Introduction
Colorectal cancer (CRC) is a common malignant tumor
with relatively poor prognosis. The highest incidence rates are
found in Australia, New Zealand, Europe and North America, whereas
the lowest rates are found in Africa and South-Central Asia.
However, CRC incidence rates are rapidly increasing in several
areas historically at low risk, including Spain, and a number of
countries within Eastern Asia and Eastern Europe (1). Approximately 608,000 mortalities from
CRC are estimated to occur every year worldwide, accounting for 8%
of all cancer-related mortalities, making it the fourth most common
cause of death from cancer (2).
Chemotherapy for CRC consists of irinotecan, oxaliplatin,
leucovorin and 5-fluorouracil (3),
providing a better prognosis for patients with advanced CRC.
However, its clinical application is greatly limited by the
resistance to chemotherapy and toxic side-effects. Therefore, an
urgent need exists to find new strategies and drugs for CRC
therapy.
Over the past years, owing to its remarkable
anticancer effect and minor toxicity, traditional Chinese medicine
has been of ever-increasing interest for cancer therapy. Oriental
Asiatic scorpion (OAS), which originates from the dried body of
Buthus martensii Karsch, family Buthidae, has been
traditionally used in China for stroke, epilepsy, spasm, migraine
and tetanus, as well as tumors, for almost 2,000 years. Various
toxic polypeptides, usually called scorpion toxins, were considered
to be the major bioactive ingredients of OAS. Recently, one of
these polypeptides, analgesic-antitumor peptide (AGAP), has been
purified and its analgesic and antitumor activities have also been
demonstrated in pharmacological studies (4,5). Small
ubiquitin-related modifier-AGAP (SUMO-AGAP) is a product of
recombinant AGAP (rAGAP) linked with a hexa-histidine tag by
Escherichia coli (E. coli). In this expression
system, SUMO was capable of significantly enhancing the expression
and efficiently improving the accurate folding of AGAP; thereby,
rAGAP significantly inhibited the proliferation of lymphoma and
glioma (5). Accordingly, rAGAP
appears to be an ideal candidate drug for antitumor therapy.
However, so far no study has been published on the antitumor
effects of rAGAP on CRC. It is well known that cell cycle arrest
and apoptosis inhibition are two major action mechanisms of
antitumor drugs. Recent studies revealed that both the p27 and
PTEN/PI3K/Akt pathways played important roles in cell cycle
progression, while Bcl-2 family proteins regulated mitochondrial
membrane permeability and played an important role in the
mitochondrial apoptosis pathway (6).
In the present study, the effects of rAGAP on
viability, proliferation and apoptosis of SW480 colon cancer cells
were analyzed to demonstrate its antitumor bioactivity in CRC.
Following this, the roles of rAGAP on protein expression of p27 and
Bcl-2/Bax, as well as regulation of PTEN/PI3K/Akt signal
transduction, were further investigated to illustrate the potential
molecular mechanism.
Materials and methods
Preparation of rAGAP
rAGAP was obtained by the expression of
pET28a/SUMO-AGAP in E. coli as previously described
(5). The activity of rAGAP was the
same as described previously (5).
The lyophilized rAGAP powder was dissolved in phosphate-buffered
saline (PBS).
Cell culture, reagents and
antibodies
The human colonic adenocarcinoma cell line SW480 was
obtained from the Type Culture collection of the Chinese Academy of
Sciences (Shanghai, China). When SW480 cells grew to ∼80% of the
whole flask, the medium was removed and washed with PBS 3 times.
Trypsin (0.25%; Invitrogen Life Technologies, Carlsbad, CA, USA)
solution was added into the flask and incubated at 37°C for 1–2
min. Then 4 ml DMEM (containing 10% FBS; Invitrogen Life
Technologies) was added into the flask to terminate digestion.
After being centrifuged for 5 min at a speed of 1,000 rpm, the
medium was replaced by fresh medium and cell suspension was divided
into 2–3 culture flasks. The flasks were incubated in a humidified
atmosphere of 5% CO2 at 37°C for 24 h. MTT and DAPI
reagents were employed (Sigma, St. Louis, MO, USA) as well as
antibodies against p27, Bcl-2, Bax, PTEN, PI3K, Akt, p-Akt and
β-actin (all purchased from Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA).
The study was approved by the Ethics Committee of
Nanjing University of Traditional Medicine (Nanjing, Jiangsu,
China).
MTT assays
SW480 cells were seeded in 96-well plastic plates
(Nunc, Roskilde, Denmark) at a density of 1×104
cells/well and incubated in a humidified atmosphere of 5%
CO2 at 37°C for 24 h. After treatment with various
concentrations of rAGAP (0, 5, 10, 20, 40 and 80 μM) for 24
h, cell viability assays were performed using the MTT method. DMEM
was added in the control group. Then, 5 mg/ml MTT solution (20
μl/well) was added to each well and cells were incubated for
an additional 4 h at 37°C. The medium was carefully removed and
formazan crystals were dissolved in 150 μl DMSO per well,
and the absorbance at 490 nm was measured on a microplate reader
(Bio-Rad Laboratories, Hercules, CA, USA) with DMSO as a blank
control. All MTT assays were conducted independently and in
triplicate. The absorbance values were presented as relative viable
cell number. The growth inhibition was calculated according to the
following formula: Growth inhibition rate (IR%) = (1 − absorbance
values of treated samples / absorbance values of untreated samples)
× 100.
