Introduction
An abundance of cyclotides have been isolated and
confirmed from Clitoria ternatea (Fabaceae family) (1,2). The
concentration of cyclotides from this plant is relatively higher
than that of the majority of cyclotide-containing plants from the
Rubiaceae and Violacecae families. C. ternatea is therefore
an ideal resource for investigating the bioactivities of
cyclotides. In previous studies, in vitro cell-based assays
have demonstrated that certain cyclotides have significant
cytotoxic activities by disrupting the cell membrane integrity
(3,4). By contrast, in an in vivo
study, even the most potent cyclotide, cycloviolacin O2, did not
demonstrate a significant anticancer effect in a xenograft model
(5). Gerlach et al revealed
that cyclotides may have potential chemosensitizing abilities when
combined with other anticancer reagents (6). Cycloviolacin O2 and psyle cyclotides
from Psychotria leptothyrsa were used to treat the
doxorubicin-resistant human breast cancer cell line MCF-7/ADR. It
was found that cyclotides were capable of enhancing the doxorubicin
effect that inhibits MCF-7/ADR proliferation; coexposure caused the
half maximal inhibitory concentration (IC50) value to
decrease 4- to 7-fold compared with single doxorubicin. The
mechanism was considered to be associated with a leakage of cell
membrane caused by cyclotides, thus enabling the chemical reagent
to pass through the cell membrane. Therefore, we hypothesize that
this type of chemosensitizating ability may be wide spectrum with
less selectivity. In the present study, the human lung cancer cell
line A549 and its sub-line A549/paclitaxel were selected to provide
support for this hypothesis. The role of the net charge status of
cyclotides on cytotoxicity and chemosentization was also
investigated.
Materials and methods
Isolation and purification of
cyclotides
Cyclotides from C. ternatea, termed
specifically as cliotides, were isolated and purified from C.
ternatea as described previously (2). Briefly, flowers and seeds were ground,
and then extracted by 20% ethanol (V/V, with water). Crude extract
was centrifuged for 30 min at 10,000 x g and the supernatant was
passed through a 0.45 μm filter to clear the debris.
Different types of cliotide peptides with different acetonitrile
concentration gradients were fractionated and purified by RP-HPLC.
Based on the amino acid sequence and net charges, a total of seven
cliotide peptide molecules were selected for use in the present
study (Table I).
 | Table I.Cliotides involved in the present
study. |
Table I.
Cliotides involved in the present
study.
| Cliotide | Sequence | MWa | Net charge | Subfamily |
|---|
| CT2 |
GEFLKCGESCVQGEC–YT–
–PGCSCDWPICKKN | 3260 | −1 | M |
| CT4 | GIP– –
CGESCVFIPC–ITAAIGCSCKSKVCYRN | 3098 | +2 | B |
| CT7 | GIP– –
CGESCVFIPCTVTALLGCSCKDKVCYKN | 3227 | +1 | B |
| CT10 | GVP–
–CAESCVWIPCTVTALLGCSCKDKVCYLN | 3251 | 0 | B |
| CT12 | GIP–
–CGESCVYIPCTVTALLGCSCKDKVCYKN | 3243 | +1 | B |
| CT19 |
GSVIKCGESCLLGKC–YT–
–PGCTCSRPICKKD | 3125 | +4 | B |
| CT20 |
GSAIRCGESCLLGKC–YT–
–PGCTCDRPICKKN | 3152 | +3 | B |
Cell culture
Human non-small lung cancer (A549) cells were
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA). An A549-derived paclitaxel-resistant sub-line,
A549/taxol, was established by the Institute of Materia Medica,
Chinese Academy of Medical Sciences (Beijing, China). For
maintenance, cells were cultured in RPMI-1640 medium (Gibco
Laboratories, Life Technologies Inc.; Grand Island, NY, USA) with
10% fetal bovine serum (FBS; Gibco) at 37°C in a humidified 5%
CO2 atmosphere.
