Introduction
Gastrointestinal stromal tumors (GISTs) are rare
neoplasms that arise from mesenchymal cells of the gastrointestinal
tract. Extragastrointestinal stromal tumors (EGISTs) have no
contact with the stomach and intestine, by definition, and
typically occur in the omentum, mesentery or retroperitoneum
(1,2). However, EGISTs are not connected to
the digestive tract (3). GISTs in
the omentum or mesentery are typically metastases from primary
sites within the gastrointestinal tract. EGISTs are often included
in large studies of stromal tumors, in which they account for less
than 10% of overall cases. EGISTs have rarely been identified in
the pancreas, diaphragm, spleen, pelvis or abdominal wall (4–7).
The most common type of carcinoma associated with
GISTs is gastric carcinoma (8). To
the best of our knowledge, primary EGIST concomitant with digestive
tract carcinoma is rarely encountered. We describe a rare case of
giant EGIST in the transverse mesocolon concomitant with gastric
adenocarcinoma, in a 78-year-old male who presented with upper
abdominal pain and a palpable mass. We discuss the specific
recommended therapies for patients with concomitant EGST and
gastric cancer, with a review of the literature.
The study was approved by the Ethics Committee of
Qilu Hospital, Shandong University, China. The patient’s family
consented to this study.
Case report
A 78-year-old male was admitted to hospital for
abdominal distension and pain. The patient had no history of
epigastralgia or peptic ulcers and there was no significant
relevant family history. A physical examination revealed a 20x12 cm
palpable mass in the middle and lower abdomen, with minimal
intrinsic mobility. Carcinoembryonic antigen (CEA), cancer antigen
(CA) 724 and carbohydrate antigen (CA) 19-9 levels were normal. A
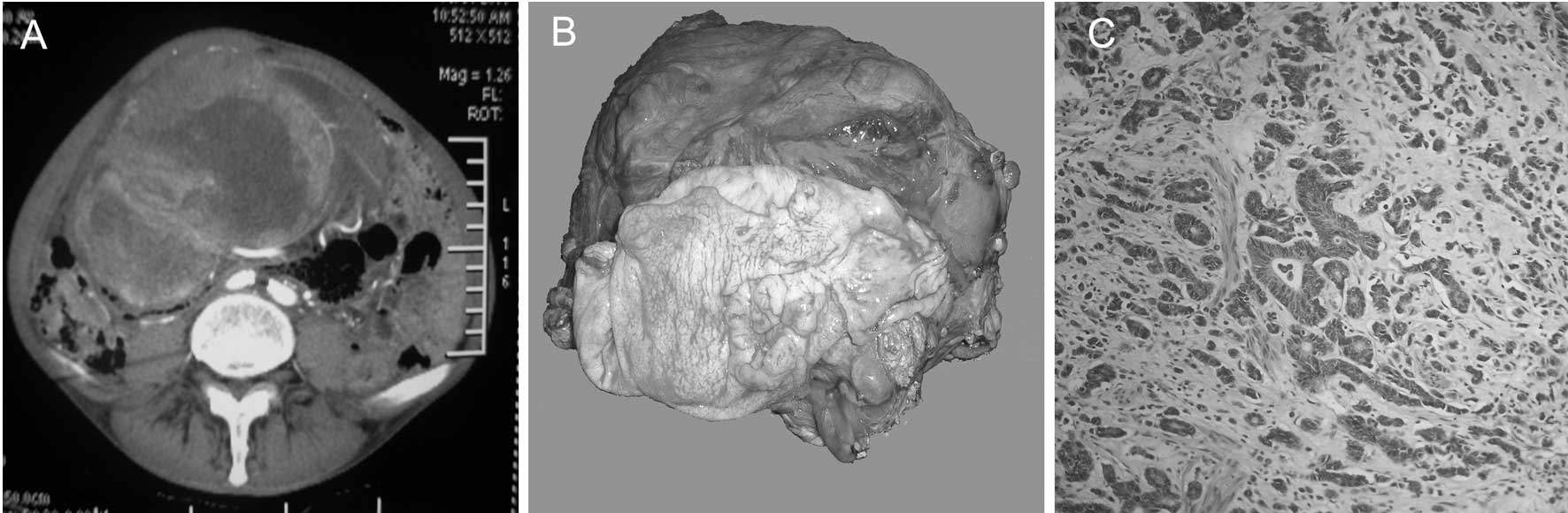
computed tomography (CT) scan revealed a 21x14 cm heterogeneous
mass extending from the fundus to the mid-abdomen, with a number of
solid and cystic components within the tumor (Fig. 1A). A gastroscopy revealed an
intraluminal ulcer in the lower posterior wall of the gastric
antrum; biopsy specimens were obtained. No metastatic lesions were
identified in any other organs in either the abdominal
ultrasonography or the CT scan. A biopsy confirmed the presence of
an adenocarcinoma of the stomach with poor to moderate
differentiation. A laparotomy revealed an extremely large tumor
(22x15 cm) arising from the transverse mesocolon and adjacent to
the greater curvature of the stomach. The mass was in close
apposition to the pancreas and duodenum. There was no evidence of
invasion or underlying peritoneal infiltration. The mass was
resected en bloc with part of the transverse colon, and distal
gastric resection was performed (Fig.