DAPI staining assay
SW480 cells (1×104 per well) were seeded
in six-well plastic plates and incubated for 24 h. Then various
concentrations of rAGAP (0, 5, 10 and 20 μM) were directly
added to the well and incubated for an additional 24 h. Cells were
washed briefly with cold PBS. A quantity of 0.5 ml 4%
paraformaldehyde was added to each well and fixed on ice for 20
min. The supernatant was aspirated and cells were washed twice with
PBS. Sodium citrate (0.1%) containing 0.1% Triton X-100 was added
to each well and cells were incubated for 2 min at 4°C. Then, DAPI
was added to each well after washing with PBS again. The final
concentration was 1 μg/ml and cells were incubated for 10
min on ice in the dark. The supernatant was aspirated and cells
were washed twice with PBS. Apoptotic cells were excitated by
Ultraviolet (UV). Then cell morphology was observed and
photographed under fluorescence microscopy (×400; Zeiss Axio
Observer A1) at 340 nm.
Cell cycle analysis
SW480 cells were seeded onto six-well plates at a
density of 1×106 cells/well and incubated for one day.
Following treatment with various concentrations of rAGAP (0, 5, 10
and 20 μM) for 24 h, the cells were collected and washed
with 1X PBS. Cell pellets were fixed in 70% cold ethanol overnight
at 4°C. The fixed cells were then resuspended in 1X PBS containing
1 mg/ml RNase A, incubated for 1 h at 37°C and the cells were
stained by adding 50 μg/ml DNA-binding dye propidium iodide
(PI) for 30 min at room temperature in the dark. The DNA contents
of the stained cells were analyzed using CellQuest Software with a
FACS Vantage SE flow cytometer (Becton Dickinson, Heidelberg,
Germany). For each sample, a minimum of 104 events were
recorded.
Western blot analysis
SW480 cells were seeded into six-well plates at a
density of 1×106 cells/well for protein extraction.
After SW480 cells were treated with various concentrations of rAGAP
(0, 5, 10 and 20 μM), the cells were lysed with ice-cold
RIPA buffer containing 15 mM Tris-HCl (pH 8.8), H2O, 30%
acrylamide, 10% SDS, 10% ammonium persulfate (AP) and
N,N,N′,N′-tetramethylethylenediamine (TEMED; Biosharp, Seattle, WA,
USA) to obtain the total cell lysates. The total cell lysates were
then centrifuged at 15,000 rpm for 15 min at 4°C to remove the
insoluble materials. Next, the protein concentrations were
determined using a BCA protein assay kit (Thermo Scientific,
Rockford, IL, USA). Protein extracts (50 μg) were analyzed
using 12% polyacrylamide gel electrophoresis and electrotransferred
to nitrocellulose membranes at 150 mA for 1 h. The polyvinylidene
fluoride (PVDF) membranes (Millipore Corporation, Bedford, MA, USA)
were then blocked for 2 h at room temperature with PBS containing
5% skimmed milk and 0.1% Tween-20 and incubated with 1:1,000
dilutions of primary antibodies including anti-p27 (1:500),
anti-Bcl-2 (1:500), anti-Bax (1:500), anti-PTEN (1:200), anti-PI3K
(1:500), anti-Akt (1:500) and anti-p-Akt (1:200) overnight at 4°C
and subsequently with a 1:10,000 dilution of horseradish
peroxidase-conjugated anti-rabbit secondary antibodies (Bioworlde,
Technology, Minneapolis, MN, USA) for 1 h at room temperature.
Peroxidase activity was visualized using the ECL kit (Thermo).
Anti-β-actin (Santa Cruz Biotechnology, Inc.) was used as a loading
control for total lysates and nuclear extracts. Immunoreactive
protein bands were detected with GelDoc 2000 System (Bio-Rad). The
relative protein content was represented through the gray value
ratio of protein bands/β-actin protein bands, and the results were
analyzed with Quantity One software.
Statistical analysis
All data were expressed as the mean ± SD from at
least three independent experiments. One-way ANOVA was used for all
statistical comparisons, and the LSD t-test was conducted for
multiple comparisons. P<0.05 was considered to indicate a
statistically significant result. All analyses were performed using
SPSS ver. 14 (SPSS, Chicago, IL, USA).
