Introduction
Synovial sarcoma (SS) is a soft tissue sarcoma of
unknown histogenesis and comprises approximately 10% of all soft
tissue sarcomas, occurring most frequently in adolescents and young
adults (1,2). Morphologically, SSs are classified
into three types, i.e., the classical biphasic type that is
composed of both epithelial and spindled-cell components, the
monophasic type composed of either epithelial or spindle-cell
components, and the poorly differentiated type composed of small
round cells (1).
A diagnosis of SS is relatively straightforward for
the classical biphasic type, however, it may be challenging for the
monophasic fibrous and poorly differentiated types. This is
partially due to the fact that, although SS usually shows a dual
mesenchymal and epithelial differentiation light-microscopically
and immunohistochemically, the differentiation may be ambiguous in
the monophasic fibrous and poorly differentiated types (1,2).
Furthermore, although SS typically arises in the deep soft tissue
of the extremities, especially in the thigh and knee joints, rare
cases have been described in a variety of anatomic sites and
organs, including the female genital tract (3,4).
However, SS of the female genital tract is extremely rare,
potentially making a diagnosis of SS difficult when this is the
primary site.
Tumor-specific chromosomal translocations and
associated fusion genes have attracted great interest due to their
possible roles as diagnostic markers and prognostic predictors in
soft tissue sarcomas (5). Over 95%
of SSs are characterized by a reciprocal chromosomal translocation
t(X;18)(p11.2;q11.2), which is not detectable as a non-random
chromosomal translocation in any sarcomas, with the exception of
SS. The translocation usually fuses SYT onto chromosome 18q with
either SSX1 or SSX2 on chromosome Xp (5). Furthermore, a rare SYT-SSX4 fusion was
also reported as an SS-specific chimeric gene (6). Currently, the detection of these
fusion genes is considered to have full diagnostic validity for
diagnosing SS. Although reverse transcription-polymerase chain
reaction (RT-PCR) is the most popular method used to detect
SS-specific fusion gene transcripts and to confirm a diagnosis of
SS, SYT break-apart rearrangement fluorescence in situ
hybridization (SYT bar-FISH) has recently been introduced as an
alternative molecular method to diagnose SS (7–9).
We report a case of SS occurring in the right vulva
of a young Japanese female. Based solely on light-microscopic and
immunohistochemical findings, the tumor was difficult to diagnose
as SS. Although RT-PCR failed to detect SS-specific SYT-SSX fusion
gene transcripts using sample RNA extracted from the
formalin-fixed, paraffin-embedded tumor tissue, SYT bar-FISH
successfully confirmed the diagnosis of SS. In the present study,
we also discuss issues concerning the diagnosis of SS, along with
the methodological considerations regarding molecular
diagnosis.
A 21-year-old Japanese female, nulligravida and
nullipara, was referred to the Yamaguchi University Hospital with a
complaint of a palpable right vulvar mass. The mass was elastic
hard in consistency, slightly tender, and smaller than a fist. A
computed tomography examination demonstrated a relatively
well-demarcated solid tumor of approximately 9x8x7 cm in size with
central cysts. A needle biopsy examination demonstrated solid nests
of poorly differentiated small round cells with distinct
vasculature. Based on a putative diagnosis of soft tissue sarcoma,
tumor resection was performed. Twenty-eight months following
surgery, the tumor recurred in the right lung, and four years
following the first surgery, the patient succumbed to multiple
recurrence of the tumor.
The present study was approved by the ethics
commettee of Yamaguchi University School of Medicine, Yamaguchi,
Japan. Informed consent was obtained from the patient.
Materials and methods
Preparation of tissues
The resected tumor tissues were forwarded for
overnight fixation in 10% neutral-buffered formalin at room
temperature (RT), routinely processed, and embedded in
paraffin-wax. Tumor tissue sections (4 μm) were cut from the
paraffin-embedded tumor tissue blocks and prepared for hematoxylin
and eosin (H&E) staining, immunohistochemistry and SYT bar-FISH
analysis. For RT-PCR, multiple 10-μm-thick tumor tissue sections
were cut from the paraffin-embedded tumor tissue blocks.
