Introduction
Leukemia is a malignant cancer in humans (1). The characteristics of leukemia include
uncontrolled cell growth and disrupted differentiation of
hematopoietic cells (2,3). In Taiwan, 3 per 100,000 individuals
succumbed to leukemia in 2011 according to the Department of
Health, Executive Yuan, R.O.C. (Taiwan; http://www.doh.gov.tw/EN2006/). The clinical therapies
for leukemia include chemotherapy, radiation and bone marrow
transplant (4–6). However, these strategies have not been
shown to be satisfactory for the treatment of leukemia, which has
led to researchers focusing on the discovery of new compounds.
Benzyloxybenzaldehyde derivatives are known for
their multiple biological effects, including antimicrobial
infection (7), anti-inflammatory
effects (8–10), phospholipase D (PLD) inhibition
(10), neutrophil superoxide anion
degeneration (11), adenylyl
cyclase activation (8) and
anticancer activities (12). In
recent years, we have designed and synthesized a new series of
2-benzyloxybenzaldehyde derivatives as potential antileukemic
agents (12). Our previous study
demonstrated that the 2-benzyloxybenzaldehyde analog CCY-1a-E2
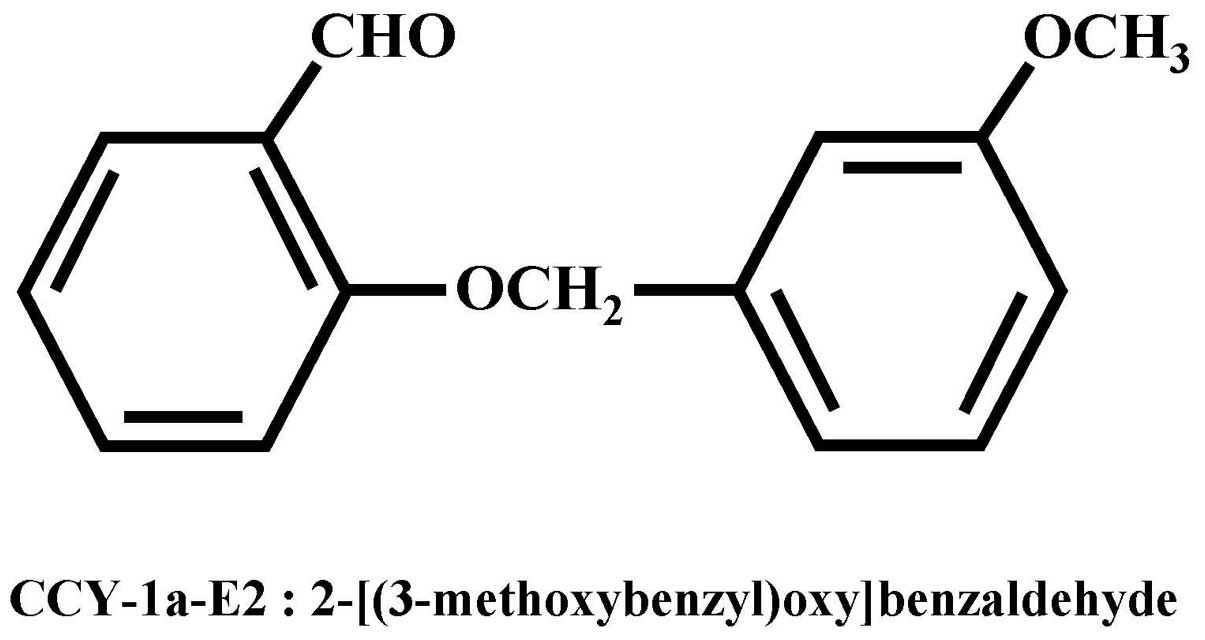
(2-[(3-methoxybenzyl)oxy]benzaldehyde) (Fig. 1) is a potent compound against HL-60
leukemia cells in vitro(12). CCY-1a-E2 induced G2/M
phase arrest and induced cell apoptosis in HL-60 cells (12). However, the cytotoxic effects of
CCY-1a-E2 on WEHI-3 leukemia cells and the antileukemic activity
in vivo have not been fully clarified. In the present study,
we demonstrated that CCY-1a-E2 induced growth inhibitory effects in
WEHI-3 leukemia cells in vitro and in vivo.
Materials and methods
Chemicals and reagents
Dimethyl sulfoxide (DMSO) was obtained from
Sigma-Aldrich Corp. (St. Louis, MO, USA). RPMI-1640 medium,
penicillin-streptomycin, trypsin-EDTA, fetal bovine serum (FBS) and
L-glutamine were obtained from Gibco/Life Technologies (Carlsbad,
CA, USA). The FITC-labeled anti-mouse CD3, PE-labeled anti-mouse
CD19, FITC-labeled anti-mouse CD11b and PE-labeled anti-mouse Mac-3
antibodies were obtained from BD Pharmingen Inc. (San Diego, CA,
USA).
Cell culture
The WEHI-3 murine myelomonocytic leukemia cell line
was purchased from the Food Industry Research and Development
Institute (Hsinchu, Taiwan). Cells were maintained in RPMI-1640
medium supplemented with 10% FBS, 2 mM L-glutamine, 100 U/ml
penicillin and 100 μg/ml streptomycin at 37°C in a
humidified atmosphere of 5% CO2(13).
