Introduction
Tumor cells may be divided into well-differentiated
mature or immature precursor cells according to different sources.
The latter are considered to be cancer stem cells (CSCs) (1,2). They
possess unlimited proliferative multi-potential differentiation,
renewal, potential tumorigenicity capacity and permanency (3,4);
however, the proportion of CSCs in adult tumor cells is small. In
1997, Bonnet and Dick confirmed for the first time that the small
proportion of human acute myeloid leukemic (AML) cells that
expressed CD34 on their membranes were AML stem cells (5). This finding provided initial insights
into the domain of stem cells. Subsequently, CSCs in breast
(6), prostate (4), liver (7) and colon (8) cancer cells were isolated through their
respective surface markers.
Based on the fact that the proportion of CSCs in
adult tumor cells is small, Collins et al purified the
prostate cancer stem cells (PCSCs) from prostate cancer by flow
cytometry (FCM). The author demonstrated that ∼0.1% of prostate
cancer cells possessed the
CD44+/α2β1hi/CD133+ phenotype,
which was independent of prostate cancer grading and staging
(4). Numerous scholars have
indicated that prostate cancer cells with the
CD133+/CD44+ phenotype demonstrate certain
stem cell characteristics, including renewal, multi-potential
differentiation, unlimited proliferative capacity and permanency
(9–11). However, little is known regarding
whether cells with the CD133+/CD44+ phenotype
are prostate cancer stem-like cells (PCSLCs). Therefore, in this
study, we aimed to isolate and enrich PC-3 PCSLC cells by magnetic
bead cell sorting (MACS) and serum-free medium (SFM) based on CD133
and CD44.
Materials and methods
Cell culture
The PC-3 cell line (derived from grade IV bone
metastases of prostate adenocarcinoma in a white, 62-year-old male)
was purchased from the Shanghai Cell Bank of the Chinese Academy of
Sciences. The cells were cultured with Dulbecco’s modified Eagle’s
medium (DMEM)/F12 (1:1) (HyClone Laboratories, Inc.; South Logan,
UT, USA) and 10% fetal calf serum (Gibco-BRL; Carlsbad, CA, USA),
in a 25-ml culture flask in an incubator at 5% CO2 and
37°C.
SFM culture of spheres
PC-3 cell spheres were cultured with DMEM/F12 (1:1)
supplemented with 20 μg/l epidermal growth factor (EGF), 20
μg/l basic fibroblast growth factor (bFGF) and 20
μg/l leukaemia inhibitory factor (LIF) (Peprotech, Inc.;
Rocky Hill, NJ, USA) in an incubator as previously described
(11). The cells were observed each
day using an inverted microscope. Medium (1 ml) was added on
alternate days and half the medium was replaced biweekly. The
spheres were suspended in the medium within 7–9 days; the
suspension was drained into another flask and the culturing process
was continued in the same way.
MACS
The CD133+/CD44+ PCSLCs were
obtained using the MACS kit according to the manufacturer’s
instructions (Miltenyi Biotech, Bergisch Gladbach, Germany)
(12). Briefly, total populations
of adherent cells were enzymatically dissociated into a single cell
suspension and counted to confirm the quantity of the whole cells.
The cells were incubated with 100 μl microbeads directly
conjugated to mouse monoclonal anti-human CD133 antibody at 4°C for
30 min. Subsequently, the suspended cells were added to a MACS
column that was placed in the magnetic field of a MACS separator
(Miltenyi Biotech). The labeled CD133+ cells were
retained on the column and the unlabeled cells were eluted; when
the column was removed from the magnetic field, the magnetically
retained CD133+ cells were collected as positively
selected cells for further research (13). The CD44+ cells were
obtained using the same method.
FCM
FCM was performed at the FCM Laboratory, Chongqing
Medical University. The collected PC-3 cells were named group 1;
the collected spheres, which were cultured in SFM, were designated
group 2 and the cells sorted by MACS formed group 3. Each group was
incubated with 20 μl mouse anti-human monoclonal antibody of
CD133-phycoerythrin (PE; eBioscience, San Diego, CA, USA) for 30
min at room temperature in the dark. This was followed by
centrifugation for 5 min at 800 rpm, extraction with 300 μl
phosphate-buffered saline (PBS) and detection on the machine. This
procedure was repeated three times for each group. The same method
was also used for the antibody of CD44-phycoerythrin. Subsequent
FCM analysis demonstrated <5% contamination by relevant
antigen-expressing cells.
