Introduction
Esophageal cancer (EC) is the eighth most common
type of cancer worldwide. In China, EC is the fourth most common
cause of mortality and is frequently located in the thorax, while
95% of EC is pathologically diagnosed as squamous cell carcinoma
(1). Surgery, as with other
thoracic malignancies (2,3), is the preferred therapeutic strategy
for EC patients. However, the majority of patients do not survive
due to recurrence, even in the presence of radical resection and
extended lymph node dissection. Numerous factors affect recurrence,
including age, gender, tumor location, local tumor stage, degree of
cell differentiation and the presence of lymph node metastases or
vascular involvement (4–6). The knowledge of patterns of recurrence
and its prevalence will be of great value in the clinic when
designing therapeutic strategies, as it would provide evidence for
determination of the extent of surgical resection and guide
effective post-operative adjuvant therapy.
The aim of the present study was to determine the
factors that influence the risk of recurrence in patients with
thoracic esophageal squamous cell carcinoma (TESCC) that have been
treated with curative surgery. The current study represents one of
the largest analyses of patterns of failure of patients undergoing
curative surgery for TESCC in Asia.
Patients and methods
Patients
From 2002–2008, patients with stage T1-4N0-3M0 EC
who had each received an esophagectomy were recruited for the
present study. Patients who had received pre-operative chemotherapy
and/or radiotherapy were excluded. In addition, only patients who
survived for >3 months following surgery were included in the
present study in favor of post-operative adjuvant therapy, as
certain patients undergoing surgery alone may not have survived the
perioperative period prior to adjuvant therapy. Thus, patients
treated with an esophagectomy with or without adjuvant therapy were
enrolled. The study was approved by the ethics committee of
Zhejiang Cancer Hospital, Hangzhou, China. Informed consent was
obtained from the patients.
Assessment of recurrence
Patterns of failure were assessed by means of
follow-up data. Following completion of treatment, the patients
were surveyed every 3–4 months by physical examination, a chest
computed tomography (CT) scan, and abdominal and cervical
ultrasonography until tumor recurrence was evident. A bone
scintigraphy was conducted if a patient presented with persistent
localized bone pain. If tumor recurrence was identified, the same
examinations were performed within 1 month and all recurrent tumors
identified were regarded as the first recurrent tumor. All patients
underwent routine post-surgical surveillance. The follow-up was
completed in August, 2010.
The mode of tumor recurrence was classified as
‘local’ if it was within the surgical field and ‘distant’ when it
was outside it. The former includes regional nodes and the surgical
margin. The latter includes hematogenic metastasis (liver, lung,
bone, skin, brain and adrenal), dissemination (pleural and
peritoneal) and distant nodes (cervical, abdominal para-aortic and
others).
Staging
Tumor size and extent was primarily coded according
to the operative medical record and pathological findings. The
number of lymph node metastases was determined based on
pathological findings. This information was used for tumor node
metastasis (TNM) classification according to the American Joint
Committee on Cancer (AJCC) classification system (7th edition)
(7).
Treatment
All patients were treated with radical resection.
The standard surgical approach consisted of a limited thoracotomy
on the right side and intrathoracic gastric tube reconstruction
(the Ivor Lewis procedure) for lesions at the middle/lower third of
the esophagus. Upper third lesions were treated by cervical
anastomosis (the McKeown procedure). The majority of patients
underwent two-field lymphadenectomy. The number of lymph nodes
harvested per case ranged from 6–96 (median 28). Pyloroplasty and
feeding jejunostomy were not routinely conducted. A nasogastric
tube was inserted for each patient until the anastomotic wound had
closed, as assessed by an esophagography on post-operative day
14.
