Introduction
Lymphoepithelioma-like gastric carcinoma (LELC) is a
rare type of gastric carcinoma with characteristic
clinicopathological features (1–3). The
majority of these tumors have been revealed to be associated with
Epstein-Barr virus (EBV) infection (4). The development of epithelioid
granulomas, named sarcoid-like reactions, is extremely rare in
LELCs (5). The mass lesion may be
misdiagnosed as a lymphoma, gastrointestinal stromal tumor (GIST)
or carcinoid tumor. In the present study, the pathological results
and CT findings of an EBV-associated LELC with epithelioid
granulomas are described.
Case report
A 53-year-old Chinese male was admitted to Nanfang
Hospital, Southern Medical University, Guangzhou, China, with a
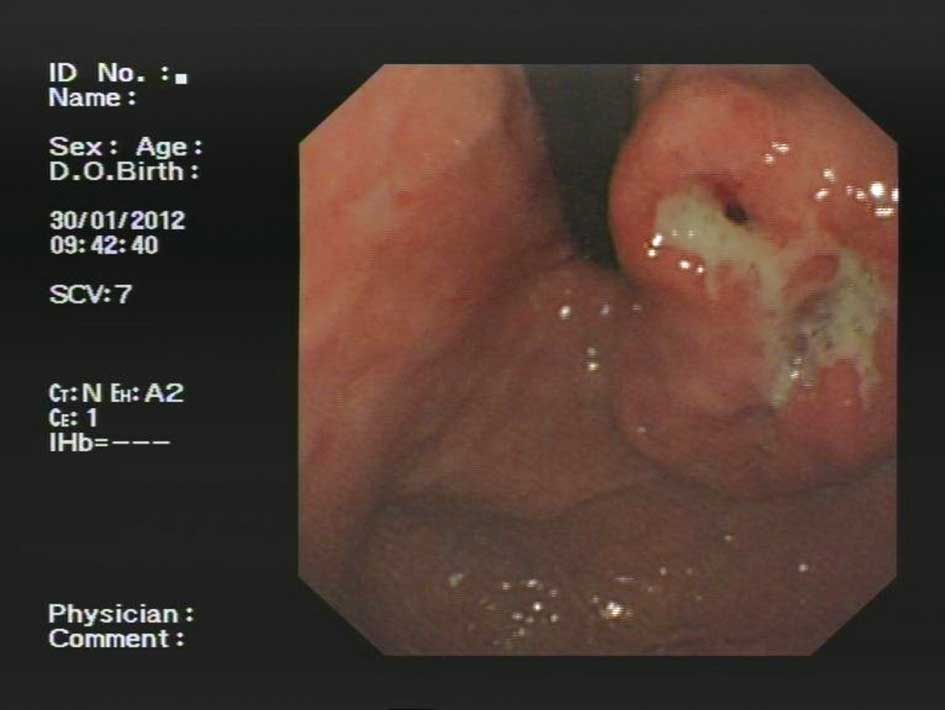
dull epigastric pain. Gastic endoscopy revealed a submucosal mass
in the lesser curvature of the upper body with scattered ulcers on
the mucosal surface (Fig. 1). The
laboratory findings were unremarkable. A total gastrectomy was
performed to remove the gastric mass. The patient remains alive
with no evidence of recurrence after a five-month follow-up
period.