Immunocytochemistry (ICC)
The expression of CD133 and CD44 was analyzed by
immunofluorescence techniques. The isolated cell suspension was
subjected to fixation for 20 min with 4% paraformaldehyde and
sealed with goat serum to avoid non-specific binding. This was then
incubated with the primary antibody overnight at 4°C, prior to
incubation with combination fluorochrome-conjugated secondary
antibody for 40 min at 37°C on the following day. Subsequently,
staining with 4′,6-diamidino-2-phenylindole (DAPI) was performed
for identification of the nuclei, and slides were sealed with 50%
glycerin.
Proliferation assay
Collected spheres, PC-3 cells, cells with a
CD133−/CD44− phenotype and differentiated
spheres comprised groups 1, 2, 3 and 4, respectively. Each group
was seeded at a low density (2×103 cells/200 μl),
the zero setting was put in place by a blank medium, and then cells
were incubated in an incubator for 7 days. The samples were
randomly removed each day and the cell number was determined by a
microplate reader. A proliferation curve was then generated
accordingly and the doubling time of cells was calculated using the
Patterson formula:
Td=T×lg2/lg(Nt/N0); where
Td indicated doubling time, T indicated days,
Nt indicated the number of cells on the last day,
N0 indicated the numbers of cells on the first day.
Differentiation assay
The PCSLCs were cultured with DMEM/F12 (1:1)
supplemented with 5 μg/l TGF-β (Peprotech, Inc.) and 1%
fetal calf serum (14). The PCSLCs
that were cultured without TGF-β were used as a control group. The
PCSLCs were incubated for 4 days and their morphology was observed
by an inverted microscope.
Western blot analysis
Cell lysates were prepared by a mixture of
phenylmethylsulfonyl fluoride (PMSF) and protease inhibitor (1:99).
Proteins were separated by gel electrophoresis and then transferred
to a 0.45-μm polyvinylidene fluoride (PVDF) membrane. The