In the present study, as the role of post-operative
adjuvant radiotherapy and/or chemotherapy in the treatment of ESCC
was controversial at the time of treatment for these patients,
post-operative adjuvant therapy was not mandatory. In each case,
the utilization of post-operative adjuvant radiotherapy and/or
chemotherapy was according to the physician’s preference and the
general physical condition of the patient. Cisplatin and
5-fluorouracil were the most frequently used agents in
post-operative chemotherapy, although several other
chemotherapeutic agents were also used. Post-operative adjuvant
radiotherapy, if administered, was initiated 4–5 weeks
post-surgery. A large T-shaped field encompassing the bilateral
supraclavicular fossa, mediastinum and tumor bed was used.
Radiation was initially administered through the anteroposterior
field to 36 Gy at 2 Gy per fraction, then through the parallel
opposing oblique fields to 14 Gy, in order to avoid the spinal
cord. Ten MV photons were used to deliver the radiation to the
mediastinum through the anteroposterior and oblique fields. In all
cases, the radiation dose was prescribed to the isocenter. The
bilateral supraclavicular fossas were treated with 9–12 MeV
electrons. In certain cases, targets were reduced on the basis of
the patient’s condition or the physician’s judgment.
Statistical analyses
Survival and recurrence curves were estimated using
the Kaplan-Meier method and compared using the log-rank test.
Multivariate analyses were performed by the Cox regression model.
The variables included two demographic variables (gender and age),
two well-known prognostic factors (depth of tumor invasion and
nodal involvement), extent of nodal dissection, length of tumor,
histological grade, vessel involvement and post-operative adjuvant
therapy. In total, 1002 patients underwent curative surgery (R0)
for TESCC. Fatalities due to causes other than TESCC were
considered censored observations at the time of death. The
χ2 test was used to compare the frequencies of different
ordinal data. Statistical analyses were performed using the
Statistical Package for the Social Sciences (SPSS) software,
version 13.0 (SPSS Inc., Chicago, IL, USA). All probability values
were two-sided and P<0.05 was considered to indicate a
statistically significant difference.
Results
General data
In total, 1002 patients were investigated. The
median follow-up period for the surviving patients was 51 months
(range, 3–93 months). Patient characteristics and
surgical/pathological details are provided in Table I. The sample consisted of 894 males
and 108 females (median age, 58 years; range, 36–86 years). The
majority of patients presented with stage T3 disease (67%,
669/1002). The majority of tumors (61%, 613/1002) were moderately
differentiated SCC with no vessel involvement (86%, 862/1002).
Among the 1002 patients, 518 patients presented with lymph node
metastasis. The average number of dissected lymph nodes was
28.2±14.4 (mean ± SD) nodes per case (median, 24; range, 2–96). The
mean number of metastatic nodes was 2.1±0.8 (median, 1; range,
0–36) in the overall series and 4.1±1.9 (median, 2; range, 1–36) in
the pathologically lymph node-positive patients. A total of 239
patients received post-operative adjuvant chemotherapy (>2
cycles); cisplatine and 5-fluorouracil were used most frequently
(63%). Post-operative adjuvant radiotherapy was administered to 255
patients. Males, and those aged <65 years, or with a tumor size
>5 cm or exhibiting a greater amount of lymph node metastasis,
were more likely to receive post-operative adjuvant chemotherapy
and/or radiotherapy.
 | Table IPatient characteristics (n=1002). |
Table I
Patient characteristics (n=1002).
