Introduction
Peripheral neuroblastic tumors (pNTs), including
neuroblastomas (NB), ganglioneuroblastomas (GNBs) and
ganglioneuromas (GNs), are rare and constitute only 6% of tumors in
children. GNs are the most differentiated and benign form of pNTs
and the majority of GNs display as slow-growing, solitary lesions
that may or may not have an effect on neighboring structures. The
most commonly affected sites are the posterior mediastinum, the
retroperitoneum and the adrenal gland, which affect 41.5, 37.5 and
21% of cases, respectively. Few GNs occur in the cervical region
(8%), of which the majority are in single form, and to the best of
our knowledge, only one case of multiple GN in one side of the neck
has been reported (1). Here, we
report a case of massive multiple GN located in the neck of a
4-year-old girl who was successfully treated using a surgical
approach. We also reviewed cases of GN located in the head and neck
region that have been reported in the English-language literature
over the past 10 years. The clinically related features,
differential diagnosis, management and pathological findings are
discussed.
The study was approved by the ethic committee of The
General Hospital of the People’s Liberation Army, Beijing, China.
The patient’s guardians consented to the publication of the
study.
Case report
A 4-year-old girl was admitted to the General
Hospital of the People’s Liberation Army, Beijing, China, with a
slow-growing, painless mass in the right lateral neck region, which