Cytotoxicity assay
Various types of cliotides were assessed for their
cytotoxicity activity in A549 cells and their chemosensitizating
capability in A549/taxol cells, as previously described by Gerlach
et al(6). Briefly, cells
were seeded in 96-well flat-bottomed microtiter plates
(1×104). After culture for 4 h, cells were treated with
cliotide T2 (CT2), CT4, CT7, CT10, CT12, CT19, CT20 and paclitaxel
at 0.4, 1, 2, 4, 10 and 20 μM, in 100 μl of media for
72 h. The medium was removed from each well and 100 μl of
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT;
0.5 mg/ml in PBS) was added in the absence of light; formazan
crystals were produced over a 4 h incubation period. To dissolve
crystals, 150 μl of 0.04 N HCl in isopropanol was added to
each well and the optical density at 540 nm was measured on a Tecan
Spectrophotometer (Tecan SPECTRAFluor, Tecan, Männedorf,
Switzerland). For coexposure experiments, the same protocol was
followed but concentrations were modified to 50:50 ratios of each
cliotide and paclitaxel at the following concentrations: 0.2, 0.5,
1, 2, 5 and 10 μM. Each test was performed with 3 replicates
and repeated in triplicate. Replicates were averaged and the blank
subtracted from control and test concentrations. Optical density of
the treated wells was compared with that of the controls (100%