| Characteristic | No. patients | % |
|---|
| Gender | | |
| Male | 894 | 89 |
| Female | 108 | 11 |
| Length of tumor
(cm) | | |
| <5 | 568 | 57 |
| ≥5 | 434 | 43 |
| Depth of
invasion | | |
| pT1 | 100 | 10 |
| pT2 | 170 | 17 |
| pT3 | 669 | 67 |
| pT4 | 63 | 6 |
| Lymph node
metastasis | | |
| pN0 | 426 | 43 |
| pN1 | 289 | 29 |
| pN2 | 192 | 19 |
| pN3 | 95 | 9 |
| Histologic
gradea | | |
| Well SCC | 200 | 20 |
| Mod. SCC | 613 | 61 |
| Poor SCC | 189 | 19 |
| Vessel
involvement | | |
| No | 862 | 86 |
| Yes | 140 | 14 |
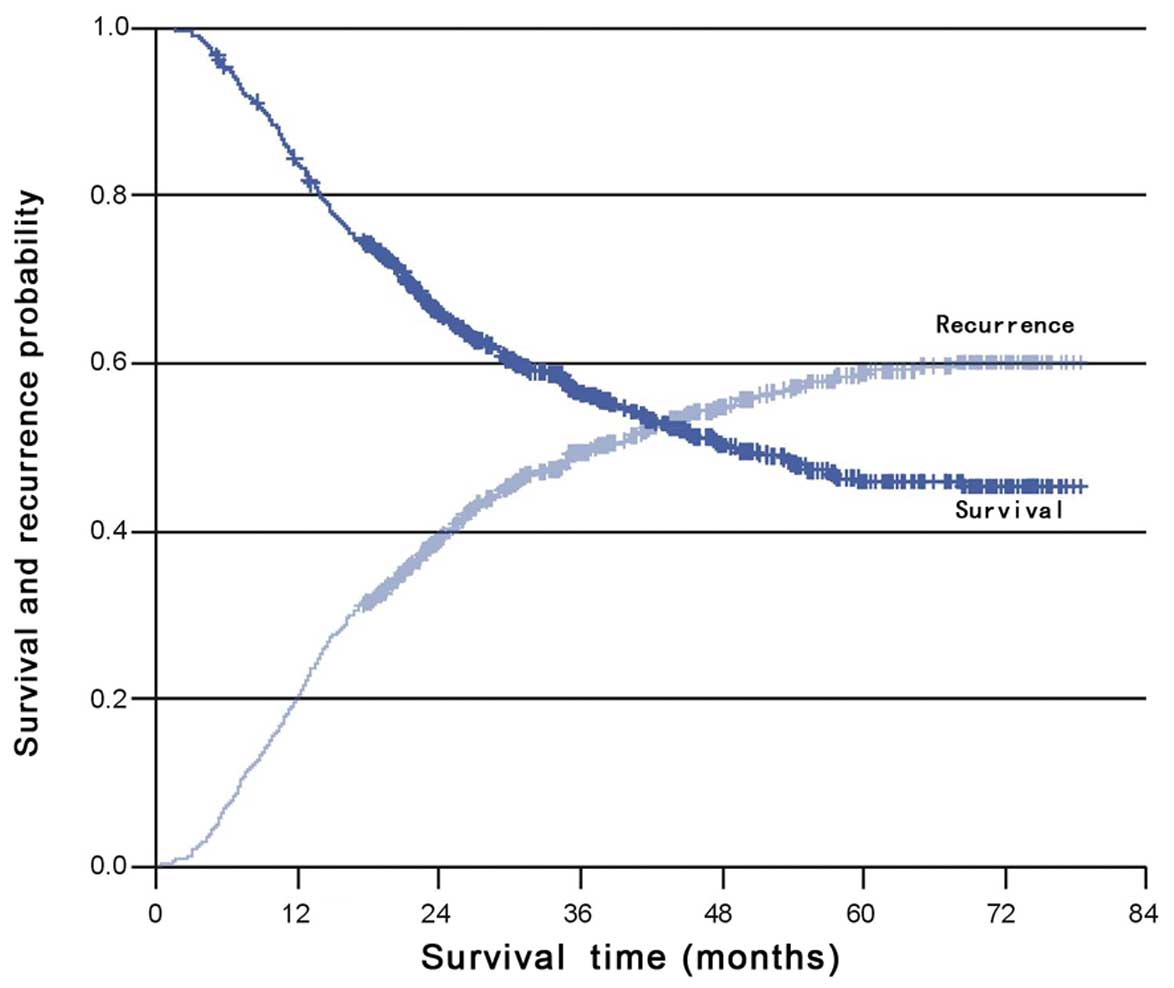
Recurrence
Almost 50% of recurrences were confirmed with a
biopsy, and the remaining recurrences were scored using imaging
studies. The data regarding survival and time to recurrence were
available for all patients. At the time of analysis, 545 of the
1002 patients had survived, 484 of which were disease-free. Tumor
recurrence was observed in 518 patients with a median follow-up
time of 51 months. Locoregional and distant recurrence were
observed in 49 and 44% of patients, respectively; while both
locoregional and distant recurrence were present in 27% of
patients. The median time to recurrence was 37.3 months (95% CI,
31.1–43.6) and the cumulative rates of recurrence at 2 and 5 years
were 39.0 and 59.2%, respectively (Fig.