Immunohistochemistry
For antigen retrieval, deparaffinized and rehydrated
sample tissue sections were pretreated by microwave irradiation for
20–30 min in 0.01 mol/l citrate-buffered saline (pH 6.0).
Endogenous peroxidase activity was blocked by incubation with 0.3%
H2O2 solution for 30 min. The tissue sections
were then incubated with mouse monoclonal antibodies against
cytokeratin (clone AE1/AE3, diluted at 1:100, Dako, Copenhagen,
Denmark), CD56 (clone 1B6, Nichirei, Tokyo, Japan), neuron-specific
enolase (NSE) (clone BBS/NC/VI-H14, diluted at 1:100, Dako),
vimentin (clone V9, diluted at 1:25, Dako), CD34 (clone NU-4A1,
diluted at 1:50, Nichirei), CD99 (clone 12E7, diluted at 1:50,
Dako), Bcl-2 (clone 124, diluted at 1:100, Dako), muscle-specific
actin (clone HHF35, diluted at 1:50, Dako) and desmin (clone D33,
diluted at 1:100, Dako), or a rabbit polyclonal antibody against
S100 protein (S100, diluted at 1:1000, Dako) at 4°C overnight.
Subsequent reactions were performed by the streptavidin-biotin
complex/horseradish peroxidase method using a Histofine SAB-PO (M)
or (R) immunohistochemical staining kit (Nichirei, Tokyo, Japan)
according to the manufacturer’s instructions.
Break-apart rearrangement FISH and
RT-PCR
SYT bar-FISH was carried out using a LSI SYT
(18q11.2) Dual Color, Break-Apart Rearrangement Probe kit (Vysis,
Downers Grove, IL, USA) according to the manufacturer’s
instructions, with minor modifications. A tumor tissue section
(4-μm) placed on a glass slide was dewaxed, rehydrated, incubated
in a 0.05% pepsin/0.1mol/l HCl solution at 37°C for 10 min, and
washed twice in phosphate-buffered saline, pH 7.4, at RT for 5 min.
The section was subjected to microwave pretreatment with 0.1 mol/l
sodium citrate buffer, pH 6.0, dehydrated in a graded series of
ethanol solutions, incubated in acetone at −20°C for 10 min, and
fixed in Carnoy’s solution at RT for 5 min. The section was
denatured in 70% formamide/2X standard sodium citrate at 73°C for 5
min. A 10 μl aliquot of the SYT bar-FISH probe (5’-SYT SO/3′-SYT
SG, Vysis) mixture was also denatured at 73°C for 5 min and applied
to the denatured sample tissue section. The section was then
covered with a cover glass, sealed with rubber cement, and
incubated at 37°C for 72 h in a humidified chamber.
Post-hybridization washes were routinely performed and the section
was counterstained with a DAPI-II antifade solution (Vysis).
The section was observed for FISH signals on each
tumor cell nucleus under a BX60 fluorescence microscope (Olympus,
Tokyo, Japan). In the enumeration of FISH signals, overlapping
cells and cells with indistinct fluorescence signals were excluded.
The number of tumor cell nuclei with three fluorescence signals,
i.e., the SpectrumOrange-SpectrumGreen overlapping yellowish
signals, the SpectrumOrange 5′-SYT signal and the SpectrumGreen
3′-SYT signal, were calculated per over 100 tumor cell nuclei for
the section. When the ratio of the number of tumor cell nuclei
carrying one overlapped and two separate fluorescent signals for
the total number of tumor cell nuclei counted was >0.7, the
tumor was considered to have a SYT gene break, being diagnostic of
SS.
Furthermore, RT-PCR for SYT-SSX1 and SYT-SSX2 fusion
gene transcripts were carried out according to the method described
in a previous study (10).
