Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignant tumors and ranks third as the leading cause of
cancer-related mortalities worldwide (1). Due to its late diagnosis,
ineffectiveness of conventional treatments and poor prognosis, the
mortality rate for HCC is extremely high (2,3).
Therefore, more effective treatments and more accurate and reliable
diagnostic markers are needed to provide earlier detection and
better therapeutic strategies.
Heat shock proteins (HSPs) are a group of proteins
whose expression is initially induced by heat. Further study
revealed the same response in HSPs exposed to other environmental
and metabolic stimulations, including hypoxia, hyperoxia, anoxia,
UV exposure and mechanical injury. In addition, HSPs have also been
found in cells under normal conditions (4,5).
Previous studies have revealed that HSPs are ubiquitous in both
prokaryotic and eukaryotic cells. They have dual functions: the
intracellular HSPs, which are known as ‘molecular chaperones’, have
cytoprotective and antiapoptotic functions, such as ensuring the
folding of proteins into the correct tertiary structure,
transporting proteins across the membranes and incorporating
polypeptides into intracellular membranes; the extracellular HSPs
have certain immunogenic functions, through the chaperoning of
antigenic peptides (6,7).
Since the molecular chaperone functions of HSPs have
been shown to be important in regulating cellular homeostasis and
promoting cell survival, the proteins have been reported to
participate in the progression of numerous types of diseases,
including autoimmune diseases and neoplastic processes (8). As the overexpression of HSP27, 70 and
90 has been detected in various types of carcinoma compared with
corresponding normal tissues, the correlation between HSPs and
cancer has attracted attention (9).
Following studies have not only focused on the correlation between
HSP expression profiles, prognosis and clinical indicators,
including disease classification, invasion, metastasis and
therapeutic resistance, but have also described the function of
HSPs in carcinogenesis (10,11).
In the present study, we investigated a novel gene
called DNAJC25, which belongs to the HSP40 subfamily C. We cloned
the gene and explored the subcellular localization of the protein
in the cytoplasm. We found that DNAJC25 had a markedly high
expression level in liver tissues and was significantly
downregulated in liver cancer tissues compared with the adjacent
normal tissues. The overexpression of DNAJC25 reduced colony
formation and limited the colony quantity and size. Flow cytometry
analysis indicated that DNAJC25 also significantly increased cell
apoptosis. The present study describes a new potential tumor
suppressor and furthers the understanding of the function of HSPs
in cancer progression.
Materials and methods
Tumor specimens
Fresh surgical specimens of HCC, including tumor
tissues and the neighboring pathologically nontumorous liver
tissues, were obtained from liver cancer patients at Zhongshan
Hospital (Shanghai, China). All the samples were immediately frozen
in liquid nitrogen after surgery and stored at −80°C before further
analysis.
Cloning and sequencing of DNAJC25
Two primers (forward, 5′-TGAGTGCTGCAGAATCGCTGG-3′;
and reverse, 5′-AAG GTTTGGCATAGTAGCATTCCATC-3′) were designed to
amplify DNAJC25 from a human testis cDNA library. PCR was performed
using a PCR kit (SH Energy, Driffield, UK) under conditions of 95°C
for 5 min, 40 cycles of 95°C for 30 sec, 64°C for 30 sec and 72°C
for 1 min 20 sec, followed by 1 cycle of 72°C for 10 min. The
product was then separated by DNA electrophoresis in 1% (w/v)
agarose gel and inserted into pMD18-T vector (Takara Bio, Inc.,
Otsu, Japan) for sequencing. The cDNA encoding DNAJC25 from the
pMD18-T vector was then subcloned into pCMV-Myc (Clontech
Laboratories, Inc., Mounatin View, CA, USA), pcDNA3.1A(-) and
pEGFP-N1 for the evaluation of eukaryotic expression and further
analysis.
RT-PCR analysis
Human multiple tissue cDNA (MTC) panels (Clontech
Laboratories, Inc.), including heart, brain, placenta, lung, liver,
skeletal muscle, kidney, pancreas, spleen, thymus, prostate,
testis, ovary, small intestine and colon, served as templates to
study the distribution of human DNAJC25 mRNA. Primer pairs designed
at the boundary between exons 1 and 2 and exons 3 and 4 (forward,
5′-AGA CACTCAAGGATGAAGAAACAC-3′; reverse, 5′-TTGCTT
GTAGACCTCATAATTCTCC-3′) were used for RT-PCR under conditions of
95°C for 5 min, 38 cycles of 95°C for 30 sec, 63°C for 30 sec and
72°C for 40 sec, followed by 1 cycle of 72°C for 10 min.
5′-ATGAGTATGCCTGCCGTG TGAAC-3′ and 5′-TGTGGAGCAACCTGCTCAGATAC-3′
were used to amplify the β2-MG gene. The products were then
separated by DNA electrophoresis in 2% (w/v) agarose gel.
