Introduction
Acute lymphoblastic leukemia (ALL) is the most
common malignancy in children. It accounts for approximately 25% of
all childhood cancers and almost 75% of childhood leukemias.
Treatment results in childhood ALL are one of the true success
stories of modern clinical oncology with an overall cure rate
currently approaching more than 85% in the developed world, mainly
through the application of intensive multi-agent chemotherapeutic
regimens (1,2).
This therapeutic progress is the result of treatment
advances that began with the identification of effective single
agent chemotherapy in the late 1940s, followed by development of
combination chemotherapy and maintenance chemotherapy in the 1950s
and early 1960s and the implementation of effective central nervous
system (CNS) preventive therapy in the 1960s and 1970s (3).
CNS-directed therapy is a key contributing factor to
improving survival among children with ALL. When cranial radiation
was linked to neurocognitive deficits, therapeutic regimens were
modified to reduce or eliminate cranial radiation and substituted
it with intensified intrathecal and systemic chemotherapy. These
CNS-directed therapies could also influence the risk of late
neurological outcomes (4).
Neurological complications are common, both during
and following completion of therapy (5). Common neurological complications
developing after completion of ALL treatment include
leukoencephalopathy and neurocognitive defects (6).
Several studies have used magnetic resonance imaging
(MRI) to detect neurologic complications in patients treated for
ALL. A wide range of results have been reported (7,8).
Hemosiderin and white matter lesions are two of the
most common neurological complications found on MRI that may be
related to cranial irradiation and intrathecal methotrexate (MTX)
therapy in childhood ALL (5).
We aimed to determine the prevalence and
characteristics of late CNS damage by MRI and clinical examination
in children treated for ALL.
Materials and methods
Patients
This study was carried out at the outpatient clinic
of the Pediatric Oncology Unit of Zagazig University Hospital and
the MRI Unit of the Radiodiagnosis Department of Zagazig University
between September 2010 and August 2011. It included 25 patients who
were consecutively enrolled and treated according to the modified
Children’s Cancer Group (CCG) 1991 protocol for standard risk ALL
and modified CCG 1961 protocol for high-risk ALL and who had
survived more than 5 years from the diagnosis. The modified CCG
1991 protocol for standard risk ALL and modified CCG 1961 protocol
for high-risk ALL have been applied as a unified protocol in Egypt
since 2004.
All relevant data were collected from patients’
medical records, specifically those concerning the initial clinical
presentation and initial brain imaging.
All patients were subjected to: i) Thorough history
and full physical examination with special emphasis on the
neurological system; ii) MRI of the brain using Philips Achieva
class II MRI 1.5-T scanner (Philips Medical Systems, Best, The
Netherlands) using T1-weighted (T1W) sagittal spin-echo [repetition
time (TR), 500 msec; echo time (TE), 15 msec], T2-weighted
transverse fast spin-echo (TR, 3,300 msec; TE, 100 msec), GE
transverse (TR, 300 msec; TE, 30 msec; flip angle, 30°), and fluid
attenuated inversion recovery (FLAIR) coronal (TR, 8,000 msec; TE,
110 msec; T1, 2,400 msec) sequences.
Definitions of abnormal MRI findings
Leukoencephalopathy
Hyperintense white matter abnormalities were graded
according to a modification of the system of Wilson et al,
1991. Grade I was defined as patchy, mildly increased signal
intensity in the periventricular white matter, grade II as moderate
changes that extend almost to the gray-white junction, sparing the
subcortical U-fibers, and grade 3 as severe changes, confluent from
the level of the frontal horns to that of the trigones, with or
without involvement of the U-fibers.
Brain atrophy
The definition was based on visual evaluation of the
width of cortical sulci and the size of the ventricles and was
divided into three grades (mild, moderate and severe).
Old infarcts
Old infarcts were diagnosed by the detection of
brain parenchymal loss pertaining to arterial territory or discrete
lesions with hypointensity on T1-weighted images and hyperintensity
on T2-weighted images.
Old hemorrhages
Old hemorrhages were defined as focal rounded areas
of very low signal intensity (attributable to the presence of
hemosiderin) detected in any part of the brain.
