Introduction
Nasopharyngeal carcinoma (NPC) is one of the most
common malignant tumors in China. Presently, the main treatment for
NPC is radiation therapy. Over the past 20 years, with the
development of imaging, radiation techniques and equipment for NPC,
the local control rate among patients in the early stage of the
disease has risen to 70–90%. However, in patients in the late
stages of the disease (stages III–IV), the local control rate is
only 50%, with a five-year overall survival rate of 40–70%
(1). The main causes of treatment
failure are local recurrence and distant metastases, which are
closely related to each other (2).
The key factor that results in the local recurrence of tumors is
the presence of tumor cells possessing a resistance to radiation.
Therefore, decreasing radiation resistance and increasing radiation
sensitivity of tumor cells are of significant clinical
relevance.
Previous studies (3,4) have
indicated that radiation therapy results in the elevated expression
of vascular endothelial growth factor (VEGF), which may contribute
to the resistance to radiation in tumors. Therefore, radiation
therapy combined with antiangiogenic therapy may be effective in
decreasing radiation resistance.
A number of studies have indicated that
neovascularization is closely related to the progression and
metastasis of tumors (5). The
growth of tumors depends on the nutrients and oxygen transported by
neovascular vessels. Hence, research has taken a new direction by
inhibiting the neovascular formation of tumors and consequently
starving the tumor to death. This is known as antiangiogenic
therapy. Thus far, endostatin is the endogenous protein which most
strongly inhibits the endothelial cells involved in vascular
formation. Endostatin inhibits growth, invasion and metastasis by
inhibiting tumor angiogenesis without inducing resistance following
repeated applications. It also possesses very low toxicity, minimal
side effects and is effective in both early- and late-stage tumors
(6). However, endostatin is
unstable in vitro, difficult to produce and expensive, which
limits its clinical application. Endostar is a new recombinant
human endostatin synthesized in China and approved by the State
Food and Drug Administration in 2005 for the treatment of non-small
cell lung cancer. Endostatin overcomes the limitations of previous
recombinant proteins in their clinical applications such as
unstable physiochemical characteristics, low water solubility and
complicated purification. Therefore, research on the large-scale
clinical application of Endostar is possible. Ling et al
showed that Endostar exerts its antiangiogenic effects by blocking
the VEGF-induced tyrosine phosphorylation of KDR/Flk-1 (7). The study suggested that tumor
treatment by radiation combined with Endostar is more effective
than radiation alone.
In the present study, the antitumor effects of the
combination of Endostar and radiation on subcutaneous
transplantation NPC tumor models in BALB/c nude mice were measured,
and the microvessel density (MVD) and VEGF levels were also
measured to explore the mechanism of the radiosensitization effect
of Endostar on NPC.
Materials and methods
Animals
Four-week-old female BALB-nu/nu
(CAnN.Cg-Foxn1<nu>/CrlCrlj nu/nu) mice (weighing 16±2 g) were
obtained from the Shanghai Laboratory Animal Center, Chinese
Academy of Sciences (Shanghai, China), and acclimated for 2 weeks
in the animal facility before use. The number of animals per
experimental group was 6, as specified in the table and figures.
All animal experiments were conducted according to the
institution’s guidelines for the care and use of laboratory
animals.
Tumor cells
The human NPC cell line CNE1, provided by the
Shanghai Institute of Biochemistry and Cell Biology, Chinese
Academy of Sciences, was cultured in RPMI-1640 medium containing
10% fetal calf serum (Gibco, Carlsbad, CA, USA) and incubated at
37˚C under a 5% CO2 atmosphere.
Human NPC xenograft model
An NPC cell suspension (0.2 ml containing
5×106 CNE1 cells in the log phase) was inoculated
subcutaneously into the right lower limb of female BALB/c nu/nu
mice. The tumor volume reached 391.270±78.787 mm3 21
days after inoculation and the mice were randomly divided into 4
groups (A, B, C and D), with 6 mice per group, and the different
treatments were initiated (day 1).
BALB-nu/nu mice treatment
Group A received 0.2 ml of normal saline once daily
around the tumor for 10 days. Group B was injected with 20 mg/kg
Endostar (Sincere Pharmaceutical Company, Nanjing, China) once
daily around the tumor for 10 days. Group C received 0.2 ml of
normal saline once daily around the tumor for 10 days and then 1 h
after administration on day 10, 20 Gy of X-rays (5 MeV) was applied
to irradiate the NPC tumor once locally (the radiation field was
2×3 cm and the non-radiation field was protected by lead bricks).