Cell culture and transfection
HEK 293, SMMC-7721, Hep3B and HeLa cells were
cultured in Dulbecco’s modified Eagle’s medium (Gibco, Invitrogen
Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal
bovine serum (Invitrogen Life Technologies) at 37°C in a 5%
CO2 humidified atmosphere. HEK 293 cells
(1.2×105) were seeded in a 6-well plate,
3.0×105 SMMC-7721 and Hep3B cells were seeded in a
6-well plate and 1.5×105 HeLa cells were seeded on
cover-slips in a 6-well plate. After overnight growth, the cells
were 60% confluent and were transfected with the plasmids using
Lipofectamine Reagent (Invitrogen Life Technologies) in the
non-serum medium. After 4 h of incubation, the medium was replaced
with fresh complete medium, and cells were cultured for an
additional 36 h before collection.
Quantitative real-time PCR
RNA was extracted with TRIzol (Invitrogen Life
Technologies) and reverse transcribed to cDNA with a reverse
transcription kit (Invitrogen Life Technologies) according to the
manufacturer’s instructions. Quantitative real-time PCR was
performed using the SYBR Green Supermix kit (Takara Bio, Inc.) with
the Light Cycler 480 (Roche Diagnostics, Mannheim, Germnay). For
both DNAJC25 and the house keeping gene β2-MG, cycle parameters
were 95°C for 1 min hot start, followed by 45 cycles of 95°C for 10
sec, 63°C for 10 sec and 72°C for 40 sec. The primers for DNAJC25
were: forward, 5′-AGACACTCAAGG ATGAAGAAACAC-3′; reverse,
5′-TTGCTTGTAGACCTC ATAATTCTCC-3′. The primers for β2-MG were:
forward, 5′-ATGAGTATGCCTGCCGTGTGAAC-3′; reverse, 5′-TGT
GGAGCAACCTGCTCAGATAC-3′. Quantified transcripts of the β2-MG gene
were used as endogenous mRNA controls. All experiments were
performed thrice for each data point.
Western blot analysis
Protein samples separated by SDS-PAGE were
electrotransferred onto a nitrocellulose membrane. The membrane was
blocked at room temperature for 1 h with TBS containing 5% (w/v)
skimmed milk. The membrane was then incubated overnight at 4°C with
mouse anti-Myc monoclonal antibody (dilution, 1:1,000; Sigma, St.
Louis, MO, USA), washed three times with a mixture of TBS with 0.1%
Tween-20 (Sigma; TBS-T) and incubated with HRP-conjugated goat
anti-mouse antibody (dilution, 1:5,000; Santa Cruz Biotechnology
Inc., Santa Cruz, CA, USA) at room temperature for 1 h. The
membrane was then washed again with TBS-T and developed using the
ECL system (Santa Cruz Biotechnology Inc.).
Immunofluorescence microscopy
SMMC-7721 and HeLa cells were plated on coverslips
and transfected with lipofectamine (Invitrogen Life Technologies).
After 36 h, the cells were washed twice with PBS (pH 7.4) and fixed
in 4% paraformaldehyde for 10 min at room temperature. Then cells
were resolved by 0.1% (v/v) Triton X-100 for 5 min, washed again
and stained with DAPI for 2 min at room temperature in the dark.
Images were viewed using LSM 710 Laser Scanning Microscopy (Carl
Zeiss, Cambridge, UK).
Colony formation assay
At 24 h post-transfection with pcDNA3.1A(-)-DNAJC25
and pcDNA3.1A(-) vector, Hep3B and SMMC-7721 were portioned into
new 6-well plates at densities of 2.5x104 and
1×105 cells/well, respectively, in triplicate. G418 (500
μg/ml; Invitrogen Life Technologies) was added to the medium
24 h later. The colonies were identified by crystal violet staining
after ∼10–14 days of culture. The results are representative of at
least three independent experiments.
Flow cytometry analysis
After transfection for 36 h, cells were harvested
and incubated with RNAase (100 μg/ml) and propidium iodide
(50 μg/ml) for 5 min at room temperature. The degree of
apoptosis was indicated by the percentage of cells in the sub-G1
fraction, using FACSCalibur (BD Biosciences, Franklin Lakes, NJ,
USA).
Statistical analysis
A two-tailed Student’s t-test was used to evaluate
group-level differences in our study. P<0.05 was considered to
indicate a difference and P<0.01 was considered to indicate a
statistically significant difference.