primary antibody (dilution, 1:200) and horse-radish peroxidase
(HRP)-labeled secondary antibody (dilution, 1:5,000) were added.
The membrane was placed in the Vilber Lourmat (Bio-Rad; Hercules,
CA, USA) for enhanced chemiluminescence (ECL) development. The
density values were determined by: Target protein/β-actin.
Statistical analysis
Data were presented as mean ± standard deviation. A
Student’s two-tailed t-test was used to compare two groups, while a
one-way analysis of variance (ANOVA) was used to compare multiple
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of CD133 and CD44 in PC-3
cells before and after MACS sorting by FCM
We investigated whether there was specific CD133 or
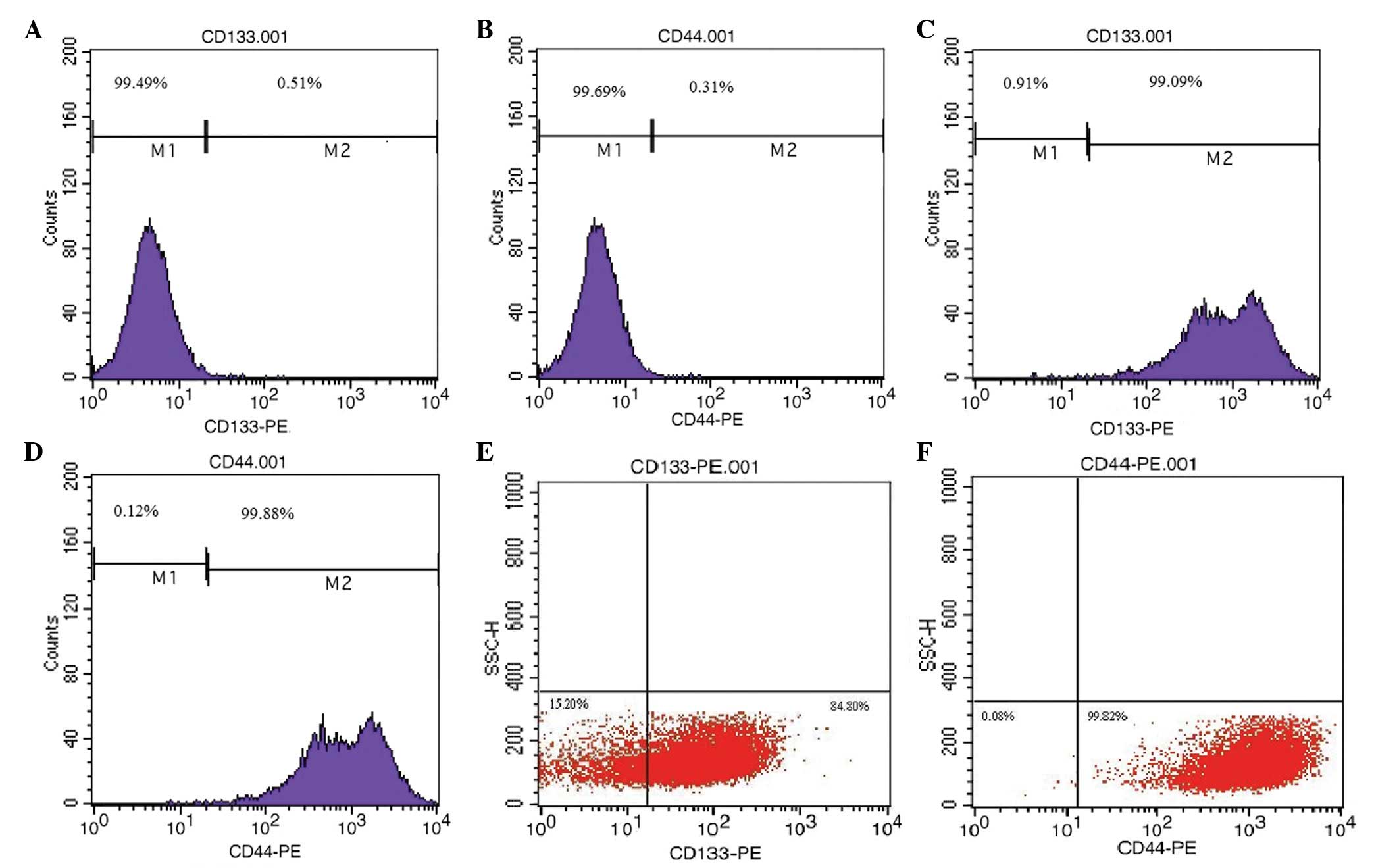
CD44 expression on the PC-3 cell membranes, and found that the
proportion of cells expressing CD133 and CD44 was 0.51 and 0.31%,
respectively, by FCM (Fig. 1A and
B). Therefore, the proportion of cells expressing CD133 and
CD44 was in accordance with a small group of cells, based on these
results.
To confirm the existence of the
CD133+/CD44+ phenotype in PC-3 cells, we
purified the cells by MACS and demonstrated that the proportion of
cells expressing CD133 and CD44 was 99.09 and 99.88%, respectively
(Fig. 1C and D). The proportion of
cells with the CD133+/CD44+ phenotype was
0.53±0.07 (Table I).
 | Table IProportion of the
CD133+/CD44+ phenotype in PC-3 cells. |
Table I
Proportion of the
CD133+/CD44+ phenotype in PC-3 cells.
| Sorting times
|
|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|
| PC-3 cell numbers
before MACS (×107) | 3.5 | 4.0 | 2.1 | 4.4 | 4.0 | 7.0 | 6.0 |
|
CD133+/CD44+ cell
number after MACS (×105) | 2.0 | 2.3 | 1.1 | 1.8 | 1.8 | 4.0 | 3.6 |
| Proportion of
CD133+/CD44+ (%) | 0.57 | 0.58 | 0.52 | 0.41 | 0.45 | 0.57 | 0.60 |
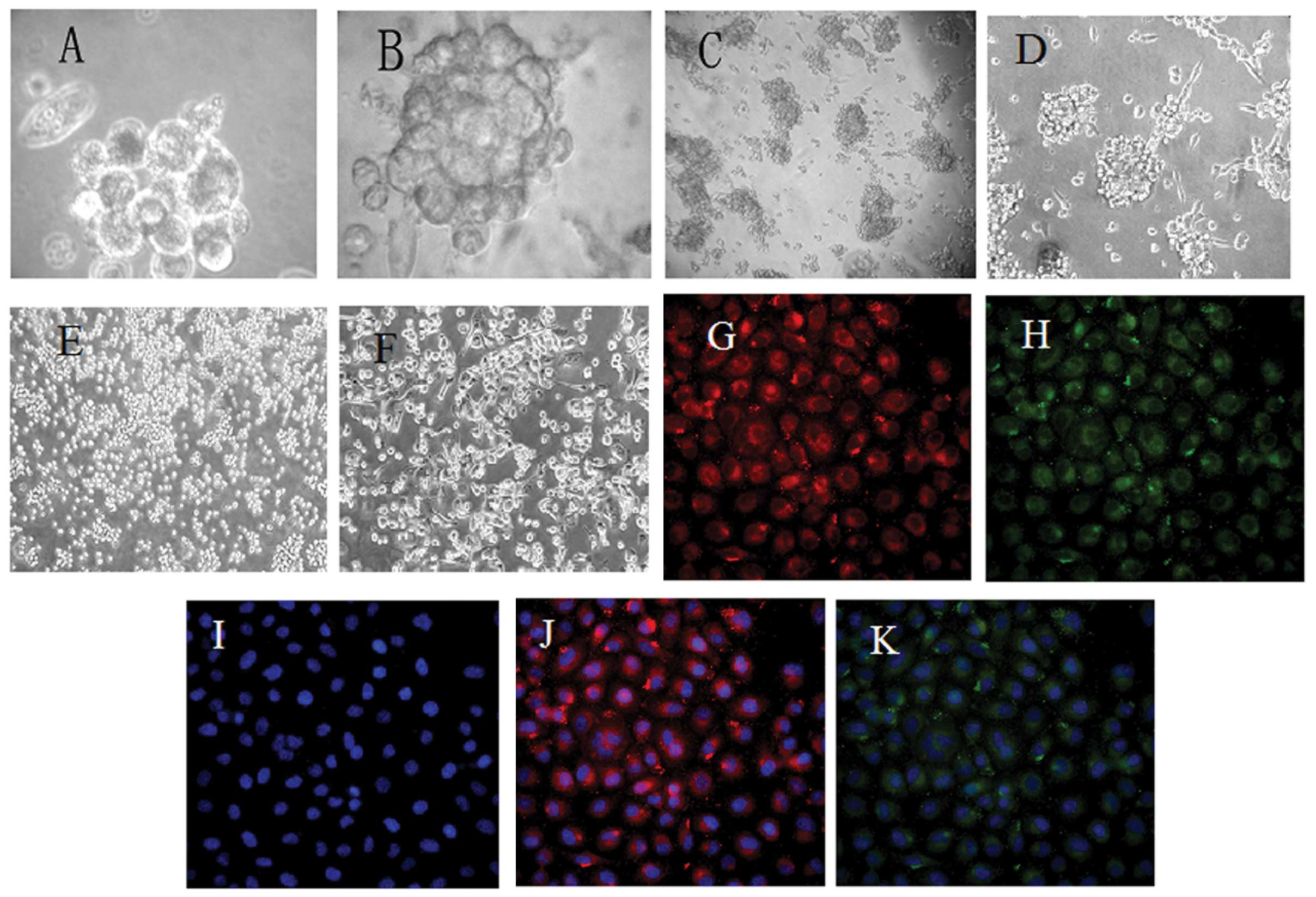
Formation of PCSLC spheres cultured in
SFM
PCSLC spheres were capable of forming in SFM. The
majority of cells with the CD133+/CD44+