Group D was injected with 20 mg/kg Endostar once daily around the
tumor for 10 days and then administered the same radical treatment
as was received by group C. Tumor volume, estimated using the
equation V = length x width2 × 0.5, was measured every 4
days after treatment. The percentage of tumor growth inhibition
(TGI) was calculated as follows: TGI(%) = [1−(mean change in the
tumor volume in each group treated with antitumor drugs/mean change
in the tumor volume in the control group)] x 100. The tumor
doubling time (DT) was calculated as follows: DT = d ×
lg2/(lgVd − lgV0), where d is the
length of time between two measurements, Vd is the
volume of the tumor treated with an antidrug and V0 is
the volume of the tumor before the treatment. The mice were
euthanized 28 days after treatment and the tumor tissues were
collected, weighed and fixed with 4% formaldehyde.
Immunohistochemistry
Immunohistochemistry was performed using standard
streptavidin-biotin complex/3,3′-diaminobenzidine (DAB) staining on
4-μm thick sections from the paraffin-embedded, formalin-fixed
tissue, with DAB acting as a chromogen. The mouse anti-human VEGF
antibodies and the rabbit anti-human CD34 antibodies (Boster
Biological Technology, Ltd., Wuhan, China) were used to identify
the VEGF expression levels and microvessels.
Microvessel density (MVD)
The MVD was determined by modification of the
methods reported by Mäkitie et al (8). The microvessels were counted from the
5 individual, most highly vascularized areas (‘hotspots’)
containing a high number of CD34 staining sites. The 5 hotspots
were selected randomly by scanning an entire immunostained tumor
section at ×100 magnification. The microvessels were subsequently
counted at ×400 magnification in each hotspot. Any immunolabeled
single or multiple endothelial cells, clearly separate from
adjacent tumor cells or interstitial cells, were counted as one
microvessel. The MVD was expressed as the mean value of the number
of microvessels in the 5 hotspots.
Levels of VEGF in tumors
The levels of VEGF were determined via the
semi-quantitative method (9). Three
replicates were selected randomly at ×200 magnification and the
proportion of the VEGF-positive cells and stain depth were scored
in each replicate. The semi-quantitative score was expressed as the
product (S) of the score for the proportion of stained cells (S1)
and the score for the stain depth (S2), i.e. S=S1×S2. The detailed
criteria for the scores are shown in Table I. The VEGF expression was designated
as negative, weakly positive, positive and strongly positive,
respectively, according to the value of the product (S≤1, 1<S≤2,
2<S≤4 and S>4). The levels of VEGF were expressed as the
product of the 2 scores in the 3 replicates.
 | Table ICriteria scores and expressions of
VEGF. |
Table I
Criteria scores and expressions of
VEGF.
| N | S1 | Stain depth | S2 | Sa | Expression |
|---|
| N=0 | 0 | No obvious
staining | 0 | S≤1 | Negative (−) |
| 0<N≤1/3 | 1 | Pale yellow | 1 | 1<S≤2 | Weakly positive
(+/−) |
| 1/3<N≤2/3 | 2 | Brownish
yellow | 2 | 2<S≤4 | Positive (+) |
| N>2/3 | 3 | Chocolate
brown | 3 | S>4 | Strongly positive
(++) |
Statistical analysis
All values are expressed as the mean ± SD. The
differences in the multiple groups were analyzed using one-way
analysis of variance (ANOVA). The changes in the inhibition rates
of the tumors between groups were compared using the Chi-square
test or Fisher’s exact test (two-tailed test, α=0.05). All
statistical analyses were performed using SPSS 15.0 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant result.