Results
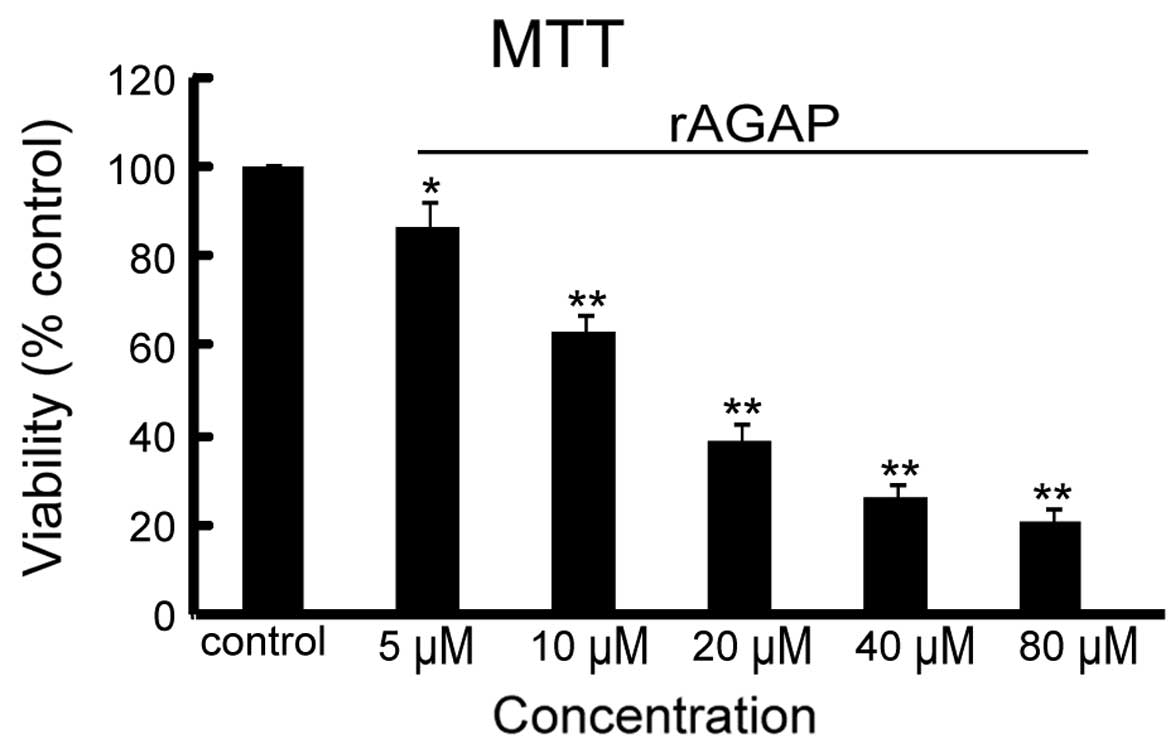
rAGAP inhibited cell viability of
SW480
To assess the effect of rAGAP on cell viability,
different concentrations (5, 10, 20, 40, 60, 80 and 100 μM)
of rAGAP were incubated with SW480 cells for 24 h followed by MTT
assay. As shown in Fig. 1, rAGAP
inhibited the viability of SW480 cells in a dose-dependent manner.
Compared with the control group, the viability of SW480 cells
decreased from 87 to 21.2% at concentrations of 5, 10, 20, 40 and
80 μM of rAGAP in a dose-dependent manner
(IC50=18.4 μM).
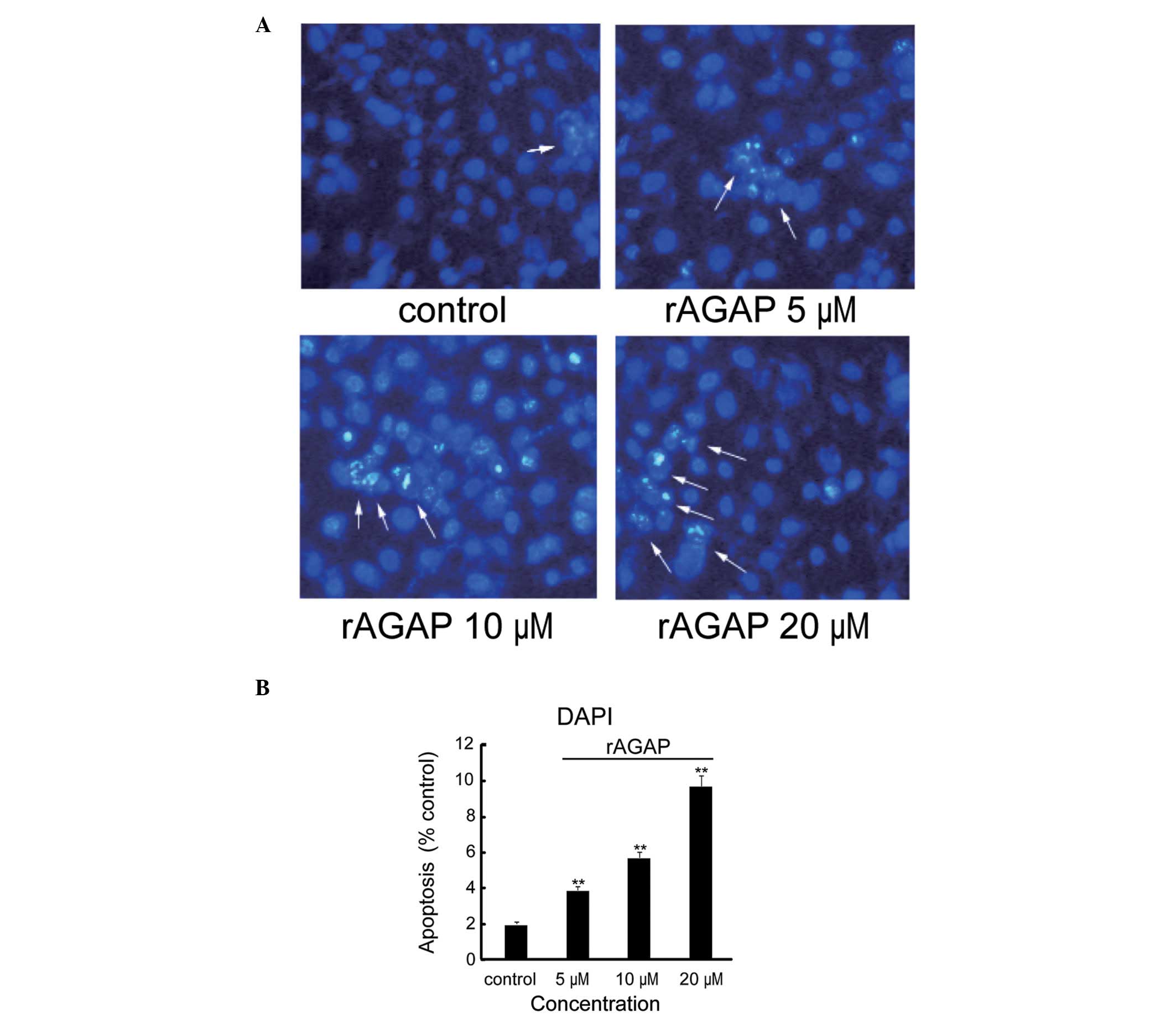
Effect of rAGAP on SW480 apoptosis
DAPI staining was used to investigate the effects of
rAGAP on SW480 cell apoptosis. As shown in Fig. 2, the DAPI dye stained
morphologically normal nuclei blue. Nuclear morphology of the cells
in the control group was round, sharp edged and uniformly stained.
