Case report
A 55-year old Caucasian male was admitted to the
University Hospital Münster, Germany, due to a solid mass in the
renal allograft observed by routine ultrasonography. The patient
had a body mass index (BMI) of 35 kg/m2 and a tobacco
consumption of 30 pack-years. Informed consent for this case report
was obtained from the patient. The study was approved by the local
ethics committee.
Examination of the patient’s medical history
revealed Goodpasture syndrome that had led to end-stage kidney
disease 27 years before. Following 14 years of hemodialysis, the
patient received a cadaveric renal transplantation, and
immunosuppression was started with a triple combination of
tacrolimus, mycophenolate mofetil and prednisolone. Two years after
transplantation, a solid tumor was incidentally detected in the
left native kidney using routine ultrasound. A native computed
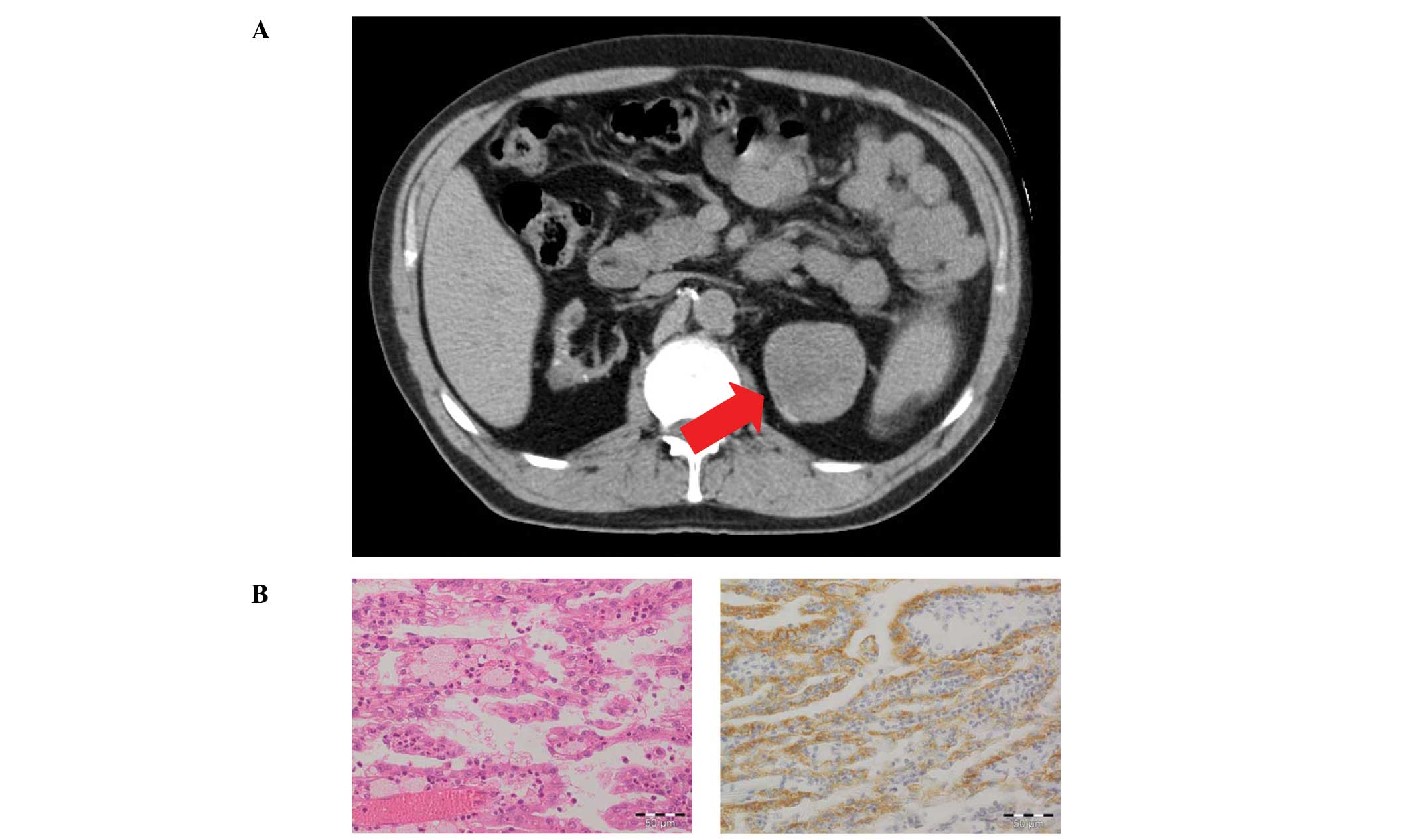
tomography (CT) scan revealed a well-defined, heterogeneous large
mass (5.8×5.0 cm) originating from the upper pole (Fig. 1A). Due to a suspected renal
malignancy, a laparoscopic radical nephrectomy of the left native
kidney was performed. Macroscopic inspection of the explanted organ
revealed acquired cystic kidney disease (ACKD) and a 5.5×4.5×4.0 cm
large tumor surrounded by a pseudocapsule, which revealed typical
characteristics of a low-grade chromophilic PRCC upon
histopathological evaluation (Fig.
1B). The tumor was limited to the kidney parenchyma, and