Summary of modified CCG 1991 protocol
for standard risk ALL
Patients were eligible for this protocol if they had
previously untreated ALL with >25% blasts (L1 or L2 morphology)
in bone marrow, age 1–9.99 years and initial WBC
<50.000/μl. Patients with CNS disease at diagnosis, overt
testicular leukemia, FAB L3 and T-cell ALL (T-ALL) were not
eligible.
Patients received induction chemotherapy for one
month (i.v. vincristine, p.o. dexamethasone, i.m. L-asparaginase,
i.t. MTX and i.t. Ara-C), consolidation therapy for 4 weeks (i.v.
vincristine, p.o. 6-mercaptopurine and i.t. MTX), interim
maintenance I for 2 months (i.v. vincristine, p.o. dexamethasone,
p.o. 6-mercaptopurine, p.o. MTX and i.t. MTX), delayed
intensification for 2 months (i.v. vincristine, i.v. doxorubicin,
p.o. dexamethasone, i.m. L-asparaginase, i.v. cylophosphamide, p.o.
6-thioguanine, i.v. or s.c. Ara-C and i.t. MTX), interim
maintenance II for 2 months (same as interim maintenance I) and
maintenance 12-week cycles (i.v. vincristine, p.o. dexamethasone,
p.o. 6-mercaptopurine, p.o. MTX and i.t. MTX). Therapy was
continued for 2 calendar years for girls and 3 calendar years for
boys.
Summary of modified CCG 1961 protocol
for high-risk ALL
Patients were eligible for this protocol if they had
previously untreated ALL with >25% blasts (L1 or L2 morphology)
in bone marrow. Patients with FAB L3 were not eligible.
Standard arm
The standard arm included patients aged 1–9.99 years
with initial WBC >50.000/μl, patients aged >10 years
with any WBC count, patients with overt testicular leukemia and
patients with T-ALL. Patients in the standard arm received
induction chemotherapy for one month (same as standard risk plus
i.v. doxorubicin), consolidation therapy for 5 weeks (i.v.
cylophosphamide, p.o. 6-mercaptopurine, i.v. or s.c. Ara-C and i.t.
MTX), interim maintenance I for 2 months (p.o. 6-mercaptopurine,
p.o. MTX and i.t. MTX), delayed intensification for 2 months (i.v.
vincristine, i.v. doxorubicin, p.o. dexamethasone, i.m.
L-asparaginase, i.v. cylophosphamide, p.o. 6-thioguanine, i.v. or
s.c. Ara-C and i.t. MTX), interim maintenance II for 2 months (same
as interim maintenance I) and maintenance 12-week cycles (same as
standard risk treatment). Therapy was continued for 2 calendar
years for girls and 3 calendar years for boys.
Augmented arm
The augmented arm included patients with CNS disease
at diagnosis and patients with poor response at day 14 (both
standard and high risk). Patients received induction chemotherapy
for one month (same as standard risk treatment plus i.v.
doxorubicin), consolidation for 9 weeks (i.v. vincristine, i.v.
cylophosphamide, i.m. L-asparaginase, p.o. 6-mercaptopurine, i.v.
or s.c. Ara-C and i.t. MTX plus cranial radiotherapy 18 Gy for
patients without CNS disease at diagnosis and 24 Gy for those with
CNS disease at diagnosis), interim maintenance I and II, each for 2
months (i.v. vincristine, i.v. MTX, i.m. L-asparaginase and i.t.
MTX), delayed intensification I and II for 8 weeks (i.v.
vincristine, i.v. doxorubicin, p.o. dexamethasone, i.m.
L-asparaginase, i.v. cylophosphamide, p.o. 6-thioguanine, i.v. or
s.c. Ara-C and i.t. MTX) and maintenance 12-week cycles (same as
standard risk treatment). Therapy was continued for 2 calendar
years for girls and 3 calendar years for boys.
Ethics
The study was performed in accordance with ethical
standards and with the Helsinki Declaration of 1964, as revised in
2000. The study was approved by the local ethics committee and
informed consent was obtained from the study participants.
Statistical analysis
Data were checked, entered and analyzed using SPSS
version 11. Data are expressed as the mean ± standard deviation for
quantitative variables, and as a number and percentage for
qualitative ones. Paired t-test and Chi-square (χ2)
tests were used when appropriate. P<0.05 was considered to
indicate a statistically significant result.