1B).
Macroscopic examination of the distal gastrectomy
specimen revealed a Borrmann type II tumor in the stomach,
measuring 3x3 cm. On histopathological examination, the tumor was
identified to be an adenocarcinoma with poor to moderate
differentiation that exhibited transmural infiltration (Fig. 1C). No nerve or vascular invasion was
evident. Two lymph node metastases were detected in 28 retrieved
lymph nodes. According to the TNM classification, the gastric
carcinoma was stage IIIA. Histopathological examination
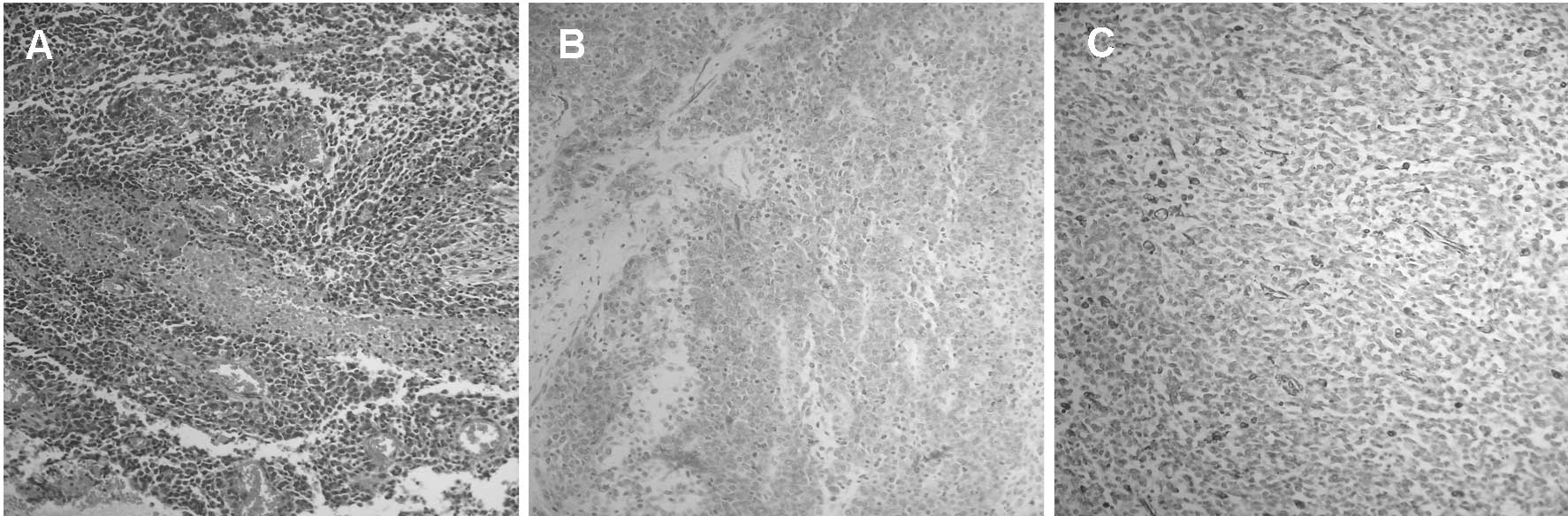
demonstrated that the extremely large mass was mainly comprised of
epithelioid cells, and revealed focal necrosis, fibrosis and
hemorrhagic areas (Fig. 2A).
Mitotic figures were recognized in 15 out of 50 high-power fields.