survival); percentage cell survival was calculated and plotted by
GraphPad Prism (GraphPad Software, Inc.; San Diego, CA, USA).
Statistical analysis
All data were analyzed with GraphPad Prism 4.0
software. The IC50 values (μM) of each treatment
were calculated and a combination of paired t-tests and analysis of
variance (ANOVA) were performed. P<0.05 was considered to
indicate a statistically significant difference.
Results
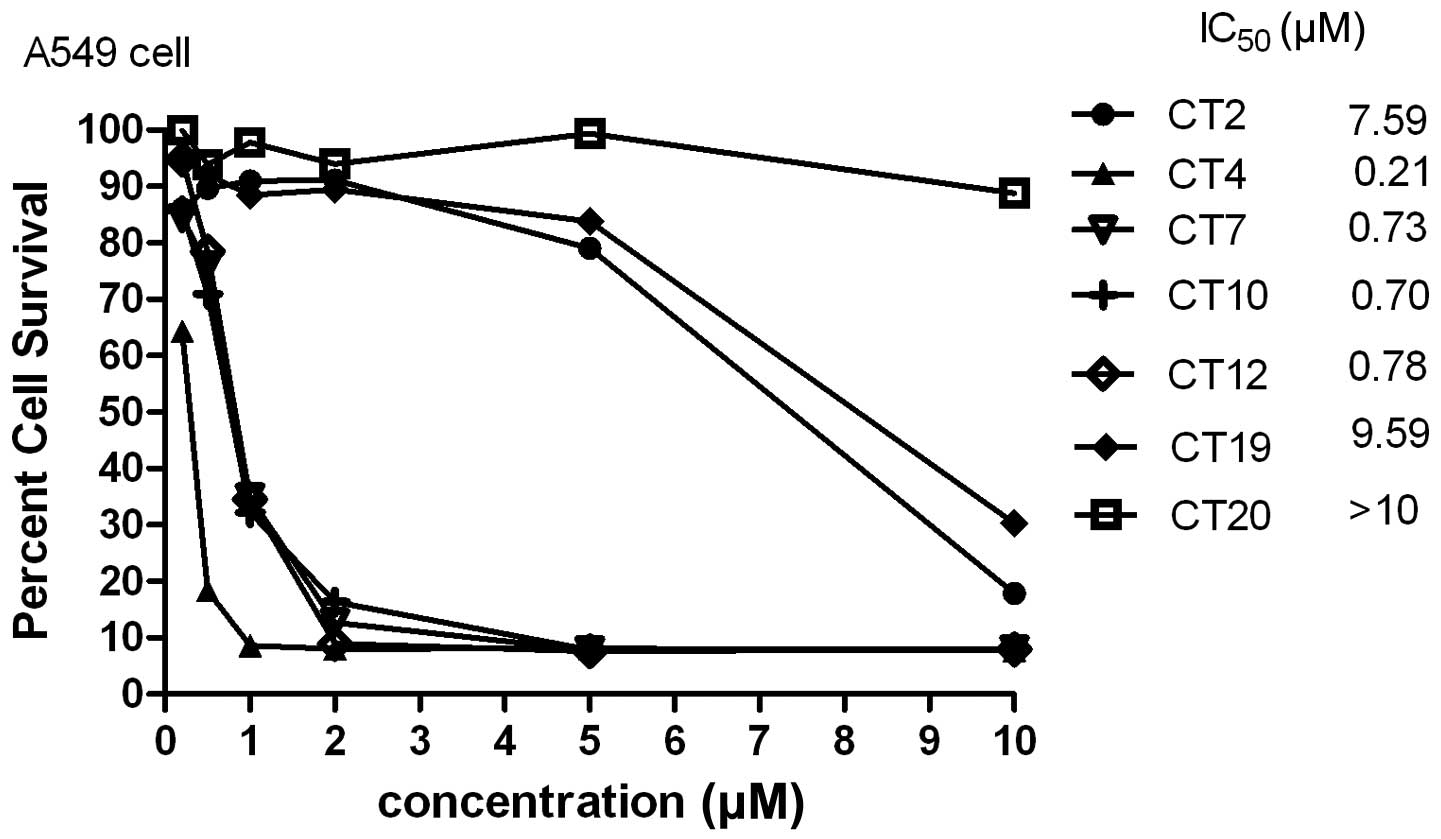
Cytotoxicity of cliotides
All cliotides in Table
I were used to test cytotoxicity in A549 cells. All of these
demonstrated significant cytotoxicity, with the exception of CT19
and CT20 whose predicted IC50 values were ∼10 μM
(Fig. 1). Previous studies have
suggested that net charge is an important factor in cytotoxic
activity, and bracelet cyclotides were generally more cytotoxic
than Möbius cyclotides (7). In the
present study, CT4, which had a net charge of +2, had the lowest
IC50 value (0.21 μM) compared with the other
cyclotides. However, a positive charge of >2 did not increase
the cytotoxicity; CT19 had a charge of +3 and its IC50
value was ∼10 μM, while CT20 had a charge of +4, with an
IC50 value above 10 μM.
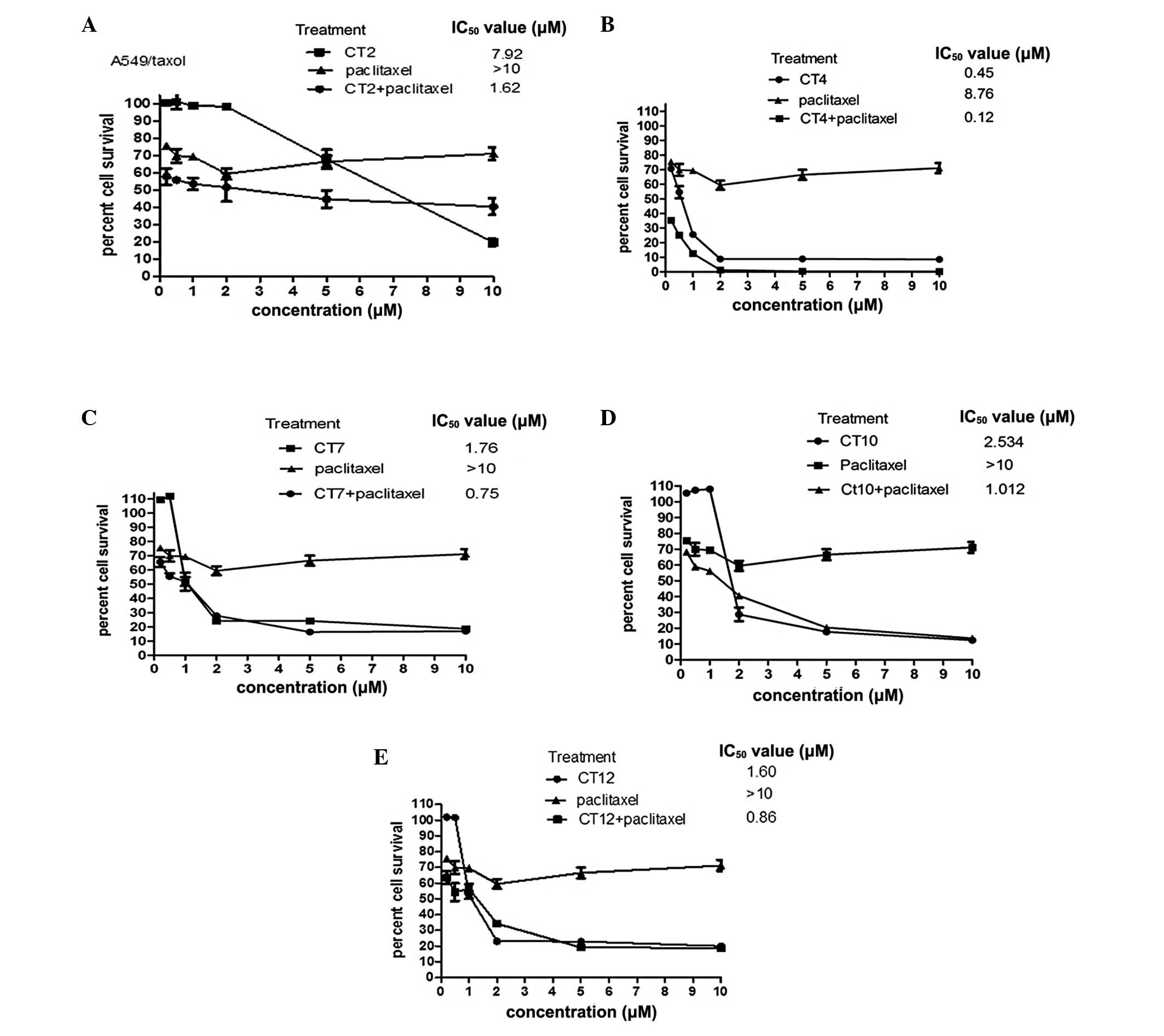
Chemosensitization of cliotides with
paclitaxel on A549/paclitaxel
Based on the results above, cliotides which had