Results
Patient characteristics
The demographic, clinical and laboratory data of the
patients are listed in Table I.
 | Table I.Demographic, clinical and laboratory
data of patients. |
Table I.
Demographic, clinical and laboratory
data of patients.
| Parameter | n | % |
|---|
| Age at diagnosis
(years) | | |
| Mean ± SD | 6.9±3.04 |
| Range | 2.5–13 |
| Age at study
(years) | | |
| Mean ± SD | 12.9±3.2 |
| Range | 8.5–20 |
| Gender | | |
| Male | 14 | 56.0 |
| Female | 11 | 44.0 |
| Risk | | |
| SR | 15 | 60.0 |
| HR | 10 | 40.0 |
| Protocol of
treatment | | |
| CCG-SR | 15 | 60.0 |
| CCG-HR-SA | 6 | 24.0 |
| CCG-HR-AA | 4 | 16.0 |
|
Immunophenotyping | | |
| Precursor
B-ALL | 22 | 88.0 |
| T-ALL | 3 | 12.0 |
| CNS manifestations at
diagnosis | | |
| Yes | 3 | 12.0 |
| No | 22 | 88.0 |
| Cranial
irradiation | | |
| Yes | 4 | 16.0 |
| No | 21 | 84.0 |
| Late neurological
complications | | |
| None | 23 | 92.0 |
| Epilepsy | 1 | 4.0 |
| Cognitive changes,
behavioral changes, epilepsy | 1 | 4.0 |
MRI findings of patients
Abnormal MRI findings were detected in six patients
(24%). The abnormalities were in the form of leukoencephalopathy in
two patients (one had grade III and the other had grade I) (8%),
brain atrophy in two patients (8%), old infarct in one patient (4%)
and old hemorrhage in one patient (4%) (Table II).
 | Table II.MRI findings of patients (n=25). |
Table II.
MRI findings of patients (n=25).
| MRI findings | n | % |
|---|
| Normal | 19 | 76.0 |
|
Leukoencephalopathy | 2 | 8.0 |
| Brain atrophy | 2 | 8.0 |
| Old infarct | 1 | 4.0 |
| Old hemorrhage | 1 | 4.0 |
Correlation between MRI findings and
demographic data of patients
There was no significant correlation between MRI
findings and the age and gender of patients (P=0.37 and P=0.89,
respectively).
Correlation between MRI findings and
immunophenotyping of leukemic cells
There was no significant correlation between MRI
findings and immunophenotyping (P=0.75).
Correlation between MRI findings and
risk group of patients
There was a significant correlation between MRI
findings and risk group, where abnormal MRI findings were
significantly higher in high-risk patients (P=0.04; Table III).
 | Table III.Correlation between MRI findings and
risk group. |
Table III.
Correlation between MRI findings and
risk group.
| MRI normal (n=19)
| MRI abnormal (n=6)
| | |
|---|
| Risk | n | % | n | % | χ2 | P-value |
|---|
| SR (n=15) | 14 | 73.7 | 1 | 16.7 | 4.03 | 0.04a |
| HR (n=10) | 5 | 26.3 | 5 | 83.3 | | |
Correlation between MRI findings and
treatment protocol
There was a significant correlation between MRI
findings and treatment protocol where abnormal MRI findings were
significantly higher in patients who received CCG high-risk
(augmented arm) protocol (P<0.001; Table IV).
 | Table IV.Correlation between MRI findings and
treatment protocol. |
Table IV.
Correlation between MRI findings and
treatment protocol.
| MRI normal (n=19)
| MRI abnormal (n=6)
| | |
|---|
| Protocol | n | % | n | % | χ2 | P-value |
|---|
| CCG-SR (n=15) | 14 | 73.7 | 1 | 16.7 | | |
| CCG-HR-SA
(n=6) | 5 | 26.3 | 1 | 16.7 | 15.31 | <0.001a |
| CCG-HR-AA
(n=4) | 0 | 0.0 | 4 | 66.6 | | |
Correlation between MRI findings and
CNS manifestations at diagnosis
There was a significant correlation between MRI
findings and CNS manifestations at diagnosis where abnormal MRI
findings were significantly higher in patients with CNS
manifestations at diagnosis (Table
V).
 | Table V.Correlation between MRI findings and
CNS manifestations at diagnosis. |
Table V.
