Introduction
Health-care workers (HCWs) may be exposed to
hepatitis C virus (HCV), one of many blood-borne pathogens, in
their work environment (1).
Previous studies (2–4) investigated the risk factors involved
in HCV. HCV transmission has been shown to occur after 3–10% of
percutaneous exposure to HCV (2–4).
Percutaneous HCV transmission occurs following needle-stick injures
or cuts from other sharp instruments. Transmission may also occur
following exposure of the eyes, nose, mouth, or broken skin to HCV
(5–7).
In a previous study, we showed a statistically
significant decrease in the number of occupational blood exposure
accidents documented in 10 years during a health surveillance
program for 403 HCWs from a single institution (8). The data suggested that the guidelines
including the standard precautions to reduce the risk of
blood-borne pathogen transmission have been shown to have a strong
efficacy among HCWs. Concomitantly, an HCV-positive HCW developed a
liver mucosa-associated lymphoma tissue (MALT) lymphoma.
Furthermore, molecular characterization of the tumour indicated
that lymphoma was associated with the HCV chronic infection
(8).
In the last two decades, studies have shown that HCV
infection may contribute to the development of non-Hodgkin lymphoma
(NHL) in addition to hepatocellular carcinoma, as previously
demonstrated (9,10). In a systematic review of 66 studies
with a focus on HCV infection and NHL development, Negri et
al(10), reported that the
majority of these studies were conducted in Italy, and their
findings indicated a high prevalence of HCV infection among NHL
cases, ranging from 8.9 to 37.1%. Accumulating evidence supports a
model in which chronic stimulation of B-cells by antigens
associated with HCV infection causes non-malignant B-cell expansion
that may evolve into B-cell NHL (11).
To clarify this issue, a tailored health
surveillance program was applied to 3,138 HCWs from four Medical
Institutions. All HCWs were screened for anti-HCV antibodies and
for HCV-related extrahepatic manifestations.
Materials and methods
Subjects
Subjects included 3,138 employees of four Italian
Public Hospitals, i.e., Policlinico Universitario,
Ferrarotto-Alessi, Santo Bambino and Ospeadali Riuniti di Torrette,
Ancona. A previous series of 403 HCWs by Marconi et
al(8) was not included in the
present study. All the subjects were HCWs at high risk for exposure
to blood-borne pathogens. Lifestyle risk factors for HCV infection
were assessed by ascertaining whether subjects had lived with an
HCV-positive partner, had been given a blood transfusion, had a
history of casual sexual intercourse, had any tattoos, or had a
history of intravenous drug abuse. Peripheral blood obtained from
the subjects was screened for anti-HCV antibodies by an
enzyme-linked immunosorbent assay (Ortho Diagnostic Systems,
Raritan, NJ, USA), as previously described (12). HCWs were also examined for
HCV-related malignancies. The study was approved by the University
of Catania Ethics Committee. Written informed consent was obtained
prior to enrolment.
Analysis of B-cell clones
To determine B-cell clonality in the NHL sample from
the HCV-positive HCW, complementary determining region-3 (CDR3) of
the Ig heavy chain gene was amplified by PCR. The upstream primer
was complementary to framework region-3 (FR3) of VH and the
downstream primer was complementary to JH. Ig heavy chain gene DNA
was amplified by PCR with upstream primers complementary to
framework region-1 (FR1) of each VH gene segment family and a
downstream primer complementary to CDR3 (12). PCR products were purified by gel
electrophoresis, then sequenced. The most similar VH and DH
germline gene segments were identified by sequence comparison to
the International Immunogenetics Database with DNAplot software
(http://imgt.cines.fr).
Analysis of t(14;18)-(IgH;Bcl-2)
translocation
DNA was isolated from tumour samples by standard
phenol-chloroform extraction. t(14;18)-(IgH;Bcl-2) translocation,
at the major break point region (MBR) and minor cluster region
(mcr), was assessed by the polymerase chain reaction (PCR), as
previously reported (13).
AccuPrime™ SuperMix (Invitrogen, Carlsbad, CA, USA) was used to
increase the specificity and sensitivity of PCR analysis. The
sensitivity of our assay was 10−5.
PCR products were separated by electrophoresis on
2.5% agarose gel. Positive and negative control samples were
included throughout all steps of the experimental procedures.
Single bands obtained by amplification of the MBR and mcr from
tumour biopsy specimens were purified from the gel and then
sequenced on an ABI 310 Genetic Analyzer (Perkin–Elmer, Foster
City, CA, USA), as previously reported (13).
Immunophenotyping
Paraffin sections were used for immunophenotyping
and lineage assignment of the NHL case. Sources and specificities
of the antibodies used in this study have been reported in detail
previously (14).