immunohistochemistry indicated positivity for α-methylacyl-CoA
racemase (AMACR), a highly sensitive diagnostic marker of PRCC
(1). There was no evidence of
metastatic disease detectable by whole-body CT. Furthermore, the
function of the renal graft remained satisfactory with a serum
creatinine level of 1.5 mg/dl.
A total of 13 years following transplantation,
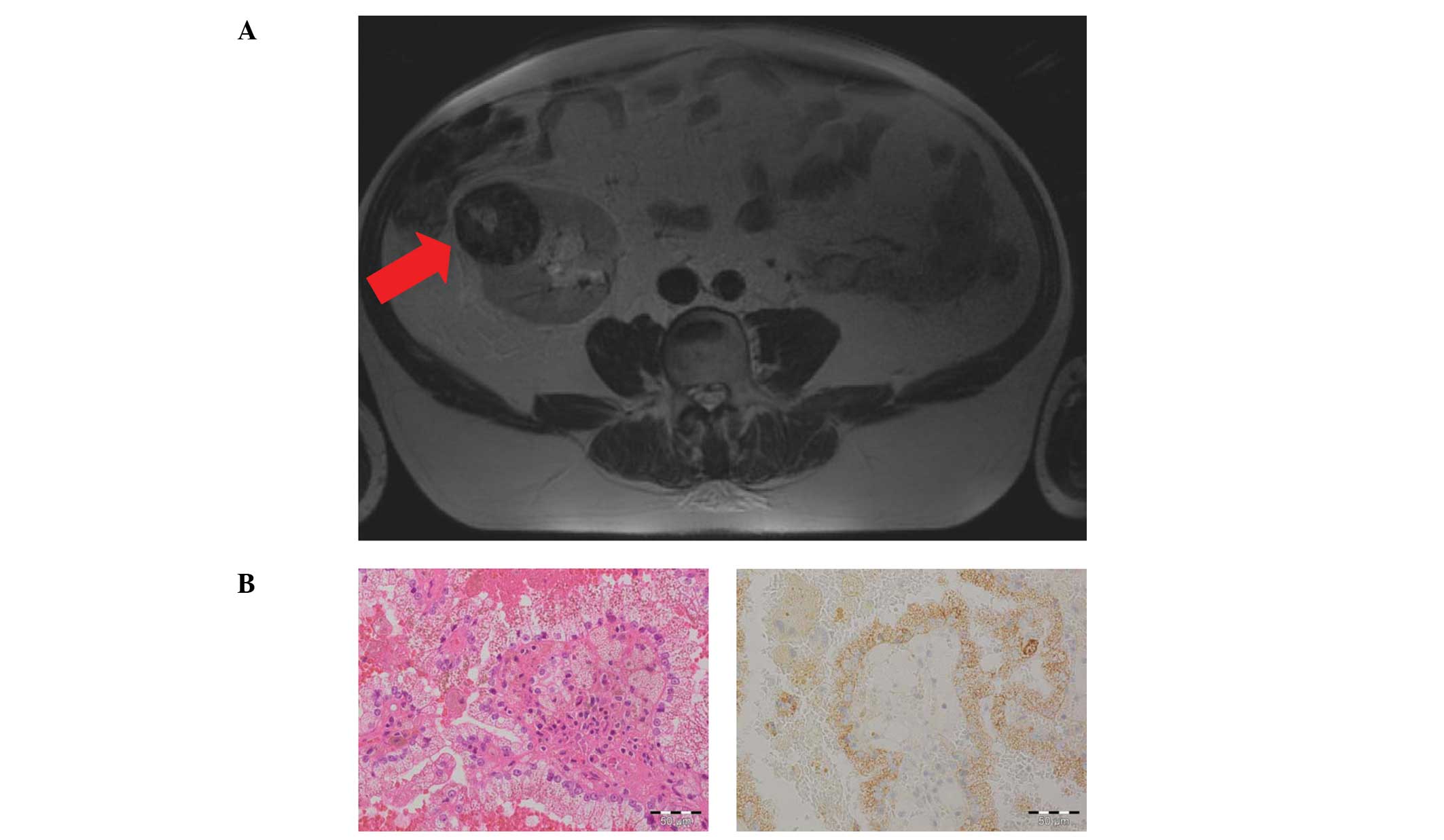
another suspicious inhomogeneous lesion (5.4×5.7×6.1 cm) was
detected at the upper pole of the allograft by routine
ultrasonography and was confirmed by magnetic resonance tomography
(MRT) imaging (Fig. 2A). Due to the
excellent physical condition of the patient and a well-functioning
allograft, an organ-preserving enucleation technique was selected.
The tumor was excised from the kidney by nephron-sparing surgery
(NSS). Notably, histolopathological examination again revealed the
rare diagnosis of a low-grade PRCC with no invasion of the
pseudocapsule. The cutting edges were free of the tumor and
histological analysis demonstrated papillary proliferating atypical
epithelial cells positive for AMACR (Fig. 2B).
A postoperative whole body CT scan and scintigraphy
of the skeletal system revealed no signs of metastasis. The graft
remained persistent and well-functioning, and tacrolimus was
replaced by everolimus to decrease the potential risk of further
tumor development. To confirm the origin of the PRCCs, a DNA
microsatellite analysis was performed with samples derived from the
two kidney tumors. Notably, PCR amplification revealed different