had been present since birth. In the past year, the mass had
demonstrated accelerated growth and tenderness, and an increasingly
heavy snore during sleep was noticed by her parents. There was no
growth retardation and no specific treatments were conducted.
The physical examination revealed two firm solid
masses extending from the left mastoid process to the clavicle,
with the frontal border of the mass extending across the midline
where throbbing of the carotid artery was observed. The overlying
skin was normal with no adhesion to the masses. No symptoms of
Horner’s syndrome, including ptosis, myosis, ipsilateral facial
anhidrosis and flushing, were observed. Additionally, routine
laboratory tests, including quantitative analysis of homovanillic
acid (HVA) and vanillylmandelic acid (VMA), revealed no
abnormality.
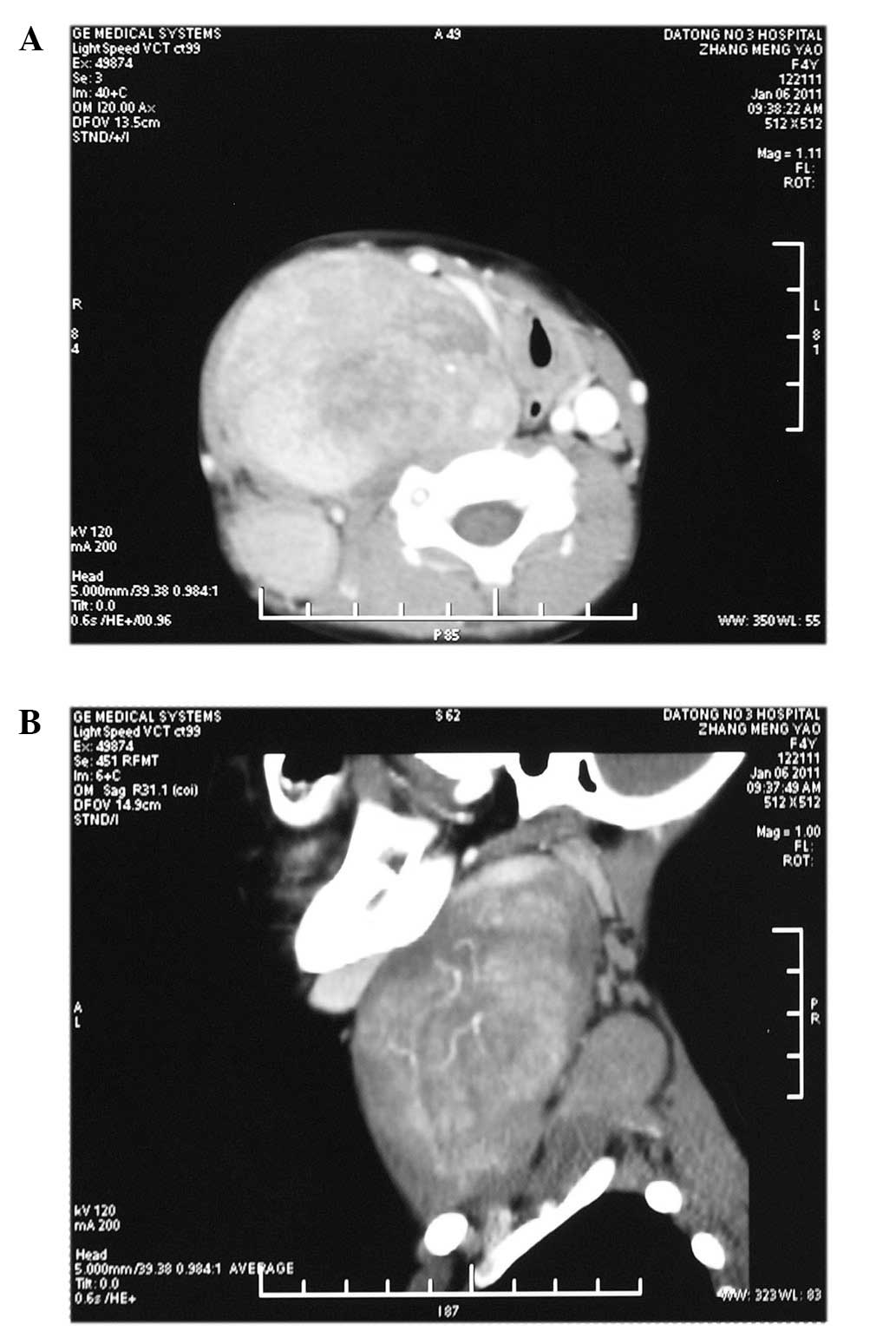
Computed tomography (CT) images identified two ovoid
and well-circumscribed masses on the right side of the neck. The
first mass extended from the scull base to the clavicle level in
the prevertebral region and across the midline and measured
10×6.4×5.7 cm. The other mass was located in the paravertebral
region from C4 to C7 level and measured 4.1×2.6×5 cm (Fig. 1A and B). A working diagnosis of
neurilemmoma was established.
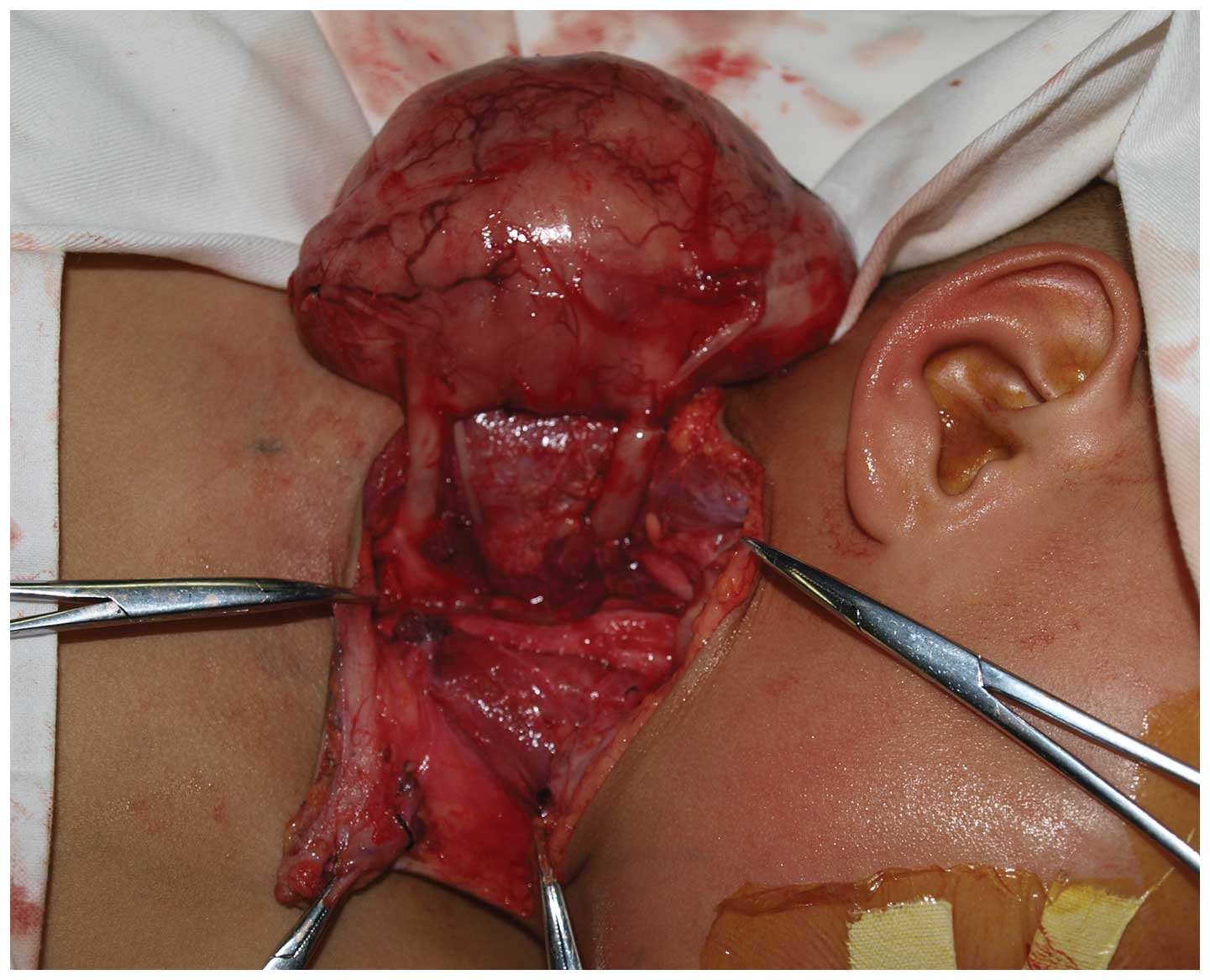
Complete surgical excision of the masses located
beneath the sternocleidomastoid and trapezius muscles was performed
under general anesthesia using a T-shaped cervical approach. Upon