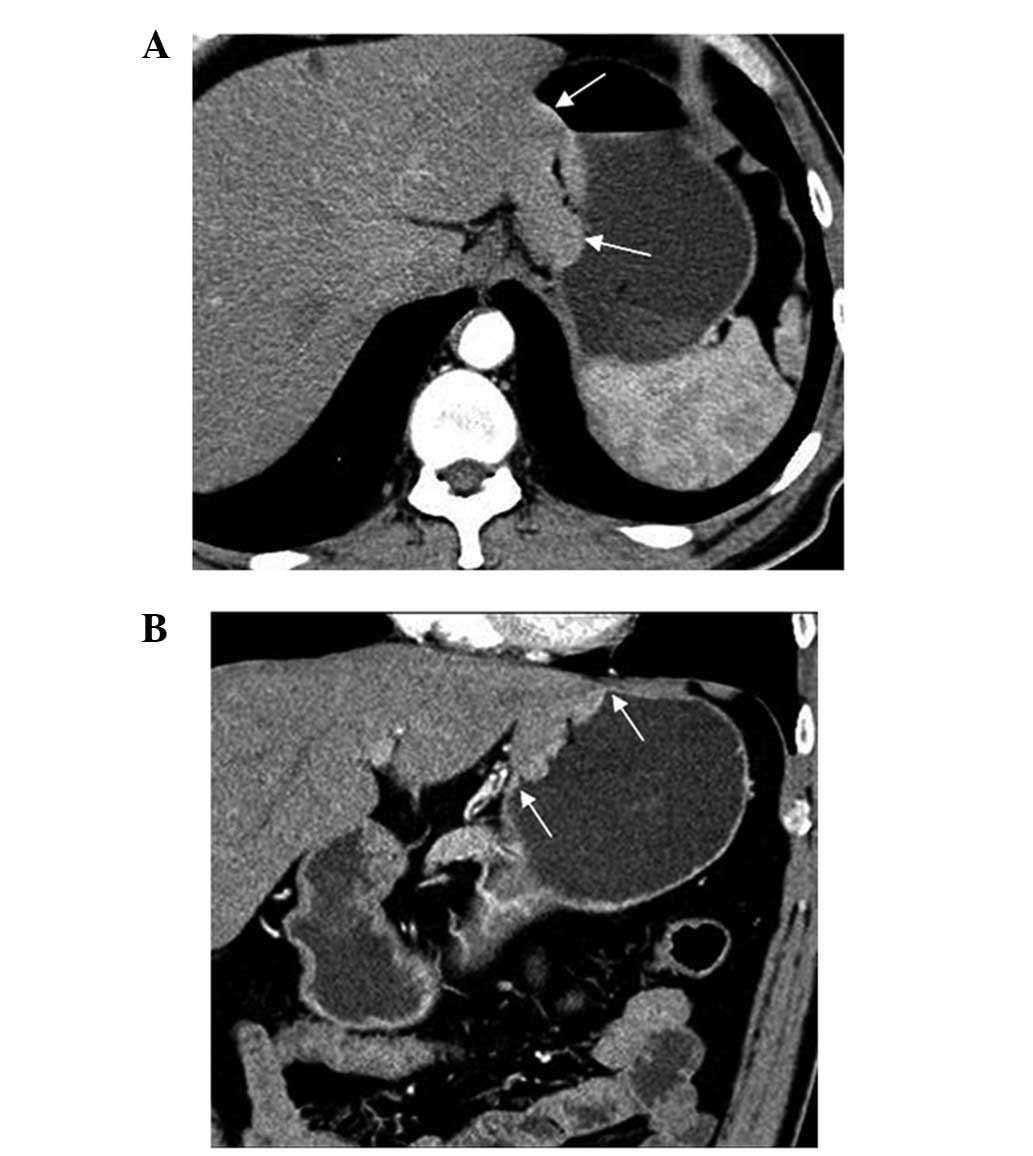
A contrast-enhanced CT of the abdomen using a CT
scanner (Siemens Somatom Plus 4; Siemens Inc., Munich, Germany) was
performed at the hospital. The contrast-enhanced CT scan and
coronal reformatted image showed a bulging mass at the lesser
curvature wall of the gastric upper body near the cardia, with a
large tumor thickness-to-width ratio (see arrows on Fig. 2A). The low-density stripe of the
normal gastric wall abruptly terminated at the edge of the lesion
(see arrows on Fig. 2B). There was
no evidence of perigastric infiltration, enlarged lymph nodes or
distant metastasis from the CT.