patterns in 5 of 7 regions in the microsatellite analysis, which
reflects the different origin of the two tumors.
Discussion
The overall prevalence of post-transplantation
malignancy is significantly increased following renal
transplantation (2,3). A total of 5% of all malignancies are
kidney tumors; twice the amount of that in the general population
(4). Kidney transplant recipients
carry a 15-fold risk of renal cell carcinoma following
transplantation (5,6). Notably, among these approximately 40%
of all renal cell carcinoma are PRCC compared to a prevalence of
only 10–15% in the general population (7–9).
The present case report demonstrates two relevant
features. Firstly, the incidence of two PRCCs of different origins
in one patient (one PRCC in the native kidney and one in the
allograft), which to the best of our knowledge, has not been
previously reported. Secondly, short ultrasonography control
intervals in a high-risk patient enable early tumor detection and
successful therapy. This demonstrates an enormous clinical
relevance.
As PRCC is a rare malignant tumor entity, the
occurrence of two PRCCs in the same patient following renal
transplantation appears to be an uncommon coincidence. There are
several well-known risk factors for the development of renal cell
carcinoma, including smoking, obesity, abuse of analgesics and ACKD
(10). Furthermore, prolonged
high-dose immunosuppression following renal transplantation leads
to a higher incidence of malignancies (3).