macroscopy, the two masses were thick and well-defined with no
adhesion to the surrounding tissues. The common carotid artery and
internal jugular vein were displaced anteromedially, and two round
and thick neurogenic pedicles were observed to originate from
underneath the prevertebral fascia (Fig. 2). Once the common carotid artery,
internal jugular vein, phrenic nerve and cranial nerves X–XII were
identified and carefully protected, the masses were
circumferentially separated using blunt dissection and the pedicles
were ligated and transected. Both masses were completely
excised.
The excised mass was smooth and well-encapsulated.
The gross sections revealed a homogeneous tan-white internal
appearance. Microscopically, the tumor was composed of large mature
ganglion cells, schwann cells and tangled masses of neurites in
bundles of schwannian stroma. Additionally, slight immaturity of
the ganglion cells was observed in part of the tumor (Fig. 3). Positivity was observed for S-100,
which is consistent with the neurogenic nature of the neoplasm. A
pathological diagnosis of GN was established for both masses.
The postoperative period was uneventful with no
observed symptoms of Horner’s syndrome with the exception of myosis
in the right eye. The diameter of the right pupil was 1.5 mm,
approximately one third of the left pupil, although it recovered to
2.5 mm by the time the patient was discharged on the 10th
postoperative day. No specific adjuvant therapy was performed and
no signs of recurrence were observed during a 1.5-year
follow-up.