Correlation between MRI findings and
CNS manifestations at diagnosis.
| MRI normal (n=19)
| MRI abnormal (n=6)
| | |
|---|
| CNS manifestations
at diagnosis | n | % | n | % | χ2 | P-value |
|---|
| Yes (n=3) | 0 | 0.0 | 3 | 50.0 | 6.58 | 0.01a |
| No (n=22) | 19 | 100.0 | 3 | 50.0 | | |
Correlation between MRI findings and
cranial irradiation
There was a significant correlation between MRI
findings and cranial irradiation where abnormal MRI findings were
significantly higher in patients who received cranial irradiation
(P<0.001; Table VI).
 | Table VI.Correlation between MRI findings and
cranial irradiation. |
Table VI.
Correlation between MRI findings and
cranial irradiation.
| MRI normal (n=19)
| MRI abnormal (n=6)
| | |
|---|
| Cranial
irradiation | n | % | n | % | χ2 | P-value |
|---|
| Yes (n=4) | 0 | 0.0 | 4 | 66.7 | 10.53 | <0.001a |
| No (n=21) | 19 | 100.0 | 2 | 33.3 | | |
Correlation between MRI findings and
late neurological complications
There was no significant correlation between MRI
findings and late neurological complications (P=0.37).
Discussion
Currently, the overall long-term survival rate in
ALL is approximately 70%, although for individual patients this
varies from 40–90%, depending on prognostic features at diagnosis
and early response to therapy (9,10).
Previously, however, CNS relapse occurred in at least 50% of
patients (11).
This phenomenon led to the introduction of CNS
prophylaxis for children with ALL. The most commonly used method in
the 1970s and 1980s involved cranial irradiation, originally at a
dose of 24 Gy but later at 18 Gy, and intrathecal chemotherapy with
MTX (12). This treatment reduces
the rate of isolated CNS relapse to 5–10% but is associated with
various forms of damage to normal brain tissue, including
leukoencephalopathy, mineralizing microangiopathy (MMA), and the
development of secondary tumors (11).
Cranial irradiation appears to be a notable cause of
long-term neuropsychological impairment (13). Protocols have used risk
stratification to avoid cranial irradiation in children with
standard and intermediate-risk ALL (14,15)
and reduced the dose to 12 Gy in those with high-risk ALL, without
compromising event-free survival (3).
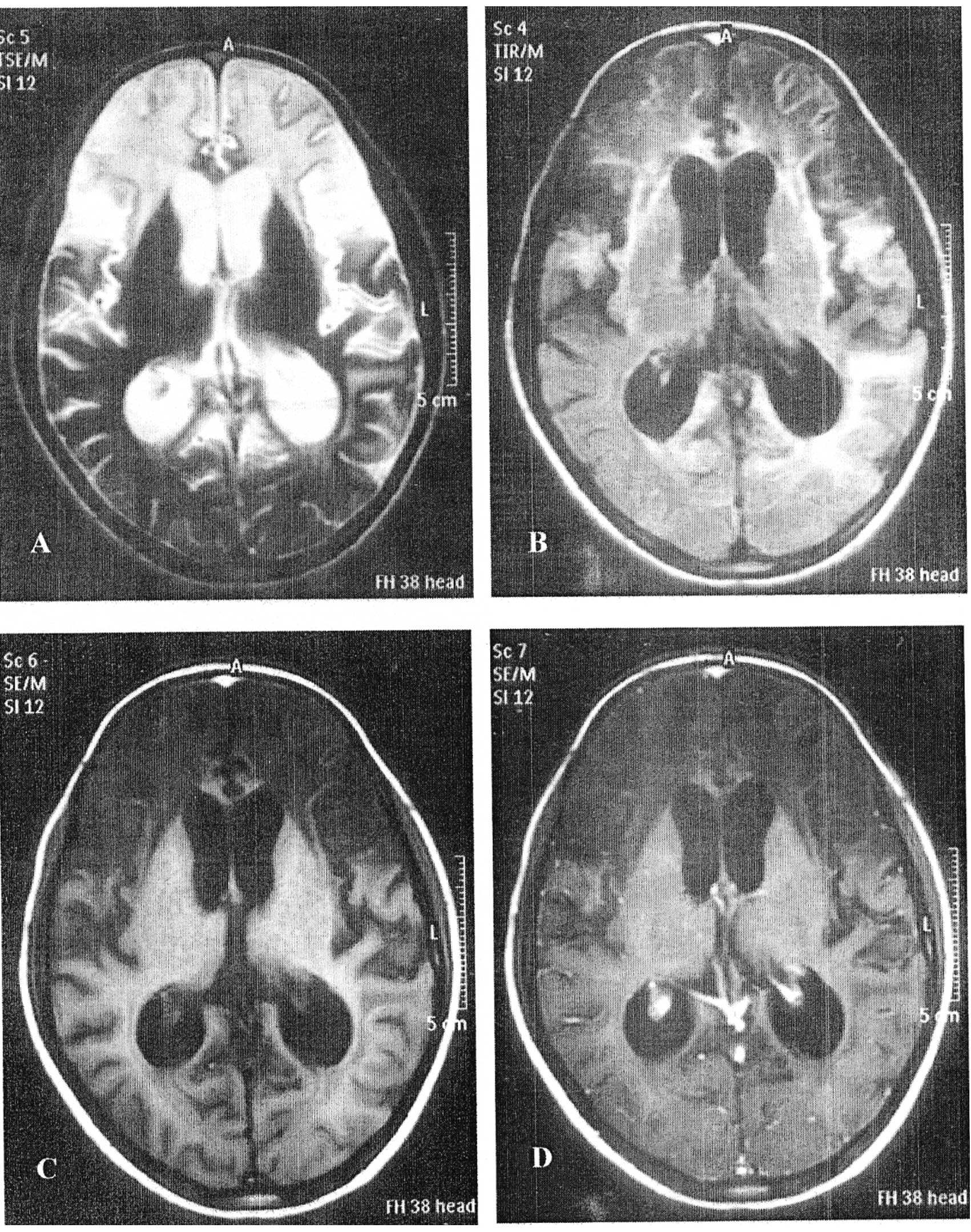
In our study, abnormal MRI findings were detected in
six patients (24%). They were in the form of leukoencephalopathy in