1). More than 85% of recurrences occurred within 36 months.
Less than 10% of patients with >10 meta-static nodes were free
from relapse 3 years post-surgery.
Univariate and multivariate analyses
Variables associated with a higher rate of
recurrence in the univariate analysis were male gender
(P<0.001), length of tumor ≥5 cm (P<0.001), deeper depth of
invasion (P<.001), greater lymph node metastasis (P<0.001),
higher histological grade (P<0.001) and positive vessel
involvement (P<0.001) (Table
II). No correlation was observed between recurrence and the
extent of lymph node dissection.
 | Table IIRecurrence according to the clinical
characteristics of the 1002 patients. |
Table II
Recurrence according to the clinical
characteristics of the 1002 patients.
| | Recurrence
| | |
|---|
| Characteristic | No. of patients | Median timea (months) | 2-year rate (%) | 5-year rate (%) | χ2 | P-value |
|---|
| Gender | | | | | 17.794 | <0.001 |
| Male | 485/894 | 34.3 | 41.3 | 61.9 | | |
| Female | 33/108 | - | 21.5 | 39.2 | | |
| Age (years) | | | | | 0.008 | 0.927 |
| <65 | 391/757 | 40.3 | 39.4 | 59.5 | | |
| ≥65 | 127/245 | 33.3 | 38.3 | 58.3 | | |
| Extent of nodal
dissection | | | | | 0.607 | 0.738 |
| Three-field | 76/139 | 34.3 | 40.7 | 63.2 | | |
| Two-field | 426/829 | 37.3 | 39.2 | 58.5 | | |
| Other | 16/34 | 55.1 | 35.7 | 57.7 | | |
| Length of tumor
(cm) | | | | | 22.553 | <0.001 |
| <5 | 258/568 | 53.9 | 34.0 | 53.5 | | |
| ≥5 | 260/434 | 26.5 | 45.8 | 66.7 | | |
| Depth of
invasion | | | | | 50.279 | <0.001 |
| pT1 | 24/100 | - | 13.3 | 39.4 | | |
| pT2 | 68/170 | | 27.7 | 49.2 | | |
| pT3 | 386/669 | 28.6 | 44.2 | 63.9 | | |
| pT4 | 40/63 | 23.1 | 56.7 | 77.3 | | |
| Lymph node
metastasis | | | | | 203.403 | <0.001 |
| pN0 | 153/426 | - | 23.9 | 43.7 | | |
| pN1 | 179/289 | 16.9 | 61.5 | 78.9 | | |
| pN2 | 121/192 | 14.9 | 63.2 | 87.1 | | |
| pN3 | 65/95 | 11.9 | 77.0 | 96.3 | | |
| Histologic
gradeb | | | | | 18.124 | <0.001 |
| Well SCC | 91/200 | 54.3 | 34.8 | 56.5 | | |
| Mod. SCC | 306/613 | 41.5 | 37.5 | 56.8 | | |
| Poor SCC | 121/189 | 24.1 | 49.7 | 71.2 | | |
| Vessel
involvement | | | | | 24.988 | <0.001 |
| No | 421/862 | 43.4 | 36.3 | 56.4 | | |
| Yes | 97/140 | 20.3 | 55.6 | 75.7 | | |
The multivariate analyses for recurrence
demonstrated that gender (HR, 1.7; 95% CI, 1.2–2.5; P=0.002), depth
of invasion (HR, 1.4; 95% CI, 1.2–1.6; P<0.001) and lymph node
involvement (HR, 1.4; 95% CI, 1.3–1.5; P<0.001) were independent
predictive factors for recurrence. Male gender, deeper depth of
primary tumor invasion and greater lymph node metastasis predicted
an increase of recurrence (Table
III).