Results
Molecular cloning and identification of
human DNAJC25
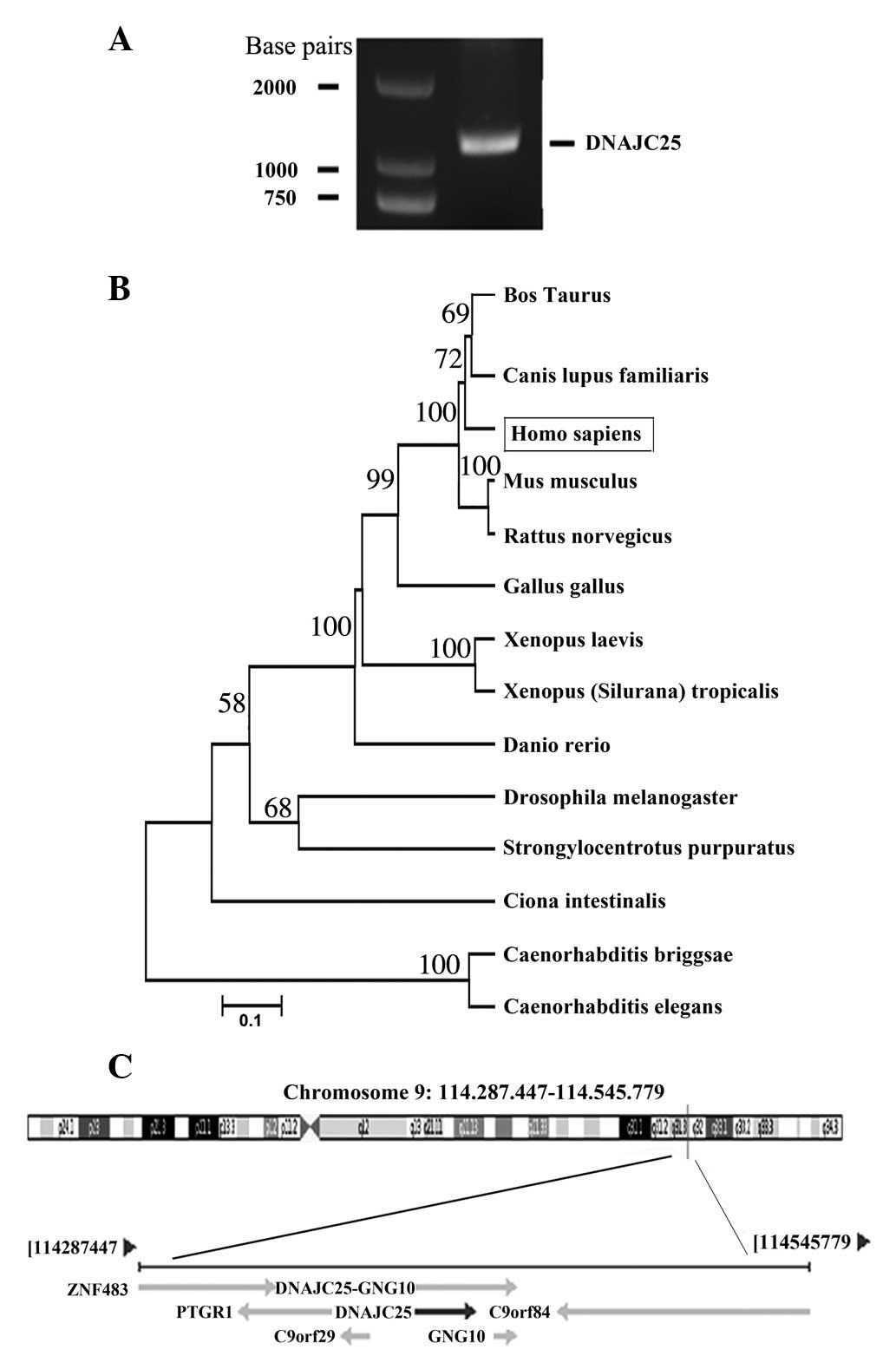
Through PCR from the human testis cDNA library with
the forward and reverse primers, an amplicon was obtained and
inserted into the pMD18-T vector (Fig.
1A). After sequencing, we found that the cDNA of the amplicon
was 1163 bp in length. Online BLAST research (http://www.ncbi.nlm.nih.gov/blast) revealed that
the amplicon sequence was identical to the mRNA sequence of DNAJC25
(GenBank ID, GI:118498346) and that they have the same ORF.
Fig. 1B shows the evolutionary
relationship of DNAJC25 with its homologous genes in other species.
Bioinformatic analysis revealed that DNAJC25 was located at
chromosome 9 (Fig. 1C) and that
DNAJC25 cDNA encodes a putative 360-amino acid protein with a
calculated molecular mass of 42.4 kDa.
Subcellular localization of DNAJC25 in
eukaryotic cells
In order to study the subcellular localization of
DNAJC25, we cloned DNAJC25 into the pEGFP-N1 vector and transfected
the construct into SMMC-7721 and HeLa cells. The pEGFP-N1 vector
was used as a control. The cells were fixed and observed under
fluorescence microscopy 36 h post-transfection. The results showed
that DNAJC25 was localized to the cytoplasm in SMMC-7721 and HeLa
cells (Fig. 2).
