Introduction
Triple-negative breast cancer (TNBC) is a type of
high-risk breast cancer in which the estrogen receptor (ER),
progesterone receptor (PR) and human epidermal growth factor
receptor (HER2/ErbB2) are all negative (1). TNBC accounts for 15–20% of all breast
cancer cases. Since the effective targeted endocrine therapy cannot
be used, patients with TNBC usually have a poor prognosis. In
addition to surgical therapy, the main treatment for TNBC is
chemotherapy. Neoadjuvant chemotherapy (NAC) has been widely
accepted in the treatment of breast cancer. Despite the relative
chemosensitivity, less than 25% of all patients with TNBC treated
with standard NAC achieve complete pathological response (pCR).
However, the evaluation of the effect of NAC is limited to the
clinical and pathological changes of the tumors and metastatic
lymph nodes. Therefore, a method to simply and accurately evaluate
the effect of NAC in the treatment of TNBC would be extremely
valuable.
A breast cancer-specific gene, BCSG1, was identified
by Ji et al (2) in 1997.
BCSG1 is highly expressed in human-infiltrating breast carcinomas
but not expressed in normal or benign breast tissues and the
expression of BCSG1 is also stage-specific for breast cancer
(3). Overexpression of BCSG1 in
breast cancer cells increases the motility and invasiveness in
vitro and stimulates metastasis in vivo (4). In a clinical trial, patients with
BCSG1-positive breast tumors generally had shorter disease-free
survival and overall survival and higher probability of mortality
compared with the patients with BCSG1-negative tumors (5). Therefore, BCSG1 may be used as a
marker for breast cancer progression and prognosis (5,6).
Although a number of studies have established the significance of
BCSG1 in breast cancer, few results concerning the correlation
between BCSG1 and TNBC have been reported (7). Therefore, we analyzed the correlation
between BCSG1 expression and the effect of NAC in the treatment of
TNBC in the present study to determine the role of BCSG1 in the
treatment of TNBC with NAC.
Patients and methods
Patients and treatment
All 32 patients (female, 27–45 years old; median
age, 40) were treated at the Center of Breast Diseases at the
Second People’s Hospital of Shenzhen between September 2009 and
August 2011. All patients were diagnosed with triple-negative
invasive non-specific cancer by pathological evaluation and hormone
receptors test. Patients underwent breasts, double axillary and
liver type-B ultrasonic scan, chest X-ray and whole body bone scan
prior to chemotherapy. The TNM stages were: IIA, 11; IIB, 14; IIIA,
5; IIIB, 2 (UICC/AJCC, 2003). A total of 18 patients were found to
have ipsilateral axillary lymph node metastasis. No other treatment
was administered prior to definite diagnosis. No serious heart,
liver or kidney damage was detected (Karnofsky score=100).
All the 32 patients were administered a combination
of 600 mg/m2 cyclophosphamide (CTX), 80 mg/m2
epirubicin (EPI) and 500 mg/m2 fluorouracil (5-FU) on
day 1 and then every 21 days. After 2 cycles of chemotherapy,
patients underwent modified radical mastectomy or breast-conserving
surgery. The changes of lesions were evaluated according to the
criteria of the World Health Organization (WHO) for anticancer
drugs prior to and following NAC-based clinical response and B
ultrasound (3). Complete response
(CR) and partial response (PR) were calculated as clinical overall
response. Breast cancer tissues were obtained through core-needle
biopsy prior to NAC or surgery following NAC. Written informed
consent was obtained from the patients. The study was approved by
the ethics committee of the First Affiliated Hospital of Shenzhen
University, Shenzhen, Guangdong, China.
Immunohistochemistry
Immunohistochemistry was performed according to the
manufacturer’s instructions with BCSG1 polyclonal antibody (Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA; 1:100 dilution).
Brain tissue was used as a positive control and PBS as a negative
control. BCSG1-positive cells were defined according to the
standard reported by Mohsin et al (8). The BCSG1 expression score was
calculated from the proportion of positive cells and the color
intensity of the cells: i) number of positive cells, grade 0,
<25%; grade 1, 26–50%; grade 2, 51–75%; grade 3, ≥75%; ii) color
intensity, grade 1, weak; grade 2, moderate; grade 3, strong. The
score for each slice is the summation of the two parts. A total
score <3 was considered to indicate low expression and ≥3 to
indicate high expression.
