlation between plasma SN-38
concentrations and neutropenia induction in patients
SN-38 is the principal agent in CPT-11-induced
neutropenia due to its potent cytotoxicity. The correlation between
plasma SN-38 concentrations obtained 15 min following completion of
a 2-h continuous infusion and neutropenia induction (the percentage
of decreased neutrophil numbers by CPT-11 treatment) was examined.
A higher plasma concentration of SN-38 resulted in a greater
decrease in neutrophil numbers as follows: y=1.34x+16.5 (r=0.486,
p=0.0481; Fig. 2). This regression
line suggested that induction of neutropenia may be related to
plasma SN-38 concentrations, although variation was observed among
patients.
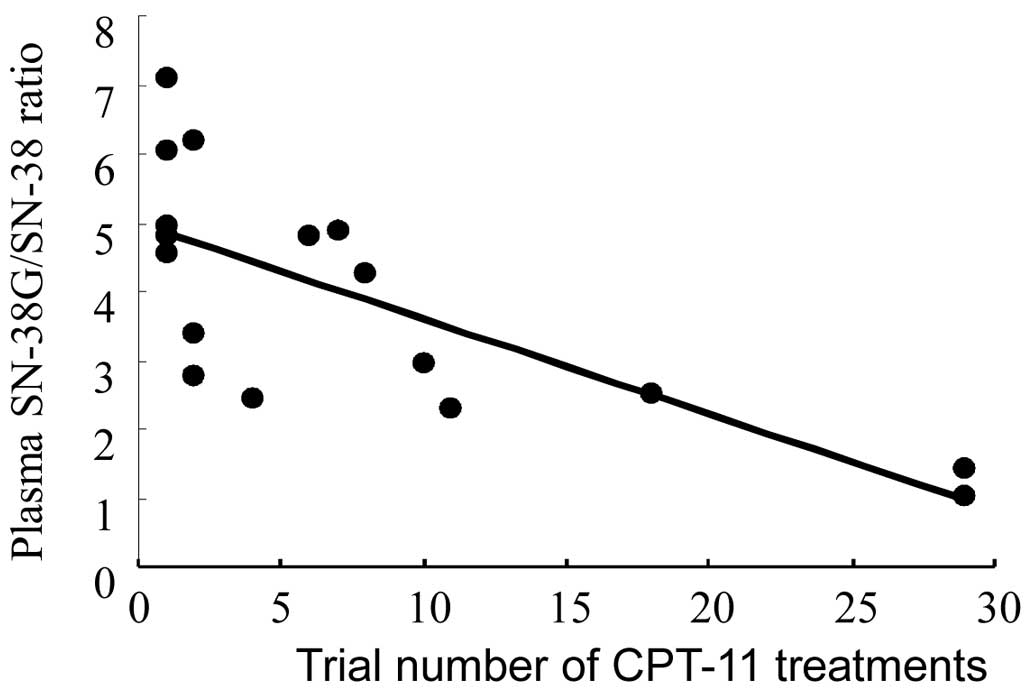
Relationship between the plasma
SN-38G/SN-38 ratio and trial numbers of CPT-11 treatments in
patients
It is generally speculated that the cumulative
amount [or value of the area under the curve (AUC)] of SN-38 in the
plasma, rather than one-point plasma SN-38 concentration, is more
directly connected to CPT-11-induced neutropenia, since cytotoxic
SN-38 is metabolized to non-toxic SN-38G by UGT1A1 at various rates
among patients. To reflect the UGT1A1 activity of each patient, a
parameter of plasma SN-38G/SN-38 ratio 15 min following a 2-h
continuous infusion of CPT-11 was introduced, and its clinical
significance in CPT-11-induced neutropenia was examined. The
median, minimum and maximum values of the plasma SN-38G/SN-38 ratio
were 4.25, 1.03 and 7.09, respectively, demonstrating a difference
of ≥6-fold, even among patients with the wild-type UGT1A1 gene. The
effect of trial numbers of CPT-11 treatments on the plasma
SN-38G/SN-38 ratio is shown in Fig.
3. The plasma SN-38G/SN-38 ratios significantly decreased with
an increase in the trial numbers of chemotherapy (r=0.741,
p=0.000669), indicating that CPT-11 treatments decrease UGT
activity serially.