DNAJC25 had a markedly high expression in
liver tissue
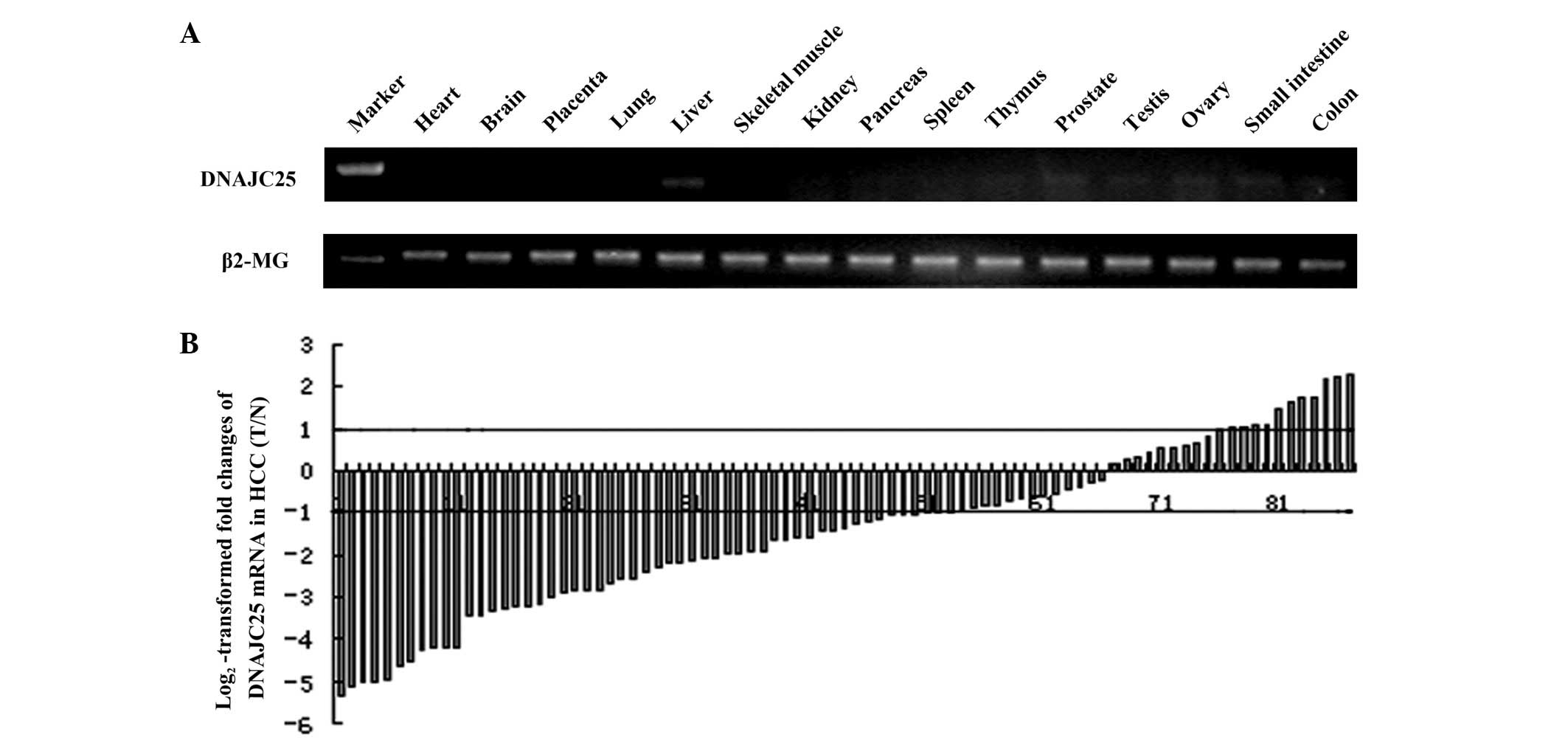
RT-PCR was performed to detect the distribution of
DNAJC25 in 15 human tissues with the primers located at the
boundary between exons 1 and 2 and exons 3 and 4. The results
showed that DNAJC25 had the highest expression level in liver
tissue and trace levels in the thymus, prostate, testis, ovary,
small intestine and colon. No amplification product was visualized
in the heart, brain, placenta, lung, skeletal muscle, kidney,
pancreas or spleen (Fig. 3A).
DNAJC25 was downregulated in HCC
Since the expression level of DNAJC25 was extremely
high in liver tissues, the mRNA level of DNAJC25 was further
evaluated in 87 pairs of HCC specimens and their corresponding
neighboring nontumorous specimens by quantitative real-time PCR.
Quantified transcripts of the β2-MG gene were used as endogenous
mRNA controls. Fig. 3B shows the
log2-transformed fold changes of DNAJC25 mRNA expression
as a ratio of tumor/nontumor levels. DNAJC25 was downregulated in
50 HCC specimens compared with adjacent normal liver tissues
(57.5%; >2-fold decrease). The DNAJC25 transcripts were normally
expressed in 26 tumours (29.9%) and overexpressed in 11 tumours
(12.6%; >2-fold increase; P<0.001).