Appropriate positive and negative controls were included in the
RT-PCR examination.
Results
Gross findings and histopathology
On the cut surface, the tumor was elastic hard to
moderately soft in consistency and brownish to tan in color,
accompanied by some areas of hemorrhaging and 0.5- to 2 cm-sized
cystic spaces filled with brownish fluid.
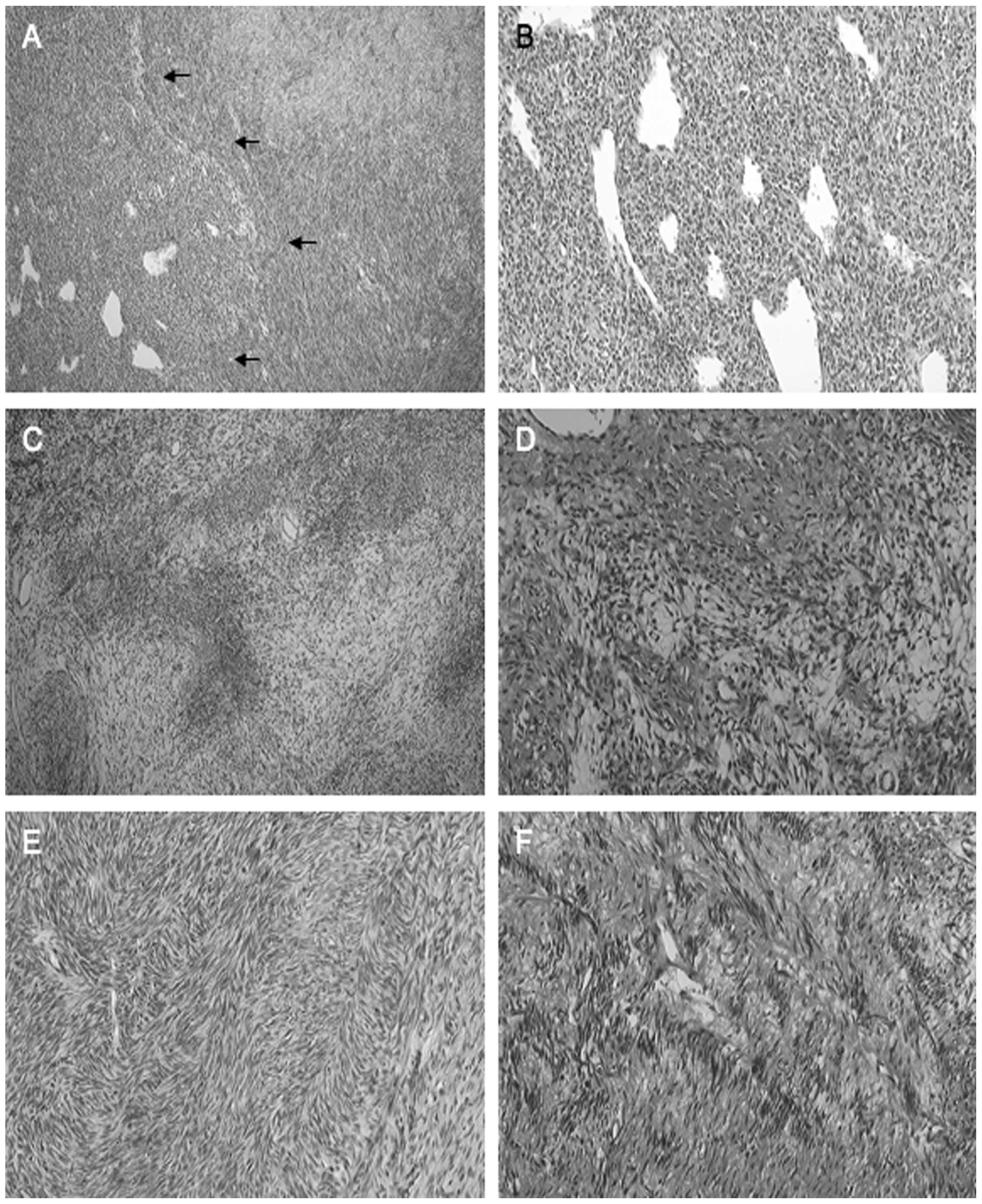
Microscopically, the tumor showed a relatively
well-demarcated nodular region composed of small rounded cells with
distinct vasculature, surrounded by a spindle-cell area composed of
fibroblastic cells, accompanied by cellular and myxoedematous
portions (Fig. 1A). The former
region was composed of a diffuse proliferation of small-rounded
tumor cells with distinct staghorn blood vessels (Fig. 1B). The rounded tumor cells were
characterized by round to oval nuclei, occasional conspicuous
nucleoli, and scant eosinophilic to pale cytoplasm. The latter
region demonstrated cellular fascicles of spindle-shaped
fibroblastic tumor cells intermingled with collagen bundles, focal
hyalinization, and geographic myxoid degeneration (Fig. 1C). The spindled tumor cells were
characterized by plump-spindled nuclei, indistinct nucleoli, and