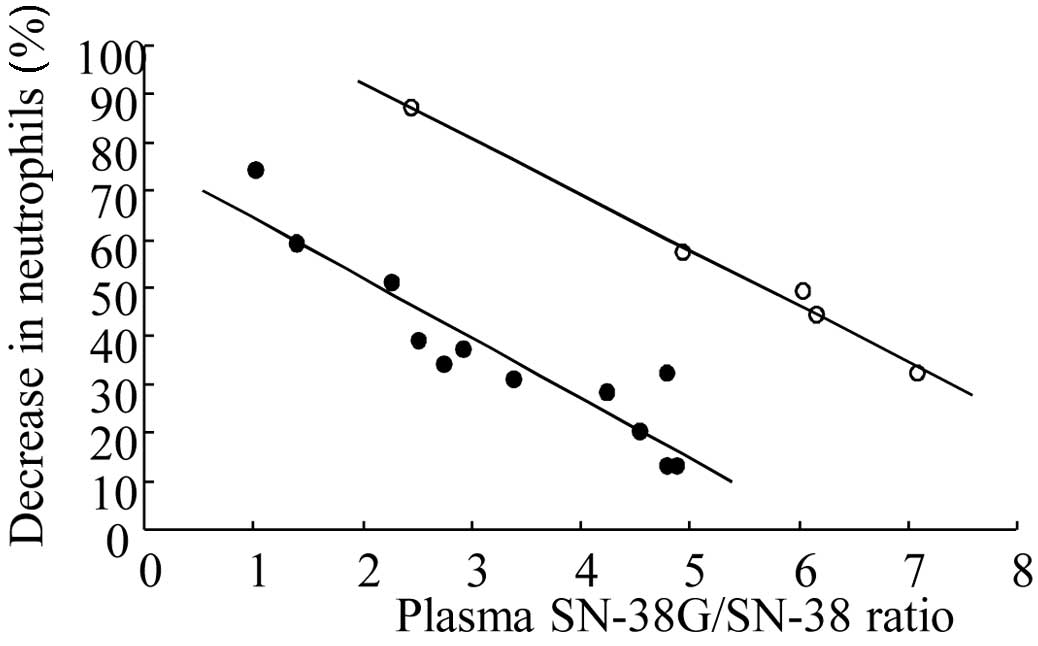
Correlation between the plasma
SN-38G/SN-38 ratio and neutropenia induction in patients
The correlation between the plasma SN-38G/SN-38
ratio and neutropenia induction in patients was examined (Fig. 4). In this analysis, 2 clearly
separated regression lines with high correlation coefficients were
obtained as follows: upper regression line (n=5), y=-11.5x+115,
(r=0.996, p=0.000366); lower regression line (n=12), y=-12.4x+76.8,
(r=0.927, p=0.0000147). These high correlations between the
regression lines suggest that low plasma SN-38G/SN-38 ratios, or
low plasma clearance of SN-38 due to low UGT activity, induce
severe neutropenia. Concurrently, it was suggested that the
variation in CPT-11-induced neutropenia among patients was not
explained by UGT activity alone in some patients.
Discussion
It is well known that the pharmacokinetics of CPT-11
as well as the incidence of CPT-11-induced side-effects are
markedly scattered among patients (1,3,5,6,8,12).
In the present study, we aimed to establish a pharmacokinetic-based
dose adjustment of CPT-11 and examined the clinical significance of
the one-point determination of the plasma SN-38G/SN-38 ratio in
predicting CPT-11-induced neutropenia in colorectal Japanese
patients with the wild-type UGT1A1 gene. The importance of
pharmacokinetic-based dose adjustment of CPT-11 has been noted by
numerous investigators, although such a method has not yet been
established (3,6,12).
For this purpose, the HPLC analytical method was
first investigated to determine SN-38, SN-38G and CPT-11
simultaneously within a relatively short period of time, in order
that pharmacokinetic-based dose adjustment of CPT-11 may be
applicable even in ambulatory patients. We modified a previously
reported HPLC method (14) by using
a mixture of 1 M zinc sulfate solution, methanol and ethylene
glycol (1:1:1) as a deproteinizing agent, instead of using column
extraction. CPT-11 is converted from the lactone to the carboxyl
form in the plasma over time following administration, which
results in a decrease of antitumor activity. Itoh et al
(15) reported that it is possible
to convert the carboxyl derivatives to lactones by adding 0.5 M
KH2PO3. However, in the present study, when
KH2PO3 was added to the plasma sample, the
peak of SN-38G overlapped with that of the endogenous substance. To
avoid the overlapping of these peaks, the composition of the mobile
phase was changed to 20%
CH3CN-KH2PO4 buffer from 30%
CH3CN-KH2PO4 buffer (14). However, in this case CPT-11 was not
eluted until 100 min or more. Thus, following the separation of
SN-38G from an endogenous substance by using 20%
CH3CN-KH2PO4 buffer, gradient
elution was performed to elute CPT-11 with 30%
CH3CN-KH2PO4 buffer. As a result,
the retention time of CPT-11 was shortened to approximately 20 min.
Similarly, a simple HPLC method with a short measurement time and a
satisfactory resolution was achieved (Fig. 1).