two patients (one had grade III and the other had grade I) (8%),
brain atrophy in two patients (8%), old infarct in one patient (4%)
and old hemorrhage in one patient (4%). The brain MRI of a
13-year-old boy with grade III leukoencephalopathy is shown in
Fig. 1.
Our results are similar to those reported by Ficek
et al(16) where white
matter changes were detected by MRI in three (11%) out of 45 ALL
survivors treated between 1994 and 2002 and examined for 6–12 years
following treatment. All children with MRI abnormalities received
CRT.
Pääkkö et al(7) carried out a prospective study on 33
children with ALL and observed high-intensity white matter changes
by MRI in 3 children (9%) who received chemotherapy only.
Aytaç et al(17) reviewed the data of 256 children with
ALL who were admitted to the Pediatric Hematology Unit of Hacettepe
University, Turkey, between March 1991 and May 2005 and who were
eligible for and treated according to the St. Jude Total XI and
XIII protocols. Abnormal MRI findings were reported in only one out
of five patients, for whom MRI was performed following cessation of
treatment. The abnormalities were in the form of an increase in
white matter intensity.
Chan et al(5)
evaluated the brains of 42 patients diagnosed more than 5 years
previously. Forty of the ALL patients had been treated with cranial
irradiation (at least 18 Gy) and intrathecal MTX, as well as
systemic chemotherapy, and two had been treated with intrathecal
MTX and systemic chemotherapy but had not received cranial
irradiation. Lesions consistent with old hemorrhage were detected
in 23 (55%) of the ALL patients, white matter abnormalities were
found in two patients (5%) while old infarcts were observed in four
patients (10%). Lesions were observed in all 40 patients who
underwent cranial irradiation.
Our results showed that there was no significant
correlation between MRI findings and age, gender or
immunophenotyping of leukemic cells.
Conversely, Pääkkö et al(7) reported that children with white matter
changes were significantly younger than those with normal MRI (mean
age 2.8 vs. 7.4 years).
In our study, cranial irradiation was administered
to only four patients who received the augmented arm of the CCG
high-risk protocol. Three of them were assigned to this arm based
on their initial CNS infiltration and one was assigned based on his
poor response to the standard arm of this high-risk protocol.