A number of risk factors may have contributed to
PRCC development in the patient. Thirteen years of triple
immunosuppressive therapy, obesity with a BMI of 35
kg/m2, ACKD and 30 pack-years tobacco consumption
identified our patient as a high-risk candidate for renal
malignancies. Therefore, routine ultrasonography screening
examinations for malignancies within the native kidney and the
allograft are necessary. Although de novo carcinomas in the
renal allograft are extremely rare, certain cases of carcinomas
detected in the graft have been reported (11,12).
Long follow-up periods should be recommended as malignancies can
occur even decades following transplantation.
Early tumor diagnosis is essential for a good
outcome. In our patient, a diagnosis at stage I of the disease
enabled an organ-preserving surgery with a persistent
well-functioning graft. In comparison to radical nephrectomy, NSS
has become a safe treatment method for low-stage renal cell
carcinomas and enables the preservation of organ function (13,14).
In conclusion, this case demonstrates the relevance
of risk factors in the development of PRCC. This strengthens the
requirement for a risk adaptive screening strategy for malignancies
in the allograft and native kidneys. For high-risk candidates,
shorter ultrasonic screening intervals may facilitate early tumor
detection and enhance the chance for successful NSS and better
outcome rates.
References
|
1
|
Szponar A, Beothe T and Kovacs G: How
useful is alpha-methylacyl-CoA racemase (AMACR)
immunohistochemistry in the differential diagnosis of kidney
cancers? Histopathology. 56:263–265. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Birkeland SA, Løkkegaard H and Storm HH:
Cancer risk in patients on dialysis and after renal
transplantation. Lancet. 355:1886–1887. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Morath C, Mueller M, Goldschmidt H,
Schwenger V, Opelz G and Zeier M: Malignancy in renal
transplantation. J Am Soc Nephrol. 15:1582–1588. 2004. View Article : Google Scholar
|
|
4
|
Penn I: Primary kidney tumors before and
after renal transplantation. Transplantation. 59:480–485. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kasiske BL, Snyder JJ, Gilbertson DT and
Wang C: Cancer after kidney transplantation in the United States.
Am J Transplant. 4:905–913. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Suson KD, Sausville JE, Sener A and Phelan
MW: Native nephrectomy for renal cell carcinoma in transplant
recipients. Transplantation. 91:1376–1379. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schwarz A, Vatandaslar S, Merkel S and
Haller H: Renal cell carcinoma in transplant recipients with
acquired cystic kidney disease. Clin J Am Soc Nephrol. 2:750–756.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ishikawa N, Tanabe K, Tokumoto T, et al:
Renal cell carcinoma of native kidneys in renal transplant
recipients. Transplant Proc. 30:3156–3158. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hoshida Y, Tsukuma H, Yasunaga Y, et al:
Cancer risk after renal transplantation in Japan. Int J Cancer.
71:517–520. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Alexander MP, Farag YM, Mittal BV, Rennke
HG, Tullius SG and Singh AK: De novo multifocal renal cell
carcinoma in the renal allograft. Kidney Int. 75:111–114. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsaur I, Obermuller N, Jonas D, et al: De
novo renal cell carci-noma of native and graft kidneys in renal
transplant recipients. BJU Int. 108:229–234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roupret M, Peraldi MN, Thaunat O, et al:
Renal cell carcinoma of the grafted kidney: how to improve
screening and graft tracking. Transplantation. 77:146–148. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Van Poppel H, Da Pozzo L, Albrecht W, et
al: A prospective, randomised EORTC intergroup phase 3 study
comparing the oncologic outcome of elective nephron-sparing surgery
and radical nephrectomy for low-stage renal cell carcinoma. Eur
Urol. 59:543–552. 2011.
|
|
14
|
Roos FC, Brenner W, Müller M, et al:
Oncologic long-term outcome of elective nephron-sparing surgery
versus radical nephrectomy in patients with renal cell carcinoma
Stage pT1b or greater in a matched-pair cohort. Urology.
77:803–808. 2011. View Article : Google Scholar
|