Immunohistochemical analysis revealed that CD117 (Fig. 2B) and desmin (Fig. 2C) were positive in the neoplastic
cells. However, tumor cells exhibited negative expression for CD34,
S-100 and smooth muscle actin (SMA). Based on these results, the
diagnosis was an EGIST of the transverse mesocolon that had not
originated from the digestive tract. The postoperative period of
the patient was uneventful; the patient was discharged two weeks
after surgery. The patient was started on adjuvant chemotherapy
with a FOLFOX regimen: oxaliplatin IV, 85 mg/m2 (on day
1); leucovorin IV, 200 mg/m2 (on days 1 and 2); 5-FU
(fluorouracil) IV, 400 mg/m2 (on days 1 and 2) and 5-FU
IV 22 hours in fusion, 600 mg/m2 (on days 1 and 2). This
regimen was repeated every 2 weeks for 6 cycles. Additionally, the
patient was simultaneously administered imatinib at a dose of 400
mg/day for 1 year. No evidence of tumor recurrence was identified
after 24 months of follow-up.
Discussion
GISTs identified outside the gastrointestinal tract
as apparent primary tumors are designated as EGISTs. EGISTs may
occur in the omentum, mesentery or retroperitoneum, adjacent to
(although separate from) the stomach and intestine. Their true
origin is uncertain; however, their histological appearance and
immunophenotypes are typically identical to those of classical
GISTs. The EGIST in the present case was located inside the
mesocolon, outside the gastrointestinal tract. EGISTs of the
mesocolon have rarely been noted in the literature (9–11). The
pathogenesis of EGIST concomitant with abdominal malignancy is
still unknown. Only sporadic cases have been described. EGISTs of
the greater omentum (10 and 8 mm in diameter, respectively) have
been identified by coincidence when a gastrectomy was performed
(12). EGISTs are often
asymptomatic as they lack mucosal participation, whereas GISTs
commonly present with gastrointestinal or intratumoral bleeding
(13,14). Due to their their anatomic site,
EGISTs typically grow larger than GISTs and only present clinical
symptoms after a significant period of time (15,16).
Certain studies have described gastric stromal tumors to be
synchronous with gastric malignancy. To the best of our knowledge,
a giant EGIST in the transverse mesocolon concomitant with gastric
carcinoma has never been studied.
The clinicopathological or immunohistochemical
features of EGISTs and GISTs were not observed to be markedly
different. Primary EGISTs of the omentum and mesentery demonstrated
clinicopathological and immunohistochemical characteristics similar
to a GIST of the digestive tract described previously in the
literature (17). This supports the
hypothesis that these tumors originate from extragastrointestinal
c-kit positive cells. Their histogenesis, criteria for diagnosis,
prognostic factors and classification have been debatable and
controversial. GISTs are accurately diagnosed by their morphology
and immunophenotyping. Histologically, there are three types of
GISTs: spindle, epithelioid and mixed. The diagnosis of an EGIST in
the present case was in accordance with the histological and
immunohistochemical criteria; the tumor was epithelioid-type, and
was immunopositive for CD117 and desmin. More than 95% of EGISTs
express CD117, the c-kit proto-oncogene protein that is a
transmembrane receptor for the stem cell growth factor; while
50–100% express CD34, the human progenitor cell antigen. It is less
common for EGISTs to stain positively for SMA, S-100 and desmin
(6,17,18).
Zheng et al demonstrated that the c-kit and PDGFRA mutation
in EGISTs was similar to that of GISTs. Therefore, from a molecular
genetics perspective, EGISTs may be a unique subtype of GISTs
(3).
The risk categories of EGISTs are contested. A tumor
size >10 cm, mitotic activity >2 per 50 high-power fields,
tumor necrosis, marked nuclear atypia, >10% Ki-67 protein
expression and epithelioid-/mixed cell-type, have acted as
significant predictors of survival (2,3).
Survival analysis indicated that mitotic count and Ki-67 protein
expression levels were significant predictors of disease-specific
survival; however, tumor size, primary location, c-kit and PDGFRA
gene mutations were not. The high risk of recurrence and prognostic
factors for survival are controversial. This may be due to a
limited number of samples, a different composition of ages and
different histologic judgements of pathologists across studies.
Therefore, it is necessary to enlarge the sample size in order to
reach a precise conclusion. EGISTs are capable of remaining
clinically silent, irrespective of their large size, which exceeded
10 cm in the majority of published cases. EGISTs are rarely
encountered due to the fact that they seldom produce symptoms that
would lead to their detection. Tumor size has not been observed to
be a reliable prognostic parameter in EGISTs. In the present case,
focal necrosis, >10% Ki-67 protein expression and a high-power
mitotic count >10 resulted in the tumor being considered
high-risk. The tumor in the present case demonstrated
epithelioid-type histology. Positive immunohistochemical staining
for CD117 is also a defining feature of EGISTs, and it correlates
with a tumor response to treatment with the KIT kinase activity
inhibitor. The present case demonstrated strong positive staining
for CD117 and desmin, as well as negative staining for CD34, S-100
and SMA, by immunohistochemistry. We propose that tumor coexistence
is a unique event in histology.