significant cytotoxicity in A549 cells (IC50<10
μM) were selected for coexposure experiments in the A549
paclitaxel-resistant cell line. These cliotides were CT2, CT4, CT7,
CT10 and CT12.
Fig. 2A–E and
Table II summarize the
chemosensitizing effect of five types of cliotide, all of which
demonstrated positive results reflected by the multi-fold decreases
in the IC50 value of cliotides in the presence of
paclitaxel. A four-fold decrease in the CT2-treated group, a
three-fold decrease in the CT4-, CT7- and CT10-treated groups and a
one-fold decrease in the CT12-treated group were observed.
 | Table II.Half maximal inhibitory concentration
(IC50μM) of cliotides in the human lung cell line
(A549) and its drug resistant cell (A549/paclitaxel). |
Table II.
Half maximal inhibitory concentration
(IC50μM) of cliotides in the human lung cell line
(A549) and its drug resistant cell (A549/paclitaxel).
| Cyclotides | A549 |
A549/paclitaxel | Coexposure to
paclitaxel |
|---|
| CT2 | 7.59 | 7.92 | 1.62 |
| CT4 | 0.21 | 0.45 | 0.12 |
| CT7 | 0.73 | 1.76 | 0.75 |
| CT10 | 0.70 | 2.53 | 1.01 |
| CT12 | 0.78 | 1.6 | 0.86 |
| Paclitaxel | 1.21 | >10 | >10 |
Discussion
At present, >200 cyclotides have been isolated
and identified from various plants of the Violaceae, Rubiaceae,
Cucurbitaceae and Fabaceae families (8–11).