Viability determination
The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay was performed to determine the cell proliferation of
CCY-1a-E2-treated WEHI-3 cells. WEHI-3 cells
(∼2×104/well) were placed into 96-well plates for 24 h.
CCY-1a-E2 was dissolved in DMSO then individually added to the
wells at final concentrations of 0.78, 1.56, 3.13, 6.25, 12.5 and
25 μM, and 0.1% of DMSO in culture medium was added to the
well as the control group. Following treatment for 24 h, cells from
each well were harvested for the determination of viability using
an MTT method as described previously (14). The data presented are from three
separate experiments.
Animal handling
A total of 60 BALB/c mice of 6 weeks of age and
22–25 g in weight were purchased from the National Laboratory
Animal Center (NLAC, Taipei, Taiwan). This study followed the
institutional guidelines (Affidavit of Approval of Animal Use
Protocol) and was approved by the Institutional Animal Care and Use
Committee (IACUC) of China Medical University (Taichung, Taiwan)
(13).
Establishment of the leukemic mice
model
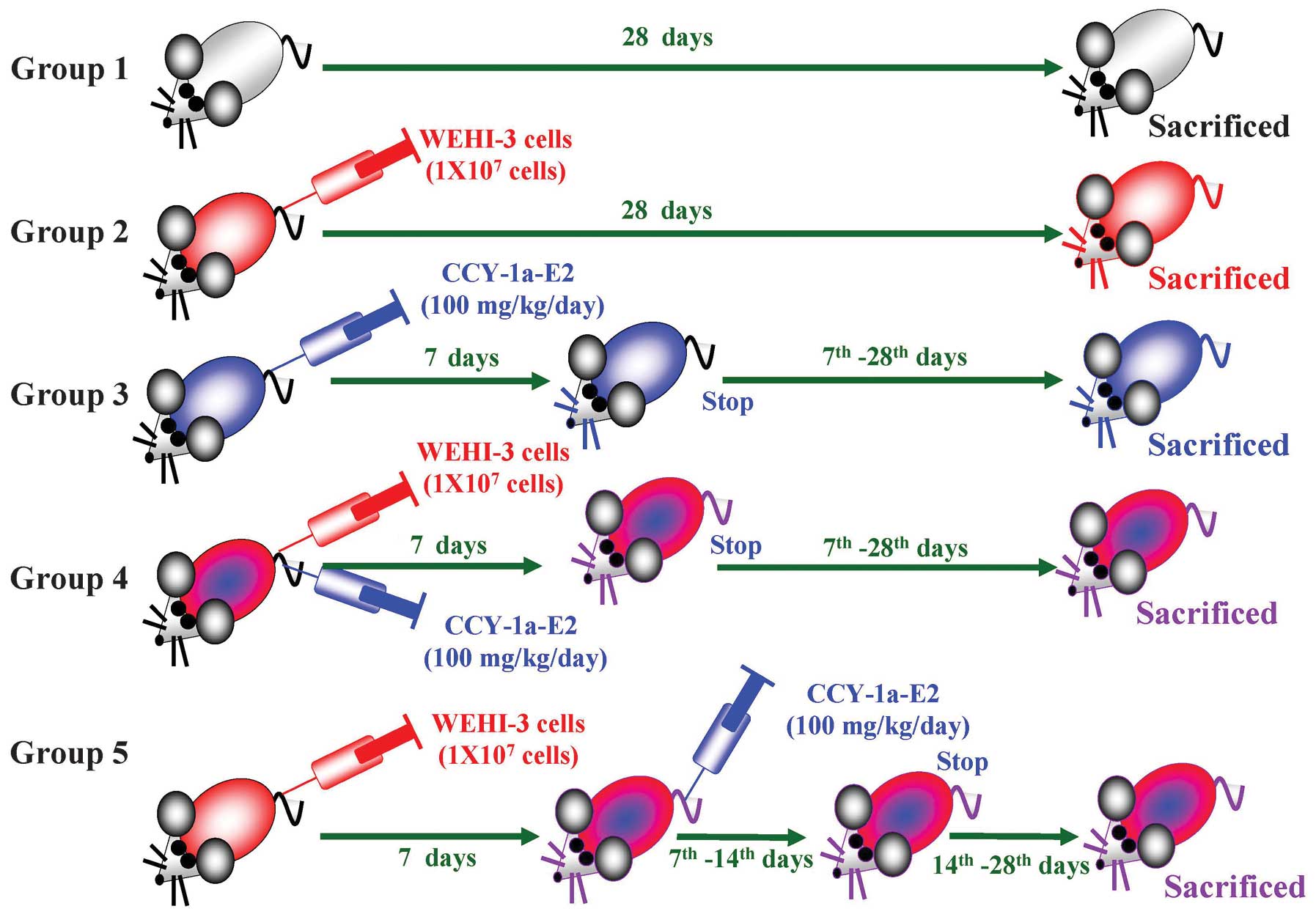
A total of 30 BALB/c mice were randomly divided into
five groups. Group 1 received an intravenous injection of the
solvent (2-glycofurol) as a control, group 2 received an
intravenous injection of 1×107 WEHI-3 cells, group 3
received an intravenous injection of CCY-1a-E2 (100 mg/kg/day) for
7 days, group 4 received an intravenous injection of
1×107 WEHI-3 cells as well as CCY-1a-E2 (100 mg/kg/day)
for 7 days, and group 5 received an intravenous injection of
1×107 WEHI-3 cells for 7 days and were then administered
an intravenous injection of CCY-1a-E2 (100 mg/kg/day) for 7 days.