Real-time RT-PCR
Total RNA was isolated from breast cancer tissue
with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to
the manufacturer’s instructions. The concentration and purity of
total RNA were determined by a spectrophotometer (Eppendorf,
Hamburg, Germany). Reverse transcription was performed using Random
primer RT mixtures with M-MLV (20 U/μl; Promega, Madison,
WI, USA) reverse transcriptase 20 U/20 μl, total RNA 1
μg/20 μl. cDNA was stored at −20°C. Quantitative
real-time PCR was performed with an ABI stepone plus Real-time PCR
system. The sequences of the primers were as follows: BCSG1,
forward 5′-AGGAGGGGGTCATGTATGTG-3′, reverse
5′-TTCTCTTTGGATGCCTCACC-3′; GAPDH forward
5′-GGAAGGTGAAGGTCGGAGT-3′, reverse 5′-CCTGGAAGATGGTGAGGG-3′. PCR
mixtures contained 1 μl cDNA, 12.5 μl
SYBR® Premix 2X (Toyobo, Osaka, Japan) and 0.16
μmol/l forward and reverse primers in a total volume of 25
μl. Reactions were started with a polymerase activation step
at 94°C for 5 min followed by 35 cycles of 94°C for 30 sec, 57°C
for 45 sec and 72°C for 30 sec. Fluorescence data were acquired
after each cycle. The amount of specific mRNA in samples was
calculated using the ΔΔCT method.
Statistical analysis
Measurement data are expressed as mean ± SD. Data
were analyzed using SPSS 13.0 for Windows (SPSS Inc., Chicago, IL,
USA). The paired Student’s t-test was used for comparing the BCSG1
expression difference before and after NAC. The χ2 test
was used for comparing the clinical overall response rate in
patients with high or low BCSG1 expression. The correlation between
the effect of NAC and BCSG1 expression was measured by Spearman
rank correlation analysis. P<0.05 was considered to indicate a