Results
Antitumor activity of Endostar in
combination with radiation
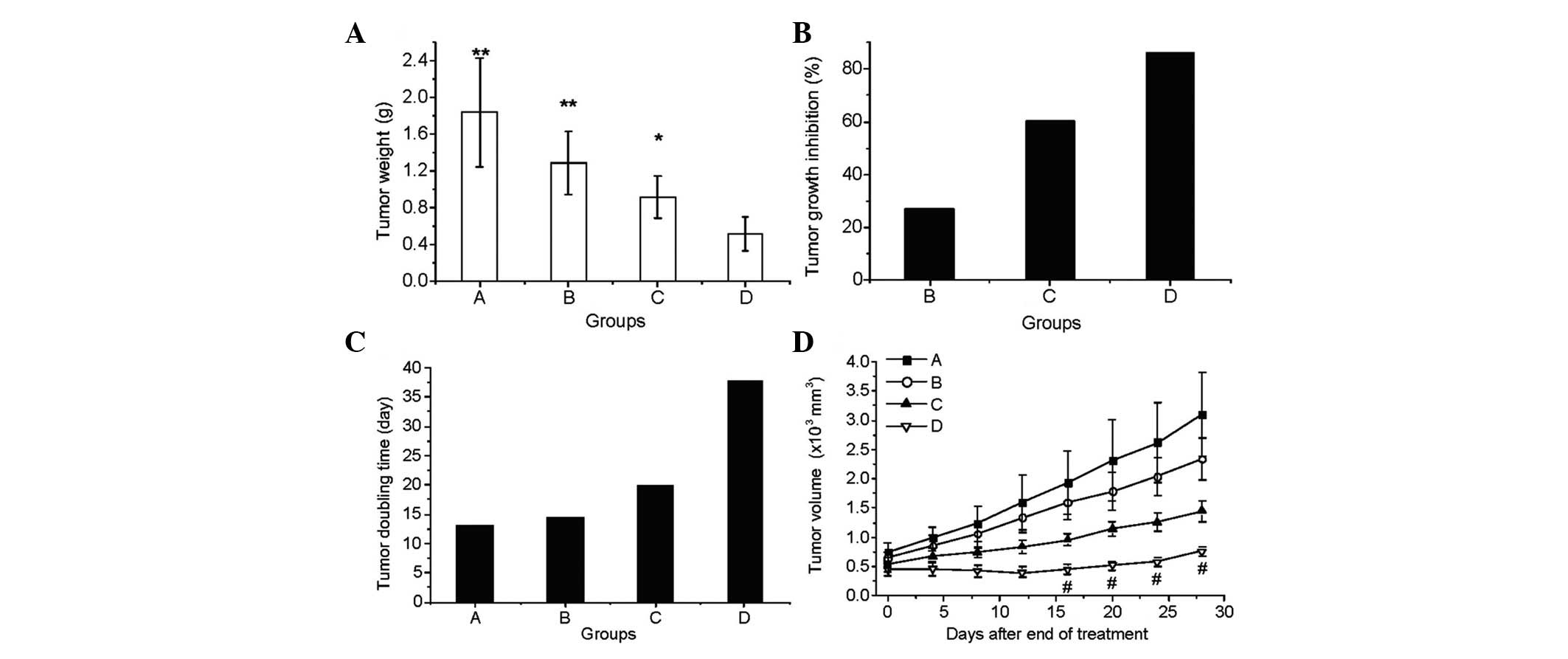
The antitumor activity was evaluated on day 39 (38
days after the treatment was initiated). Statistically significant
differences were found in tumor weight (Fig. 1A), tumor growth inhibition (Fig. 1B) and tumor doubling time (Fig. 1C) between the combination treatment
group D and the other three groups (P<0.05; Fig. 1). The average tumor weights of the
normal saline (A), Endostar (B), radiation (C) and Endostar +
radiation (D) groups were 1.849±0.596, 1.293±0.346, 0.926±0.233 and
0.523±0.180 g, respectively (Fig.
1A). The tumor inhibition rates, calculated using the tumor
volumes, in groups B, C and D were 27.12, 60.45 and 86.11%,
respectively (Fig. 1B). The tumor
doubling time was also prolonged dramatically, particularly in the
combined treatment group (Fig. 1C).
The therapeutic effects on the growth of the tumor (Fig. 1D) showed that the tumor size in the
Endostar + radiation group was significantly smaller than that of
the other groups 16, 20, 24 and 28 days after treatment (on days
26, 30, 34 and 38).