The body weight of each mouse was measured once every 7 days. At
day 28, all animals were sacrificed by euthanasia with
CO2. Blood was collected and spleen and liver samples
were obtained and weighed individually as previously described
(13,15).
Immunofluorescent staining
Blood (∼500 μl) was collected from each mouse
in different groups and then added to Pharm Lyse lysing buffer (BD
Biosciences, San Jose, CA, USA) for lysing of the red blood cells
followed by centrifugation for 5 min at 1,500 rpm at 4°C. The
isolated leukocytes were examined for cell markers based on being
stained with FITC-conjugated anti-mouse CD3, PE-conjugated
anti-mouse CD19, PE-conjugated anti-mouse Mac-3 and FITC-conjugated
anti-mouse CD11b antibodies (BD Pharmingen Inc., San Diego, CA,
USA). Subsequently, cells were analyzed for the levels of specific
cell surface markers by flow cytometry as described previously
(13,15).
Safety evaluation
A total of 30 BALB/c mice were randomly divided into
five groups. Group 1 received an intravenous injection of PBS as a
control. Group 2 received an intravenous injection of the solvent
(2-glycofurol) as the solvent control. Group 3 received an
intravenous injection of CCY-1a-E2 (5 mg/kg/day) for 7 days. Group
4 received an intravenous injection of CCY-1a-E2 (50 mg/kg/day) for
7 days. Group 5 received an intravenous injection of CCY-1a-E2 (100
mg/kg/day) for 7 days. At day 7, all animals were sacrificed by
euthanasia with CO2. The body weight, liver weight,
spleen weight and biochemical profiles of blood analysis [lactate
dehydrogenase (LDH), albumin (ALB), total protein (PRO), serum
glutamicpyruvic transaminase (sGPT), serum glutamic-oxaloacetic
transaminase (sGOT) and blood urea nitrogen (BUN)] were analyzed as
described previously (15–17).
Statistical analysis
The results are presented as mean ± SEM, and the
difference between the CCY-1a-E2-treated and control groups was
analyzed by Student’s t-test. P≤0.05 was considered to indicate a
statistically significant result.
Results
CCY-1a-E2 reduces the percentage of
viable WEHI-3 cells
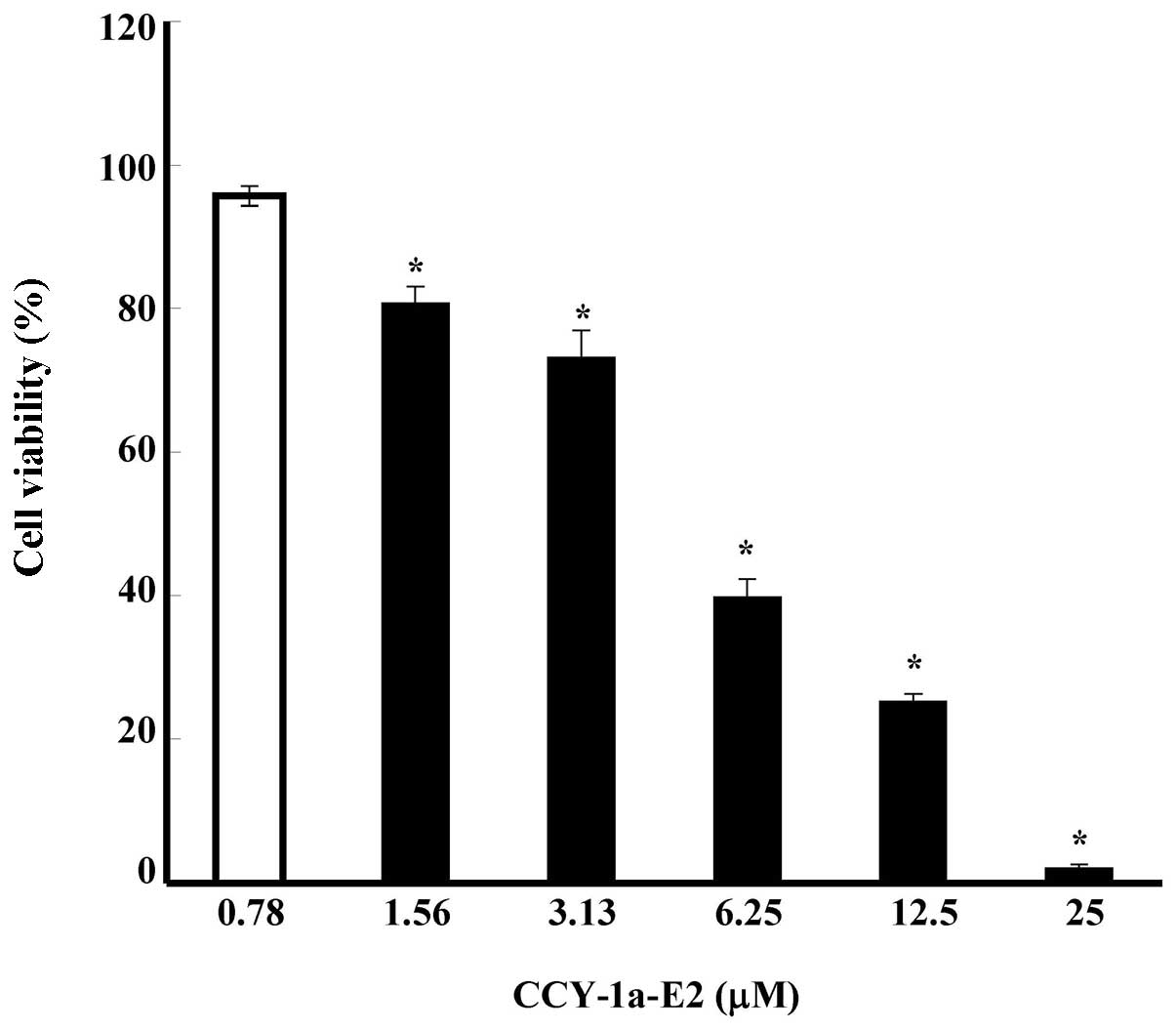
To evaluate the effect of CCY-1a-E2 on the viability
of WEHI-3 cells, we treated WEHI-3 cells with various
concentrations of CCY-1a-E2 (0.78, 1.56, 3.13, 6.25, 12.5 and 25
μM) for 24 h. The percentage of viable cells was measured by
MTT assay. The results shown in Fig.