The contribution of plasma SN-38 concentrations in
CPT-11-related side-effects is well recognized (5,6). In
the present study, a significant correlation was obtained between
plasma SN-38 concentrations and neutropenia induction (Fig. 2). However, the correlation
coefficient of the regression line was not very high, indicating
that CPT-11-induced neutropenia cannot be predicted correctly from
one-point plasma SN-38 concentration alone. Thus, to consider the
clearance ability for SN-38 in each patient, the parameter of
plasma SN-38G/SN-38 ratio was introduced, since the plasma
SN-38G/SN-38 ratio is reported to correlate with the AUC ratio 2 h
following the completion of CPT-11 treatment (16). In this study, the plasma
SN-38G/SN-38 ratio was scattered by ≥6-fold among patients with the
wild-type UGT1A1 gene. This result indicates that the variation in
the pharmacokinetics of CPT-11 and CPT-11-induced neutropenia among
patients cannot be predicted by genetic polymorphisms of the UGT1A1
gene alone. Minami et al (10) reported that the median and
interquartile range (IQR) of the AUC of the SN-38G/SN-38 ratio were
5.55 and 4.13–7.26, respectively. In the present study, the median
value of the plasma SN-38G/SN-38 concentration ratio was 4.25 and
the IQR was 1.03–7.09. In the present study, the plasma
SN-38G/SN-38 ratios significantly decreased with an increase in the
trial numbers of CPT-11-based chemotherapy (Fig. 3), indicating that SN-38 may suppress
UGT activity on each occasion. In other words, the plasma clearance
of SN-38 decreases and the intensity of neutropenia induction
increases, with an increase in the trial number of CPT-11
treatments. Finally, the correlation between the plasma
SN-38G/SN-38 ratio and neutropenia induction in patients was
examined (Fig. 4). In this
analysis, 2 clearly separated regression lines with high
correlation coefficients were obtained from the two lines. A total
of 5 (numbers 7, 10, 11, 15 and 16) out of 17 patients belonged to
the upper regression line, indicating that a greater neutropenia
was induced in this patient group, even at the same plasma
SN-38G/SN-38 ratio. These patients received CPT-11 chemotherapy for
the first time (3 out of 5 patients) or the second time (1
patient), suggesting that, in certain cases, neutropenia induction
cannot be predicted by UGT activity alone. In the clearances of
CPT-11 and SN-38, multiple enzymes and transporters are involved,
including carboxyesterases, UGT1A1, CYP3A4, P-glycoprotein, MRP2,
BCRP and/or OATP1B1, although the contribution of OATP1B1 remains
under discussion (12,17–22).
To predict CPT-11-induced neutropenia in all patients systemically
(Fig. 4), the contribution of the
above enzymes and/or transporters involved in the clearance of
SN-38 should be further studied. In addition, the contribution of
5-FU included in the FOLFIRI regimen on neutropenia induction
should also be studied carefully. Since standardized dose-reduction
criteria are not yet available for CPT-11, even when the risk is
considered to be high, excessive dose reduction should be avoided,
since it may lead to a decrease of antitumor activity and loss of
therapeutic benefits to the patient. By contrast, even if the risk
to the patient is considered to be low, serious adverse reactions
may occur and specific treatment may be necessary. To determine the
most effective and safe doses of CPT-11 based on
pharmacokinetic-based dose adjustment, future studies should
evaluate the effect of the ‘complex hetero’, ‘*28 hetero’, ‘*6
hetero’ and ‘*6 homo’ patterns of UGT1A1 on plasma SN-38G/SN-38
ratios in a larger number of Japanese patients (10,18,19,22).
In conclusion, UGT activity involved in SN-38
metabolism is variable among patients, even with the wild-type
UGT1A1 gene, and each CPT-11 treatment suppresses UGT activity.
One-point determination of the plasma SN-38G/SN-38 ratio may
provide evidence for predicting CPT-11-induced neutropenia and
adjustment of optimal dose, although further studies are
required.
References
|
1
|
Shimoyama S: Pharmacogenetics of
irinotecan: An ethnicity- based prediction of irinotecan adverse
events. World J Gastrointest Surg. 2:14–21. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hasegawa Y, Ando Y and Shimokata K:
Screening for adverse reactions to irinotecan treatment using the
invader UGT1A1 molecular assay. Expert Rev Mol Diagn. 6:527–533.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ratain MJ and Innocenti F: Individualizing
dosing of irinotecan. Clin Cancer Res. 16:371–372. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Glimelius B, Garmo H, Berglund A, et al:
Prediction of irinotecan and 5-fluorouracil toxicity and response
in patients with advanced colorectal cancer. Pharmacogenomics J.