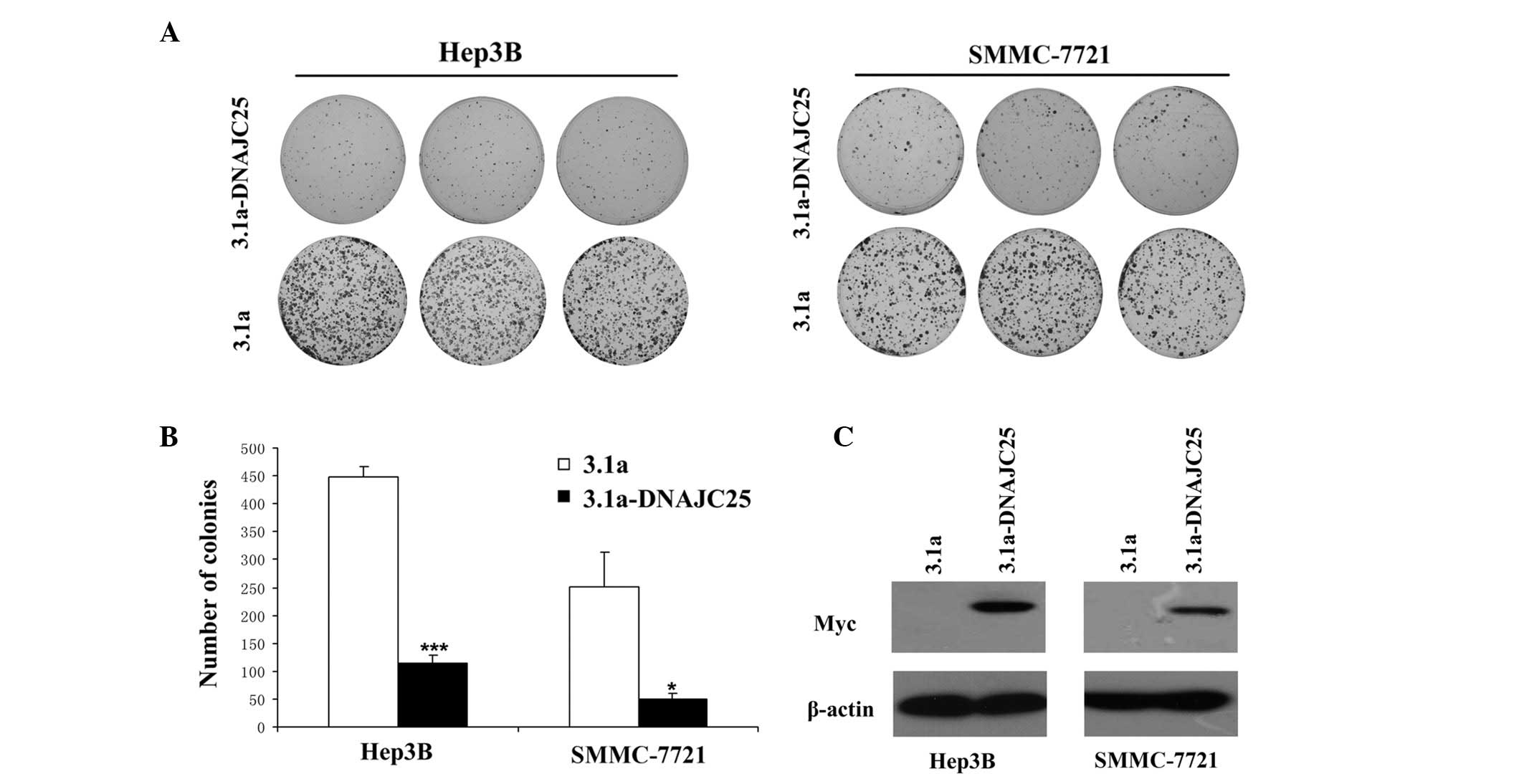
Colony formation assay
Since DNAJC25 was downregulated in HCC, we aimed to
determine whether DNAJC25 was able to inhibit tumor growth. We
performed a colony formation assay with HCC cells overexpressing
DNAJC25. As shown in Fig. 4A, the
overexpression of DNAJC25 markedly reduced the number of surviving
colonies of Hep3B and SMMC-7721 cells. The mean reduction in colony
formation was 74.67% for Hep3B (P<0.001) and 79.00% for
SMMC-7721 cells (P<0.05) from three independent experiments
(Fig. 4B). Colonies which formed in
the group transfected with pcDNA3.1A(-)-DNAJC25 were smaller in
size than those formed in the group transfected with the
pcDNA3.1A(-) vector. Our results suggest a suppressive role of
DNAJC25 in cell survival or proliferation.