2 indicate that CCY-1a-E2 decreased the percentage of viable
cells in a concentration-dependent manner for 24 h after the
exposure to 0.78–25 μM of CCY-1a-E2 (Fig. 2). The IC50 for the 24-h
CCY-1a-E2 treatment of WEHI-3 cells was 5 μM.
WEHI-3 cell allograft model
The experimental design and protocol of the leukemic
mice model are shown in Fig. 3.
Representative mouse images are shown in Fig. 4. At day 28, all animals were
sacrificed.
CCY-1a-E2 reduces leukemia formation in
WEHI-3 leukemic BALB/c mice
We examined the in vivo antileukemic
activities of CCY-1a-E2 in a BALB/c mouse WEHI-3 allograft model.
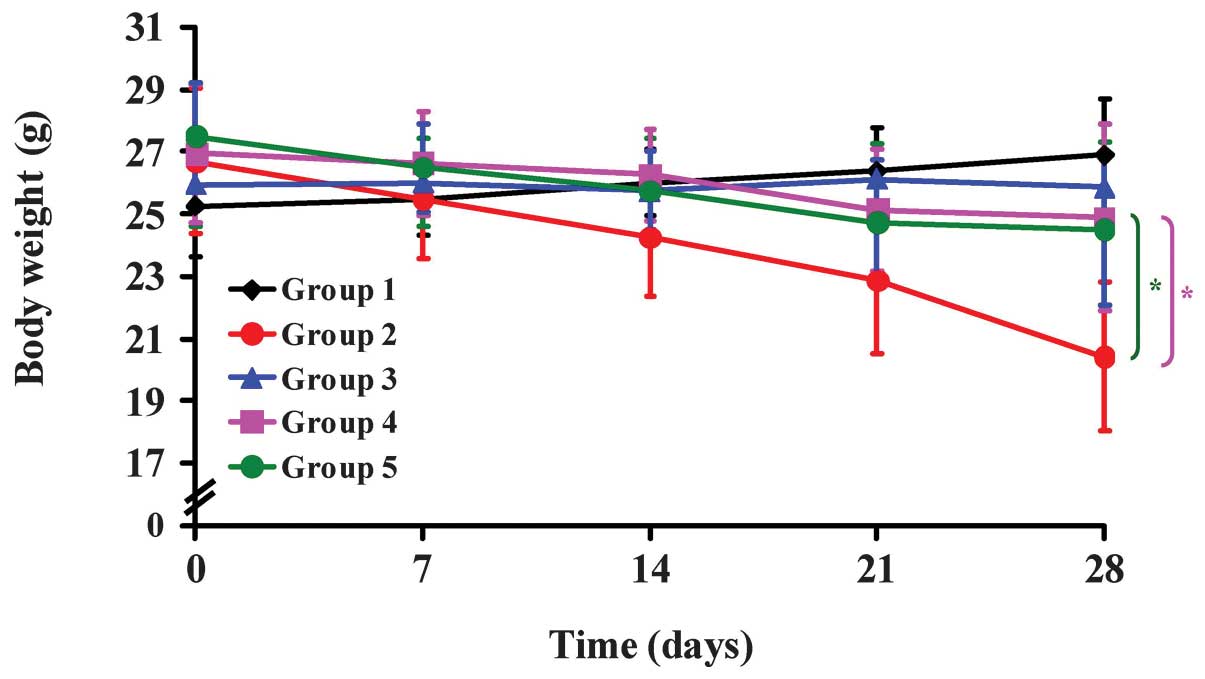
Representative body weights from the WEHI-3 allograft mice treated
with or without CCY-1a-E2 are shown in Fig. 5. The body weight of mice in the
CCY-1a-E2 (100 mg/kg) group was not significantly decreased
compared with that of the control mice; however, the body weight of
mice in the leukemia group was significantly decreased compared
with that of the control treatment group. Furthermore, the average
body weight in the CCY-1a-E2 (100 mg/kg)-treated leukemic mice
increased slightly. As shown in Fig.