eosinophilic to pale cytoplasm (Fig.
1D). Herringbone (Fig. 1E) and
nuclear palisading (Fig. 1F)
arrangements of the tumor cells were focally observed.
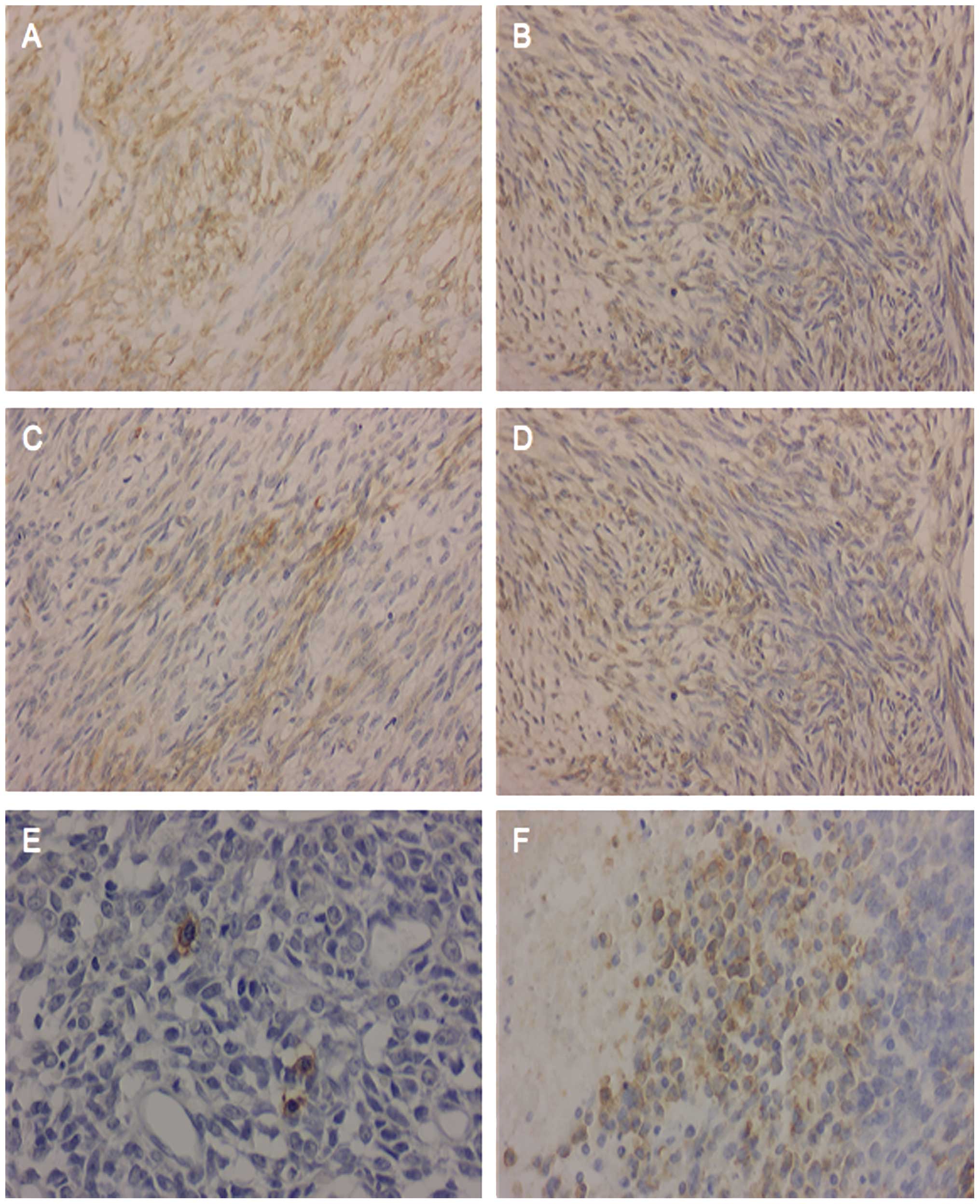
Immunohistochemistry
Immunohistochemically, the tumor cells were
diffusely positive for vimentin (Fig.
2A), S100 (Fig. 2B), Bcl-2
(Fig. 2C), NSE (Fig. 2D) and CD56 in both the spindle-cell
and round-cell components, and were focally positive for
cytokeratin (Fig. 2E) and CD99
(Fig. 2F) in the round-cell region.
The tumor cells were negative for CD34 and muscle-specific actin in
both the spindle-cell and round-cell regions.
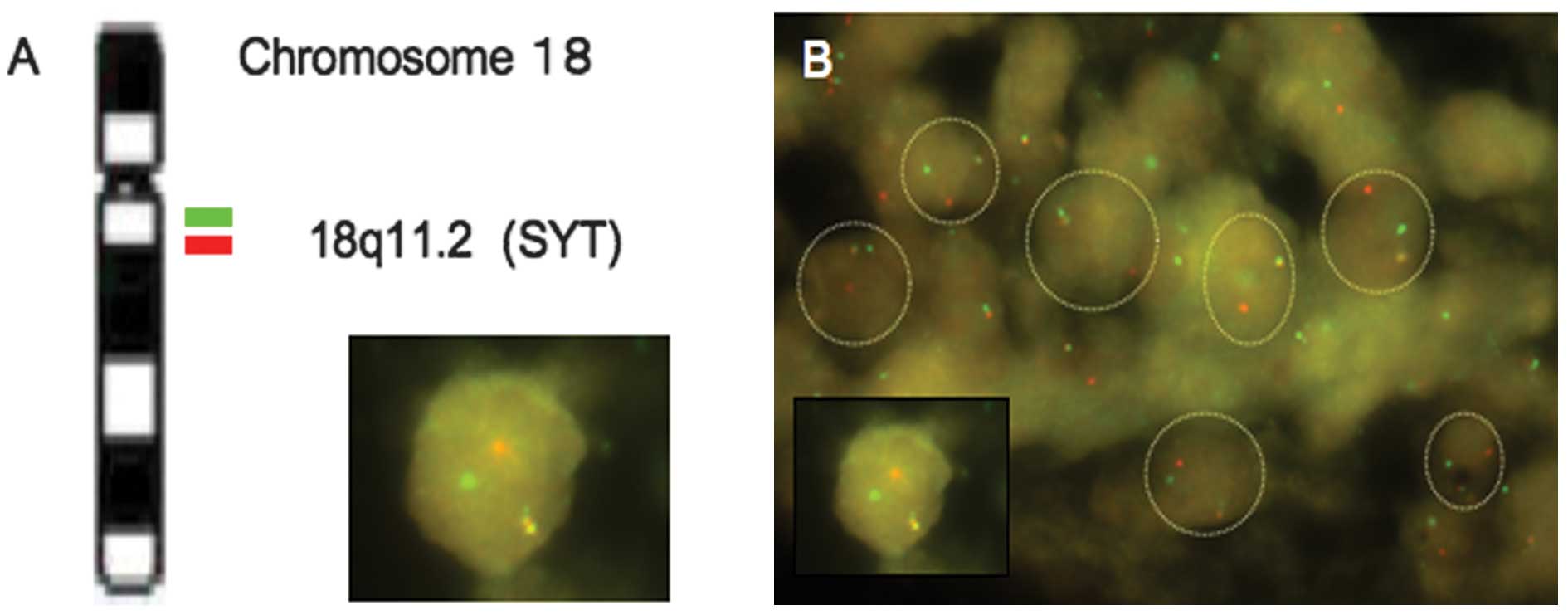
FISH and RT-PCR
The LSI SYT bar-FISH probe consists of a mixture of
two FISH DNA probes. The first probe labeled with SpectrumOrange
extends distally from the SYT gene. The second probe labeled with
SpectrumGreen lies 3′- or proximal to the SYT gene. In normal
metaphase cells without the SYT gene break, the two fusion orange
signals are observed. However, in abnormal cells carrying a SYT
gene break, the cells usually demonstrate one fusion orange, one
red and one green signal pattern (Fig.
