Introduction
The development of eosinophilia within solid tumors
is a rare manifestation, accounting for ∼1% of all cancer patients
(1). Since a number of medical
conditions are associated with eosinophilia (2), paraneoplastic eosinophilia is
diagnosed by exclusion. Depending on the etiology, the consequences
of paraneoplastic eosinophilia may range in severity from
asymptomatic to life-threatening. Eosinophilia is usually treated
successfully with corticosteroids. Paraneoplastic eosinophilia has
been reported in a few cases of lung cancer, including lung
squamous cell carcinoma (3,4), non-small-cell lung carcinoma (5) and lung adenocarcinoma (6). In the latter case, the patient
succumbed rapidly following a tumor relapse associated with rapidly
evolving eosinophilia. These studies emphasize the importance of
identifying the early signs of aggressive paraneoplastic
eosinophilia to initiate corticosteroid treatment prior to
end-organ failure.
The transition from asymptomatic to life-threatening
paraneoplastic eosinophilia is rapid and difficult to diagnose upon
summary examination of the patient, particularly in lung cancer
patients who are expected to suffer from respiratory complications.
While paraneoplastic eosinophilia is often linked with the
overexpression of interleukin (IL)-5 in tumor cells, this type of
diagnosis is impractical for such a rapidly evolving and
life-threatening complication (4).
In this report, we present a case of paraneoplastic
eosinophilia in a patient diagnosed with lung adenocarcinoma. The
condition of the 82-year-old male degenerated suddenly, as
circulating eosinophil counts increased 4-fold over a few days. The
patient experienced cognitive disturbance and shortness of breath,
which may represent new diagnostic tools for early corticosteroid
treatment to avoid organ damage. The study was approved by the
Ethics Committee of the Tri-Service General Hospital, National
Defense Medical Center, Taiwan, R.O.C. Informed consent was
obtained from the patient’s family.
Case report
An 82-year-old male was admitted to our hospital on
October 5, 2011, with a 2-week history of right-sided flank pain
and abdominal fullness. An abdominal sonogram revealed a huge liver
mass and the patient was then admitted to our gastrointestinal (GI)
section. The patient had a history of well-controlled chronic
obstructive pulmonary disease (COPD), hypertension and benign
prostate hyperplasia. The patient had herniorrhaphy 1 year earlier
and had received amlodipine, tamsulosin and PRN
ipratropium/albuterol turbu-haler. The patient had no known