 | Table IIIMultivariate analysis of predictors
for tumor recurrence. |
Table III
Multivariate analysis of predictors
for tumor recurrence.
| Characteristic | Hazard ratio | 95% confidence
interval | P-valuea |
|---|
| Gender | 1.732 | 1.214–2.471 | 0.002 |
| Depth of
invasion | 1.353 | 1.181–1.550 | <0.001 |
| Lymph node
metastasis | 1.420 | 1.319–1.528 | <0.001 |
Rate of recurrence in relation to
post-operative adjuvant therapy
The rate of recurrence in relation to post-operative
adjuvant therapy (including post-operative radiotherapy and/or
post-operative chemotherapy) was also studied in detail.
Postoperative radiotherapy was administered to 255 patients, while
239 patients received post-operative chemotherapy. Post-operative
radiotherapy and/or chemotherapy did not significantly prolong
failure-free survival (FFS), particularly in patients with
early-stage disease (Table IV). In
patients with pT3-4 disease, post-operative adjuvant radiotherapy
and/or chemotherapy had a similar median recurrence-free time
compared with surgery alone. However, in patients with pT1-2
disease, the post-operative adjuvant radiotherapy and/or
chemotherapy arms had a shorter median recurrence-free time. For
patients with pN3, the median recurrence-free time was prolonged by
∼2 months following post-operative radiotherapy (P=0.072) and by ∼3
months following post-operative chemo-therapy (P= 0.094); however,
the difference was not significant.
 | Table IVStratified analysis according to T
and N stages for different post-operative adjuvent therapies. |
Table IV
Stratified analysis according to T
and N stages for different post-operative adjuvent therapies.
| Median
recurrence-free time (months)
| | Median
recurrence-free time (months)
| |
|---|
| Characteristic | No post-op. RT | Post-op. RT | P-value | No post-op. CT | Post-op CT | P-value |
|---|
| Depth of
invasion | | | | | | |
| pT1 | - | 52.7 | 0.121 | - | - | 0.049 |
| pT2 | - | 30.2 | <0.001 | - | 30.1 | <0.001 |
| pT3 | 27.3 | 24.5 | 0.278 | 28.7 | 23.1 | 0.355 |
| pT4 | 15.7 | 18.7 | 0.692 | 16.3 | 17.6 | 0.887 |
| LN metastais | | | | | | |
| pN0 | - | 27.4 | <0.001 | - | 24.1 | <0.001 |
| pN1 | 41.0 | 25.4 | 0.002 | 41.2 | 25.2 | 0.003 |
| pN2 | 16.2 | 15.9 | 0.360 | 17.1 | 13.4 | 0.449 |
| pN3 | 11.0 | 12.8 | 0.072 | 9.8 | 12.8 | 0.094 |
Discussion
Esophageal cancer is a highly lethal disease.
Despite best efforts, the 5-year survival rate following curative
surgery rarely exceeds 30%. Even patients with early-stage disease
are at a relatively high risk of disease recurrence. A large
proportion of patients do not survive due to disease recurrence
either at the surgical site or at extrathoracic sites. The overall
recurrence rate following curative surgery ranges from 34–79%,
while the locoregional, distant and both locoregional and distant
recurrence rates range from 21–68, 18–63 and 5–47%, respectively
(4,5,8–10). In
the present study, the rate of tumor recurrence was similar to the
findings mentioned previously in such a Chinese TESCC population
treated with surgery. The present study also demonstrated that
lymph node involvement and depth of tumor invasion are both
significant predictive indicators of tumor recurrence; greater
lymph node metastasis and deeper depth of invasion may suggest a
greater tumor burden or a more aggressive tumor biology, and so the
likelihood of locoregional or systemic recurrence may be speculated
to be higher.