Results
Subjects
The HCWs examined included 1,352 (43%) nurses, 953
(30%) physicians and surgeons, 833 (27%) other employees
(laboratory technicians, midwives and rehabilitation therapists).
HCV infection was detected in 229 (7.3%) HCWs. The frequency of HCV
infection according to professional categories revealed that 8.18%
of physician and surgeons, 7.25% of nurses, and 6.36% of other
employees were HCV-positive. The remaining 2,826 HCWs were
HCV-negative (Table I). None of the
HCV-positive HCWs experienced accidental blood exposure while
working in the last 10 years.
 | Table I.Hepatitis C virus infection status
according to professional categories. |
Table I.
Hepatitis C virus infection status
according to professional categories.
| Professional
categories | Age (years) median
(range) |
HCV-positive/tested | Prevalence (%) |
|---|
| Nurse | 53 (31–64) | 98/1,352 | 7.25 |
|
Physician-surgeon | 54 (26–69) | 78/953 | 8.18 |
| Other (32–62
years) | 46 (32–65) | 53/833 | 6.36 |
| Total HCWs | 51 (26–69) | 229/3,138 | 7.26 |
The case of a 58-year-old HCV-positive male
physician diagnosed in 2011 with a gastric MALT lymphoma was
examined. To determine whether HCV infection is associated with the
development of the B-cell lymphoproliferation, a molecular analysis
of the B-cell clone was performed. Neoplastic B-cell expansion
expressed VH3-7-DH6-6-JH4 Ig heavy chain genes according to the
IMGT database. Furthermore, the t(14;18)-(IgH;Bcl-2) translocation,
recently detected in a fraction of HCV-associated Malt lymphomas as
an additional molecular marker, was analysed (14). Accordingly, a positive PCR reaction
was obtained using the MBR-specific primers, indicating that the
translocation involved the MBR. Nucleotide sequence analysis
confirmed that Bcl-2 was joined to JH6. The breakpoint was detected
at position 3,128 of the Bcl-2 gene and at position 1,504 of the
JH6 gene.
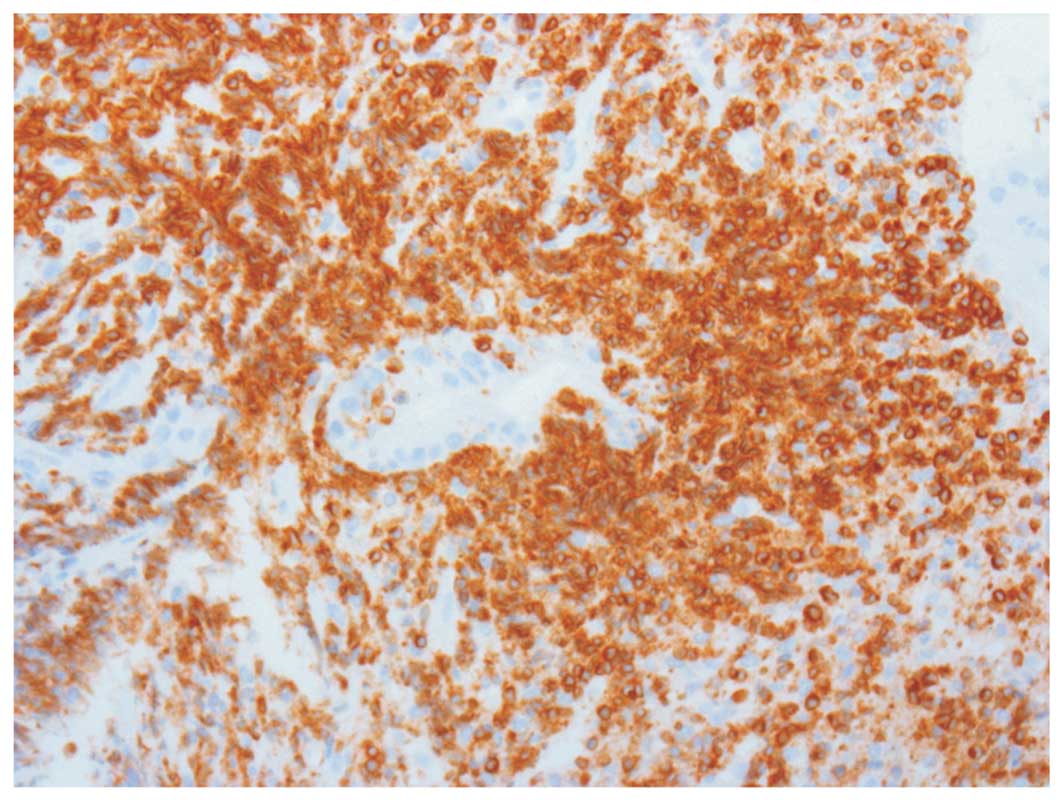
Immunophenotyping
Tumours analyzed were CD20+, cyclin
D1−, CD23−, CD5−,
Bcl-6−, CD43−, and CD10−,
supporting the diagnosis of MALT lymphoma. In particular, the null
expression of CD10 and Bcl-6 excluded the follicular origin of this
tumour. Moreover, the overexpression of Bcl-2 suggested that
t(14;18) translocation, usually linked to HCV-associated lymphomas,
may sustain survival of B-cells preventing apoptosis (Fig. 1).