There are few data regarding the clinicopathological
factors of EGISTs that predict the patient’s prognosis. In a study
by Reith et al, 39% of patients with EGISTs had an adverse
outcome, which suggested that EGISTs were aggressive and were more
similar to GISTs located in the distal gastrointestinal tract
(2). Barros et al analyzed 9
EGISTs and revealed that the average overall survival was 26.4
months (6). Llenas-García et
al demonstrated that 66.6% of mesenteric cases with a high
mitotic rate exibited hepatic metastasis at 6 and 32 months,
respectively (17). It was
suggested that EGISTs that originated from a mesenteric location
had an aggressive course that was more similar to that of small
intestinal, as opposed to gastric stromal tumors.
Surgery is the standard treatment for non-metastatic
EGISTs. A preoperative diagnosis is difficult, and the patient
undergoes an operation based on the diagnosis of an abdominal mass.
Where possible, en bloc resection with contiguous tissues and
regional lymph nodes is conducted. It is crucial for the
pathologist to examine whether the tumor is adhesive to the
gastrointestinal wall or other tissues. A histological diagnosis of
EGIST is often unsatisfactory. In the present case, the patient
underwent an en bloc resection of the tumor and partial transverse
mesocolon, accompanied with a distal gastrectomy with a D2
lymphadenectomy. Positive immunohistochemical staining for CD117 is
a defining feature of EGISTs. C-kit-positive tumors are most
responsive to treatment with a c-kit selective tyrosine kinase
inhibitor. Due to the advent of targeted therapy, imatinib, an
inhibitor of the tyrosine kinase activity of c-kit, has
revolutionized the treatment of this disease. The recommended
first-line treatment of advanced GIST is 400 mg/day imatinib
(19). In certain patients, an
escalated dose (800 mg/day) has achieved tumor control and
conferred further survival benefits after failure with 400 mg/day
(20). Adjuvant chemotherapy
(FOLFOX) and imatinib were applied in the present case. The patient
was followed up and was free of recurrence 24 months after
surgery.
The coexistence of EGIST with other abdominal
malignancies is a rare phenomenon; we suggest that their
synchronous occurrence may be a coincidence. Although there are
data regarding the co-occurrence of EGISTs and other malignant
tumors, the mechanism of synchronous tumorigenesis remains unclear.
Surgical resection is currently the mainstay of treatment for
EGISTs. The existing data on the coexistence of EGISTs with other
types of gastric carcinoma are insufficient to reach a final
conclusion regarding the treatment, prognosis and recurrence.
In summary, the present case was a giant EGIST in
the mesocolon accompanied with gastric cancer in an elderly male.
We stress that, although their prevalence is very low,
surgery-based multimodal treatment is the preferred strategy for
compound tumors. Adjuvant chemotherapy and targeted therapy are
important for high-risk EGISTs accompanied with digestive tract
malignancies. Due to the limited number of cases, the existence of
a common tumorigenesis factor among the different tumors cannot be
identified. Further studies are required to expound the possible
association.
Acknowledgements
The authors would like to thank Mr.
Xinjun Li for critically reading the manuscript.
References
|
1.
|
Miettinen M, Monihan JM, Sarlomo-Rikala M,
Kovatich AJ, Carr NJ, Emory TS and Sobin LH: Gastrointestinal
stromal tumors/smooth muscle tumors (GISTs) primary in the omentum
and mesentery: clinicopathologic and immunohistochemical study of
26 cases. Am J Surg Pathol. 23:1109–1118. 1999. View Article : Google Scholar
|
|
2.
|
Reith JD, Goldblum JR, Lyles RH and Weiss
SW: Extragastrointestinal (soft tissue) stromal tumors: an analysis
of 48 cases with emphasis on histologic predictors of outcome. Mod
Pathol. 13:577–585. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Zheng S, Huang KE, Tao DY and Pan YL: Gene
mutations and prognostic factors analysis in extragastrointestinal
stromal tumor of a Chinese three-center study. J Gastrointest Surg.