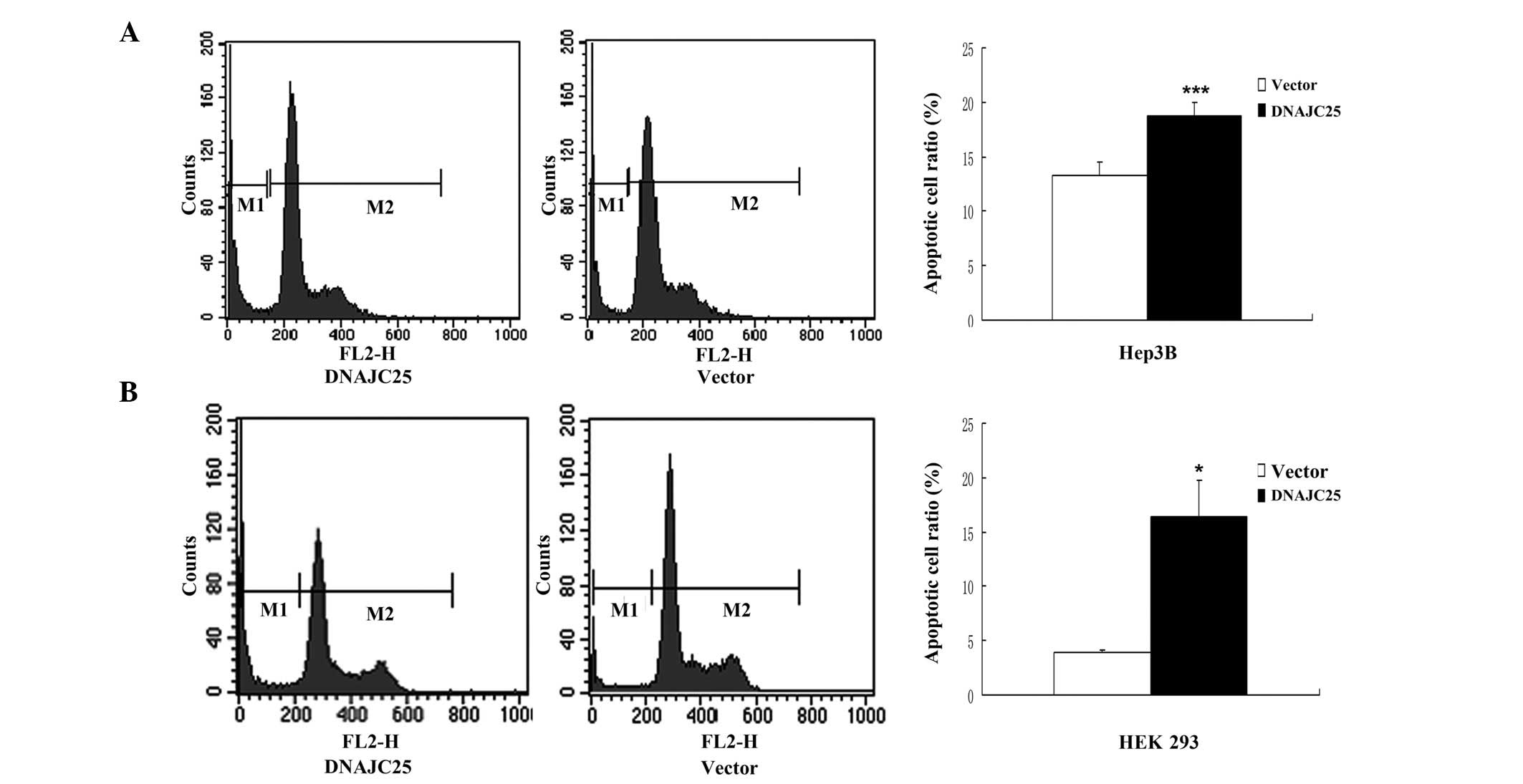
Overexpression of DNAJC25 induced
apoptotic cell death
In order to study the role of DNAJC25 in cell
proliferation, survival and cell cycle blocking, Hep3B and HEK 293
cells were transfected with DNAJC25 and analyzed by flow cytometry.
Compared with the control group, Hep3B and HEK 293 cells
transfected with DNAJC25 showed a marked sub-G1 peak, indicating a
significantly increased apoptotic cell population. The sub-G1 ratio
of the Hep3B cells transfected with pCMV-Myc-DNAJC25 was 18.80%,
while that of the control group was 13.23% (P<0.001; Fig. 5A). The sub-G1 ratio of the HEK 293
cells transfected with pCMV-Myc-DNAJC25 was 16.41% and that of the
control group was 3.92% (P<0.05; Fig. 5B). However, there was no marked
difference between cells transfected with pCMV-Myc-DNAJC25 and the
control group in cell cycle progression. Our results indicate that
DNAJC25 was able to induce apoptosis, but not cycle arrest.
Discussion
HSPs are best known as a group of proteins whose
expression is increased when cells are exposed to stresses, such as
high temperatures or oxygen deprivation (12). Further study revealed that HSPs are
also found in cells under normal conditions (13,14).