Effect of Endostar combined with
radiation on MVD
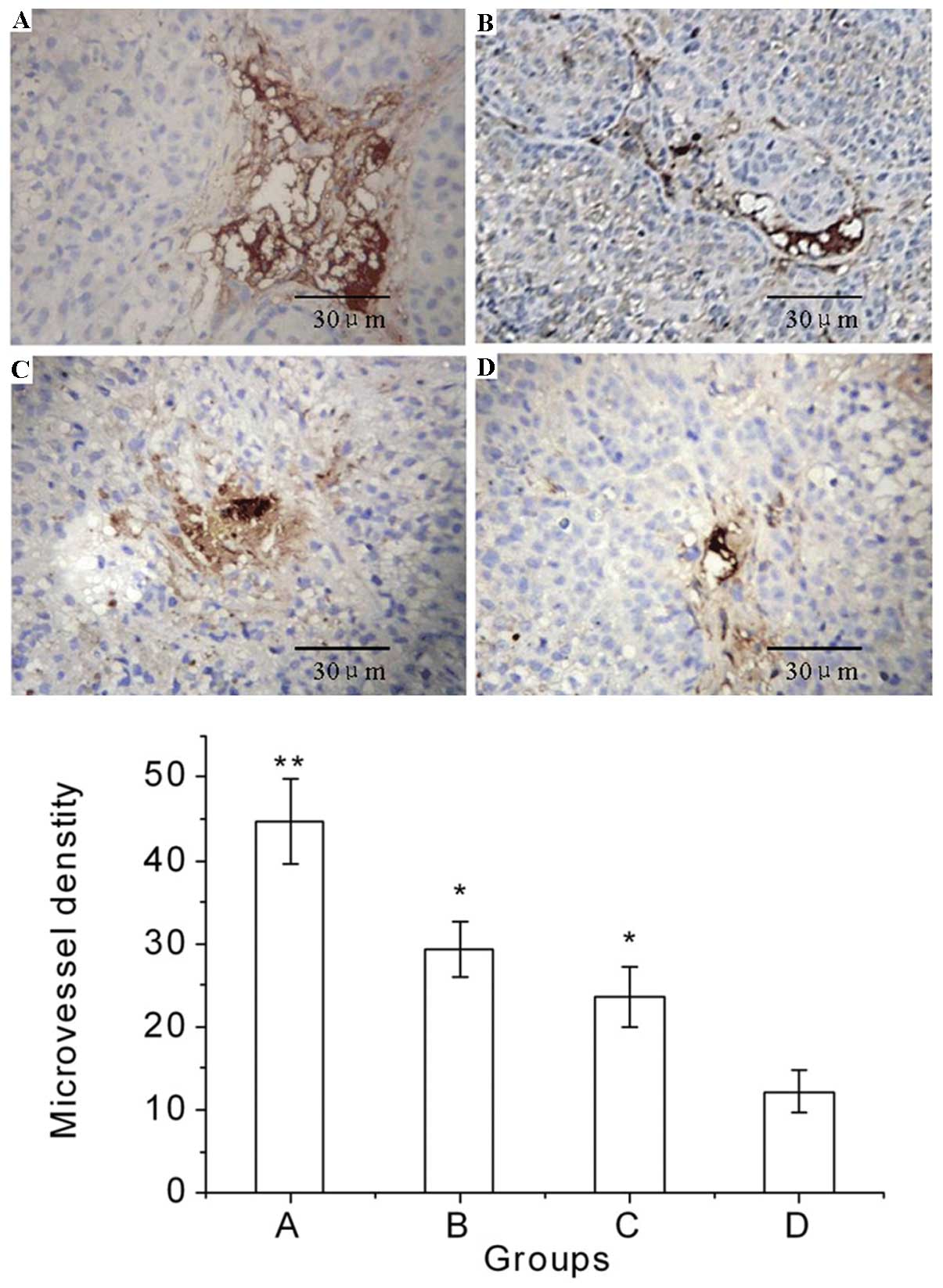
The typical immunohistochemical staining images of
CD34 and the effects of the various treatments on the MVD are shown
in Fig. 2. These results indicate
that Endostar or radiation monotherapy decreased the MVD, and the
combination of Endostar and radiation significantly decreased the
MVD of the tumor tissue in group D compared with that in group A
(P<0.01), group B (P<0.05) and group C (P<0.05).