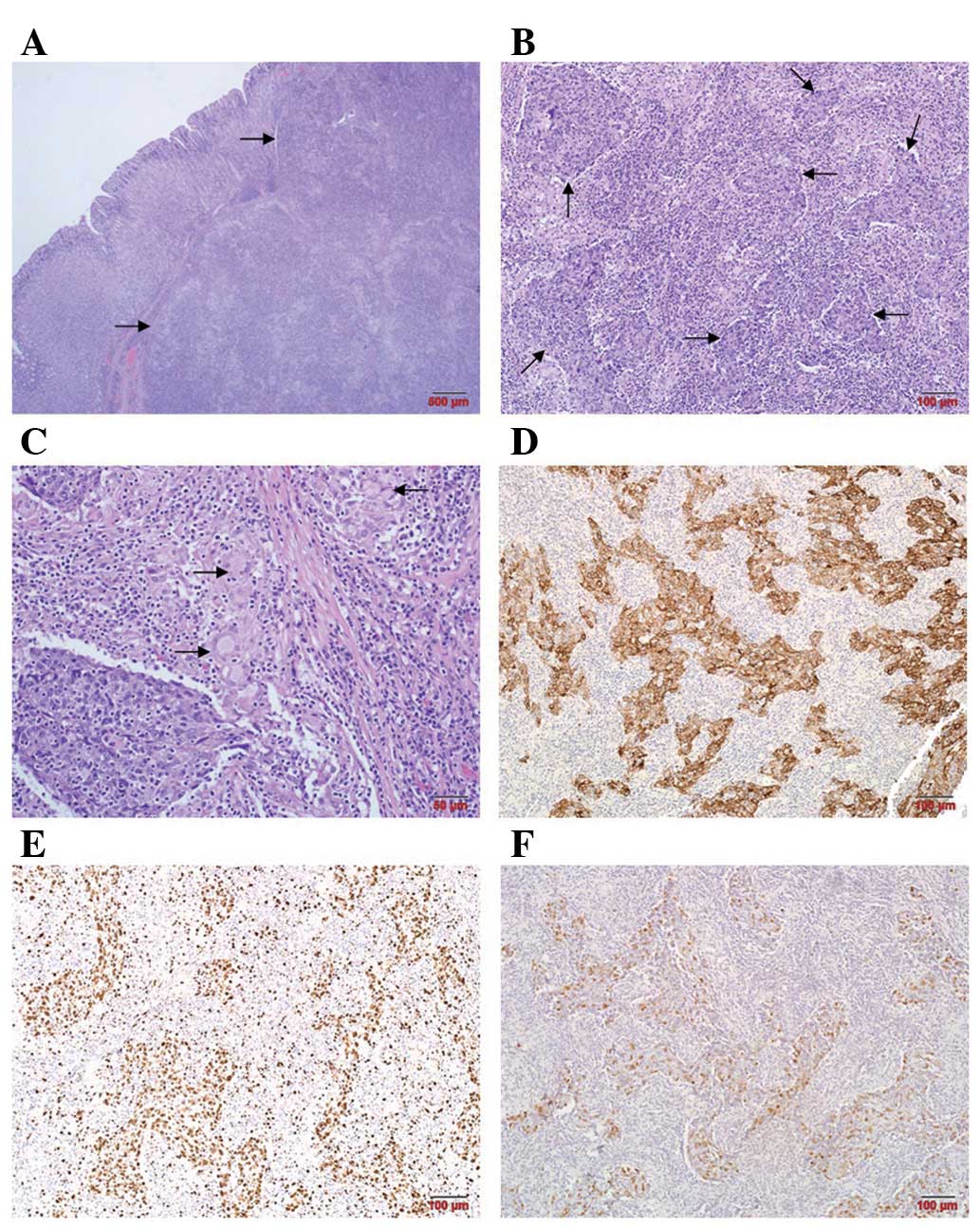
Macroscopically, the resected specimen appeared as a
7.0×5.0×2.0-cm mass with ulcerations on the mucosa. Using light
microscopy, a cross-section of the resected specimen at low
magnification showed a circumscribed, superficially depressed
ulcerated mass. The majority of the lesion was located in the
submucosa (Fig. 3A). The tumor had
invaded the subserosal adipose tissue. Histological examination
revealed a poorly differentiated adenocarcinoma with marked
peritumoral infiltration by lymphoid cells (Fig. 3B). Epithelioid granulomas with
Langhans-type giant cells were noted in the lymphoid stroma
(Fig. 3C). No lymph node metastasis
was exhibited in 31 regional lymph nodes. Immunohistochemical
staining indicated that the tumor cells were strongly positive for
Ckpan (Fig. 3D).
Immunohistochemistry for Ki67 revealed a high proliferation index
(>90%; Fig. 3E). No expression
of chromogranin (CgA), synaptophysin (SYN) or neuron-specific
enolase (NSE) was observed (data not shown). In situ
hybridisation for EBV-encoded RNA-1 (EBER-1) revealed strong
nuclear staining in the tumor cells, while the background
lymphocytes were negative (Fig.