allergies and had smoked half a pack of cigarettes per day for 40
years, after which the patient quit for 20 years.
Laboratory data revealed the following: white blood
cells, 52,310 cells/μl with 46.3% neutrophils and 45.4%
eosinophils; 13.3 g/dl hemoglobin and 242,000 cells/μl
platelets; renal functional insufficiency with 36 mg/dl blood urea
nitrogen (BUN) and 1.4 mg/dl creatinine; a routine stool test
revealed no evidence of parasite infection; immunoglobulin E level
was 99.1 IU/ml and the levels of tumor markers in the blood,
including carcinoembryonic antigen (CEA; 6.47 ng/ml) and cancer
antigen (CA) 19-9 (49.81 U/ml), were elevated.
On admission, crackles were heard in the right lower
lung field. Abdominal palpation revealed mild epigastric tenderness
without muscle guarding. A chest radiograph revealed an ill-defined
mass lesion ∼5 cm in size in the right middle lung zone (Fig. 1A). Computed tomography of the chest
revealed a right middle lung lobe mass and multiple variable-sized
nodules in the two lung fields. Computed tomography of the abdomen
demonstrated several peripherally enhancing lesions in the lobes of
the liver. Magnetic resonance imaging of the brain revealed no
evidence of metastasis. Whole-body bone scan revealed multiple bone
metastases. Biopsies of the liver and lung mass were performed and
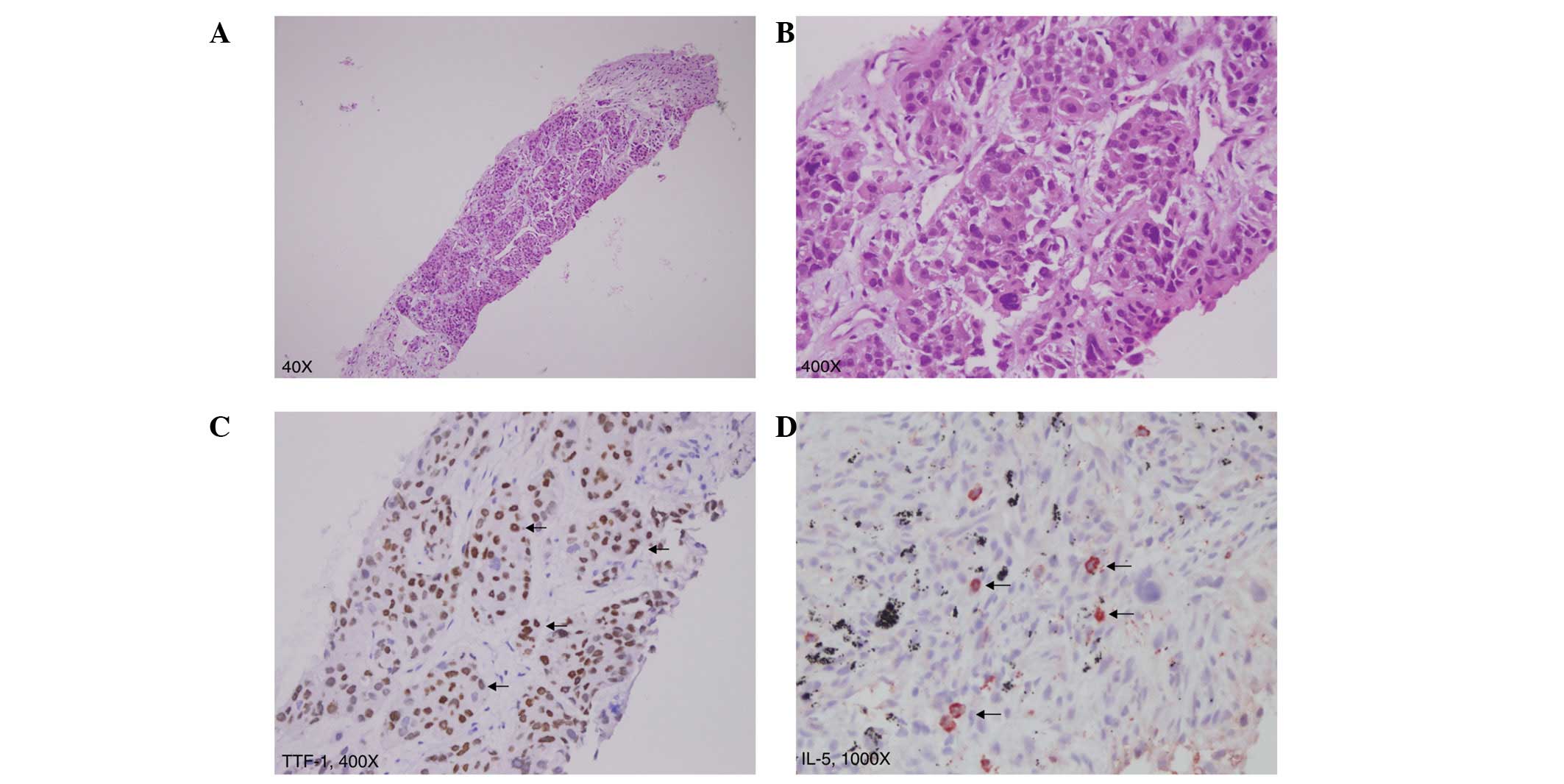
pathology revealed poorly differentiated adenocarcinoma of the
lung, positive for thyroid transcription factor-1 (TTF-1; Fig. 2A–C). Definitive oral-targeted
therapy was advised if epidermal growth factor receptor (EGFR)
abnormality was present due to the patient’s end stage and old
age.
The patient was discharged following completion of
the staging work-up and waited for the result of the EGFR analysis.
One week later, the patient was readmitted for cognitive
disturbance and shortness of breath. On arrival, the patient was
noted to be agitated and disoriented and had disorganized speech.
Physical examination revealed diffuse wheezing over all lung
fields. Pitting edema was noted on the legs. The peripheral white
blood cell count had increased 4-fold over a week (168,800
cells/ml), with a proportional increase in eosinophil counts
(55.2%). Elevated potassium (5.6 mmol/l), uric acid (13.8 mg/dl),
creatinine (2.7 mg/dl) and lactate dehydrogenase (LDH; 420 U/l)
levels were also noted. Chest radiography demonstrated diffuse
infiltration and ground glass opacities over the two lung fields in
addition to the previous finding (Fig.
1B). Brain computed tomography presented no special findings.