In our study, there was a significant (P<0.001)
correlation between MRI findings and cranial irradiation. All
patients who received cranial irradiation developed abnormal MRI
findings while only two out of 21 (9.5%) who received systemic
chemotherapy and intrathecal methotrexate without cranial
irradiation developed abnormal MRI findings. Our results augment
and support the idea that cranial irradiation should be avoided in
patients with standard risk criteria. In support of this theory,
Chan et al(5) and Ficek
et al(16) reported that all
abnormal MRI lesions were observed in patients who underwent
cranial irradiation.
Radiation-induced brain toxicity was explained by
Kim et al(18) who found
that radiation injures the supportive tissues and neurogenic
microenvironment of the nervous system and leads to neuronal loss
or damage. Oxygen-free-radical damage and altered cytokine
responses may influence the development of late delayed damage.
Glial and neuronal stem-cell damage may result in a progressive
demyelination and/or neuronal cell loss.
In our study, there was a significant correlation
between MRI findings and risk group (P=0.04) where five out of six
patients who developed abnormal MRI findings belonged to the
high-risk group and only one belonged to the standard risk group.
Also, there was a significant (P<0.001) correlation between MRI
findings and treatment protocol where the four patients (100%) who
received the CCG high-risk augmented arm protocol developed
abnormal MRI findings while one out of 15 (6.7%) who received the
CCG high-risk standard arm protocol developed abnormal MRI findings
and one out of six (16.7%) who received the CCG standard risk
protocol developed abnormal MRI findings. This finding can be
attributed to the fact that patients who received the CCG high-risk
augmented arm underwent cranial irradiation. In our study, there
was a significant correlation (P= 0.01) between MRI findings and
CNS infiltration at diagnosis where all patients with CNS
infiltration at initial diagnosis developed abnormal MRI findings
and only three out of 22 (13.6%) without CNS infiltration at
diagnosis developed abnormal MRI findings. This can once more be
attributed to patients with CNS infiltration at initial diagnosis
receiving the CCG high-risk augmented arm protocol and undergoing
cranial irradiation.
In our study, only two of our patients developed
late neurological complications. One patient developed recurrent
seizures and proved to have epilepsy. This patient belonged to the
high-risk group and received the CCG high-risk standard arm
protocol. He did not receive cranial irradiation and his MRI
examination was completely normal. The second patient developed
recurrent seizures, abnormal behavioral changes and severe
cognitive changes. This patient belonged to the high-risk group and
received CCG high-risk augmented arm protocol. He received cranial
irradiation and he had grade III leukoencephalopathy by MRI
examination.
The lower incidence (8%) of late neurological
complications in our study can be explained by the fact that most
(84%) of our patients did not receive cranial irradiation. Cranial
irradiation is reserved for patients with CNS infiltration at
initial diagnosis and for those with poor response to the standard
risk protocol and the standard arm of the high-risk protocol. It
can also be explained by the fact that 60% of our patients belonged
to the standard risk group who received tolerable doses of
chemotherapeutics.
In our study, there was no significant correlation
between MRI findings and late neurological complications. This can
be attributed to the small number of patients with late
neurological complications.
We conclude that cranial irradiation is associated
with higher incidence of MRI changes in children treated for ALL.
Limitation of cranial irradiation to selected patients contributed
to the lower incidence of neurological complications in our study.
MRI is a sensitive radiological tool to detect structural changes
in children treated for ALL, even in asymptomatic cases.
References
|
1.
|
Pui CH and Evans WE: Treatment of
childhood acute lymphoblastic leukemia. N Engl J Med. 354:166–178.
2006. View Article : Google Scholar
|
|
2.
|
Tucci F and Arico M: Treatment of
pediatric acute lymphoblastic leukemia. Haematologica.
93:1124–1128. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Schrappe M, Reiter A and Ludwig WD:
Improved outcome in childhood acute lymphoblastic leukemia despite
reduced use of anthracyclines and cranial radiotherapy: Results of
trial ALL-BFM90. German-Austrian-Swiss ALL-BFM study group. Blood.