Apoptotic cells were characterized by shrunken and fragmented
nuclei with condensed chromatin. The ratio of apoptotic cells was
2.0, 3.9, 5.7 and 9.8% in the control, 5, 10 and 20 μM rAGAP
groups, respectively [F(3,8)=276.69, P<0.01].
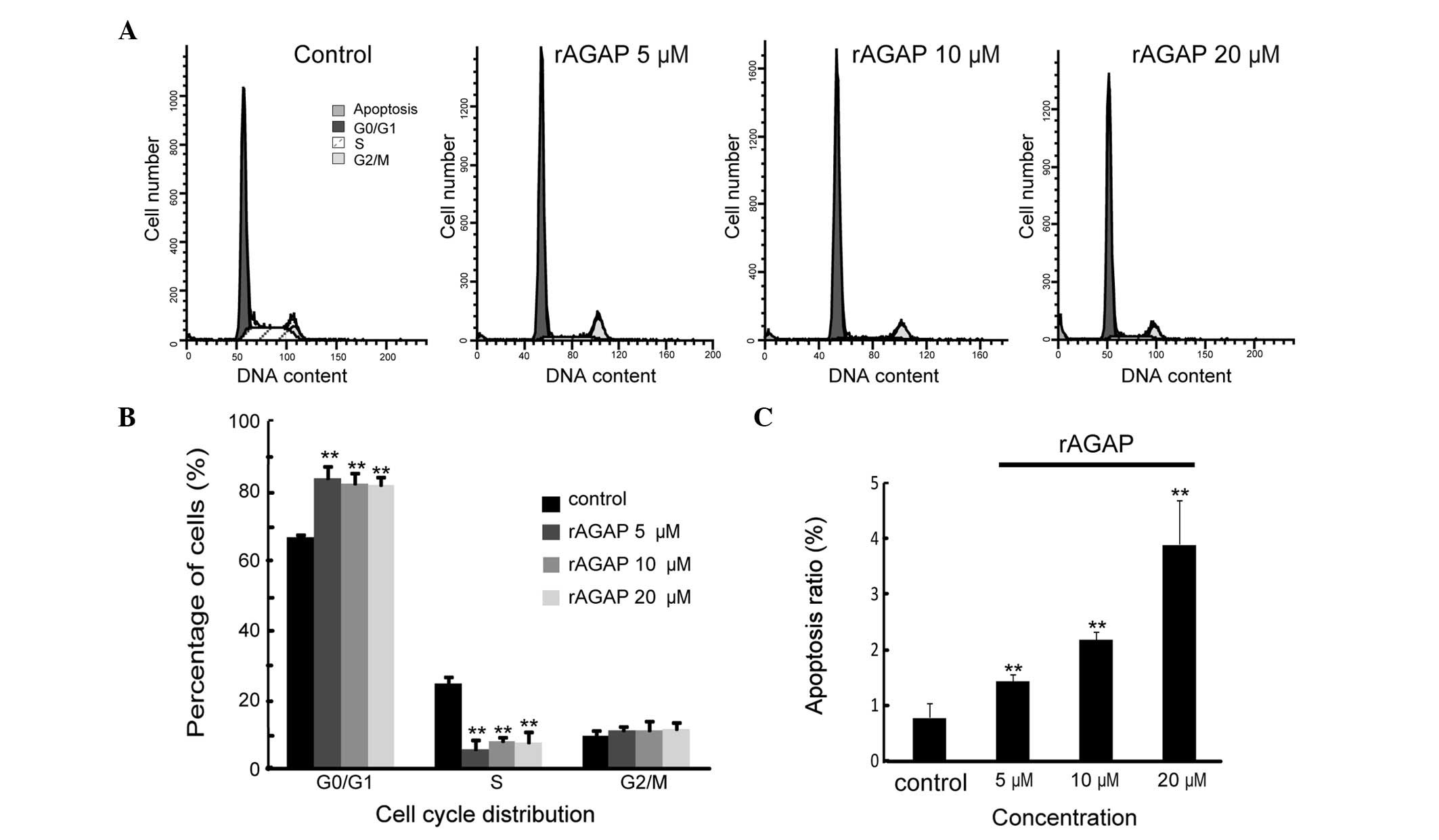
Effects of rAGAP on cell cycle of SW480
cells
To determine the growth inhibitory effect of rAGAP,
the cell cycle distribution of SW480 cells was assessed by flow
cytometry. In the control group, the proportion of G0/G1 phase
cells was 66.5%. Treatment for SW480 cells with 5, 10 and 20
μM rAGAP for 24 h resulted in a significantly increased
proportion of G1 phase cells of 83.42, 81.79 and 81.34%,
respectively [F(3,8)=23.7, P<0.01]. However, there was no
difference in the three drug treatment groups (LSD-t, P<0.05).