statistically significant result.
Results
Response to neoadjuvant chemotherapy
The tumor softened in 62.5% of patients (20/32) 10
days after NAC and reduced in size in 71.9% of patients (23/32)
after one cycle of NAC. Tumor size shrank markedly in 84.4% (27/32)
patients after 2 cycles of NAC. There were 3 CR, 24 PR and 5 stable
disease (SD) with an overall response rate of 84.4% (27/32). In 28
cases of postoperative cancer tissue specimens, a clear boundary
between the cancer tissue and the breast tissue and white cut
surface and little necrosis on tumor tissue were observable with
the naked eye. Various degrees of cell degeneration and necrosis of
the tumor cells, nuclear contraction, rupture and cytoplasmic
coagulation necrosis of the cells surrounding the tumor tissue and
vascular endothelial hyperplasia and blood vessel narrowing or
occlusion were observed with a light microscope. In the remaining
four cases, various degrees of cell degeneration were observed, but
necrosis was not clear. Various degrees of nuclear contraction and
fragmentation, necrosis, calcification and fibrosis were also
observed in the cancer cells in the axillary lymph nodes.
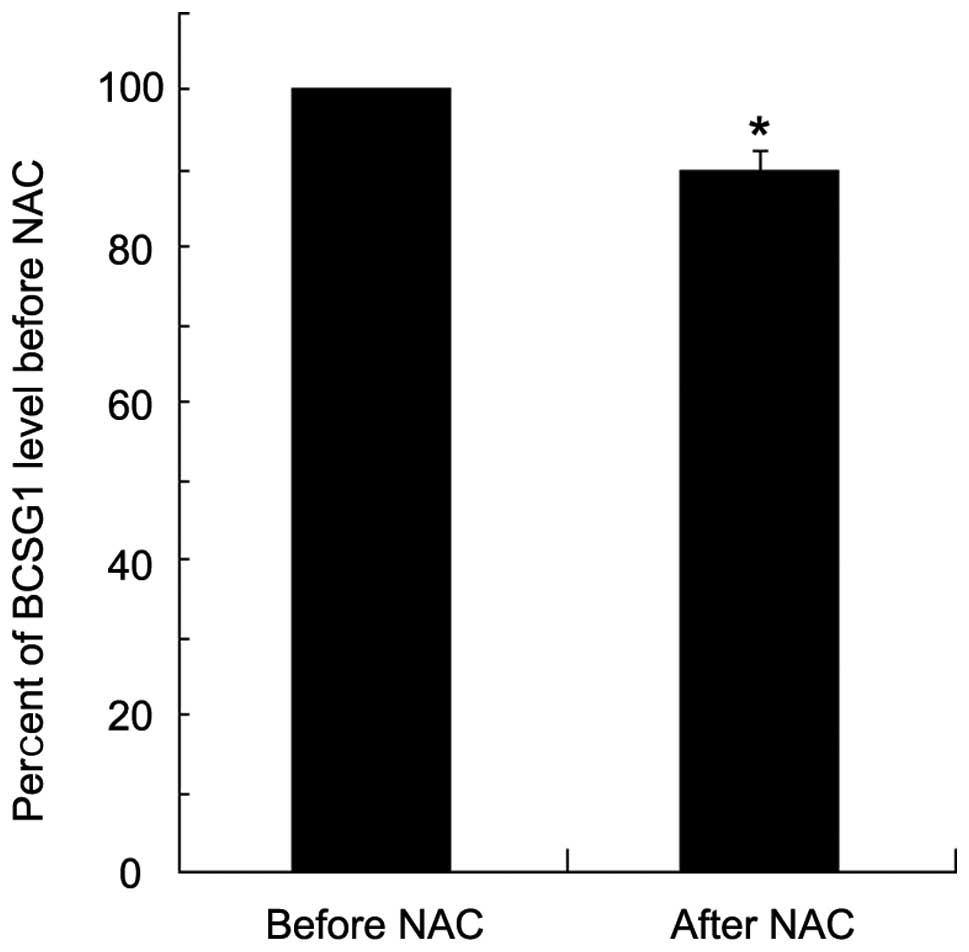
BCSG1 mRNA expression
Real time RT-PCR was performed to examine the BCSG1
mRNA level in the cancer tissues of each patient prior to and
following NAC (Fig. 1). The BCSG1
mRNA level in breast cancer tissues after NAC was decreased
significantly compared with that before NAC (a decrease of
∼10%).
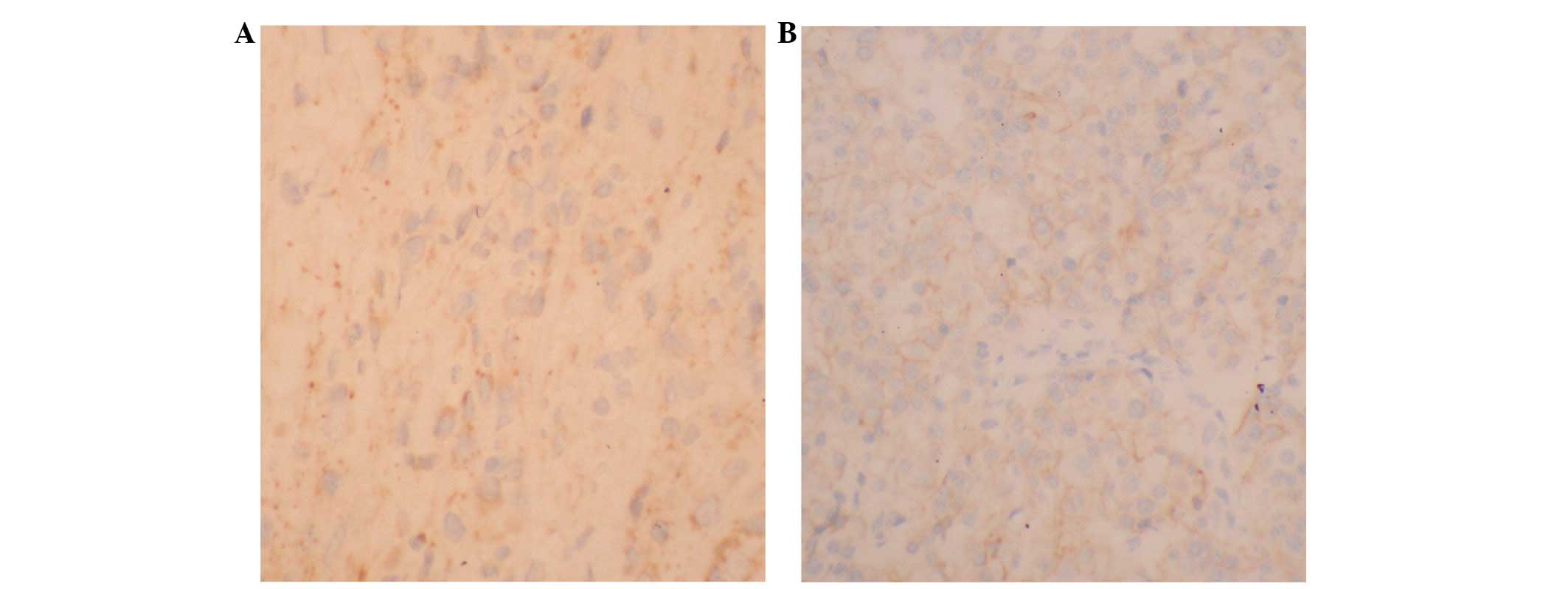
Correlation of the BCSG1 level and the
effect of NAC
The expression of BCSG1 protein was determined with
immunohistochemistry (Fig. 2).