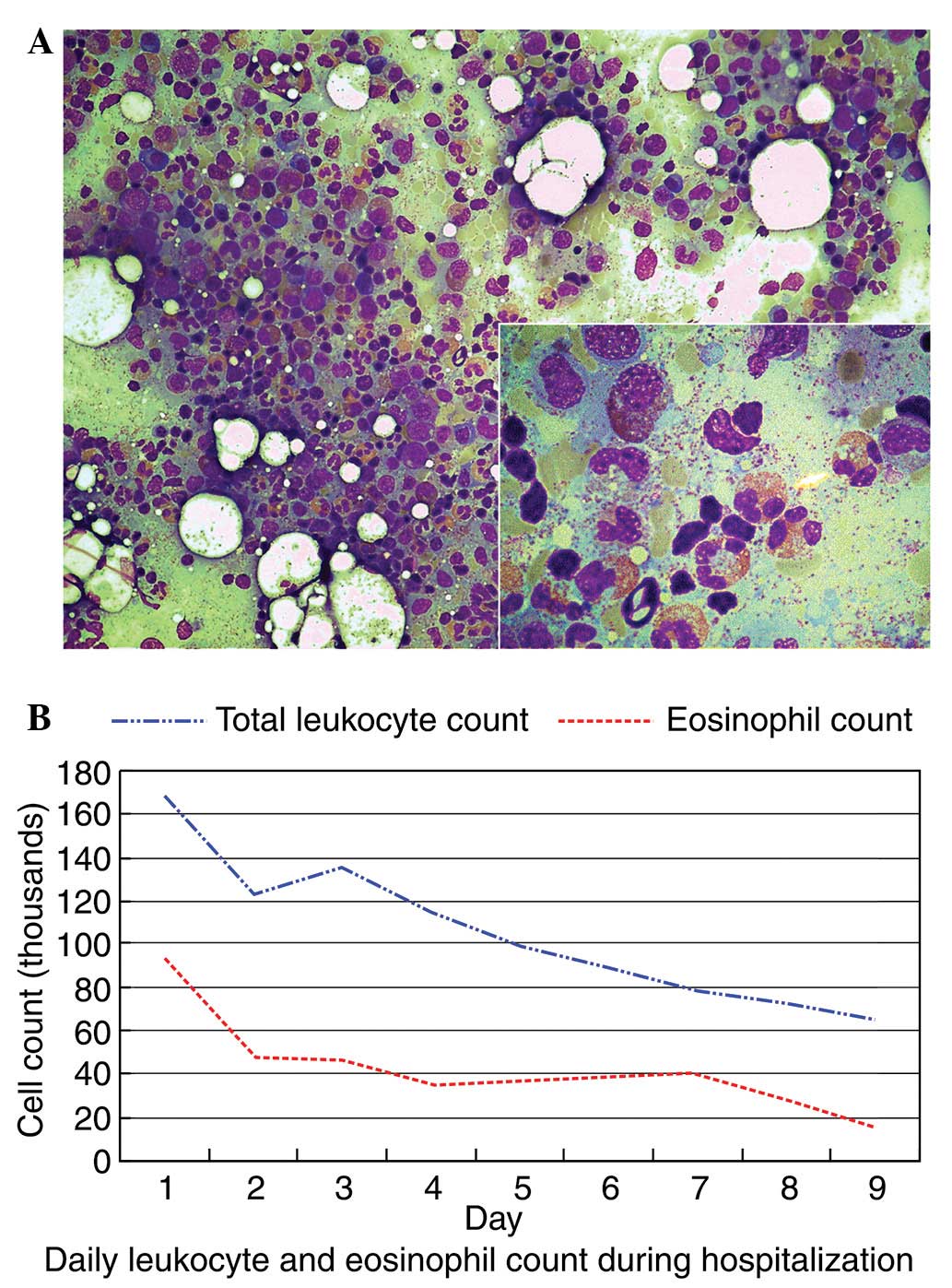
Bone marrow biopsy was performed, which revealed reactive bone
marrow hypercellularity with a markedly high eosinophil count
(Fig. 3A). The average percentage
of eosinophils was 39%, compared to 1–5% in normal bone marrow.
Chromosomal analysis demonstrated normal karyotype.
Immunohistochemical analysis using the monoclonal mouse anti-human
IL-5 antibody (R&D Systems, Minneapolis, MN, USA) demonstrated
that IL-5 was specifically expressed in tumor cells (Fig. 2D). Lung cancer-associated
paraneoplastic eosinophilia and acute renal dysfunction were
diagnosed.
The patient was treated with hydration and
allopurinol to control extreme hyperuricemia. Due to the old age
and weak condition of the patient, anticancer treatment was not
provided. Since eosinophilia-related organ damage was suspected,
hydroxyurea and corticosteroid were administered to reduce the
number of eosinophils. The white blood cell count was reduced
significantly after 9 days of treatment to 65,500 cells/μl
with 23% eosinophils (Fig. 3B). The
patient’s conscious state, kidney function and blood cell count
improved following treatment. However, the dyspnea persisted and
the patient acquired pneumonia 4 days after the second admission.