3F).
Discussion
EBV-associated gastric carcinoma is defined by the
presence of EBV in gastric carcinoma cells and its absence in
normal epithelium or dysplastic lesions (6). EBV-associated gastric carcinoma
constitutes ∼8.7% of all cases of gastric carcinoma (3). It occurs in two histological patterns,
LELC and ordinary gastric carcinoma. Of LELCs, 86–91% are positive
for EBV, compared with ∼6% of diffuse and 7% of intestinal
adenocarcinomas (3,6–9).
LELC, which constitutes ∼4% of all gastric
carcinomas, is a relatively rare type of gastric carcinoma,
characterized by morphological features similar to undifferentiated
nasopharyngeal carcinoma (6). It
occurs in old age, predominantly in males, and arises in the cardia
or middle portion of the stomach. Pathological findings indicate
that the cancer cells, which are arranged primarily in
microalveolar, thin trabecular and primitive tubular patterns or as
isolated cells, are surrounded by a dense population of
non-neoplastic small lymphocytes (10–12).
LELCs are characteristically accompanied by
prominent lymphocyte infiltration, particularly in the submucosa.
EBV is considered to be the main cause of the lymphocytic response
(13,14). The pathogenesis of the abundant
lymphoid stroma in the submucosal layer remains unknown, but it is
hypothesized that the formation of the submucosal mass is induced
by the lymphocytic reaction. Granulomatous reactions, or sarcoid
reactions, have been reported to occur in the regional lymph nodes
of malignant tumors. However, numerous epithelioid granulomas with
multinucleated giant cells are extremely rare in the tumor tissue,
particularly of LELCs. To the best of our knowledge, only two
studies have been published in English concerning epithelioid
granulomas occurring in LELC of the stomach (5,6). In
the present case, there was no lymph node or distant organ
metastasis. This is because the spread of tumors through the
gastric wall may be prevented by abundant lymphocytic reactions and
granulomatous reactions. LELC has a more favorable prognosis than
other forms of EBV-associated gastric carcinoma, as well as
ordinary gastric carcinomas, although the tumor cells are often of
the poorly differentiated type (14,15).
The presence of epithelioid granulomas in the stroma with prominent
lymphocyte infiltration may represent a host defense reaction
against the cancer and is recognized as a favorable prognostic
factor with regard to the immune response to the tumor (5).
Although LELCs have distinct clinicopathological
features, they are not familiar to most radiologists. The CT
appearance of LELCs with epithelioid granulomas has not been
reported previously. In the present case, the advanced lesion was
located in the gastric upper body near the cardia and appeared as a
bulging mass with a large thickness-to-length ratio. The
low-density stripe of the normal gastric wall abruptly terminated
at the edge of the lesion. The majority of the lesion was revealed
to be located in the submucosa by pathological investigation. It
has been reported that the growth pattern with a larger
thickness-to-length ratio is the characteristic appearance of
EBV-associated lesions (16).
Various factors, including epithelioid granulomas and lymphocytic
induction, may contribute to the formation of the submucosal mass.
Maeda et al reported that EBV-associated gastric carcinomas
had various appearances in a CT study, including focal mucosal
thickening, marked wall thickening with contrast enhancement and
bulky portions projecting from the gastric wall (17). It is difficult to differentiate
between LELCs presenting as a bulging mass and lymphomas, GISTs,
neurogenic tumors and glomus tumors by imaging alone. It has been
reported that the ulcerated shape of advanced EBV-associated
gastric carcinomas is associated with the superficial depressed
shape of the early lesions (18).
However, in the present case, the tumor tissue with numerous
epithelioid granulomas exhibited expansive growth and formed a
nodule after invading the submucosa. The preoperative CT features
may suggest the presence of LELCs. When LELC is suspected,
detection of EBV may be performed using gastroscope biopsy
specimens.
In summary, the CT findings of an LELC with
epithelioid granulomas that developed in the gastric body revealed
that it appeared as a bulging mass with abundant lymphoid stroma.
The LELC tumor is a distinct entity associated with a good
prognosis. The case in the present study is under follow-up at
present. Therefore, understanding the radiological and clinical
features of LELC is important in the preoperative diagnosis and in
differentiating this entity from other tumors.