phenotype were growing cultured in SFM, while few cells exhibited
apoptosis. After three days, 5–15 differently sized spheres with a
capacity of refraction had formed and were loosley combined
(Fig. 2A). Subsequently, the
spheres enlarged and the connection between the cells became
tighter. With the extension of culture time, new cells were born by
budding on the spheres that became rounder within four days
(Fig. 2B). The spheres passaged and
formed new irregular spheres, which became regular after one week
(Fig. 2C).
Expression of CD133 and CD44 on
sphere-forming cell membranes detected by FCM
To determine whether the spheres that we had
cultured in SFM were PCSLCs, we detected the expression of CD133
and CD44 on sphere-forming cell membranes by FCM; the proportion of
cells expressing CD133 and CD44 was 84.8 and 99.82%, respectively
(Fig. 1E and F). The expression
rate of CD133 on sphere-forming cell membranes was close to that in
PC-3 cells isolated by MACS (15),
but the difference between them was significant (P<0.05).
However, the expression of CD44 on sphere-forming cell membranes
was almost equivalent to that in PC-3 cells isolated by MACS, and
the difference was not significant (P>0.05).
ICC analyses of CD133 and CD44 expression
on sphere-forming cell membranes
To further assess whether CD133 and CD44 were
expressed on sphere-forming cell membranes, following several
subcultures, a high expression level of CD133 and CD44 was observed
by ICC (Fig. 2G and H), and the
nuclei had stained blue (Fig. 2I).
In the merge phase (Fig. 2J and K),
CD133 and CD44 were mainly expressed on the membranes. On
incubation with rhodamine (TRITC)-conjugated anti-rabbit IgG
antibody, CD133 expressed a red fluorescence, while CD44 exhibited
a green fluorescence when incubated with FITC-conjugated
anti-rabbit IgG antibody. Thus, the
CD133+/CD44+ subpopulation could be enriched
and subcultured with permanent retention.