The family refused further treatment and intervention due to the
poor prognosis. The patient succumbed to healthcare-acquired
pneumonia with severe sepsis due to Pseudomonas aeruginosa,
10 days after admission.
Discussion
The present report describes a case of lung
adenocarcinoma complicated by severe and aggressive eosinophilia. A
number of medical conditions, including allergic disorders,
parasitic and fungal infections, vasculitis and drug reactions, as
well as hematologic and non-hematologic malignancies are associated
with eosinophilia (2). The fact
that our patient did not present any of these conditions supports
the paraneoplastic nature of the eosinophilia. The pathogenesis of
paraneoplastic eosinophilia is unclear. Numerous mechanisms have
been postulated and bone marrow stimulation by cytokines secreted
by tumor tissues, including granulocyte macrophage-colony
stimulating factor (GM-CSF), G-CSF, IL-3 and IL-5, is most commonly
reported (4,7–11). In
our case, the immunoreactivity of tumor cells to IL-5 is consistent
with that reported in these previous studies.
Patients with paraneoplastic eosinophilia are
typically asymptomatic. However, in a number of cases, a markedly
elevated eosinophil count may be associated with shortness of
breath and wheezing. In the present case, the patient exhibited
shortness of breath and cognitive disturbance in the form of
agitation, disorientation and disorganized speech. Normally,
anticancer therapies also resolve the eosinophilia. Matsumoto et
al reported a return to normal hematologic status with
chemotherapy (12) and Pandit et
al demonstrated that leukocytosis and eosinophilia normalize
following tumor removal (4).
Primary eosinophilic syndromes are managed
successfully with corticosteroid therapy (13–15).
However, a number of patients are non-responsive to
corticosteroids, but respond well to hydroxyurea (16). Hydroxyurea is also reported to be an
effective first-line agent in hypereosinophilic syndrome (15). A combination of hydroxyurea and
corticosteroid increases the response rate (15). However, there is no standard
treatment for paraneoplastic eosinophilia. To prevent potential
harmful effects from chronic exposure of organs to excessive
eosinophils, we used a combination of corticosteroid and
hydroxyurea, which led to a marked improvement in blood cell
counts. The significant effect of corticosteroid and hydroxyurea in
reducing the eosinophil count may play a role in improving and
stabilizing paraneoplastic eosinophilia and act as a bridge to more
anticancer therapies.
The clinical significance of eosinophilia in cancer
patients is undefined. Iwasaki et al report that
tumor-associated eosinophilia is associated with a good prognosis
(17). However, more studies
support the view that paraneoplastic eosinophilia reflects a more
extensive disease and poor prognosis (7,18–21).
Anagnostopoulos et al suggested that the return of
eosinophilia may be an indicator of tumor recurrence (10). In our case, the extremely high
eosinophil count and its rapid rise suggested aggressive disease
progression and poor prognosis. The addition of combination
therapies (corticosteroid and hydroxyurea) to anticancer drugs in
paraneoplastic eosinophilia may be beneficial to patient
prognosis.
In conclusion, this is the first report of cognitive
impairment in combination with respiratory insufficiency as
symptoms of rapidly worsening paraneoplastic eosinophilia
(eosinophil surge) in cancer patients. This condition may be used
for an early diagnosis to initiate corticosteroid treatments and
avoid organ damage. This case also suggests that lung cancer
patients who present abnormally high counts of eosinophils, should
receive a combination of corticosteroids, hydroxyurea and
anticancer drugs to prevent the development of aggressive and
life-threatening eosinophilia, even if they are asymptomatic
initially. This is likely to also enhance the benefits of the
anticancer treatment.
References
|
1.
|
Jameson JL and Johnson BE: Paraneoplastic
syndromes: endocrinologic/hematologic. Harrison’s Principles of
Internal Medicine. Fauci AS, Braunwald E, Kasper DL, Hauser SL,
Longo DL, Jameson JL and Loscalzo J: 17th edition. McGraw Hill
Medical; New York, NY: pp. 617–622. 2008
|
|
2.
|
Brito-Babapulle F: The eosinophilias,
including the idiopathic hypereosinophilic syndrome. Br J Haematol.