Discussion
GN is a rare, benign, non-invasive and neurogenic
tumor. We reviewed cases of GN located in the head and neck region
that were reported in the English-language literature during the
past 10 years. A total of 16 papers and 26 cases were included
(2–17). The epidemiology and clinical
characteristics are summarized in Table
I. At presentation, 18 cases were under 18 years, the mean age
was 20.6 years and females demonstrated a slight preponderance,
accounting for 61.5% of all cases.
 | Table ICharacteristics of GN located in the
head and neck region reported in the English-language literature
over the past 10 years. |
Table I
Characteristics of GN located in the
head and neck region reported in the English-language literature
over the past 10 years.
| Characteristics | No. of cases | % |
|---|
| Gender |
| Female | 16 | 61.5 |
| Male | 9 | 34.6 |
| Location |
| Parapharyngeal
space | 6 | 23.1 |
| Retropharyngeal
space | 3 | 11.5 |
| Spine | 2 | 7.7 |
| Mandible | 1 | 3.8 |
| Superior orbit | 1 | 3.8 |
| Internal auditory
canal | 1 | 3.8 |
| Other | 13 | 50.0 |
| Form |
| Solitary | 23 | 88.5 |
| Multiple | 3 | 11.5 |
| Unilateral mass | 23 | 88.5 |
| Bilateral mass | 3 | 11.5 |
| Symptoms |
| Asymptomatic
mass | 15 | 57.7 |
| Dysphagia
snoring | 5 | 19.2 |
| Pain | 2 | 7.7 |
| Proptosis of the
right eye | 2 | 7.7 |
| Spinal cord
compression symptoms | 2 | 7.7 |
| Hearing loss | 1 | 3.8 |
| Mandibular
asymmetry | 1 | 3.8 |
| VMA/HVA
abnormality | 0/5 | 0.0 |
| Treatment |
| Complete
excision | 23 | 88.5 |
| Partial
excision | 3 | 11.5 |
| Postoperative
Horner’s syndrome | 10 | 38.5 |
| Recurrence | 1 | 3.8 |
GN in the head and neck region usually presents as a
slowly enlarging mass, predominantly in single form. Only one case
of the multiple form in one side of the neck has been reported;
thus, it is extremely rare (1). Of
the 26 reviewed cases of GN, 3 demonstrated bilateral masses in the
neck, 2 of which were presented in the spine (2,3). The
majority of cases reviewed were primary lesions (34.5%), occurring
in the parapharyngeal and retropharyngeal space. The cervical
sympathetic chain is the most frequent structure of origin in the
neck. Other sites of origin include the larynx, pharynx and
ganglion nodosum of the vagus nerve. In rare circumstances, GN
evolves from differentiating NBs or GNBs, either spontaneously or
due to treatment. One case of GN resulted from metastasis of NB in
the adrenal gland (5), which
extends the scope of differential diagnosis of masses in the head
and neck region.
Most GN cases we reviewed in the head and neck
region presented as an asymptomatic mass (57.7%), and only 2 cases
experienced pain (7.7%). The present case also demonstrated
tenderness. When masses compress the pharyngeal wall, cranial
nerves, eustachian tubes or vascular structures, dysphagia,
dyspnea, snoring and obstructive sleep apnea may be reported
(19.2%). Symptoms of Horner’s syndrome may ensue from the
compression of the cervical sympathetic chain. In this review, no
symptoms of Horner’s syndrome at presentation were reported;
however, 1 case in the internal auditory canal presented hearing
loss (6).
It has been reported that GNs are secretory in up to
39% of patients releasing catecholamines (18). The elevated catecholamines increase
the levels of VMA or HMA in the plasma or urine, causing
hypertension, diarrhea, sweating, flushing, renal acidosis and
other symptoms of catecholamine excess. However, in our review,
including the present case, no secretory function-related symptoms
were observed, and none of the VMA/VHA tests identified any
abnormality. Therefore, quantitative analysis of VMA/VHA is not
indispensable for the differential diagnosis of GN in this
region.
Due to the scarcity of GN and the lack of specific
signs and symptoms, it is often difficult to reach a definite
diagnosis prior to pathological examination. CT and magnetic
resonance imaging (MRI) provide valuable information on the size,
location, composition of the mass and its relationship to adjacent
significant structures, which is of great aid in determining a
surgical plan. GN usually appears as a defined oval or irregular
mass with a hypodense appearance. Punctate and coarse calcification
could also be observed in certain cases in a disseminated sprinkled
pattern, and administration of contrast media could lead to low or
moderate enhancement. MRI is superior to CT in defining the
intraspinal involvement; low intensity on T1-weighted images
(T1WI), marked high intensity on T2-weighted images (T2WI) and
gradual increasing enhancement on dynamic MR images are typical
appearances of GN (19).