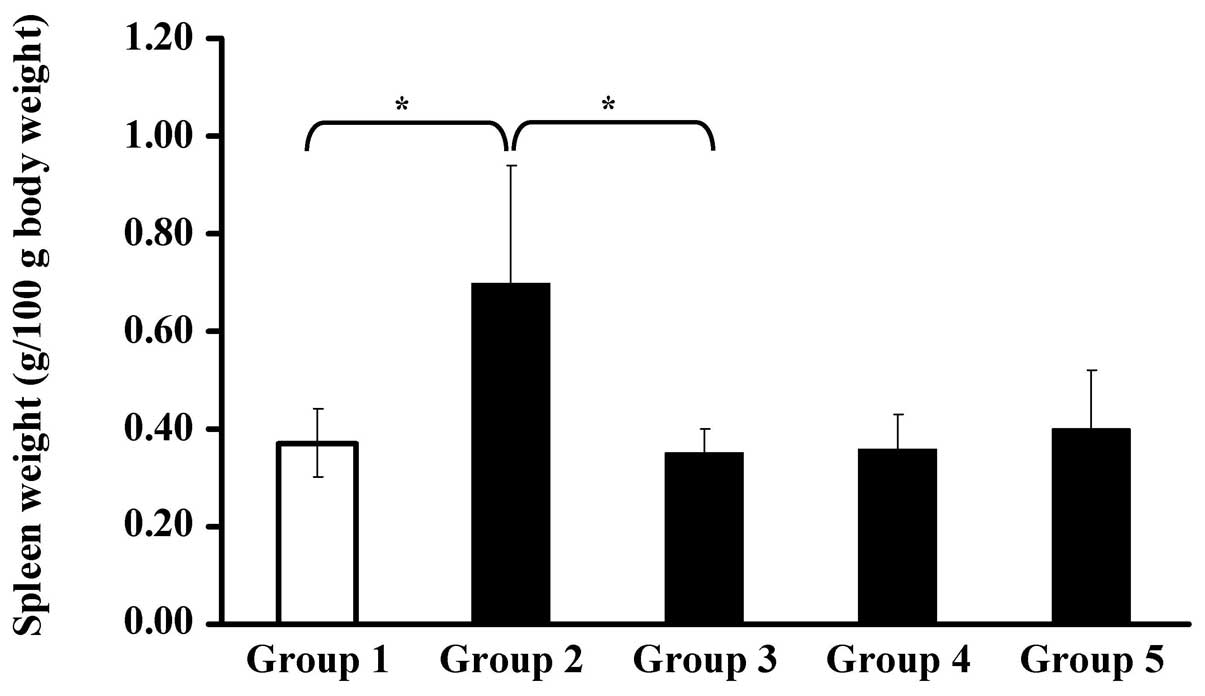
6, the spleen weight of group 2 increased significantly; while
the spleen weights of groups 3, 4 and 5 did not differ
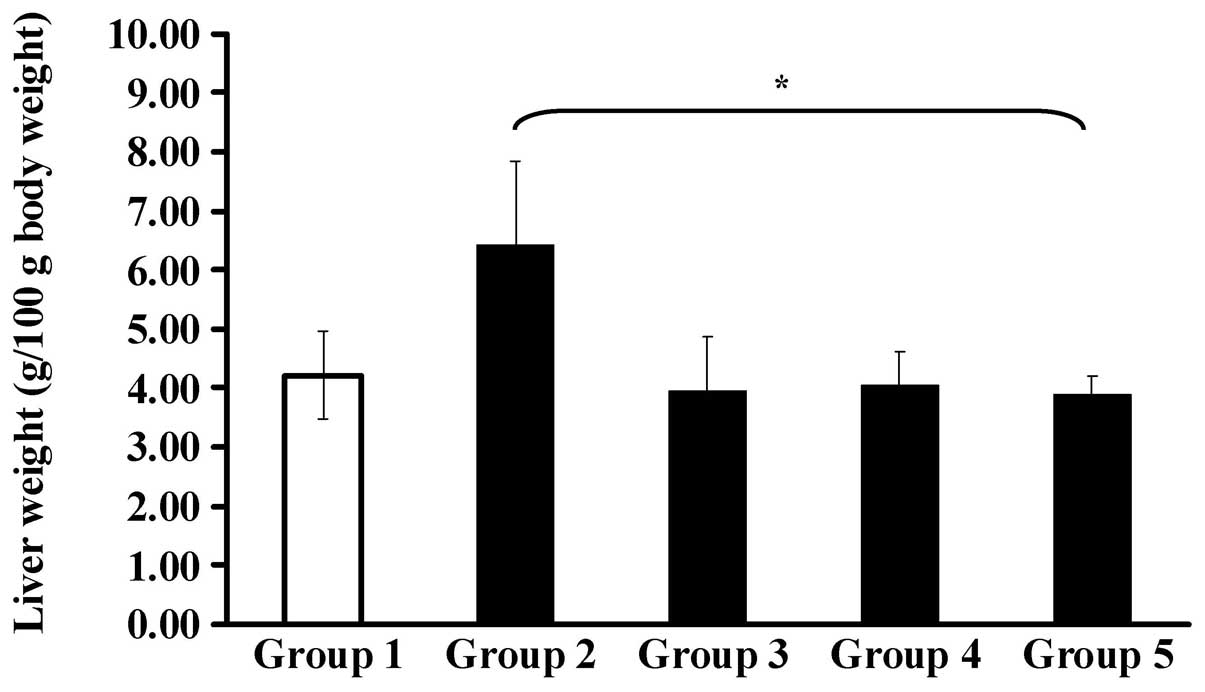
significantly from group 1 (the control group). Fig. 7 shows that the liver weights of the
allograft mice were significantly different following treatment
with CCY-1a-E2 (100 mg/kg) at day 28.