Expressions of VEGF in NPC tumor
tissues
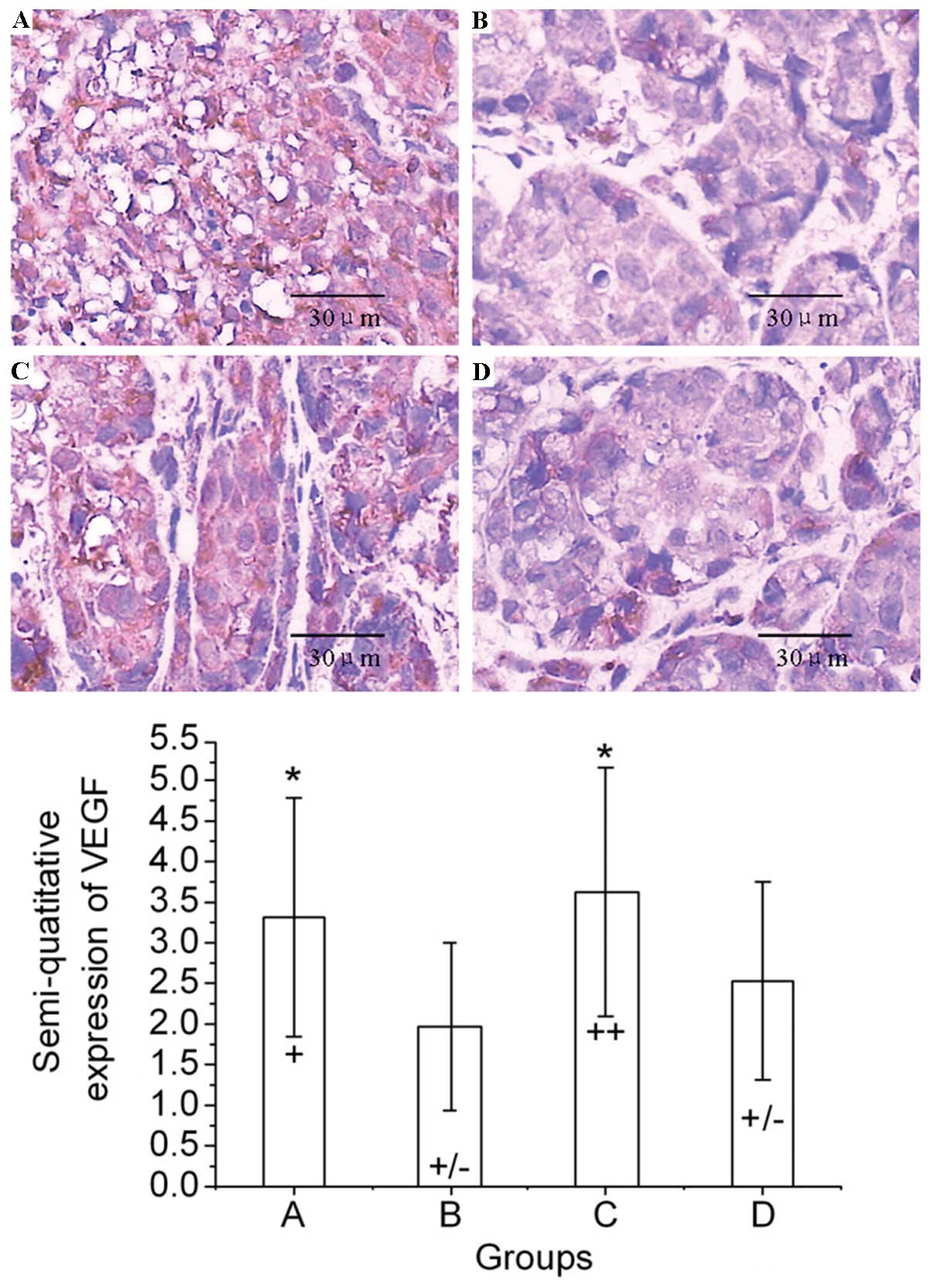
The typical immunohistochemical staining images of
VEGF are shown in Fig. 3. The
results indicate that the VEGF expression in mice treated with
normal saline, Endostar, radiation and Endostar + radiation were
positive, weakly positive, strongly positive and weakly positive,
respectively (Fig. 3).