3A). This makes it easy to differentiate the sarcoma cells from
inflammatory and non-neoplastic stromal cells under fluorescence
microscopy. In the present study, over 70% of the tumor cell nuclei
carried one red, one green, and one fusion orange signal in the
tumor cells of both the poorly differentiated areas and monophasic
fibrous areas (Fig. 3B). A small
proportion of the tumor cells had a reduced number of FISH signals,
probably due to the nuclear truncation that occurred during the
preparation of the tissue sections.
The RT-PCR examination failed to detect the
SS-specific SYT-SSX fusion gene transcripts specific for SS,
probably due to the poor quality of the RNA samples used in the
RT-PCR examination from the formalin-fixed, paraffin-embedded
sarcoma tissue (data not shown).
Discussion
SS rarely arises in the female genital tract. To the
best of our knowledge, only 13 SSs of the female genital tract have
been reported previously, including 8 tumors in the vulva (3,4), 3 in
the vagina (4,11), and one each in the fallopian tube
(12) and the ovary (13). Recently, various techniques have
been used to detect a t(X;18)(p11.2;q11.2) translocation or SYT-SSX
fusion gene transcripts as a molecular marker to confirm a
diagnosis of SS. These techniques include short-term tumor cell
culture and karyotyping, RT-PCR for SS-specific SYT-SSX fusion gene
transcripts, and SYT bar-FISH. Among these methods, RT-PCR is the
most popular, and appears to be regarded as a standard method for
diagnosing SS (4). Among the 13 SSs
arising in the female genital tract, eight were monophasic fibrous
or poorly differentiated SSs and were diagnostically confirmed by
RT-PCR to have the SYT-SSX fusion gene transcripts (3,4,11–13).
The present tumor was difficult to diagnose based
solely on the light-microscopic and immunohistochemical findings.
Microscopically, the tumor was composed of well-demarcated,
spindled-cell areas and poorly differentiated small round cell
areas. The former showed cellular fascicles of spindle-shaped
cells, focally exhibiting a herringbone pattern and a nuclear
palisading arrangement of the tumor cells with focal myxoedematous
to collagenous portions. The latter were characterized by distinct
vasculature with a staghorn configuration. Furthermore, the tumor
cells were, immunohistochemically at least, focally positive for
cytokeratin, vimentin, CD99, Bcl-2, and NSE (1,2).