With these image characteristics it remains
difficult to discriminate GN from other lesions in the cervical
region, including salivary gland tumors (pleomorphic adenoma),
other neurogenic tumors (neurolemmoma, neurofibroma and
fibrosarcomas) and soft tissue lesions (fibrosarcomas,
rhabdomyosarcomas and malignant lymphomas). When GN is in close
proximity to the thyroid gland, these lesions may be mistaken for
thyroid swellings (7). Recently,
fine-needle aspiration biopsy (FNAB) has been conducted in the
diagnosis of GN (7–11). It is a rapid, cost-effective and
safe diagnostic procedure, particularly for the pediatric age
group; however, it is not always reliable. In certain cases, it was
inconclusive and even misleading (7,8,11).
Mature ganglion cells are characteristic of GN, and if FNA fails to
take the ganglion cells the diagnosis of other neurogenic
neoplasms, including schwannomas and neurofibromas, may be
mistakenly made. Moreover, GNBs may only contain small foci of
neuroblasts and FNA may easily miss this immature component,
leading to the wrong diagnosis. Thus, the results of FNA should be
taken cautiously (9).
Peripheral neuroblastic tumors represent a spectrum
of diseases from undifferentiated and malignant NB to
well-differentiated and benign GN (8). According to the international
neuroblastoma pathology classification (the Shimada System),
neuroblastic tumors are classified into 4 groups: NB (schwannian
stroma-poor), intermixed GNB (schwannian stroma-rich), nodular GNB
(schwannian stroma-rich/stroma-dominant and stroma-poor) and GN
(schwannian stroma-dominant), which is further divided into 2
subtypes (maturing and mature). Typical GN is composed of mature
ganglion cells and schwannian stroma; however, complete maturation
of ganglion cells is rare (approximately 7%) and slightly atypical
ganglion cells could be detected in the present case. Immaturity of
ganglion cells did not influence the diagnosis of GN (20).
Complete surgical excision is the treatment of
choice, in which a transcervical or transoral approach may be
employed depending on the location of the tumor. The risks are
mainly related to the intraoperative sacrifice of the neural
structures and the vasculature associated with the tumor. The
prognosis of GN is usually good. Complete excision was achieved in
23 (88.5%) cases in this review and no recurrences were reported in
those patients. In the 3 cases of partial excision, 2 cases
demonstrated no growth in 2–4 years and recurrence was reported in
1 case. Symptoms of Horner’s syndrome are often detected following
surgery, but these symptoms are usually completely resolved within
several months. Further therapy, chemotherapy or radiotherapy, is
not usually required, even for cases with partial excision. Retrosi
et al (21) studied 10
patients that underwent incomplete excision, and after 33.5±40
months’ follow-up, no tumor progression or recurrence occurred.
However, if slight immaturity is present in GN, close follow-up is
recommended.
References
|
1
|
Shotton JC, Milton CM and Allen JP:
Multiple ganglioneuroma of the neck. J Laryngol Otol. 106:277–278.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miyakoshi N, Hongo M, Kasukawa Y, Misawa A
and Shimada Y: Bilateral and symmetric C1–C2 dumbbell
ganglioneuromas associated with neurofibromatosis type 1 causing
severe spinal cord compression. Spine J. 10:e11–e15. 2010.