Meanwhile, rAGAP treatment significantly decreased the proportion
of S phase SW480 cells (24.25% of control, 5.47, 7.75 and 7.42% of
5, 10 and 20 μM rAGAP groups, respectively) [F(3,8)=44.85,
P<0.01]. The percentage of cells in G2/M phase was 10.87, 10.72
and 11.23% in the 5, 10 and 20 μM rAGAP treatment groups,
respectively, compared with that of the control group (9.24%)
[F(3,8)=0.59, P<0.05]. Moreover, the rate of apoptotic cells was
increased by rAGAP (0.79% for control, 1.45, 2.2 and 3.91% for the
5, 10 and 20 μM rAGAP groups, respectively) [F(3,8)=30.03,
P<0.01] (Fig. 3). These results
demonstrated that rAGAP treatment resulted in cell cycle arrest in
G0/G1 phase and increased apoptosis of SW480 cells.
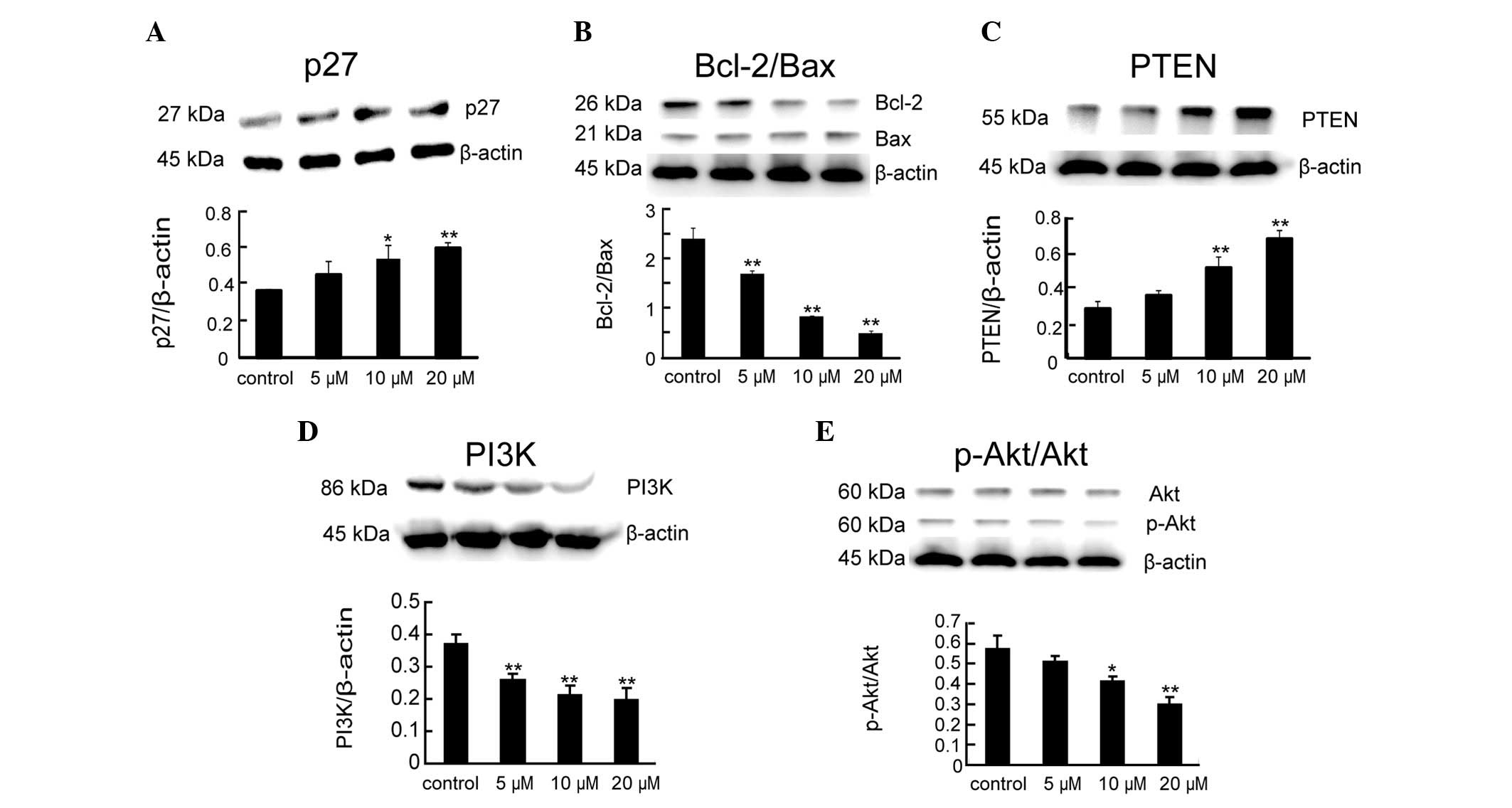
Expression of proliferation and
apoptosis-related protein by rAGAP treatment
Compared with the control group, the protein
expression of p27, a key effector of cell cycle arrest, was
significantly increased by rAGAP treatment for 24 h [F(3,8)=9.6,
P<0.05] (Fig. 4A). To explore
the mechanism of rAGAP on SW480 cell apoptosis, the levels of Bcl-2
and Bax was measured following rAGAP treatment. The results showed
that anti-apoptotic gene Bcl-2 was significantly downregulated. By
contrast, the expression of pro-apoptotic gene Bax was upregulated.
Thus, the ratios of Bcl-2/Bax in SW480 cells with 24 h treatment of
different concentrations of rAGAP was significantly reduced
compared to the control group [F(3,8)=145.1, P<0.01] (Fig. 4B).