Taking these findings into consideration, the major
differential diagnoses of the present tumor were considered to be
fibrosarcoma, malignant peripheral nerve sheath tumor (MPNST),
myopericytoma, solitary fibrous tumor (SFT) and SS. Although
regions mimicking fibrosarcoma and myopericytoma were present in
the tumor, they were observed only sporadically. Therefore, these
diagnoses could be excluded. SFT and MPNST were more problematic
differential diagnoses. The present tumor was immunohistochemically
negative for CD34 and there were no histories of neurofibromatosis
in the patient or her family. Therefore, we eventually excluded SFT
and MPNST when making a differential diagnosis.
Although SS was selected as the putative diagnosis
of the present tumor from the differential diagnoses presented,
evidence supporting diagnostic confirmation was required. For this
purpose, RT-PCR for the SYT-SSX fusion gene transcripts was
performed. Since there were no fresh-frozen tumor tissues available
for the RT-PCR examination, the archival formalin-fixed,
paraffin-embedded tumor tissue blocks were used to extract RNA.
However, it was difficult to retrieve a sufficient quality and
quantity of sample RNA suitable for RT-PCR from the
paraffin-embedded tissues. Although RT-PCR is a sensitive method
that may be used to detect target RNA from a small amount of sample
RNA, it is sometimes difficult to obtain RNA samples suitable for
RT-PCR from archival formalin-fixed paraffin-embedded tissues
(8,14). This is partially due to the fact
that RNA tends to be degraded by prolonged formalin fixation and/or
ubiquitously present RNases when there is inappropriate tissue
handling. Such inappropriate RNA samples may generate a
false-negative or even false-positive result in a RT-PCR
analysis.
Since FISH may be more easily and reliably used,
even with an archival formalin-fixed, paraffin-embedded sample
compared to RT-PCR (8,9), SYT bar-FISH may be more suitable for a
diagnosis of SS than RT-PCR. There have been few studies dealing
with the methodological comparison between RT-PCR and SYT bar-FISH
for the diagnosis of SS based on a considerable number of SS cases.
For example, Amary et al reported that a combination of
molecular methods, including RT-PCR and SYT bar-FISH, had a 96%
sensitivity and 100% specificity (8). Sun et al compared the
diagnostic efficiency of SYT bar-FISH and RT-PCR in diagnosing 255
cases of SS, and concluded that the efficiency of FISH was
comparable to or even higher than that of RT-PCR (9).
In the present case, unlike RT-PCR, SYT bar-FISH
successfully confirmed the diagnosis of SS by detecting a pair of
split SYT signals for the present tumor. In a typical pathology
laboratory unfamiliar with the potential issues regarding RNA
experiments, SYT bar-FISH may be more suitable for detecting a
tumor-specific chromosomal translocation and diagnosing difficult
cases of SS. Furthermore, in the current tumor, a pair of split SYT
signals was exclusively observed in the tumor cells of the
monophasic fibrous and poorly differentiated components, supporting
the hypothesis that these components are derived from the same
tumor stem cell in spite of their morphologic differences.
In conclusion, we have described a diagnostically
difficult case of vulvar SS that was composed of monophasic fibrous
and poorly differentiated components. In the present case of SS,
SYT bar-FISH, but not RT-PCR, was used to successfully validate our
diagnosis of SS. For the diagnosis of SS arising in unusual
anatomic sites, frozen tumor tissue samples may be difficult to
prepare for the molecular diagnosis. In such a situation, FISH may
be more suitable for the detection of the SS-specific chromosomal
translocation and the diagnosis of SS using archival
formalin-fixed, paraffin-embedded tumor tissue specimens than
RT-PCR.
References
|
1.
|
SW WeissJR GoldblumMalignant soft tissue
tumors of uncertain typeSW WeissJR GoldblumEnzinger and Weiss’s
Soft Tissue TumorsMosbySt. Louis148315712001
|
|
2.
|
C FisherDHR de BruijinA Geurts van
KesselSynovial sarcomaCDM FletcherKK UnniF MertensWorld Health
Organization Classification of Tumours. Pathology and Genetics.