Surgical resection with radical esophagectomy and
lymphadenectomy is currently the only well-established curative
treatment methodology for patients with resectable ESCC when the
patient is fit to undergo major surgery. Due to longitudinal
lymphatic drainage through the extensive submucosal plexus, lymph
node metastases may occur relatively early in all three body
compartments (the abdomen, chest and neck), regardless of the
location of the primary tumor. Matsubara et al(11) and Altorki et al(12) revealed a 30% incidence of
pre-operatively unsuspected cervical nodal metastases, irrespective
of the stage of the tumor, implying that cervical dissemination may
be an early event. Therefore, certain surgeons perform a formal
cervical lymphadenectomy as well as a two-field lymphadenectomy
(abdomen and chest). Notably, a three-field lymphadenectomy
achieves a higher rate of R0 resections, which may also lead to an
improved oncological outcome. Cancer recurrence originates from
micro-residues of cancer cells at the local or other sites. The
surgical outcome may be improved in the case of an extended
lymphadenectomy in patients with ESCC; however, there is no
conclusive evidence thus far. Shiozaki et al(13) demonstrated that the prognosis of
patients with recurrent nerve chain node metastasis was
significantly better in a three-field dissection group than in a
two-field dissection group. In our institution, the Ivor-Lewis
approach with a two-field lymphadenectomy (extended mediastinal
lymphadenectomy and lymph node dissection of the upper abdominal
compartment) was the most frequently performed procedure (82.7%).
Our results indicated that tumor recurrence following extensive
three-field dissection was similar to that following the less
extensive two-field dissection (P=0.738). However, in our study the
number of patients who received a three-field lymphadenectomy was
only 139; the limited number of events made it difficult to
evaluate recurrence differences. Therefore, a prospective
observational study is required. Extended esophagectomy with
three-field lymphadenectomy in treating operable TESCC has been
demonstrated with a large number of patients in China (14). We may observe a recurrence of the
three-field lymphadenectomy in this group of patients.
Another concern with this type of study is the
multidisciplinary treatment approaches. An accurate assessment of
factors influencing failure post-surgery may guide postoperative
adjuvant therapy. Given the recognized risk of local recurrence,
even in patients with early-stage disease, we advise a re-analysis
of post-operative radiotherapy and chemotherapy. The role of
adjuvant therapy of ESCC has been addressed in a number of clinical
trials; however, until presently, whether post-operative
radiotherapy and/or chemotherapy affect the therapeutic outcomes
has remained controversial (15–20).
Zhang et al(21) conducted a
meta-analysis comprising a total of 1000 patients with esophageal
cancer (ESCC and adenocarcinoma) in 2008. The results indicated
that adjuvant chemotherapy did not significantly improve survival.
Only the patients with pathologically positive lymph nodes
demonstrated a positive trend towards improved survival, but this
was not significant (OR, 0.76; 95% CI, 0.538–1.083). Lu et
al conducted a meta-analysis to study the clinical value of
prophylactic radiotherapy for esophageal carcinoma after curative
resection. The randomized trials did not demonstrate an influence
of prophylactic radiotherapy on ESCC following curative resection
(5-year survival OR, 1.26; 95% CI, 0.687–2.290). However, patients
with lymph node involvement had a statistically significant
survival benefit 5 years after prophylactic radiotherapy, compared
with surgery alone (5-year survival OR, 2.20; 95% CI, 1.331–3.632)
(22). In the present study,
post-operative adjuvant radiotherapy and/or chemotherapy did not
prolong FFS significantly in the whole study population. However,
for patients with pT3-4 or pN3, the median recurrence-free time
marginally increased following post-operative radiotherapy and/or
chemotherapy (Table IV). Adjuvant
radiotherapy and chemotherapy are theoretically capable of treating
microscopic disease that remains following an incomplete surgical
procedure and of increasing local control. However, caution should
be taken in practice, as there is a lack of evidence in support of
post-operative adjuvant therapy. Recently, Macdonald et al
indicated that post-operative chemoradiation therapy significantly
improved overall survival and relapse-free survival for patients at
a high risk of recurrence of adenocarcinoma of the stomach or GE
junction (23); however, the
combination with the best outcome for ESCC is yet to be defined.