Since PTEN-PI3K-Akt signaling plays a critical role
in cell survival, the expression of PTEN, PI3K and Akt in SW480
cells after 24 h rAGAP treatment were measured by western blot
analysis. As shown in Fig. 4C–E,
rAGAP increased the levels of PTEN [F(3,8)=46.14, P<0.01] but
decreased the levels of PI3K [F(3,8)=73.74, P<0.01] and
p-Akt/Akt [F(3,8)=25.19, P<0.01] compared with the control
group. However, the expression of total Akt did not impact
significantly on the protein levels [F(3,8)=4.02, P<0.05].
Discussion
Currently, resistance to chemotherapy is becoming a
major issue in the long-term treatment of CRC patients. Increasing
evidence shows that metastatic CRC patients with v-Ki-ras2 Kirsten
rat sarcoma viral oncogene homolog (KRAS) mutations are resistant
to treatment with monoclonal antibodies such as cetuximab and
panitumumab that target the epidermal growth factor receptor
(7,8). Notably, a number of chemotherapy drugs
currently in use are natural products or derived directly from
natural sources. Therefore, it is feasible to explore natural
products as a potential choice for novel anti-cancer therapeutics
(9,10).
As a traditional Chinese medicine, Quan Xie (the
scorpion Buthus martensii Karsch) has been used in the
treatment of numerous diseases, including tetanus, tuberculosis,
apoplexy, epilepsy, spasm and migraine. Scorpion venom is a complex
mixture of low molecular weight bioactive molecules, small peptides
and enzymes (11,12). Various different toxic peptides
extracted from scorpion venom have different functions. Buthus
martensii Karsch chlorotoxin-like toxin (BmKCT) has a similar
function to CTX, which inhibits the growth and metastasis of glioma
cells (13). Indian black scorpion
(Heterometrus bengalensis) venom inhibits the growth of
leukemic U937 and K562 cells by inducing cell apoptosis. Flow
cytometric assay revealed that Heterometrus bengalensis
venom blocked the cell cycle at sub-G1 phase (14). BMK-CBP, a serine proteinase-like
protein isolated from the venom of Chinese scorpion (Buthus
martensii Karsch) dose-dependently inhibits the proliferation
of the MCF-7 cancer cell line (15). AGAP is a peptide extracted from
scorpion venom. Previous studies showed that AGAP inhibited the
mRNA transcription of Nav1.5. Therefore, it is suggested that AGAP
may be a Na+-channel specific inhibitor (4). rAGAP, a fusion protein consisting of a
hexa-histidine (His6) tag, SUMO and AGAP, was overexpressed in
E. coli(5). The antitumor
activity of rAGAP has been confirmed in several studies (4,5).
The cell cycle consists of the pre-DNA synthesis
phase (G1), DNA synthesis phase (S), DNA post-synthetic phase (G2)
and the phase of mitosis (M). In addition, cells are able to stop
mitosis and move from G1 phase of the cell cycle into G0 phase
(stationary phase) temporarily. Cell cycle arrest is one of the
most important mechanisms of antitumor drug action. If the cell
cycle is blocked in G1 phase, unlimited proliferation of tumor
cells would be controlled (16,17).
Our MTT experimental results indicated the notable ability of rAGAP
to inhibit the variability of SW480 cells. Moreover, flow cytometry
assay showed that rAGAP induced SW480 cell cycle arrest at G0/G1
phase, accompanied by the reduction in S phase but no significant
change in G2/M phase. As a result, the cell cycle could not pass
through the G1/S restriction point and was prevented from G1 to S
phase transition (18,19).
To determine the molecular mechanism of G0/G1 cell
cycle arrest induced by rAGAP, we further examined the expression
of cell cycle regulatory protein p27 and the PTEN/PI3K/Akt pathway.
p27, a member of the CDK inhibitor family, is a tumor suppressor
gene which blocks phosphorylation of the Rb protein, thus
inhibiting cell growth and proliferation. When growth signals are
lacking, p27 accumulates in cells and combines with cyclin E-CDK2
to inhibit its function. Thereby, p27 is a potential mediator of
extracellular stimulation signals to regulate the cell cycle and
negatively regulates the cell cycle to inhibit cell growth and
tumor formation. p27 protein stability can also be regulated by the
PTEN/PI3K/Akt pathway (20–22). The PI3K pathway, which is activated
in G1/S transition, contributes to cell proliferation, growth and
resistance to therapy for a number of cancers. An accumulation of
evidence supports a key role for the PI3K pathway in cell cycle
progression (23,24). PTEN is the only tumor suppressor
gene involved in the PI3K/Akt pathway. The PTEN/PI3K/Akt pathway
not only inhibits G1/S cell cycle progression but also plays a key
role in G2/M transition and its constitutive activation may lead to
defects in DNA damage checkpoint control (25). Our results showed that rAGAP
upregulated the expression of p27 and PTEN, while it downregulated
PI3K and p-Akt expression. Therefore, we proposed that rAGAP was
capable of increasing the amount of p27 protein and inhibiting
PI3K/Akt signal transduction, subsequently leading to G0/G1 cell
cycle arrest in SW480 cells.