Tumours of Soft Tissue and BoneIARC PressLyon2002042002
|
|
3.
|
DS AmbaniB WhiteAL KaplanA AlbertoA case
of monophasic synovial sarcoma presenting as a vulvar massGynecol
Oncol100433436200610.1016/j.ygyno.2005.09.01316226798
|
|
4.
|
VP SumathiC FisherA WilliamsJM MeisR
GanesanLG KindblomWG McCluggageSynovial sarcoma of the vulva and
vagina: a clinicopathologic and molecular genetic study of 4
casesInt J Gynecol
Pathol308491201010.1097/PGP.0b013e3181f0c51021131827
|
|
5.
|
ES MelzerMolecular genetics of soft tissue
tumorsSW WeissJR GoldblumEnzinger and Weiss’s Soft Tissue
TumorsMosbySt. Louis1251462001
|
|
6.
|
B SkyttingG NilssonB BrodinY XieJ
LundebergM UhlénO LarssonA novel fusion gene, SYT-SSX4, in synovial
sarcomaJ Natl Cancer
Inst91974975199910.1093/jnci/91.11.97410359553
|
|
7.
|
J TerryTS BarryDE HorsmanFD HsuAM GownDG
HuntsmanTO NielsenFluorescence in situ hybridization for the
detection of t(X;18)(p11.2;q11.2) in a synovial sarcoma tissue
microarray using a breakapart-style probeDiagn Mol
Pathol147782200510.1097/01.pas.0000155021.80213.c915905690
|
|
8.
|
MF AmaryF BerishaC Bernardi FdelA HerbertM
JamesJS Reis-FilhoC FisherAG NicholsonR TiraboscoTC DissAM
FlanaganDetection of SS18-SSX fusion transcripts in formalin-fixed
paraffin-embedded neoplasms: analysis of conventional RT-PCR,
qRT-PCR and dual color FISH as diagnostic tools for synovial
sarcomaMod Pathol20482496200710.1038/modpathol.3800761
|
|
9.
|
B SunY SunJ WangX ZhaoS ZhangY LiuX LiY
FengH ZhouX HaoThe diagnostic value of SYT-SSX detected by reverse
transcription-polymerase chain reaction (RT-PCR) and fluorescence
in situ hybridization (FISH) for synovial sarcoma: a review and
prospective study of 255 casesCancer
Sci9913551361200810.1111/j.1349-7006.2008.00830.x
|
|
10.
|
S KawauchiT FukudaY ChochiT KondoA OgaK
SasakiReverse transcription-polymerase chain reaction in situ
hybridization for SYT-SSX fusion gene transcripts in synovial
sarcomasInt J Mol Med16763766200516142418
|
|
11.
|
G PelosiF LuzzattoF LandoniN StaffaA
MaggioniP BraidottiA CabrasA AielloB Del CurtoG VialePoorly
differentiated synovial sarcoma of the vagina: first reported case
with immunohistochemical, molecular and ultrastructural
dataHistopathology50808810200710.1111/j.1365-2559.2007.02647.x17355275
|
|
12.
|
A MitsuhashiY NagaiK SuzukaK YamazawaT
NojimaT NikaidoH IshikuraH MatsuiM ShozuPrimary synovial sarcoma in
fallopian tube; a case report and literature reviewInt J Gynecol
Pathol263437200610.1097/01.pgp.0000225841.13880.3a17197895
|
|
13.
|
CJ SmithAJ FerrierP RussellS
DanielettoPrimary synovial sarcoma of the ovary: first reported
casePathology37385387200510.1080/0031302050025433916194852
|
|
14.
|
C SuraceI PanagopoulosE PålssonM RocchiN
MandahlF MertensA novel FISH assay for SS18-SSX fusion type in
synovial sarcomaLab
Invest8411851192200410.1038/labinvest.370014215208645
|