Differentiation assays of prostate cancer
stem-like spheres (PCSLSs)
The process of differentiation of spheres was
evident following treatment with TGF-β. The morphological changes
in the sphere-forming cells were marked in the one-, two- and
four-day phases. The spheres remained suspended for the first 12 h,
then after one day certain cells became adherent to the wall, with
spindles and deformation evident (Fig.
2D). After two days, numerous cells were adherent and no marked
differences in the morphology were observed, compared with the
control group (Fig. 2E). All
spheres had adhered to the wall after 4 days, with various forms
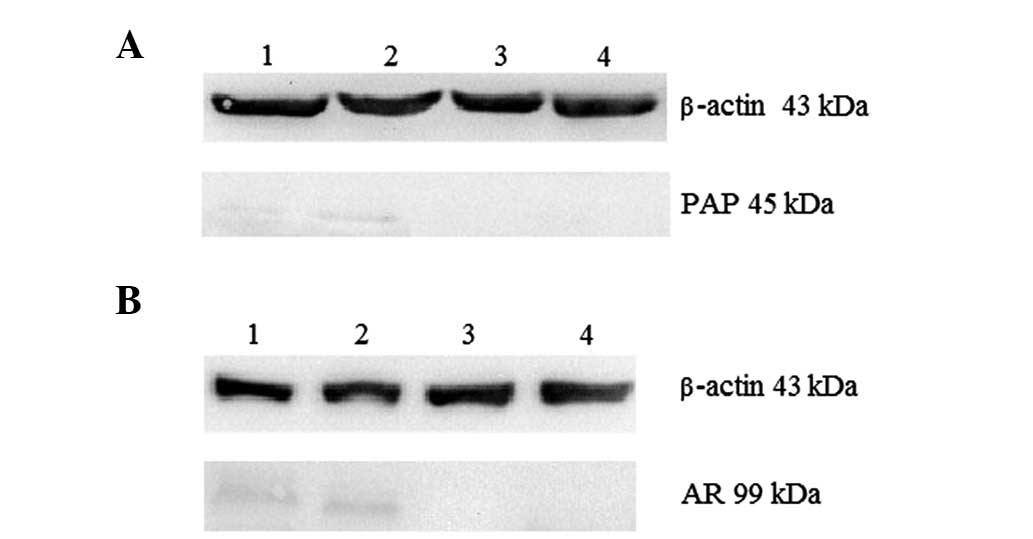
evident (Fig. 2F). The results of
the western blot analysis revealed that cells with the
CD133+/CD44+ phenotype and spheres that had
both been induced by TGF-β co-expressed PAP and AR (Fig. 3A and B); the values of PAP and AR
expression were 0.192±0.011 and 0.473±0.064, respectively, while
the control groups expressed neither of these proteins.
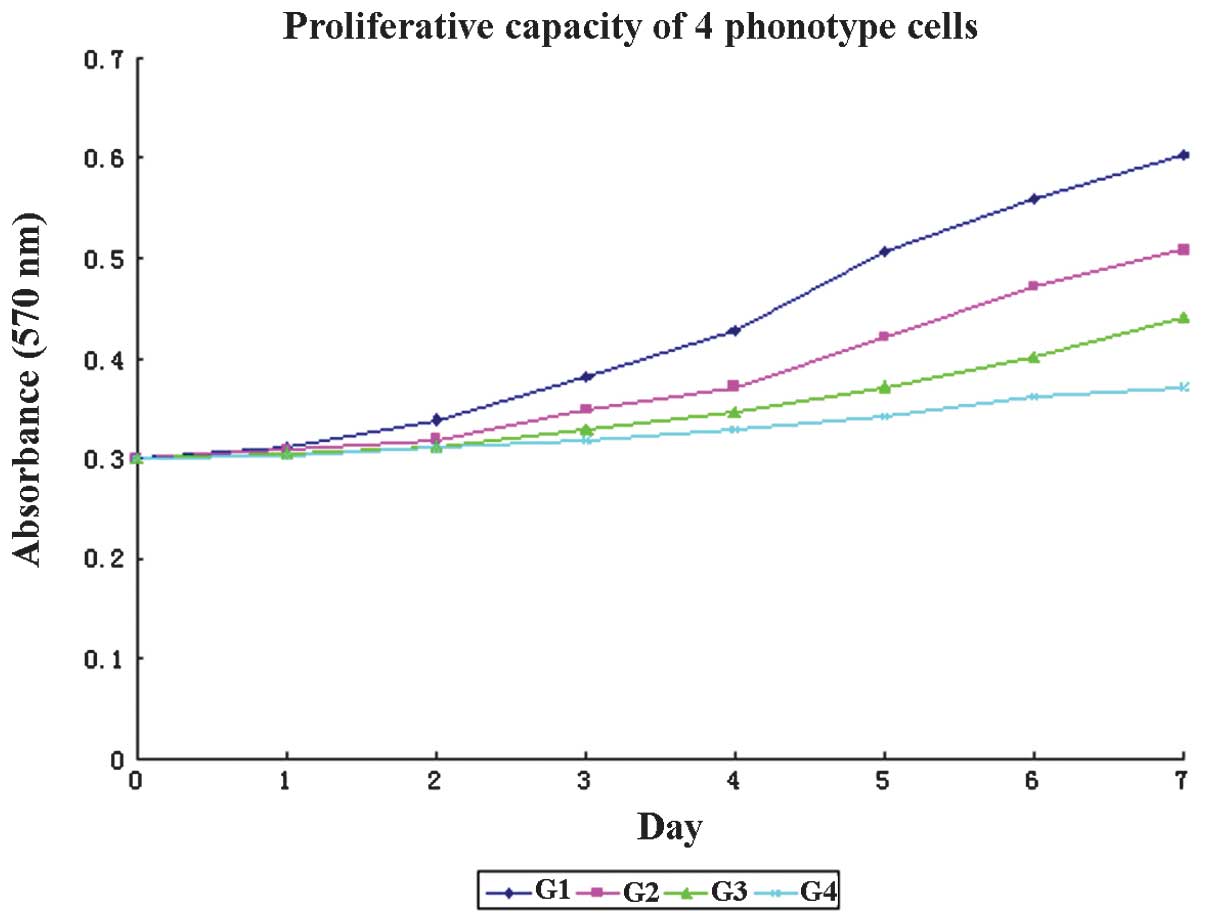
PCSLSs exhibit a high proliferative
capacity in vitro
To determine whether PCSLSs were different from PC-3
cells, we next observed the proliferative properties of four
independent phenotypes in vitro. As demonstrated in Fig. 4, the categories comprised spheres,
PC-3 cells, cells with a CD133−/CD44−
phenotype and differentiated spheres, as groups 1–4 (G1–4). G1 was
the group with the highest proliferative capacity. A significant
difference was observed between the absorbance levels of G1, 2 and
3 between the 1st and 7th days (P<0.05), while that of G4 was
not signifcantly different, although the absorbance was higher on
the 7th day (P>0.05). Significant differences were identified in
the doubling times and cell numbers among G1–4; the doubling times
were 23.24, 38.87, 57.79 and 133.01 h, while the cell numbers were
3×105, 4×104, 1.5×104 and