121:203–223. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Zhu YL, Tong ZH, Jin ML and Wang C: Lung
cancer with marked blood eosinophilia: case report and literature
review. Zhonghua Jie He He Hu Xi Za Zhi. 32:369–372. 2009.(In
Chinese).
|
|
4.
|
Pandit R, Scholnik A, Wulfekuhler L and
Dimitrov N: Non-small-cell lung cancer associated with excessive
eosinophilia and secretion of interleukin-5 as a paraneoplastic
syndrome. Am J Hematol. 82:234–237. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Verstraeten AS, De Weert A, van Den Eynden
G, Van Marck E, Snoeckx A and Jorens PG: Excessive eosinophilia as
paraneoplastic syndrome in a patient with non-small-cell lung
carcinoma: a case report and review of the literature. Acta Clin
Belg. 66:293–297. 2011.PubMed/NCBI
|
|
6.
|
Andriamanantena D, Boye T, Gervaise A,
Vieu C, Splingard B, Dot JM, Veran Y, et al: An unusual
paraneoplastic manifestation in lung cancer: eosinophilic
erythroderma. Rev Pneumol Clin. 65:32–35. 2009.PubMed/NCBI
|
|
7.
|
Watanabe M, Ono K, Ozeki Y, Tanaka S, Aida
S and Okuno Y: Production of granulocyte-macrophage
colony-stimulating factor in a patient with metastatic chest wall
large cell carcinoma. Jpn J Clin Oncol. 28:559–562. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Sawyers CL, Golde DW, Quan S and Nimer SD:
Production of granulocyte-macrophage colony-stimulating factor in
two patients with lung cancer, leukocytosis, and eosinophilia.
Cancer. 69:1342–1346. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Nakada T, Sato H, Inoue F, Mizorogi F,
Nagayama K and Tanaka T: The production of colony-stimulating
factors by thyroid carcinoma is associated with marked neutrophilia
and eosinophilia. Intern Med. 35:815–820. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Anagnostopoulos GK, Sakorafas GH,
Kostopoulos P, Margantinis G, Tsiakos S, Terpos E, Pavlakis G, et
al: Disseminated colon cancer with severe peripheral blood
eosinophilia and elevated serum levels of interleukine-2,
interleukine-3, interleukine-5, and GM-CSF. J Surg Oncol.
89:273–275. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Kato H, Kohata K, Yamamoto J, Ichikawa S,
Watanabe M, Ishizawa K, Ichinohasama R, et al: Extreme eosinophilia
caused by interleukin-5-producing disseminated colon cancer. Int J
Hematol. 91:328–330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Matsumoto S, Tamai T, Yanagisawa K,
Kawamura S and Fujita S: Lung cancer with eosinophilia in the
peripheral blood and the pleural fluid. Intern Med. 31:525–529.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Ogbogu PU, Bochner BS, Butterfield JH,
Gleich GJ, Huss-Marp J, Kahn JE, Leiferman KM, et al:
Hypereosinophilic syndrome: a multicenter, retrospective analysis
of clinical characteristics and response to therapy. J Allergy Clin
Immunol. 124:1319–1325. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Tefferi A, Patnaik MM and Pardanani A:
Eosinophilia: secondary, clonal and idiopathic. Br J Haematol.
133:468–492. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Gotlib J: World Health
Organization-defined eosinophilic disorders: 2011 update on
diagnosis, risk stratification, and management. Am J Hematol.
86:677–688. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Srinivasan A, Lavanya R and Sankar J:
Steroid-unresponsive hypereosinophilic syndrome. Ann Trop Paediatr.
31:273–277. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Iwasaki K, Torisu M and Fujimura T:
Malignant tumor and eosinophils: Prognostic significance in gastric
cancer. Cancer. 58:1321–1327. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Teoh SC, Siow WY and Tan HT: Severe
eosinophilia in disseminated gastric carcinoma. Singapore Med J.
41:232–234. 2000.PubMed/NCBI
|
|
19.
|
Chang WC, Liaw CC, Wang PN, Tsai YH and
Hsueh S: Tumor-associated hypereosinophilia: report of four cases.
Changgeng Yi Xue Za Zhi. 19:66–70. 1996.PubMed/NCBI
|
|
20.
|
Reddy SS, Hyland RH, Alison RE, Sturgeon
JF and Hutcheon MA: Tumor-associated peripheral eosinophilia: two
unusual cases. J Clin Oncol. 2:1165–1169. 1984.PubMed/NCBI
|
|
21.
|
El-Osta H, El-Haddad P and Nabbout N: Lung
carcinoma associated with excessive eosinophilia. J Clin Oncol.
26:3456–3457. 2008. View Article : Google Scholar : PubMed/NCBI
|