Discussion
NPC animal models from nude mice are a viable
research tool showing a high activity of NK cells, B cells and T
cell-independent cells. The selection of nude mice for constructing
animal models is appropriate due to their short experimental
period, high tumor formation rate and good data comparability. The
present study succeeded in the subcutaneous inoculation of the NPC
cell line CNE into the right lower limb of nude mice, which
developed into squamous cell carcinoma, suggesting the successful
construction of an NPC animal model. The results of the animal
model show that the combination of Endostar with radiation is more
effective than monotherapy (either Endostar or radiation) in the
treatment of NPC tumors.
Several studies have indicated that antiangiogenesis
therapy combined with chemotherapy increases the local control rate
and long-term survival rate (10,11).
Wang et al reported that endostatin combined with adriamycin
chemotherapy significantly controls liver cancer in mice (12). However, studies investigating the
application of combined antiangiogenesis and radiation therapy are
scarce. Several researchers believe that the inhibition of vascular
formation decreases the blood supply to tumors and increases the
ratio of hypoxic cells, resulting in a weakening of the effects of
radiation. The results of the present study show that the
combination of Endostar with radiation significantly inhibits the
growth and metastasis of the NPC tumors inoculated with CNE1 cells,
which is consistent with the studies by Teicher et al
(13), Hansen-Algenstaedt et
al (14), Luo et al
(15) and Itasaka et al
(16). In the present study, the
sensitivity of NPC tumors to irradiation, recurrence time and local
control rate were significantly increased in the combined treatment
group that received Endostar for 10 days and were irradiated
locally once on day 10. These results suggest that the combination
of local fractionated radiation with Endostar achieves better tumor
control rates.
The present study also shows a decrease in MVD in
the Endostar and combination groups compared with the other two
groups (P<0.05). The present study suggests that Endostar
improves tumor oxygenation (17)
and sensitivity to radiation mainly by decreasing tumor microvessel
density, depriving premature vessels of blood and increasing the
blood flow to major vessels.
Radiation therapy controls the growth of tumor cells
by killing them but it also induces an increase in VEGF expression
in tumor cells. This increases tumor resistance to radiation and
promotes the formation of tumor vessels (18). It also increases their ability to
invade other tissues by increasing the activity of matrix lyase in
tumor cells (19). Therefore,
radiation can protect tumors and decrease their sensitivity to
further radiation therapy. Topolovec et al also verified
that VEGF and MVD act as prognostic factors in endometrial cancer
(20). The current study
demonstrates that Endostar lowers VEGF expression in NPC tumors,
which lowers the ability of vascular endothelial cells to repair
radiation damage and increases the sensitivity of NPC tumor cells
to radiation. Hence, the combination of Endostar with radiation has
a synergistic effect on killing NPC tumors.
Combining Endostar with radiation inhibits the
growth of NPC xenograft tumors and increases the sensitivity of
tumor cells to radiation. This strategy provides a new clinical
treatment method for NPC. However, angiogenesis is a complicated
process that is influenced by many factors. Radiation therapy may
be classified according to exposure time and intensity of
radiation. The present study is only a preliminary exploration into
combining Endostar with radiation for treating NPC. The underlying
mechanisms and other alternatives require further study.
Acknowledgements
This study was supported by the Social
Development Fund of Suzhou Science and Technology Bureau (grant
number SYS201013).
Abbreviations:
|
NPC
|
nasopharyngeal carcinoma
|
|
VEGF
|
vascular endothelial growth factor
|
|
MVD
|
microvessel density
|
References
|
1.
|
WB YinXZ GuNasopharyngeal
carcinomaRadiation Oncology4th editionPeking Union Medical College
PressBeijing4802007
|
|
2.
|
D KwongJ ShamD ChoyThe effect of
loco-regional control on distant metastastic dissemination in
nasopharyngeal carcinoma: an analysis of 1301 patientsInt J Radiat
Oncol Biol
Phys3010291036199410.1016/0360-3016(94)90306-97961008
|
|
3.
|
AB Langeland MarthinsenA Dybdahl WanderasS
LundgrenT StrickertEffects of growth factors on growth and
radiation sensitivity of the human breast cancer cell line
T-47DOncol Rep9397403200211836616
|
|
4.