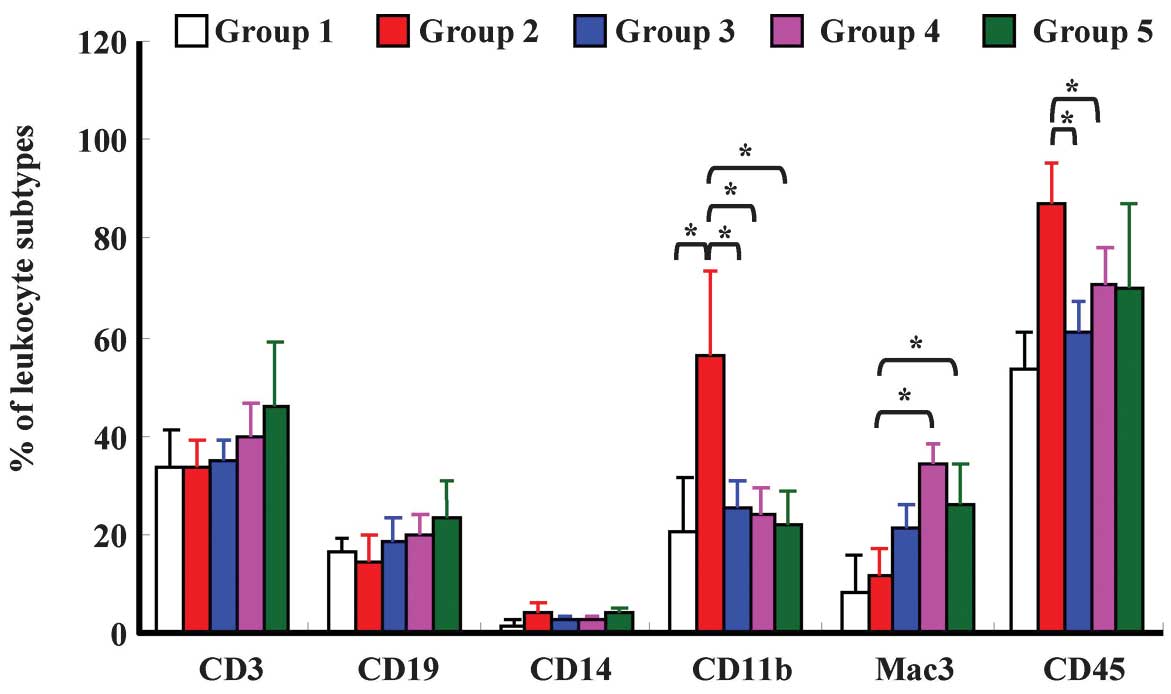
CCY-1a-E2 affects surface markers on
whole blood cells from WEHI-3 leukemic BALB/c mice
In order to investigate whether CCY-1a-E2 affects
the levels of cell surface markers, leukocytes from
CCY-1a-E2-treated and untreated (control) groups were isolated and
levels of CD3, CD19, CD14, CD11b, Mac-3 and CD45 were measured. The
data from each treatment indicate that CCY-1a-E2 (100 mg/kg)
significantly decreased the levels of CD11b and CD45 when compared
with the leukemia group (Fig.
8).
CCY-1a-E2 shows no adverse effects on
renal, hepatic and hematological parameters
The safety profile of CCY-1a-E2 (5, 50 and 100
mg/kg) was investigated by pathological examinations and clinical
chemistry. Body weight, spleen weight and liver weight were not
affected by treatment with CCY-1a-E2 (Table I). The spleen and liver were
examined by histopathology. No significant microscopic aberrations
were noted compared with the vehicle-treated controls (data not
shown). The biochemical measurements of sGPT, sGOT and BUN are
shown in Table II. The sGPT and
sGOT levels were within the normal value range, suggesting that
these groups of mice had normal hepatic function. The BUN assays
yielded biochemical values that reflect normal kidney functions.
Notably, mice apparently tolerated treatment with CCY-1a-E2,
showing no adverse effects on splenic, hepatic or renal
parameters.
 | Table IChange in body, spleen and liver
weight in the BALB/c mice following treatment with CCY-1a-E2 by
intravenous injection once every day for 7 days. |
Table I
Change in body, spleen and liver
weight in the BALB/c mice following treatment with CCY-1a-E2 by
intravenous injection once every day for 7 days.
| Item | Control | 2-glycofurol (solvent
control) | CCY-1a-E2 (5
mg/kg) | CCY-1a-E2 (50
mg/kg) | CCY-1a-E2 (100
mg/kg) |
|---|
| Body weight (g) | 24.58±0.66 |
28.20±1.10a |
28.42±1.13a |
28.88±1.52a |
28.90±0.51a |
| Liver weight (g/100 g
body weight) | 4.43±0.47 | 3.98±0.30 | 4.42±0.47 | 4.18±0.33 | 4.17±0.26 |
| Spleen weight (g/100
g body weight) | 0.33±0.05 | 0.28±0.04 | 0.35±0.06 | 0.31±0.06 | 0.37±0.04 |
 | Table IIBiochemical profiles of blood analysis
in the BALB/c mice following treatment with CCY-1a-E2 by
intravenous injection once every day for 7 days. |
Table II
Biochemical profiles of blood analysis
in the BALB/c mice following treatment with CCY-1a-E2 by
intravenous injection once every day for 7 days.