4.8×103 in G1–4, respectively (P<0.05).
Discussion
CSCs are not only the origin of tumors but also the
basis of tumor progression, resistance to therapy, metastasis and
subsequent tumor recurrence (16,17).
An increasing number of studies confirm that solid tumors and
established cancer cell lines are organized in a hierarchy of
heterogeneous cell populations, and that CSCs sustain the growth of
cancer cells overall (18). Methods
to identify and isolate CSCs include cell sorting based on surface
markers and staining of cell subpopulations forming tumor cell
spheres (19–21).
Although the majority of tumor cells may be
eliminated with the current treatment methods, difficulty remains
in treating androgen-independent prostate cancer (AIPC), which
develops in the majority of prostate cancer patients. If the stem
cell hypothesis is accepted, traditional treatment is not capable
of preventing tumor recurrence, as only the differentiated mature
cells and not a small population of cells may be killed (11). It has been suggested that prostate
cancer is a stem cell disease (22)
and that human PCSCs exhibit the same traits as CSCs, including
renewal, multi-potential differentiation, unlimited proliferation
capacity and permanency. Therefore, effective therapy for prostate
cancer should target PCSCs. However, a wide gap in the knowledge
remains, as fewer cells with the CD133+/CD44+
phenotype are available and there is a lack of powerful methods of
identification. Herein, we established an effective scheme whereby
PCSLCs may be isolated and enriched.
CD133 and CD44 are considered to be PCSC biomarkers.
Guo et al demonstrated that Epcam+CD44+ prostate
basal cells are able to form abundant spheres, while
Epcam+CD44− cells are not (23). Patrawala et al revealed that
CD44+ prostate cancer cells possessed stem-like cell
characteristics, including increased tumorigenicity, clonogenicity
and metastatic potential (24).