Discussion
Infection by HCV leads to the development of hepatic
(9) and extra-hepatic disorders
(11). In a previous study
conducted in a group of 403 HCWs from a single institution a case
of NHL was identified among HCV-infected employees (8). Consequently, we analysed the
occurrence of B-cell lymphoma in HCV-positive HCWs from four
Medical Institutions, i.e., Policlinico Universitario,
Ferrarotto-Alessi, Santo Bambino and Ospeadali Riuniti di Torrette,
Ancona.
The HCV prevalence rate in HCWs was 7.3%. Among the
professional subgroups, the category of physician-surgeon had the
highest prevalence at 8.18%, followed by that of nurse at 7.25%.
The prevalence detected in the HCWs was lower than that observed in
the general population suggesting that HCV infection is common in
the elder population as a result of past iatrogenic transmission
such as blood transfusion or surgical intervention (15). However, our results are comparable
to those from other studies on the seroprevalence of HCV among
HCWs, and show that the prevalence is similar among subgroups of
HCWs (8,16).
Epidemiological and experimental studies have
demonstrated that HCV infection contributes to the development of
B-cell NHL. The prevalence of anti-HCV Abs in NHL patients was
19.7%, ranging from 8.3 to 37.1% (see 11 for a review). Based on a
previous observation by Marconi et al(8), results of the present study conducted
on 229 HCV-infected HCWs demonstrated the occurrence of B-cell
lymphoma. It was a gastric MALT lymphoma, diagnosed in a physician
after a long history of HCV chronic infection. Of note, the heavy
chain gene combinations detected in the DNA from MALT tissue were
those usually found in the HCV-associated lymphomas sustaining the
role of HCV infection in the mechanism of lymphomagenesis (17). The sequence analysis of rearranged
Ig genes in malignant B-cells from HCV-positive patients revealed
that certain combinations of heavy and light chain genes are
frequently present. These common combinations include:
IGHV3-23/IGHD3-22/IGHJ4, IGHV1-69/IGHD3-22/IGHJ4 or
IGHV4-59/IGHD2-15/IGHJ2 with either IGKV3-20/IGKJ1 or
IGKV3-20/IGKJ2, and IGHV3-7/IGHD3-16/IGHJ3 or
IGHV3-7/IGHD3-22/IGHJ3 with IGKV3-15/IGKJ1 (18–20).
The overexpression of Bcl-2 detected in this MALT tissue suggests
that t(14;18) translocation, linked to HCV-associated lymphomas
(14), may sustain the survival of
B-cells preventing apoptosis. Additionally, the immunohistochemical
evaluation revealed that the null expression of CD10 and Bcl-6
excludes the follicular origin of this tumour. This finding is in
agreement with previous studies as follicular lymphoma histotype is
uncommon among HCV-infected patients (11,21).
Overall, these findings support the hypothesis that
HCV infection affects the pathway of transformation and progression
of lymphoma cells. The occurrence of B-cell NHL, among HCV-positive
HCWs, is an additional reason to apply the standard precautions to
reduce the risk of blood-borne pathogen transmission (22–24).
References
|
1.
|
Butsashvili M, Kamkamidze G, Kajaia M,
Morse DL, Triner W, Dehovitz J and McNutt LA: Occupational exposure
to body fluids among health care workers in Georgia. Occup Med
(Lon). Aug 6–2012.(Epub ahead of print).
|
|
2.
|
Riddell LA and Sherrard J: Blood-borne
virus infection: the occupational risks. Int J STD AIDS.
11:632–639. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Torbati SS and Guss DA: Emergency
department management of occupational exposures to HIV-infected
fluids. J Emerg Med. 17:261–264. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Beltrami EM, Williams IT, Shapiro CN and
Chamberland ME: Risk and management of blood-borne infections in
health care workers. Clin Microbiol Rev. 13:385–407. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Klein RS, Freeman K, Taylor PE and Stevens
CE: Occupational risk for hepatitis C virus infection among New
York City dentists. Lancet. 338:1539–1542. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Kiyosawa K, Sodeyama T, Tanaka E, et al:
Hepatitis C in hospital employees with needlestick injuries. Ann
Intern Med. 115:367–369. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Mitsui T, Iwano K, Masuko K, et al:
Hepatitis C virus infection in medical personnel after needlestick
accident. Hepatology. 16:1109–1114. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Marconi A, Candido S, Talamini R, Libra M,
Nicoletti F, Spandidos DA, Stivala F and Proietti L: Prevalence of
hepatitis C virus infection among health-care workers: A 10-year
survey. Mol Med Rep. 3:561–564. 2010.PubMed/NCBI
|
|
9.
|
Bartosch B, Thimme R, Blum HE and Zoulim
F: Hepatitis C virus-induced hepatocarcinogenesis. J Hepatol.