15:675–681. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Vij M, Agrawal V and Pandey R: Malignant
extra-gastrointestinal stromal tumor of the pancreas. A case report
and review of literature. JOP. 12:200–204. 2011.PubMed/NCBI
|
|
5.
|
Yeung CK, Yuen CH, Chan IK and Chu RW:
Malignant extra-gastrointestinal stromal tumour of diaphragm. ANZ J
Surg. 78:923–924. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Barros A, Linhares E, Valadão M, Gonçalves
R, Vilhena B, Gil C and Ramos C: Extragastrointestinal stromal
tumors (EGIST): a series of case reports. Hepatogastroenterology.
58:865–868. 2011.PubMed/NCBI
|
|
7.
|
Alkhatib L, Albtoush O, Bataineh N,
Gharaibeh K, Matalka I and Tokuda Y: Extragastrointestinal Stromal
Tumor (EGIST) in the abdominal wall: Case report and literature
review. Int J Surg Case Rep. 2:253–255. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Agaimy A, Wünsch PH, Sobin LH, Lasota J
and Miettinen M: Occurrence of other malignancies in patients with
gastrointestinal stromal tumors. Semin Diagn Pathol. 23:120–129.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Jakobs K, de Gheldere CD and Vanclooster
P: A ruptured gastrointestinal stromal tumor of the transverse
mesocolon: a case report. Acta Chir Belg. 106:218–221.
2006.PubMed/NCBI
|
|
10.
|
Paparelli C, Cavallaro G, Basso L,
Polistena A, Mingazzini PL and De Toma G: Giant GIST of the
mesocolon: report of a case. Minerva Chir. 61:537–540.
2006.PubMed/NCBI
|
|
11.
|
Terada T: Primary extragastrointestinal
stromal tumor of the transverse mesocolon without c-kit mutations
but with PDGFRA mutations. Med Oncol. 26:233–237. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Terada T: Primary multiple
extragastrointestinal stromal tumors of the omentum with different
mutations of c-kit gene. World J Gastroenterol. 14:7256–7259. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Cruz RJ Jr, Vincenzi R, Ketzer BM, Cecilio
AL and Cepeda LA: Spontaneous intratumoral bleeding and rupture of
giant gastric stromal tumor (> 30 cm) in a young patient. World
J Surg Oncol. 6:762008.PubMed/NCBI
|
|
14.
|
Dedemadi G, Georgoulis G, Kontopanos D,
Anagnostou E, Morphopoulos G, Emile JF and Christopoulos C:
Extragastrointestinal stromal tumors of the omentum: review apropos
of a case with a novel gain-of-function KIT mutation. J
Gastrointest Cancer. 40:73–78. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Miettinen M, Makhlouf H, Sobin LH and
Lasota J: Gastrointestinal stromal tumors of the jejunum and ileum:
a clinicopathologic, imunohistochemical, and molecular genetic
study of 906 cases before imatinib with long-term follow-up. Am J
Surg Pathol. 30:477–489. 2006. View Article : Google Scholar
|
|
16.
|
Castillo-Sang M, Mancho S, Tsang AW,
Gociman B, Almaroof B and Ahmed MY: A malignant omental
extra-gastrointestinal stromal tumor on a young man: a case report
and review of the literature. World J Surg Oncol. 6:502008.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Llenas-García J, Guerra-Vales JM, Moreno
A, et al: Primary extragastrointestinal stromal tumors in the
omentum and mesentery: a clinicopathological and
immunohistochemical study. Hepatogastroenterology. 55:1002–1005.
2008.PubMed/NCBI
|
|
18.
|
Kim KH, Nelson SD, Kim DH, et al:
Diagnostic relevance of overexpressions of PKC-θ and DOG-1 and
KIT/PDGFRA gene mutations in extragastrointestinal stromal tumors:
A Korean six-centers study of 28 cases. Anticancer Res. 32:923–937.
2012.PubMed/NCBI
|
|
19.
|
Casali PG, Jost L, Reichardt P, et al:
ESMO Guidelines Working Group: Gastrointestinal stromal tumors:
ESMO clinical recommendations for diagnosis, treatment and follow
up. Ann Oncol. S2:ii35–38. 2008.PubMed/NCBI
|
|
20.
|
Li J, Gong JF, Li J, Gao J, Sun NP and
Shen L: Efficacy of imatinib dose escalation in Chinese
gastrointestinal stromal tumor patients. World J Gastroenterol.
18:698–703. 2012. View Article : Google Scholar : PubMed/NCBI
|