However, whether CD44 was the specific biomarker of CSC is unknown.
Our results demonstrated that there was no significant difference
in the expression of CD44 between PC-3 cells, following MACS, and
sphere cells, while that of CD133 exhibited a significant
difference. Therefore, we concluded that the hypothesis that CD133
is the specific biomarker of PCSCs remains controversial. Vander
et al demonstrated that CD133+ prostate cancer
cells exhibited stem cell properties, such as renewal and
multi-potential differentiation, even after several subcultures
(25).
Li et al found that prostate cancer cell
holoclones contained self-renewing cells and could be continuously
propagated (26). Holoclones have
been demonstrated to exhibit a high renewing capacity under SFM
culture, whilst retaining their holoclone morphology and
demonstrating high tumorigenicity (27). These findings are concordant with
our results, in which spheres that formed in SFM could be
continuously propagated, whilst retaining their CSC-like
characteristics. This shows that the method that we designed is
able to isolate and enrich PCSLCs.
To further identify the PCSLCs that possessed the
characteristics of CSCs, we conducted proliferation and
differentiation assays. Certain studies have concluded that TGF-β
regulates multiple cellular functions and influences normal
prostate growth and differentiation (28,29).
The untreated spheres exhibited the highest absorbance and the
shortest doubling time; while spheres induced by TGF-β had the
lowest absorbance, which may reflect that the proliferative rate of
PCSLCs was higher than the proliferative rate of the cancer cells
in a stage after differentiation. Following induction with TGF-β,
the spheres exhibited various morphologies, while the PC-3 cells
demonstrated no morphological changes. The PC-3 cell line has been
demonstrated to possess characteristics of prostatic small cell
carcinoma and to not express AR, while this was observed in the
DU145 cells (30), and the mRNA
level of PAP was almost undetectable in the PC-3 cells (31). In our study, the PAP and AR proteins
were not expressed on PC-3 cells with the
CD44+/CD133+ phenotype and sphere-forming
cell membranes. However, following treatment with TGF-β, these
cells co-expressed PAP and AR. Therefore, the PCSLCs may be induced
into other differentiative cells, of which the subpopulations have
not been confirmed in this study; thus, PCSCs may be considered to
be the progenitor cells of these mature cells.
To conclude, PCSLCs may be considered to be PCSCs,
which possess certain characteristics of all types of CSCs. The
CD133+/CD44+ phenotype of PC-3 cells represented a small
subpopulation of cells (∼0.53%) within the whole population of
tumor cells. Additionally, the spheres were able to continue to
proliferate and self-renew, while retaining their morphology.
Furthermore, the PCSLCs exhibited multi-potential differentiation.
Therefore, the processes of serum-free suspension culture and MACS
may be used effectively to enrich and isolate PCSLCs, and to
provide an inexhaustible source of genetically stable PCSCs for
studies. Understanding how to eliminate cancer cells in the
stationary phase that are considered to be PCSCs will lead to an
effective treatment for prostate cancer, and bring benefit to AIPC
patients.
Acknowledgements
This study was supported by the
Natural Science Foundation of China (NSFC; Grant No. 30972999). The
contents of this study are solely the responsibility of the authors
and do not necessarily represent the official views of the
NSFC.
References
|
1
|
Dean M, Fojo T and Bates S: Tumor stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar
|
|
2
|
Hadnagy A, Gaboury L, Beaulieu R and
Balicki D: SP analysis may be used to identify cancer stem cell
populations. Exp Cell Res. 312:701–710. 2006. View Article : Google Scholar
|
|
3
|
Piccirillo SG and Vescovi AL: Brain tumor
stem cells: possibilities of new therapeutic strategies. Expert
Opin Biol Ther. 7:1129–1135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Collins AT, Berry PA, Hyde C, Stower MJ
and Maitland NJ: Prospective identification of tumorigenic prostate
cancer stem cells. Cancer Res. 65:10946–10951. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Clarke MF, Morrison SJ, Wicha MS, et al:
Isolation and use of solid tumor stem cells. US Patent Application
119565. Filed December 28, 2002; issued April 21, 2011.
|
|
7
|
Suetsugu A, Nagaki M, Aoki H, et al:
Characterization of CD133+ hepatocellular carcinoma
cells as cancer stem/progenitor cells. Biochem Biophys Res Commun.
351:820–824. 2006.
|
|
8
|
O’Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110.