Apoptosis is an active, programmed and
self-destructive process of gene regulation. In general, the
initiation of apoptosis is divided into intrinsic and extrinsic
apoptotic pathways, which differ in the activation of the signaling
pathways that lead to death. Mitochondria and cell surface
receptors mediate the two main pathways of apoptosis. In the
current study, the intrinsic apoptosis pathway involved with
mitochondria was examined by DAPI and flow cytometry, and the
results indicated that rAGAP had some induction effect on
apoptosis. Expression of cell apoptosis related proteins such as
Bcl-2 and Bax as well as PTEN/PI3K/Akt pathway were examined to
explore the molecular mechanism of apoptosis induction. It is
reported that Bcl-2 family proteins regulate mitochondrial membrane
permeability and play an important role in the mitochondrial
apoptosis pathway (6). Bcl-2 family
proteins includes anti-apoptotic protein (Bcl-xl, Bcl-2, KSHV-Bcl-2
and Bcl-w) and pro-apoptotic proteins (Bax, Bad and Bid) (26). The Bcl-2/Bax ratio may play a key
role in deciding whether the cell switches towards proliferation or
apoptosis (27,28). It has been reported that the
PTEN/PI3K/Akt pathway is constitutively activated in several types
of cancer (29). The PTEN/PI3K/Akt
pathway is also involved in cell apoptosis progression. PTEN plays
a significant role not only in inducing cell cycle arrest but also
programming apoptosis (30). Loss
of PTEN expression is present in 20–40% of CRC tumors assessed by
immunohistochemistry (31–33). PI3K is a lipid kinase that plays an
important role in cell growth, survival and resistance to therapy
in a number of different solid and liquid cancers. Furthermore, Akt
is able to suppress cell apoptosis by inhibiting Bax activation
(34,35). Once activated, p-Akt phosphorylates
other proteins such as NFκB, mTOR, Bad, GSK-3β and MDM-2 to enhance
cell growth, metabolism, survival and proliferation (36–38).
Certain studies suggest that second mitochondria-derived activator
of caspase (Smac) release is suppressed by Akt, Bcl-2 and Bcl-xl,
but promoted by Bax, Bad, and Bid (34,35,39,40).
This evidence suggests that PTEN/PI3K/Akt is not only involved in
the regulation of the apoptotic process directly but also has
interaction with Bcl-2 family members. Our study revealed that
rAGAP upregulated the expression of Bax and caused simultaneous
downregulation of Bcl-2, thereby decreasing the Bcl-2/Bax ratio.
The decrease of Bcl-2 protein level and binding by
nonphosphorylated Bad caused the release of Bax, which was
translocated into the mitochondria, where it activated the
mitochondrial apoptosis pathway (41). As a result, reduction of the ratio
of Bcl-2/Bax subsequently enhanced cell apoptosis. Moreover, our
study showed that rAGAP significantly increased expression of PTEN,
and decreased expression of PI3K and phosphorylation activation of
Akt. Therefore, the intrinsic apoptotic pathway (regulating the
Bcl-2 family) and the PTEN/PI3K/Akt pathway were involved in
rAGAP-induced SW480 colon cancer cell apoptosis.
Taken together, rAGAP inhibits proliferation and
induces apoptosis of SW480 human colon cancer cells. The antitumor
mechanism of rAGAP may contribute to the increase in p27
expression, reduced ratio of Bcl-2/Bax and inhibition of
PTEN/PI3K/Akt signal transition. Our results suggest that rAGAP has
the potential to be developed into new therapeutic agents for
CRC.
Acknowledgements
The authors are grateful to Dr Hui
Kong for the research assistance and useful suggestions. This
research was supported by the Priority Academic Program Development
of Jiangsu Higher Education Institutions.
References
|
1.
|
Center MM, Jemal A, Smith RA and Ward E:
Worldwide variations in colorectal cancer. CA Cancer J Clin.
59:366–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Weekes J, Lam AK, Sebesan S and Ho YH:
Irinotecan therapy and molecular targets in colorectal cancer: a
systemic review. World J Gastroenterol. 15:3597–3602. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Zhao Y, Cai X, Ye T, et al:
Analgesic-antitumor peptide inhibits proliferation and migration of
SHG-44 human malignant glioma cells. J Cell Biochem. 112:2424–2434.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Cao P, Yu J, Lu W, et al: Expression and
purification of an antitumor-analgesic peptide from the venom of
Mesobuthus martensii Karsch by small ubiquitin-related modifier
fusion in Escherichia coli. Biotechnol Prog. 26:1240–1244.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Guo Y, Srinivasula SM, Druilhe A,
Fernandes-Alnemri T and Alnemri ES: Caspase-2 induces apoptosis by
releasing proapoptotic proteins from mitochondria. J Biol Chem.
277:13430–13437. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Amado RG, Wolf M, Peeters M, et al:
Wild-type KRAS is required for panitumumab efficacy in patients
with metastatic colorectal cancer. J Clin Oncol. 26:1626–1634.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Karapetis CS, Khambata-Ford S, Jonker DJ,
et al: K-ras mutations and benefit from cetuximab in advanced
colorectal cancer. N Engl J Med. 359:1757–1765. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Deorukhkar A, Krishnan S, Sethi G and
Aggarwal BB: Back to basics: how natural products can provide the
basis for new therapeutics. Expert Opin Investig Drugs.
16:1753–1773. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the last 25 years. J Nat Prod.
70:461–477. 2007.PubMed/NCBI
|
|
11.
|
du Plessis LH, Elgar D and du Plessis JL:
Southern African scorpion toxins: an overview. Toxicon. 51:1–9.
2008.PubMed/NCBI
|
|
12.
|
Rodriguez de la Vega RC and Possani LD:
Overview of scorpion toxins specific for Na+ channels and related
peptides: biodiversity, structure-function relationships and
evolution. Toxicon. 46:831–844. 2005.PubMed/NCBI
|
|
13.
|
Fan S, Sun Z, Jiang D, et al: BmKCT toxin
inhibits glioma proliferation and tumor metastasis. Cancer Lett.