|
KE HovingaLJ StalpersC Van
BreeRadiation-enhanced vascular endothelial growth factor (VEGF)
secretion in glioblastoma multiforme cell lines - a clue to
radioresistanceJ
Neurooncol7499103200510.1007/s11060-004-4204-716193379
|
|
5.
|
J FolkmanRole of angiogenesis in tumor
growth and metastasisSemin
Oncol291518200210.1053/sonc.2002.3726312516034
|
|
6.
|
AL FeldmanNP RestifoHR
AlexanderAntiangiogenic gene therapy of cancer utilizing a
recombinant adenovirus to elevate systemic endostatin levels in
miceCancer Res6015031506200010749112
|
|
7.
|
Y LingY YangN LuEndostar, a novel
recombinant human endostatin, exerts antiangiogenic effect via
blocking VEGF-induced tyrosine phosphorylation of KDR/Flk-1 of
endothelial cellsBiochem Biophys Res
Commun3617984200710.1016/j.bbrc.2007.06.155
|
|
8.
|
T MäkitieP SummanenA
TarkkanenMicrovascular density in predicting survival of patients
with choroidal and ciliary body melanomaInvest Ophthalmol Vis
Sci4024712480199910509639
|
|
9.
|
M TokurakuH SatoS MurakamiActivation of
the precursor of gelatinase A/72 kDa type IV collagenase/MMP-2 in
lung carcinomas correlates with the expression of membrane-type
matrix metalloproteinase (MT-MMP) and with lymph node metastasisInt
J Cancer64355359199510.1002/ijc.29106405137591310
|
|
10.
|
A GergerA El-KhoueiryW
ZhangPharmacogenetic angiogenesis profiling for first-line
Bevacizumab plus oxaliplatin-based chemotherapy in patients with
metastatic colorectal cancerClin Cancer
Res1757835792201110.1158/1078-0432.CCR-11-111521791631
|
|
11.
|
J MaCS ChenT BluteDJ
WaxmanAntiangiogenesis enhances intratumoral drug retentionCancer
Res7126752685201110.1158/0008-5472.CAN-10-324221447737
|
|
12.
|
ZX WangSM WangQ ZhouEndostatin in
different administration routes combined with adriamycin
chemotherapy in the treatment of liver cancer xenograft in miceNan
Fang Yi Ke Da Xue Xue Bao30190319052010(In Chinese)
|
|
13.
|
BA TeicherN DupuisT KusomotoAntiangiogenic
agents can increase tumor oxygenation and response to radiation
therapyRad Oncol Invest2269276199410.1002/roi.2970020604
|
|
14.
|
N Hansen-AlgenstaedtBR StollTP PaderaTumor
oxygenation in hormone-dependent tumors during vascular endothelial
growth factor receptor-2 blockade, hormone ablation, and
chemotherapyCancer Res60455645602000
|
|
15.
|
X LuoJM SlaterDS GridleyRadiation and
endostatin gene therapy in a lung carcinoma model: pilot data on
cells and cytokines that affect angiogenesis and immune
statusTechol Cancer Res
Treat5135146200610.1177/15330346060050020716551133
|
|
16.
|
S ItasakaR KomakiRS HerbstEndostatin
improves radioresponse and blocks tumor revascularization after
radiation therapy for A431 xenograft in miceInt J Radiat Oncol Biol
Phys67870878200710.1016/j.ijrobp.2006.10.03017293237
|
|
17.
|
CG LeeM HeijnE di TomasoAnti-vascular
endothelial growth factor treatment augments tumor radiation
response under normoxic or hypoxic conditionCancer
Res6055655570200011034104
|
|
18.
|
A AbdollahiKE LipsonX HanSU5416 and SU6668
attenuate the angiogenic effects of radiation-induced tumor cell
growth factor production and amplify the direct antiendothelial
action of radiation in vitroCancer Res63375537632003
|
|
19.
|
A KaliskiL LassauL
MaggiorellaAntiangiogenic effect and tumor growth control achieved
by an MMP-inhibitor combined to radiation in vivo, targeting
radio-induced MMP-2 enhancement and VEGF modulationInt J Radiat
Oncol Biol Phys60368369200410.1016/j.ijrobp.2004.07.218
|
|
20.
|
Z TopolovecA CorusićD BabićVascular
endothelial growth factor and intratumoral microvessel density as
prognostic factors in endometrial cancerColl
Antropol34447453201020698116
|