11:61–71. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chabot GG, Abigerges D, Catimel G, et al:
Population pharmacokinetics and pharmacodynamics of irinotecan
(CPT-11) and active metabolite SN-38 during phase I trials. Ann
Oncol. 6:141–151. 1995.PubMed/NCBI
|
|
6
|
Chabot GG: Clinical pharmacokinetics of
irinotecan. Clin Pharmacokinet. 33:245–259. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ando Y, Saka H, Ando M, et al:
Polymorphisms of UDP-glucuronosyltransferase gene and irinotecan
toxicity: a pharmacogenetic analysis. Cancer Res. 60:6921–6926.
2000.PubMed/NCBI
|
|
8
|
Innocenti F, Undevia SD, Iyer L, et al:
Genetic variants in the UDP-glucuronosyltransferase 1A1 gene
predict the risk of severe neutopenia of irinotecan. J Clin Oncol.
22:1382–1388. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Araki K, Fujita K, Ando Y, et al:
Pharmacogenetic impact of polymorphisms in the coding region of the
UGT1A1 gene on SN-38 glucuronidation in Japanese patients with
cancer. Cancer Sci. 97:1255–1259. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Minami H, Sai K, Saeki M, et al:
Irinotecan pharmacokinetics/pharmacodynamics and UGT1A genetic
polymorphisms in Japanese: roles of UGT1A1*6 and *28. Pharmacogenet
Genomics. 17:497–504. 2007.PubMed/NCBI
|
|
11
|
Sai K, Sawada J and Minami H: Irinotecan
pharmacogenetics in Japanese cancer patients: roles of UGT1A1*6 and
*28. Yakugaku Zasshi. 128:575–584. 2008.
|
|
12
|
Mathijssen RH, van Alphen RJ, Verweij J,
et al: Clinical pharmacokinetics and metabolism of irinotecan
(CPT-11). Clin Cancer Res. 7:2182–2194. 2001.PubMed/NCBI
|
|
13
|
Koo DH, Lee JL, Kim TW, et al: A phase II
study of cetuximab plus FOLFIRI for irinotecan and
oxaliplatin-refractory metastatic colorectal cancer. J Korean Med
Sci. 22:98–103. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kurita A and Kaneda N: High-performance
liquid chromatographic method for the simultaneous determination of
the camptothecin derivative irinotecan hydrochloride, CPT-11, and
its metabolites SN-38 and SN-38 glucuronide in rat plasma with a
fully automated on-line solid-phase extraction system, PROSPEKT. J
Chromatogr B. 724:335–344. 1999.
|
|
15
|
Itoh T, Takemoto I, Hata Y, et al:
Determination of the lactone forms and carboxylate forms of
irinotecan and its active metabolite, glucuronide by
high-performance liquid chromatography. Jpn J Ther Drug Monit.
17:383–389. 2000.
|
|
16
|
Takane H, Miyata M, Burioka N, et al:
Severe toxicities after irinotecan-based chemotherapy in a patient
with lung cancer: a homozygote for the SLCO1B1*15 allele. Ther Drug
Monit. 29:666–668. 2007.PubMed/NCBI
|
|
17
|
Nozawa T, Minami H, Sugiura S, Tsuji A and
Tamai I: Role of organic anion transporter OATP1B1 (OATP-C) in
hepatic uptake of irinotecan and its active metabolite,
7-ethyl-10-hydroxycamptothecin: in vitro evidence and effect of
single nucleotide polymorphisms. Drug Metab Dispos. 33:434–439.
2005. View Article : Google Scholar
|
|
18
|
Tanaka H, Saito K, Mino K, et al:
Assessment of total bilirubin or SN-38/SN-38G ratio as a predictor
of severe irinotecan toxicity. Jpn J Cancer Chemother.
36:1505–1509. 2009.PubMed/NCBI
|
|
19
|
Onoue M, Terada T, Kobayashi M, et al:
UGT1A1*6 polymorphism is most predictive of severe neutropenia
induced by irinotecan in Japanese cancer patients. Int J Clin
Oncol. 14:136–142. 2009.
|
|
20
|
Oostendorp RL, van de Steeg E, van der
Kruijssen CM, et al: Organic anion-transporting polypeptide 1B1
mediates transport of Gimatecan and BNP1350 and can be inhibited by
several classic ATP-binding cassette (ABC) B1 and/or ABCG2
inhibitors. Drug Metab Dispos. 37:917–923. 2009. View Article : Google Scholar
|
|
21
|
Marsh S and Hoskins JM: Irinotecan
pharmacogenomics. Pharmacogenomics. 11:1003–1010. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sai K, Saito Y, Maekawa K, et al: Additive
effects of drug transporter genetic polymorphisms on irinotecan
pharmacokinetics/pharmacodynamics in Japanese cancer patients.
Cancer Chemother Pharmacol. 66:95–105. 2010. View Article : Google Scholar
|