The high frequency of recurrence and the short survival time
following relapse are indicative of the need for aggressive initial
treatment.
However, although almost 50% of recurrences were
confirmed with a biopsy in this study, the remainder were scored
using imaging studies that may overestimate/underestimate the
extent of disease. In addition, only patients with squamous cell
carcinoma were recruited in this study. In contrast, the incidence
of adenocarcinoma is dramatically increasing in Western countries.
Therefore, the results of this study may not be applicable to North
American and European patients. Moreover, the use of neoadjuvant
chemoradiotherapy has been an increasingly used treatment approach.
The results of a multicenter phase III randomized trial (CROSS
study) demonstrated that neoadjuvant chemoradiotherapy improved OS
compared with surgery alone in patients with resectable (T2-3 N0-1
M0) esophageal or EGJ cancers. The median survival time was 49
months in the neoadjuvant chemoradiotherapy arm compared to 26
months in the surgery alone arm (24). Further development of the
multidisciplinary management for patients with locally advanced
esophageal cancer post-surgery using an adjuvant treatment as
opposed to neoadjuvant chemoradiotherapy is warranted. The approach
is currently being explored in China by investigators of the
ZTOG1201 trial, a multicenter phase II trial of neoadjuvant and
adjuvant chemoradiotherapy in locally advanced EC
(NCT01463501).
In conclusion, this study demonstrated that data
concerning the depth of primary tumor invasion and the number of
lymph nodes involved may aid doctors in evaluating the recurrence
risk of patients with TESCC that have been treated with curative
surgery in a Chinese population. Further studies are required to
clarify the correlation between recurrence and the different
multidisciplinary treatment approaches.
Acknowledgements
This study was presented in part as a
poster during the annual meeting of the 2011 European
Multidisciplinary Cancer Congress (Abstract 6.558).
References
|
1
|
Guo M, Zhao YD, Yang HJ and Yan XF:
Analysis of clinicopathological characteristics for 5406 cases of
esophageal neoplasm. Chin J Cancer Prev Treat. 15:54–56. 2008.
|
|
2
|
Debevec L, Jeric T, Kovac V, et al: Is
there any progress in routine management of lung cancer patients? A
comparative analysis of an institution in 1996 and 2006. Radiol
Oncol. 43:47–53. 2009. View Article : Google Scholar
|
|
3
|
Kovac V, Zwitter M and Zagar T:
Population-based survey of malignant pleural mesothelioma in
Slovenia: improved survival after introduction of chemotherapy.
Radiol Oncol. 46:136–144. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Law SY, Fok M and Wong J: Pattern of
recurrence after oesophageal resection for cancer: clinical
implications. Br J Surg. 83:107–111. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bhansali MS, Fujita H, Kakegawa T, et al:
Pattern of recurrence after extended radical esophagectomy with
three-field lymph node dissection for squamous cell carcinoma in
the thoracic esophagus. World J Surg. 21:275–281. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Osugi H, Takemura M, Takada N, et al:
Prognostic factors after esophagectomy and extended lymphadenectomy
for squamous esophageal cancer. Br J Surg. 89:909–913. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rice TW, Rusch VW, Ishwaran H, et al;
Worldwide Esophageal Cancer Collaboration: Cancer of the esophagus
and esophagogastric junction: data-driven staging for the seventh
edition of the American Joint Committee on Cancer/International
Union Against Cancer Staging Manuals. Cancer. 116:3763–3773. 2010.