291:158–166. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Das Gupta S, Debnath A, Saha A, et al:
Indian black scorpion (Heterometrus bengalensis Koch) venom
induced antiproliferative and apoptogenic activity against human
leukemic cell lines U937 and K562. Leukemia. 31:817–825. 2007.
|
|
15.
|
Gao R, Zhang Y and Gopalakrishnakone P:
Purification and N-terminal sequence of a serine proteinase-like
protein (BMK-CBP) from the venom of the Chinese scorpion (Buthus
martensii Karsch). Toxicon. 52:348–353. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Chaudhry MA: Base excision repair of
ionizing radiation-induced DNA damage in G1 and G2 cell cycle
phases. Cancer Cell Int. 7:152007. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Sitko JC, Yeh B, Kim M, et al: SOCS3
regulates p21 expression and cell cycle arrest in response to DNA
damage. Cell Signal. 20:2221–2230. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Narbonne-Reveau K and Lilly M: The
Cyclin-dependent kinase inhibitor Dacapo promotes genomic stability
during premeiotic S phase. Mol Biol Cell. 20:1960–1969. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Liu S and Yamauchi H: p27-Associated G1
arrest induced by hinokitiol in human malignant melanoma cells is
mediated via down-regulation of pRb, Skp2 ubiquitin ligase, and
impairment of Cdk2 function. Cancer Lett. 286:240–249. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Connor MK, Kotchetkov R, Cariou S, et al:
CRM1/Ran-mediated nuclear export of p27(Kip1) involves a nuclear
export signal and links p27 export and proteolysis. Mol Biol Cell.
14:201–213. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Yakes FM, Chinratanalab W, Ritter CA, King
W, Seelig S and Arteaga CL: Herceptin-induced inhibition of
phosphatidylinositol-3 kinase and Akt is required for
antibody-mediated effects on p27, cyclin D1, and antitumor action.
Cancer Res. 62:4132–4141. 2002.PubMed/NCBI
|
|
22.
|
Kerkhoff E, Simpson JC, Leberfinger CB, et
al: The Spir actin organizers are involved in vesicle transport
processes. Curr Biol. 11:1963–1968. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Jones SM and Kazlauskas A:
Growth-factor-dependent mitogenesis requires two distinct phases of
signalling. Nat Cell Biol. 3:165–172. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Liang J, Zubovitz J, Petrocelli T, et al:
PKB/Akt phosphorylates p27, impairs nuclear import of p27 and
opposes p27-mediated G1 arrest. Nat Med. 8:1153–1160. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Liang J and Slingerland JM: Multiple roles
of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell
Cycle. 2:339–345. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Petros AM, Olejniczak ET and Fesik SW:
Structural biology of the Bcl-2 family of proteins. Biochim Biophys
Acta. 1644:83–94. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Chowdhury I, Tharakan B and Bhat GK:
Caspases - an update. Comp Biochem Physiol B Biochem Mol Biol.
151:10–27. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
D'Amelio M, Tino E and Cecconi F: The
apoptosome: emerging insights and new potential targets for drug
design. Pharm Res. 25:740–751. 2008.PubMed/NCBI
|
|
29.
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Di Cristofano A and Pandolfi PP: The
multiple roles of PTEN in tumor suppression. Cell. 100:387–390.
2000.PubMed/NCBI
|
|
31.
|
Laurent-Puig P, Cayre A, Manceau G, et al:
Analysis of PTEN, BRAF, and EGFR status in determining benefit from
cetuximab therapy in wild-type KRAS metastatic colon cancer. J Clin
Oncol. 27:5924–5930. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Loupakis F, Pollina L, Stasi I, et al:
PTEN expression and KRAS mutations on primary tumors and metastases
in the prediction of benefit from cetuximab plus irinotecan for
patients with metastatic colorectal cancer. J Clin Oncol.
27:2622–2629. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Frattini M, Saletti P, Romagnani E, et al:
PTEN loss of expression predicts cetuximab efficacy in metastatic
colorectal cancer patients. Br J Cancer. 97:1139–1145. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Vyas S, Juin P, Hancock D, et al:
Differentiation-dependent sensitivity to apoptogenic factors in
PC12 cells. J Biol Chem. 279:30983–30993. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Majewski N, Nogueira V, Robey RB and Hay
N: Akt inhibits apoptosis downstream of BID cleavage via a
glucose-dependent mechanism involving mitochondrial hexokinases.
Mol Cell Biol. 24:730–740. 2004. View Article : Google Scholar
|
|
36.
|
Engelman JA, Luo J and Cantley LC: The
evolution of phosphatidylinositol 3-kinases as regulators of growth
and metabolism. Nat Rev Genet. 7:606–619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Carnero A: The PKB/AKT pathway in cancer.
Curr Pharm Des. 16:34–44. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Song G, Ouyang G and Bao S: The activation
of Akt/PKB signaling pathway and cell survival. J Cell Mol Med.
9:59–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Liu J, Yin S, Reddy N, Spencer C and Sheng
S: Bax mediates the apoptosis-sensitizing effect of maspin. Cancer
Res. 64:1703–1711. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Maianski NA, Geissler J, Srinivasula SM,
Alnemri ES, Roos D and Kuijpers TW: Functional characterization of
mitochondria in neutrophils: a role restricted to apoptosis. Cell
Death Differ. 11:143–153. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Kang MH and Reynolds CP: Bcl-2 inhibitors:
targeting mitochondrial apoptotic pathways in cancer therapy. Clin
Cancer Res. 15:1126–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|