| Item | Control | 2-glycofurol (solvent
control) | CCY-1a-E2 (5
mg/kg) | CCY-1a-E2 (50
mg/kg) | CCY-1a-E2 (100
mg/kg) |
|---|
| LDH (U/l) | 255.50±105.18 | 327.00±162.48 | 298.00±62.22 | 244.83±46.99 | 270.83±42.09 |
| ALB (g/dl) | 1.63±0.28 | 1.71±0.08 | 1.58±0.38 | 1.73±0.26 | 1.84±0.13 |
| PRO (g/dl) | 3.81±0.69 | 3.90±0.95 | 3.68±0.60 | 4.06±0.58 | 4.77±0.46 |
| sGPT (U/l) | 38.25±7.49 | 38.58±10.31 | 41.83±8.94 | 43.92±16.02 | 41.83±10.02 |
| sGOT (U/l) | 85.58±12.24 | 92.25±37.19 | 105.83±22.16 | 79.98±26.10 | 115.12±15.08 |
| BUN (mg/dl) | 26.83±2.09 | 27.58±1.80 | 30.33±5.02 | 26.17±2.42 | 28.92±2.20 |
Discussion
In a previous study, our groups synthesized several
benzyloxybenzaldehyde analogs as novel adenyl cyclase activators
and studied the mechanism of action (8). The results showed that
2-benzyloxybenzaldehyde exhibited antiproliferative effects on the
vascular smooth muscle cells through the inhibition of the Ras/MAPK
signal pathway and its downstream effectors (9). It has also been demonstrated that
2-benzyloxybenzaldehyde inhibits superoxide anion generation
through the suppression of Akt and PLD activation in rat
neutrophils (10,11). More recently, we have designed and
synthesized a new series of 2-benzyloxybenzaldehyde derivatives as
anticancer agents (12). In the
present study, our results demonstrated that cell viability
decreased following treatment with various concentrations of
CCY-1a-E2 in a concentration-dependent manner in WEHI-3 leukemia
cells (Fig. 2). The IC50
of CCY-1a-E2 was 5 μM for the 24-h treatment of WEHI-3
cells. This is in agreement with the previous studies from our
investigators, which showed that CCY-1a-E2 decreased the percentage
of viable cells in HL-60 cells (12). In addition, CCY-1a-E2 exerts low
cytotoxicity on human normal human peripheral blood mononuclear
cells (PBMCs; IC50 >20 μM). This result shows
that CCY-1a-E2 was less toxic for PBMCs than for WEHI-3 cells.
In vivo model systems of leukemia were
established for the evaluation of new antileukemic agents (15,18).
The murine allograft model is frequently used for experimental
antileukemic therapy as it is quick and easy to induce leukemia
(13,19). The murine WEHI-3 myelomonocytic
leukemia cell line originally derived from the BALB/c mouse was
first established in 1969 and it has been used to induce leukemia
in BALB/c mice for evaluating the antileukemic effects of drugs
(13,20,21).
There is no information concerning the effects of CCY-1a-E2 on
leukemia cells in vivo. In the present study, the
antileukemic effects of CCY-1a-E2 on WEHI-3 leukemia cells were
first investigated in vivo. Our results suggest that
CCY-1a-E2 had a growth inhibitory effect in WEHI-3 cells in
vitro and also affected leukemia formation in vivo. In
addition, treatment with CCY-1a-E2 significantly inhibited the loss
of body weight compared with the leukemia group (Fig. 5). CCY-1a-E2 also inhibited the
spleen and liver growth of leukemic mice (Figs. 6 and 7). The results of histopathological
examination indicate that the infiltration of immature myeloblastic
cells into the splenic red pulp in spleen sections was eliminated
in CCY-1a-E2-treated leukemic mice when compared with the untreated
leukemia group (data not shown). Moreover, CCY-1a-E2 reduced the
levels of CD11b (monocytic marker) in comparison to the leukemia
group. Therefore, intraperitoneal administration with CCY-1a-E2 to
leukemic mice altered the specific surface markers from PBMCs in
vivo. Our results show that CCY-1a-E2 is a promising candidate
as an antileukemic agent and our studies provide useful information
for the development of a new therapeutic strategy against
leukemia.
In conclusion, our study is the first to demonstrate
that CCY-1a-E2 has growth inhibitory effects in WEHI-3 leukemia
cells. In vivo results indicate that CCY-1a-E2 has no
adverse effects on renal, hepatic or hematological parameters and
exerts the ability of antileukemic activity in WEHI-3 allograft
model of leukemia.
Acknowledgements
This study was supported by the grant
NSC-101-2313-B-039-008 from the National Science Council, Republic
of China (Taiwan).
References
|
1
|
Yildirim R, Gundogdu M, Ozbıcer A, Kiki I,
Erdem F and Dogan H: Acute promyelocytic leukemia, centre,
experience, Turkey. Transfus Apher Sci. Aug 11–2012.(Epub ahead of
print).
|
|
2
|
Guo J, Chang CK and Li X: Recent advances
of molecular mechanisms influencing prognosis of myelodysplastic
syndrome. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 20:1020–1024.