HSPs are important in the metabolism of cells, immunological
processes, cell cycle regulation, transcriptional activation and
signal transduction (15–17). They act as ‘chaperones’, ensuring
that their target proteins (client proteins) are in the right place
and right shape at the right time. Therefore, HSPs aid the folding
of proteins into the proper shapes and shuttle proteins from one
compartment to another inside the cell, which is essential for
their function (18,19).
HSPs are broadly classified into six major families
based on their relative molecular weights: HSP100, HSP90, HSP70,
HSP60, HSP40 and small HSPs (such as HSP27) (20). Numerous HSPs are conserved
throughout evolution, implying that their roles are important in
the physiology of the cell.
To date, HSPs have been reported to be involved in
the progression of a number of diseases. For instance, in
autoimmune diseases, HSPs play indispensable roles in stimulating
T-cell reactivity and protecting against disease (8). The upregulation of the synthesis of
HSPs is considered to lead to tolerance of ischemia in certain
animals (21). Additionally, HSPs
are involved in the regulation of tumorigenesis. The overexpression
of HSP27, 70 and 90 has been detected in various types of
carcinoma, including HCC (9,22).
According to the domain similarity to the E.
coli DnaJ protein, the HSP40 (DnaJ) family is further
classified into three subclasses: DNAJA, DNAJB and DNAJC. In
contrast to the well-studied HSP90 and HSP70 (10,23,24),
little is known concerning the role of HSP40 in tumor progression
and metastasis. Only a few members of HSP40, including HLJ1
(DNAJB4), Tid1 (DNAJA3), MRJ (DNAJB6), JDP1 (DNAJC12) and HDJ2
(DNAJA1), have been determined to be associated with cancer in
previous studies (25).
Previous studies have indicated that in canines,
DNAJC25 is an ER-resident membrane protein. Its amino-terminal
signal peptide comprised an ER-lumenal J-domain plus two
transmembrane regions and another ER-lumenal domain (26). Our data showed that in HeLa cells
and the HCC cell line SMMC-7721, the exogenous DNAJC25 fused with
EGFP located in the cytoplasm.
To study the expression profile of this novel gene,
we used RT-PCR to detect the distribution of DNAJC25 in 15 human
tissues. The expression level of DNAJC25 in the liver was markedly
higher than that in the other 14 tissues. In the thymus, prostate,
testis, ovary, small intestine and colon, DNAJC25 was only
expressed at trace levels. The markedly high expression of DNAJC25
in the liver led us to investigate the possibility of its
correlation with the occurrence of diseases in the liver and study
its function. The expression of DNAJC25 in HCC was further
evaluated in 87 pairs of HCC specimens and adjacent normal liver
tissues by quantitative real-time PCR. Our result indicated DNAJC25
was significantly downregulated in HCC, suggesting that DNAJC25 is
involved in hepotocellular carcinogenesis and acts as a suppressor
of HCC.
To explore the function of DNAJC25, we performed a
colony formation assay on the HCC cell lines SMMC-7721 and Hep3B
overexpressing DNAJC25. Our data indicate that the ectopic
expression of DNAJC25 resulted in an inhibition of colony growth,
which was consistent with the properties of a tumor suppressor to
inhibit the ability of cells to initiate colonies and inhibit cell
proliferation. Flow cytometry analysis further indicated that
overexpression of DNAJC25 induced cell apoptosis in the HCC cell
line Hep3B. A similar and also significant result was observed in
HEK 293 cells, which may be attributed to the higher transfection
efficiency. We also performed a cell cycle analysis, and no marked
effect of DNAJC25 on the cell cycle was observed. This implies that
the proapoptotic property may be the cause of the inhibition of
cell growth by ectopic DNAJC25 expression.
In summary, we cloned and identified a new member of
the DNAJC family, DNAJC25, explored its subcellular localization
and tissue distribution and revealed its function as a candidate
tumor suppressor in HCC for the first time. Notably, our
description of both the downregulated expression of DNAJC25 in HCC
and its proapoptotic function is opposite to the previous findings
of certain other HSPs, such as HSP27 and HSP70, which have been
reported to be upregulated in tumors and have antiapoptotic
properties (9,22). The present study has not only
provided a new candidate suppressor of HCC, but also furthered the
understanding of the HSP family. Further studies are required to
validate its proapoptotic function and explore its potential role
in cancer therapy.
Acknowledgements
This study was supported by the
National Key Sci-Tech Special Project of China (grant no.
2008ZX10002-020).