Cyclotides comprise a family of circular mini-proteins and have a
wide range of bioactivities. They have a characteristic
head-to-tail cyclized backbone typically composed of 28–37 amino
acids, and a knotted disulfide topology involving six conserved
cysteine residues (12–14). The cyclic backbone and conserved
cysteine residues form a unique structure termed the cyclic cystine
knot (CCK), in which two of the disulfide bonds and their
connecting backbone segments form an embedded ring that is
penetrated by the third disulfide bond (14). Aside from being a characteristic
structural feature of the cyclotide family, the CCK motif enables
these mini-proteins to be exceptionally resistant to chemical,
enzymatic and thermal degradation (15). Therefore, cyclotides have become an
ideal scaffold for drug delivery (15,16).
Cyclotides exhibit a range of biological activities, including
uterotonic (17), hemolytic
(18), antineurotensin (19), anti-HIV (20), cytotoxic (3) and antibacterial (18) activities; however, their natural
function is considered to be as plant defence molecules, based on
their insecticidal (21) and
molluscidal (22) properties.
Among all the bioactivities of cyclotides,
cytotoxicity was was one of the first to be identified and has been
investigated widely. However, the sharp dose-response profile and
poor in vivo anticancer results affected the usage of
cyclotides in clinical treatment (5). In the present study, consistent with
previous research, cliotides were less cytotoxic against
A549/paclitaxel compared to A549 (Table
II). Additionally concordant, cliotides with a charge of +2
were the most cytotoxic; CT4 had the lowest IC50 value.
The cytotoxic ranking was IC50 CT4 (+2)<IC50 CT7 (+1)
and CT12 (+1)<IC50 CT10 (0)<IC50 CT2
(−1); cytotoxicity increased with an increase in net positive
charge. However, CT19 and CT20, which had charges of +3 and 4,
respectively, did not show significant cytotoxicity. With regard to
why higher cliotide positive charges may decrease cytotoxicity, we
hypothesized that although multiple positive charges made it easier
for the cliotides to adhere to the cell membrane, they also changed
the peptide space structure and reduced the hydrophobicity, thus
reducing the ability to disrupt the cell membrane.
Drug resistance is one of the greatest obstacles
limiting chemotherapy in clinical treatment. The chemosensitizing
ability of the cliotides in the A549/paclitaxel cells in the
present study is notable; all five tested cyclotides demonstrated a
two- to four-fold decrease in IC50 value in the presence
of paclitaxel.
In summary, this study demonstrated that cyclotides
from C. ternatea have the potential to be used in
chemosensitization for treating cancer. We are optimistic that
cyclotides are suitable drug candidates after modification and
their charge status plays a significant role in their
bioactivities.
Acknowledgements
The authors would like to thank
Professor J. P. Tam (Nanyang Technological University, Singapore)
for providing instructions for the project, and Dr G. K. Nguyen
(Nanyang Technological University) for help in preparing the
cyclotide samples.
References
|
1.
|
Poth AG, Colgrave ML, Philip R, et al:
Discovery of cyclotides in the fabaceae plant family provides new
insights into the cyclization, evolution, and distribution of
circular proteins. ACS Chem Biol. 6:345–355. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Nguyen GK, Zhang S, Nguyen NT, et al:
Discovery and characterization of novel cyclotides originated from
chimeric precursors consisting of albumin-1 chain a and cyclotide
domains in the Fabaceae family. J Biol Chem. 286:24275–24287. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Lindholm P, Göransson U, Johansson S, et
al: Cyclotides: a novel type of cytotoxic agents. Mol Cancer Ther.