Acknowledgements
The authors are grateful for the
sincere help and excellent technical support provided by the
Laboratory of Pathology at Southern Medical University.
References
|
1.
|
Arikawa J, Tokunaga M, Satoh E, Tanaka S
and Land CE: Morphological characteristics of Epstein-Barr
virus-related early gastric carcinoma: a case-control study. Pathol
Int. 47:360–367. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Chen JN, He D, Tang F and Shao CK:
Epstein-Barr virus-associated gastric carcinoma: a newly defined
entity. J Clin Gastroenterol. 46:262–271. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Murphy G, Pfeiffer R, Camargo MC and
Rabkin CS: Meta-analysis shows that prevalence of Epstein-Barr
virus-positive gastric cancer differs based on sex and anatomic
location. Gastroenterology. 137:824–833. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Fukayama M and Ushiku T: Epstein-Barr
virus-associated gastric carcinoma. Pathol Res Pract. 207:529–537.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Tamura T, Hamada T, Sako T, et al:
Lymphoepithelioma-Like Carcinoma of the Stomach with Epithelioid
Granulomas. Case Rep Gastroenterol. 4:361–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Herath CH and Chetty R: Epstein-Barr
virus-associated lymphoepithelioma-like gastric carcinoma. Arch
Pathol Lab Med. 132:706–709. 2008.PubMed/NCBI
|
|
7.
|
Lee JH, Kim SH, Han SH, An JS, Lee ES and
Kim YS: Clinicopathological and molecular characteristics of
Epstein-Barr virus-associated gastric carcinoma: a meta-analysis. J
Gastroenterol Hepatol. 24:354–365. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Torlakovic G, Snover DC and Torlakovic E:
Simultaneous EBV-positive lymphoepithelioma-like carcinoma and
EBV-negative intestinal-type adenocarcinoma in a patient with
Helicobacter pylori-associated chronic gastritis. Am J Clin
Pathol. 121:237–243. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Sousa H, Pinto-Correia AL, Medeiros R and
Dinis-Ribeiro M: Epstein-Barr virus is associated with gastric
carcinoma: the question is what is the significance? World J
Gastroenterol. 14:4347–4351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Matsunou H, Konishi F, Hori H, et al:
Characteristics of Epstein-Barr virus-associated gastric carcinoma
with lymphoid stroma in Japan. Cancer. 77:1998–2004. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Oda K, Tamaru J, Takenouchi T, et al:
Association of Epstein-Barr virus with gastric carcinoma with
lymphoid stroma. Am J Pathol. 143:1063–1071. 1993.PubMed/NCBI
|
|
12.
|
Lü BJ, Lai M, Cheng L, Xu JY and Huang Q:
Gastric medullary carcinoma, a distinct entity associated with
microsatellite instability-H, prominent intraepithelial lymphocytes
and improved prognosis. Histopathology. 45:485–492. 2004.
|
|
13.
|
Shah KM and Young LS: Epstein-Barr virus
and carcinogenesis: beyond Burkitt’s lymphoma. Clin Microbiol
Infect. 15:982–988. 2009.
|
|
14.
|
Song HJ, Srivastava A, Lee J, et al: Host
inflammatory response predicts survival of patients with
Epstein-Barr virus-associated gastric carcinoma. Gastroenterology.
139:84–92. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Wu MS, Shun CT, Wu CC, et al: Epstein-Barr
virus-associated gastric carcinomas: relation to H. pylori
infection and genetic alterations. Gastroenterology. 118:1031–1038.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Nishikawa J, Yanai H, Mizugaki Y, Takada
K, Tada M and Okita K: Case report: hypoechoic submucosal nodules:
a sign of Epstein-Barr virus-associated early gastric cancer. J
Gastroenterol Hepatol. 13:585–590. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Maeda E, Akahane M, Uozaki H, et al: CT
appearance of Epstein-Barr virus-associated gastric carcinoma.
Abdom Imaging. 34:618–625. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Yanai H, Nishikawa J, Mizugaki Y, et al:
Endoscopic and pathologic features of Epstein-Barr virus-associated
gastric carcinoma. Gastrointest Endosc. 45:236–242. 1997.
View Article : Google Scholar : PubMed/NCBI
|