References
|
1.
|
FX BoschJ RibesM DiazR ClériesPrimary
liver cancer: worldwide incidence and
trendsGastroenterology127Suppl
1S5S16200410.1053/j.gastro.2004.09.01115508102
|
|
2.
|
FX BoschJ RibesR ClériesM DíazEpidemiology
of hepatocellular carcinomaClin Liver
Dis9191211200510.1016/j.cld.2004.12.00915831268
|
|
3.
|
DM ParkinF BrayJ FerlayP PisaniGlobal
cancer statistics, 2002CA Cancer J
Clin5574108200510.3322/canjclin.55.2.74
|
|
4.
|
S LindquistEA CraigThe heat-shock
proteinsAnnu Rev
Genet22631677198810.1146/annurev.ge.22.120188.003215
|
|
5.
|
JJ CottoRI MorimotoStress-induced
activation of the heat-shock response: cell and molecular biology
of heat-shock factorsBiochem Soc Symp64105118199910207624
|
|
6.
|
E SchmittM GehrmannM BrunetG MulthoffC
GarridoIntracellular and extracellular functions of heat shock
proteins: repercussions in cancer therapyJ Leukoc
Biol811527200710.1189/jlb.030616716931602
|
|
7.
|
P KopecekK AltmannováE WeiglStress
proteins: nomenclature, division and functionsBiomed Pap Med Fac
Univ Palacky Olomouc Czech
Repub1453947200110.5507/bp.2001.01012426770
|
|
8.
|
M RaskaE WeiglHeat shock proteins in
autoimmune diseasesBiomed Pap Med Fac Univ Palacky Olomouc Czech
Repub149243249200510.5507/bp.2005.03316601763
|
|
9.
|
AA KhalilNF KabapySF DerazC SmithHeat
shock proteins in oncology: diagnostic biomarkers or therapeutic
targets?Biochim Biophys Acta181689104201121605630
|
|
10.
|
ET SooGW YipZM LwinSD KumarBH BayHeat
shock proteins as novel therapeutic targets in cancerIn
Vivo22311315200818610741
|
|
11.
|
DR CioccaSK CalderwoodHeat shock proteins
in cancer: diagnostic, prognostic, predictive, and treatment
implicationsCell Stress
Chaperones1086103200510.1379/CSC-99r.116038406
|
|
12.
|
MN RylanderY FengJ BassKR DillerThermally
induced injury and heat-shock protein expression in cells and
tissuesAnn NY Acad
Sci1066222242200510.1196/annals.1363.00916533928
|
|
13.
|
C SchäferJA WilliamsStress kinases and
heat shock proteins in the pancreas: possible roles in normal
function and diseaseJ Gastroenterol3519200010632533
|
|
14.
|
L PirkkalaP NykänenL SistonenRoles of the
heat shock transcription factors in regulation of the heat shock
response and beyondFASEB
J1511181131200110.1096/fj00-0294rev11344080
|
|
15.
|
M JäätteläHeat shock proteins as cellular
lifeguardsAnn Med312612711999
|
|
16.
|
Z MatijasevicJE SnyderDB LudlumHypothermia
causes a reversible, p53-mediated cell cycle arrest in cultured
fibroblastsOncol Res10605610199810367942
|
|
17.
|
LA SonnaJ FujitaSL GaffinCM LillyInvited
review: Effects of heat and cold stress on mammalian gene
expressionJ Appl
Physiol9217251742200210.1152/japplphysiol.01143.200111896043
|
|
18.
|
FU HartlMolecular chaperones in cellular
protein foldingNature381571579199610.1038/381571a08637592
|
|
19.
|
S LeeFT TsaiMolecular chaperones in
protein quality controlJ Biochem Mol
Biol38259265200510.5483/BMBRep.2005.38.3.259
|
|
20.
|
HH KampingaChaperones in preventing
protein denaturation in living cells and protecting against
cellular stressHandb Exp
Pharmacol172142200610.1007/3-540-29717-0_116610353
|
|
21.
|
LH SnoeckxRN CornelussenFA Van
NieuwenhovenRS RenemanGJ Van Der VusseHeat shock proteins and
cardiovascular pathophysiologyPhysiol Rev8114611497200111581494
|
|
22.
|
MM FieldsE ChevlenOvarian cancer
screening: a look at the evidenceClin J Oncol
Nurs107781200610.1188/06.CJON.77-8116482731
|
|
23.
|
SC BishopJA BurlisonBS BlaggHsp90: a novel
target for the disruption of multiple signaling cascadesCurr Cancer
Drug Targets7369388200710.2174/15680090778080977817979631
|
|
24.
|
C DidelotD LanneauM BrunetAnti-cancer
therapeutic approaches based on intracellular and extracellular
heat shock proteinsCurr Med
Chem1428392847200710.2174/09298670778236007918045130
|
|
25.
|
A MitraLA ShevdeRS SamantMulti-faceted
role of HSP40 in cancerClin Exp
Metastasis26559567200910.1007/s10585-009-9255-x
|
|
26.
|
RP ZahediC VölzingA SchmittAnalysis of the
membrane proteome of canine pancreatic rough microsomes identifies
a novel Hsp40, termed
ERj7Proteomics934633473200910.1002/pmic.20080072219579229
|