1:365–369. 2002.PubMed/NCBI
|
|
4.
|
Herrmann A, Svangård E, Claeson P, Gullbo
J, Bohlin L and Göransson U: Key role of glutamic acid for the
cytotoxic activity of the cyclotide cycloviolacin O2. Cell Mol Life
Sci. 63:235–245. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Burman R, Svedlund E, Felth J, et al:
Evaluation of toxicity and antitumor activity of cycloviolacin O2
in mice. Biopolymers. 94:626–634. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Gerlach SL, Rathinakumar R, Chakravarty G,
et al: Anticancer and chemosensitizing abilities of cycloviolacin
O2 from Viola odorata and psyle cyclotides from
Psychotria leptothyrsa. Biopolymers. 94:617–625. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Svangård E, Göransson U, Hocaoglu Z,
Gullbo J, Larsson R, Claeson P and Bohlin L: Cytotoxic cyclotides
from Viola tricolor. J Nat Prod. 67:144–147. 2004.
|
|
8.
|
He W, Chan LY, Zeng G, Daly NL, Craik DJ
and Tan N: Isolation and characterization of cytotoxic cyclotides
from Viola philippica. Peptides. 32:1719–1723. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Kaas Q and Craik DJ: Analysis and
classification of circular proteins in CyBase. Biopolymers.
94:584–591. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Mulvenna JP, Wang C and Craik DJ: CyBase:
a database of cyclic protein sequence and structure. Nucleic Acids
Res. 34:D192–D194. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Wang CK, Kaas Q, Chiche L and Craik DJ:
CyBase: a database of cyclic protein sequences and structures, with
applications in protein discovery and engineering. Nucleic Acids
Res. 36:D206–D210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Yeshak MY, Burman R, Asres K and Göransson
U: Cyclotides from an extreme habitat: characterization of cyclic
peptides from Viola abyssinica of the Ethiopian highlands. J
Nat Prod. 74:727–731. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Craik DJ: Applications of NMR in drug
design: Structure-activity relationships in disulfide-rich
peptides. Protein Peptide Lett. 6:341–350. 1999.
|
|
14.
|
Göransson U and Craik DJ: Disulfide
mapping of the cyclotide kalata B1. Chemical proof of the cystic
cystine knot motif. J Biol Chem. 278:48188–48196. 2003.PubMed/NCBI
|
|
15.
|
Colgrave ML and Craik DJ: Thermal,
chemical, and enzymatic stability of the cyclotide kalata B1: The
importance of the cyclic cystine knot. Biochemistry. 43:5965–5975.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Wong CT, Taichi M, Nishio H, Nishiuchi Y
and Tam JP: Optimal oxidative folding of the novel antimicrobial
cyclotide from Hedyotis biflora requires high alcohol
concentrations. Biochemistry. 50:7275–7283. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Gran L, Sandberg F and Sletten K:
Oldenlandia affinis (R&S) DC. A plant containing
uteroactive peptides used in African traditional medicine. J
Ethnopharmacol. 70:197–203. 2000. View Article : Google Scholar
|
|
18.
|
Tam JP, Lu YA, Yang JL and Chiu KW: An
unusual structural motif of antimicrobial peptides containing
end-to-end macrocycle and cystine-knot disulfides. Proc Natl Acad
Sci USA. 96:8913–8918. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Witherup KM, Bogusky MJ, Anderson PS, et
al: Cyclopsychotride A, a biologically active, 31-residue cyclic
peptide isolated from Psychotria longipes. J Nat Prod.
57:1619–1625. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Gustafson KR, McKee TC and Bokesch HR:
Anti-HIV cyclotides. Curr Protein Pept Sci. 5:331–340. 2004.
View Article : Google Scholar
|
|
21.
|
Barbeta BL, Marshall AT, Gillon AD, Craik
DJ and Anderson MA: Plant cyclotides disrupt epithelial cells in
the midgut of lepidopteran larvae. Proc Natl Acad Sci USA.
105:1221–1225. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Plan MR, Saska I, Cagauan AG and Craik DJ:
Backbone cyclised peptides from plants show molluscicidal activity
against the rice pest Pomacea canaliculata (golden apple
snail). J Agric Food Chem. 56:5237–5241. 2008. View Article : Google Scholar : PubMed/NCBI
|