|
|
3
|
Bacci C, Sestini R, Ammannati F, Bianchini
E, Palladino T, Carella M, Melchionda S, Zelante L and Papi L:
Multiple spinal ganglioneuromas in a patient harboring a pathogenic
NF1 mutation. Clin Genet. 77:293–297. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zebing Z, Jianwei S, Yan C and Yan G:
Clinicopathological characteristics of neck ganglioneuroma. Oral
Med Pathol. 12:131–134. 2008. View Article : Google Scholar
|
|
5
|
Patterson AR, Barker CS, Loukota RA and
Spencer J: Ganglioneuroma of the mandible resulting from metastasis
of neuroblastoma. Int J Oral Maxillofac Surg. 38:196–198. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ozluoglu LN, Yilmaz I, Cagici CA, Bal N
and Erdogan B: Ganglioneuroma of the internal auditory canal: a
case report. Audiol Neurootol. 12:160–164. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Leonardis M, Sperb D, Alster C, Campisi C
and Herter NT: Ganglioneuroma of the neck, masquerading as a
goiter. Eur J Surg Oncol. 29:929–930. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cannady SB, Chung BJ, Hirose K, Garabedian
N, Van Den Abbeele T and Koltai PJ: Surgical management of cervical
ganglioneuromas in children. Int J Pediatr Otorhinolaryngol.
70:287–294. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ponce-Camacho MA, Diaz de Leon-Medina R,
Miranda-Maldonado I, Garza-Guajardo R, Hernandez-Salazar J and
Barboza-Quintana O: A 5-year-old girl with a congenital
ganglioneuroma diagnosed by fine needle aspiration biopsy: a case
report. Cytojournal. 5:52008.PubMed/NCBI
|
|
10
|
Kolte SS: Ganglioneuroma presenting as a
neck mass diagnosed by fine needle aspiration cytology.
Cytopathology. 22:205–206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Müller A, Förster G, Behrendt W and
Kosmehl H: Headache as an unusual presenting symptom of
retropharyngeal ganglioneuroma. Acta Otolaryngol. 122:565–568.
2002.PubMed/NCBI
|
|
12
|
Kaufman MR, Rhee JS, Fliegelman LJ and
Costantino PD: Ganglioneuroma of the parapharyngeal space in a
pediatric patient. Otolaryngol Head Neck Surg. 124:702–704. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Choi HY, Lee JH, Park JM and Shin MK:
Orbital ganglioneuroma in a young healthy person. Arch Ophthalmol.
127:223–225. 2009.PubMed/NCBI
|
|
14
|
Katilmis H, Ozturkcan S, Adadan I, Ozdemir
I, Algin H and Tunakan M: Cervical ganglioneuroma. Int J Pediatr
Otorhinolaryngeal Extra. 1:157–159. 2006. View Article : Google Scholar
|
|
15
|
Gary C, Robertson H, Ruiz B, Zuzukin V and
Walvekar RR: Retropharyngeal ganglioneuroma presenting with neck
stiffness: report of a case and review of literature. Skull Base.
20:371–374. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cannon TC, Brown HH, Hughes BM, Wenger AN,
Flynn SB and Westfall CT: Orbital ganglioneuroma in a patient with
chronic progressive proptosis. Arch Ophthalmol. 122:1712–1714.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Friedlander PL, Hunt JP and Palacios E:
Ganglioneuroma of the neck. Ear Nose Throat J. 81:4352002.
|
|
18
|
Geoerger B, Hero B, Harms D, Grebe J,
Scheidhauer K and Berthold F: Metabolic activity and clinical
features of primary ganglioneuromas. Cancer. 91:1905–1913. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ichikawa T, Ohtomo K, Araki T, Fujimoto H,
Nemoto K, Nanbu A, Onoue M and Aoki K: Ganglioneuroma: computed
tomography and magnetic resonance features. Br J Radiol.
69:114–121. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shimada H, Ambros IM, Dehner LP, Hata J,
Joshi VV, Roald B, Stram DO, Gerbing RB, Lukens JN, Matthay KK and
Castleberry RP: The international neuroblastoma pathology
classification (the Shimada system). Cancer. 86:364–372. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Retrosi G, Bishay M, Kiely EM, Sebire NJ,
Anderson J, Elliott M, Drake DP, Coppi P, Eaton S and Pierro A:
Morbidity after ganglioneuromaexcision: is surgery necessary? Eur J
Pediatr Surg. 21:33–37. 2011. View Article : Google Scholar : PubMed/NCBI
|