51:810–820. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Negri E, Little D, Boiocchi M, La Vecchia
C and Franceschi S: B-cell non-Hodgkin’s lymphoma and hepatitis C
virus infection: a systematic review. Int J Cancer. 111:1–8.
2004.
|
|
11.
|
Libra M, Polesel J, Russo AE, De Re V,
Cinà D, Serraino D, Nicoletti F, Spandidos DA, Stivala F and
Talamini R: Extrahepatic disorders of HCV infection: A distinct
entity of B-cell neoplasia? Int J Oncol. 36:1331–1340. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Libra M, Gloghini A, De Re V, et al:
Aggressive forms of non-Hodgkin’s lymphoma in two patients bearing
coinfection of Epstein-Barr and hepatitis C viruses. Int J Oncol.
26:945–950. 2005.
|
|
13.
|
Libra M, De Re V, De Vita S, Gasparotto D,
Gloghini A, Rupolo M, Degan M, Marzotto A, Stivala F, Carbone A and
Boiocchi M: Low frequency of bcl-2 rearrangement in HCV-associated
non-Hodgkin’s lymphoma tissue. Leukemia. 7:1433–1436.
2003.PubMed/NCBI
|
|
14.
|
Libra M, Gloghini A, Malaponte G, et al:
Association of t(14;18) translocation with HCV infection in
gastrointestinal MALT lymphomas. J Hepatol. 49:170–174. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Di Stefano R, Stroffolini T, Ferraro D,
Usticano A, Valenza LM, Montalbano L, Pomara G and Craxì A: Endemic
hepatitis C virus infection in a Sicilian town: further evidence
for iatrogenic transmission. J Med Virol. 67:339–344.
2002.PubMed/NCBI
|
|
16.
|
Montella M, Crispo A, Grimaldi M, Ruffolo
P, Ronga D, Izzo F and Mastro AA: An assessment of hepatitis C
virus infection among health-care workers of the National Cancer
Institute of Naples, Southern Italy. Eur J Public Health.
15:467–469. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Libra M, Gasparotto D, Gloghini A,
Navolanic PM, De Re V and Carbone A: Hepatitis C virus (HCV)
infection and lymphoproliferative disorders. Front Biosci.
10:2460–2471. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
18.
|
De Vita S, De Re V, Sansonno D, Sorrentino
D, Corte RL, Pivetta B, Gasparotto D, Racanelli V, Marzotto A,
Labombarda A, Gloghini A, Ferraccioli G, Monteverde A, Carbone A,
Dammacco F and Boiocchi M: Gastric mucosa as an additional
extrahepatic localization of hepatitis C virus: viral detection in
gastric low-grade lymphoma associated with autoimmune disease and
in chronic gastritis. Hepatology. 31:182–189. 2000.
|
|
19.
|
Ivanovski M, Silvestri F, Pozzato G, Anand
S, Mazzaro C, Burrone OR and Efremov DG: Somatic hypermutation,
clonal diversity, and preferential expression of the VH 51p1/VL
kv325 immunoglobulin gene combination in hepatitis C
virus-associated immunocytomas. Blood. 91:2433–2442. 1998.
|
|
20.
|
Gasparotto D, De Re V and Boiocchi M:
Hepatitis C virus, B-cell proliferation and lymphomas. Leuk
Lymphoma. 43:747–751. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Talamini R, Montella M, Crovatto M, et al:
Non-Hodgkin’s lymphoma and hepatitis C virus: a case-control study
from northern and southern Italy. Int J Cancer. 110:380–385.
2004.
|
|
22.
|
Centers for Disease Control and Prevention
(CDC): Recommendations 5 for prevention and control of hepatitis
virus (HCV) infection and HCV-related chronic disease. MMWR Recomm
Rep. 47:1–39. 1998.
|
|
23.
|
Centers for Disease Control and Prevention
(CDC): Recommendations for follow-up of health-care workers after
occupational exposure to hepatitis C virus. MMWR Morb Mortal Wkly
Rep. 46:603–606. 1997.PubMed/NCBI
|
|
24.
|
Haiduven DJ, DeMaio TM and Stevens DA: A
five-year study of needlestick injuries: significant reduction
associated with communication, education, and convenient placement
of sharps containers. Infect Control Hosp Epidemiol. 13:265–271.
1992.
|