95:3310–3322. 2000.
|
|
4.
|
Pullen J, Boyett J, Shuster J, et al:
Extended triple intrathecal chemotherapy trial for prevention of
CNS relapse in good-risk and poor-risk patients with B-progenitor
acute lymphoblastic leukemia: A Pediatric Oncology Group study. J
Clin Oncol. 11:839–849. 1993.
|
|
5.
|
Chan MSM, Roebuck DJ, Yuen MP, Li CK and
Chan YL: MR imaging of the brain in patients cured of acute
lymphoblastic leukemia - the value of gradient echo imaging. Am J
Neuroradiol. 27:548–552. 2006.PubMed/NCBI
|
|
6.
|
Gay CT, Bodensteitter JB, Nitschke R,
Sexauer C and Wilson D: Reversible treatment-related
leukoencephalopathy. Child Neurol. 4:208–213. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Pääkkö E, Harila-Saari A, Vanionpää L,
Himanen S, Pyhtinen J and Lanning M: White matter changes on MRI
during treatment in children with acute lymphoblastic leukemia:
correlation with neuropsychological findings. Med Pediatr Oncol.
35:456–461. 2000.PubMed/NCBI
|
|
8.
|
Koike S, Aida N, Hata M, Fujita K, Ozawa Y
and Inoue T: Asymptomatic radiation-induced telangiectasia in
children after cranial irradiation: frequency, latency, and dose
relation. Radiology. 230:93–99. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Forestier E, Johansson B, Gustafsson G,
Borgström G, Kerndrup G, Johannsson J and Heim S: Prognostic impact
of karyotypic findings in childhood acute lymphoblastic leukemia: a
Nordic series comparing two treatment periods. Br J Haematol.
110:147–153. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Donadieu J and Hill C: Early response to
chemotherapy as a prognostic factor in childhood acute
lymphoblastic leukemia: a methodological review. Br J Haematol.
115:34–45. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Schroeder H, Garwicz S, Kristinsson J,
Siimes MA, Wesenberg F and Gustafsson G: Outcome after first
relapse in children with acute lymphoblastic leukemia: a
population-based study of 315 patients from the Nordic Society of
Pediatric Hematology and Oncology. Med Pediatr Onco1. 25:372–378.
1995. View Article : Google Scholar
|
|
12.
|
Nesbit ME Jr, Sather HN, Robison LL,
Ortega J, Littman PS, D’Angio GJ and Hammond GD: Presymptomatic
central nervous system therapy in previously untreated childhood
acute lymphoblastic leukaemia: comparison of 1800 rad and 2400 rad.
A report for Children’s Cancer Study Group. Lancet. 1:461–466.
1981.PubMed/NCBI
|
|
13.
|
Hill JM, Kornblith AB, Jones D, et al: A
comparative study of the long term psychosocial functioning of
childhood acute lymphoblastic leukemia survivors treated by
intrathecal methotrexate with or without cranial radiation. Cancer.
82:208–218. 1998. View Article : Google Scholar
|
|
14.
|
Kamps WA, Bokkerink JP, Hahlen K, et al:
Intensive treatment of children with acute lymphoblastic leukemia
according to ALL-BFM-86 without cranial radiotherapy: results of
Dutch Childhood Leukemia Study Group Protocol ALL-7 (1988–1991).
Blood. 94:1226–1236. 1999.PubMed/NCBI
|
|
15.
|
Hill FG, Richards S, Gibson B, et al:
Successful treatment without cranial radiotherapy of children
receiving intensified chemotherapy for acute lymphoblastic
leukaemia: results of the risk-stratified randomized central
nervous system treatment trial MRC UKALL XI (ISRC TN 16757172). Br
J Haematol. 124:33–46. 2004. View Article : Google Scholar
|
|
16.
|
Ficek K, Blamek S, Syguła D, Miszczyk L,
Sońta-Jakimczyk D and Tarnawski R: Evaluation of the late effects
of CNS prophylactic treatment in childhood acute lymphoblastic
leukemia (ALL) using magnetic resonance spectroscopy. Acta
Neurochir Suppl. 106:195–197. 2010. View Article : Google Scholar
|
|
17.
|
Aytaç S, Yetgin S and Tavil B: Acute and
long-term neurologic complications in children with acute
lymphoblastic leukemia. Turk J Pediatr. 48:1–7. 2006.PubMed/NCBI
|
|
18.
|
Kim JH, Brown SL, Jenrow KA and Ryu S:
Mechanisms of radiation-induced brain toxicity and implications for
future clinical trials. J Neurooncol. 87:279–286. 2008. View Article : Google Scholar : PubMed/NCBI
|