View Article : Google Scholar
|
|
8
|
Dresner SM and Griffin SM: Pattern of
recurrence following radical esophagectomy with two-field
lymphadenectomy. Br J Surg. 87:1426–1433. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Isono K, Sato H and Nakayama K: Results of
a nationwide study on the three-field lymph node dissection of
esophageal cancer. Oncology. 48:411–420. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dixit S, Tilston M and Peter WM: Risk
stratification for recurrence in patients with esophageal and
junctional carcinoma treated with neoadjuvant chemotherapy and
surgery. Med Oncol. 27:242–248. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matsubara T, Ueda M, Nagao N, et al:
Cervicothoracic approach for total mesesophageal dissection in
cancer of the thoracic esophagus. J Am Coll Surg. 187:238–245.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Altorki NK and Skinner DB: Occult cervical
nodal metastasis in esophageal cancer: preliminary results of
three-field lymphadenectomy. J Thorac Cardiovasc Surg. 113:540–544.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shiozaki H, Yano M, Tsujinaka T, et al:
Lymph node metastasis along the recurrent nerve chain is indication
for cervical lymph node dissection in thoracic esophageal cancer.
Dis Esophagus. 14:191–196. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen J, Liu S, Pan J, et al: The pattern
and prevalence of lymphatic spread in thoracic oesophageal squamous
cell carcinoma. Eur J Cardiothorac Surg. 36:480–486. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ando N, Iizuka T, Ide H, et al; Japan
Clinical Oncology Group: Surgery plus chemotherapy compared with
surgery alone for localized squamous cell carcinoma of the thoracic
esophagus: a Japan Clinical Oncology Group Study - JCOG9204. J Clin
Oncol. 21:4592–4596. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pouliquen X, Levard H, Hay JM, et al:
5-Fluorouracil and cisplatin therapy after palliative surgical
resection of squamous cell carcinoma of the esophagus. A
multicenter randomized trial French Associations for Surgical
Research. Ann Surg. 223:127–133. 1996. View Article : Google Scholar
|
|
17
|
Lee J, Lee KE, Im YH, et al: Adjuvant
chemotherapy with 5-fluorouracil and cisplatin in lymph
node-positive thoracic esophageal squamous cell carcinoma. Ann
Thorac Surg. 80:1170–1175. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Heroor A, Fujita H, Sueyoshi S, et al:
Adjuvant chemotherapy after radical resection of squamous cell
carcinoma in the thoracic esophagus: who benefits? A retrospective
study. Dig Surg. 20:229–235; discussion. 236–237. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shiozaki A, Yamagishi H, Itoi H, et al:
Long-term administration of low-dose cisplatin plus 5-fluorouracil
prolongs the postoperative survival of patients with esophageal
cancer. Oncol Rep. 13:667–672. 2005.PubMed/NCBI
|
|
20
|
Bystricky B, Okines AF and Cunningham D:
Optimal therapeutic strategies for resectable oesophageal or
oesophagogastric junction cancer. Drugs. 71:541–555. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang J, Chen HQ, Zhang YW, et al:
Adjuvant chemotherapy in oesophageal cancer: a meta-analysis and
experience from the Shanghai Cancer Hospital. J Int Med Res.
36:875–882. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu JC, Qian PD, Zha WW, et al: The
meta-analysis of randomized controlled trial of prophylactic
radiotherapy for esophageal carcinoma after curative resection. J
Evid Based Med. 5:166–171. 2005.
|
|
23
|
Macdonald JS, Smalley SR, Benedetti J, et
al: Chemoradiotherapy after surgery compared with surgery alone for
adenocarcinoma of the stomach or gastroesophageal junction. N Engl
J Med. 345:725–730. 2001. View Article : Google Scholar
|
|
24
|
Gaast AV, van Hagen P, Hulshof M, et al:
Esophagogastric junction cancer: Results from a multicenter
randomized phase III study. J Clin Oncol (Meeting Abstracts).
28:40042010.
|