2012.(In Chinese).
|
|
3
|
Kinoshita K and Funauchi M: Therapeutic
effect of retinoic acid in lupus nephritis. Nihon Rinsho Meneki
Gakkai Kaishi. 35:1–7. 2012.(In Japanese).
|
|
4
|
Flatt T, Neville K, Lewing K and Dalal J:
Successful treatment of fanconi anemia and T-cell acute
lymphoblastic leukemia. Case Report Hematol.
2012:3963952012.PubMed/NCBI
|
|
5
|
Estey EH: Acute myeloid leukemia: 2012
update on diagnosis, risk stratification, and management. Am J
Hematol. 87:89–99. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang TT and Chen BA: Leukemia
stem/progenitor cells and target therapy for leukemia - review.
Zhongguo Shi Yan Xue Ye Xue Za Zhi. 18:1654–1658. 2010.(In
Chinese).
|
|
7
|
Krauss J and Unterreitmeier D: Synthesis
and antimicrobial activity of hydroxyalkyl- and hydroxyacyl-phenols
and their benzyl ethers. Arch Pharm (Weinheim). 335:94–98. 2002.
View Article : Google Scholar
|
|
8
|
Chang C, Kuo S, Lin Y, Wang J and Huang L:
Benzyloxybenzaldehyde analogues as novel adenylyl cyclase
activators. Bioorg Med Chem Lett. 11:1971–1974. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pan SL, Guh JH, Huang YW, et al:
Inhibition of Ras-mediated cell proliferation by
benzyloxybenzaldehyde. J Biomed Sci. 9:622–630. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang JP, Chang LC, Hsu MF, Huang LJ and
Kuo SC: 2-Benzyloxybenzaldehyde inhibits
formyl-methionyl-leucylphenylalanine stimulation of phospholipase D
activation in rat neutrophils. Biochim Biophys Acta. 1573:26–32.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang JP, Chang LC, Lin YL, et al:
Investigation of the cellular mechanism of inhibition of
formyl-methionyl-leucylphenylalanine-induced superoxide anion
generation in rat neutrophils by 2-benzyloxybenzaldehyde. Biochem
Pharmacol. 65:1043–1051. 2003. View Article : Google Scholar
|
|
12
|
Lin CF, Yang JS, Chang CY, Kuo SC, Lee MR
and Huang LJ: Synthesis and anticancer activity of
benzyloxybenzaldehyde derivatives against HL-60 cells. Bioorg Med
Chem. 13:1537–1544. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chung JG, Yang JS, Huang LJ, et al:
Proteomic approach to studying the cytotoxicity of YC-1 on U937
leukemia cells and antileukemia activity in orthotopic model of
leukemia mice. Proteomics. 7:3305–3317. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsai SC, Yang JS, Peng SF, et al: Bufalin
increases sensitivity to AKT/mTOR-induced autophagic cell death in
SK-HEP-1 human hepatocellular carcinoma cells. Int J Oncol. Jul
31–2012, (Epub ahead of print).
|
|
15
|
Lu CC, Yang JS, Chiang JH, et al: Novel
quinazolinone MJ-29 triggers endoplasmic reticulum stress and
intrinsic apoptosis in murine leukemia WEHI-3 cells and inhibits
leukemic mice. PLoS One. 7:e368312012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chiang JH, Yang JS, Ma CY, et al:
Danthron, an anthraquinone derivative, induces DNA damage and
caspase cascades-mediated apoptosis in SNU-1 human gastric cancer
cells through mitochondrial permeability transition pores and
Bax-triggered pathways. Chem Res Toxicol. 24:20–29. 2011.
View Article : Google Scholar
|
|
17
|
Hsu SC, Ou CC, Li JW, et al: Ganoderma
tsugae extracts inhibit colorectal cancer cell growth via
G(2)/M cell cycle arrest. J Ethnopharmacol. 120:394–401. 2008.
View Article : Google Scholar
|
|
18
|
Yang JS, Hour MJ, Huang WW, Lin KL, Kuo SC
and Chung JG: MJ-29 inhibits tubulin polymerization, induces
mitotic arrest, and triggers apoptosis via cyclin-dependent kinase
1-mediated Bcl-2 phosphorylation in human leukemia U937 cells. J
Pharmacol Exp Ther. 334:477–488. 2010. View Article : Google Scholar
|
|
19
|
Yang JS, Wu CC, Kuo CL, et al: Solanum
lyratum Extracts Induce Extrinsic and Intrinsic Pathways of
Apoptosis in WEHI-3 Murine Leukemia Cells and Inhibit Allograft
Tumor. Evid Based Complement Alternat Med.
2012:2549602012.PubMed/NCBI
|
|
20
|
Li J and Sartorelli AC: Synergistic
induction of the differentiation of WEHI-3B D+ myelomonocytic
leukemia cells by retinoic acid and granulocyte colony-stimulating
factor. Leuk Res. 16:571–576. 1992.
|
|
21
|
Barr RD and Harnish D: Induction of
differentiation of HL-60 and WEHI-3B D+ leukemia cells by lithium
chloride. Leuk Res. 17:1017–1018. 1993.
|