2007.PubMed/NCBI
|
|
9
|
Gong C, Liao H, Guo F, et al: Implication
of expression of Nanog in prostate cancer cells and their stem
cell. J Huazhong Univ Sci Technolog Med Sci. 32:242–246. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dubrovska A, Elliott J, Salamone RJ, et
al: CXCR4 expression in prostate cancer progenitor cells. PLoS One.
7:e312262012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fan X, Liu S, Su F, et al: Effective
enrichment of prostate cancer stem cells from spheres in a
suspension culture system. Urol Oncol. 30:314–318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu RQ, Wu JG, Zhou GC, et al: Sorting of
CD133(+) subset cells in human gastric cancer and the
identification of their tumor initiating cell-like properties.
Zhonghua Wei Chang Wai Ke Za Zhi. 15:174–179. 2012.(In
Chinese).
|
|
13
|
He JQ, Vu DM, Hunt G, et al: Human cardiac
stem cells isolated from atrial appendages stably express c-kit.
PLoS One. 6:e277192011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang B, Yoo N, Vu M, et al: Transforming
growth factor-beta can suppress tumorigenesis through effects on
the putative cancer stem or early progenitor cell and committed
progeny in a breast cancer xenograft model. Cancer Res.
67:8643–8652. 2007. View Article : Google Scholar
|
|
15
|
Richardson GD, Robson CN, Lang SH, et al:
CD133, a novel marker for human prostatic epithelial stem cells. J
Cell Sci. 117:3539–3545. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mittal S, Mifflin R and Powell DW: Cancer
stem cells: the other face of Janus. Am J Med Sci. 338:107–112.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Scopelliti A, Cammareri P, Catalano V, et
al: Therapeutic implications of cancer initiating cells. Expert
Opin Biol Ther. 9:1005–1016. 2009. View Article : Google Scholar
|
|
18
|
Uchida N, Buck DW, He D, et al: Direct
isolation of human central nervous system stem cells. Proc Natl
Acad Sci. 97:14720–14725. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ponti D, Costa A, Zaffaroni N, et al:
Isolation and in vitro propagation of tumorigenic breast
cancer cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005.PubMed/NCBI
|
|
20
|
Bao S, Wu Q, Li Z, et al: Targeting cancer
stem cells through L1CAM suppresses glioma growth. Cancer Res.
68:6043–6048. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Woodward WA, Chen MS, Behbod F, et al: On
mammary stem cells. J Cell Sci. 118:3585–3594. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rizzo S, Attand G and Hudson DL: Prostate
epithelial stem cells. Cell Prolif. 38:363–374. 2005. View Article : Google Scholar
|
|
23
|
Guo C, Liu H, Zhang BH, et al: Epcam,
CD44, and CD49f distinguish sphere-forming human prostate basal
cells from a subpopulation with predominant tubule initiation
capability. PLoS One. 7:e342192012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Patrawala L, Calhoun T,
Schneider-Broussard R, et al: Highly purified CD44+
prostate cancer cells from xenograft human tumors are enriched in
tumorigenic and metastatic progenitor cells. Oncogene.
25:1696–1708. 2006.PubMed/NCBI
|
|
25
|
Vander Griend DJ, Karthaus WL, Dalrymple
S, et al: The role of CD133 in normal human prostate stem cells and
malignant cancer-initiating cells. Cancer Res. 68:9703–9711.
2008.PubMed/NCBI
|
|
26
|
Li H, Chen X, Calhoun-Davis T, Claypool K
and Tang DG: PC3 human prostate carcinoma cell holoclones contain
self-renewing tumor-initiating cells. Cancer Res. 68:1820–1825.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang K and Waxman DJ: PC3 prostate
tumor-initiating cells with molecular profile
FAM65Bhigh/MFI2low/LEF1low increase tumor angiogenesis. Mol Cancer.
9:3192010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Diener KR, Need EF, Buchanan G, et al:
TGF-beta signalling and immunity in prostate tumourigenesis. Expert
Opin Ther Targets. 14:179–192. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li X, Wang Y, Sharif-Afshar AR, et al:
Urothelial transdifferentiation to prostate epithelia is mediated
by paracrine TGF-beta signaling. Differentiation. 77:95–102. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tai S, Sun Y, Squires JM, et al: PC3 is a
cell line characteristic of prostatic small cell carcinoma.
Prostate. 71:1668–1679. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zeng Y, Kakehi Y, Nouh MA, et al: Gene
expression profiles of lysophosphatidic acid-related molecules in
the prostate: relevance to prostate cancer and benign hyperplasia.
Prostate. 69:283–292. 2009. View Article : Google Scholar : PubMed/NCBI
|