BCSG1 protein was highly expressed in 22 patients (68.8%) before
NAC, but only in nine patients after NAC (28.1%). The difference
was significant. There was a negative correlation between the BCSG1
level and the effect of NAC (r= −0.584, P<0.01; Table I).
 | Table I.Correlation of BCSG1 expression and
curative effect of NAC (n=32). |
Table I.
Correlation of BCSG1 expression and
curative effect of NAC (n=32).
| BCSG1 expression
|
|---|
| Curative effect of
NAC | High expression | Low expression | P-value | r |
|---|
| CR + PR | 5 | 22 | <0.01 | −0.584 |
| SD | 4 | 1 | | |
Discussion
In the present study, we found that the expression
of BCSG1 was decreased following NAC in TNBC patients. There was a
negative correlation between the BCSG1 level and the effect of NAC
in TNBC. Our results indicate that there is a correlation between
the BCSG1 levels and the effect of NAC in patients with TNBC.
BCSG1, also known as SNCG (γ-synuclein gene), was
identified in 1997 by direct sequencing of cDNA gene in breast
cancer (2). BCSG1 is not expressed
in normal breast tissue but is highly expressed in most invasive
and metastatic breast cancers. It has been shown that
overexpression of BCSG1 promoted the invasion and metastasis of
breast cancer cells (4).
Overexpression of BCSG1 is also an event in advanced breast cancer
and predicts poor clinical outcome in breast cancer (5,6) These
results suggest that BCSG1 is a predicator for the tumor invasion
and metastasis and a target for gene therapy. Therefore, detecting
BCSG1 may aid the evaluation of the invasive and metastatic ability
and the prognosis of breast cancer. In our study, we found that
BCSG1 was also highly expressed in TNBC patients before NAC,
suggesting a potential therapy target for the TNBC. Our results
showed that TNBC patients who gained more benefit from NAC had
lower BCSG1 expression, indicating that BCSG1 is involved in the
NAC treatment. TNBC is a subtype of breast cancer. Although TNBC is
an initially chemosensitive disease, less than 25% of patients with
TNBC who received standard NAC achieved pCR and the remaining
patients usually have a poor prognosis (9). Several approaches have been reported
to improve the NAC efficacy in previous studies, including
different anthracycline-based regimens, anthracycline-taxane
combinations, sequential regimens and dose-dense schedules
(9). Furthermore, certain
researchers have revealed other characteristics of TNBC, including
overexpression of EGFR and c-KIT, increased proliferative rate
through MAP kinase and Akt pathways (10) and providing some basis for the
targeted therapy in those patients. Poly-ADP-ribose polymerase
(PARP), a DNA-repair nuclear enzyme, has gained attention as a
therapeutic target for cancer. Inhibition of PARP may improve the
efficacy of certain DNA-damaging chemicals, including platinum
compounds and topoisomerase inhibitors (11,12).
However, lack of consistency and the complexity of the analysis and
interpretation of molecular classification data hindered the
application of these targets in the treatment of TNBC (9). Since TNBC patients cannot benefit from
target endocrine therapy and anti-HER-2 treatment, systemic
chemotherapy treatment is the only one option in combination with
local surgery and radiotherapy. In the present study, we showed
that patients with lower BCSG1 levels after NAC gained more benefit
from NAC than patients with high BCSG1 levels. Since the expression
level of BCSG1 has been indicated to correlate with the effect of
chemotherapy, BCSG1 would be used as a new target in NAC and a
screening compound for TNBC.
The current study has certain limitations. First,
the number of patients enrolled in the study was small and further
research is required to support the conclusion. Second, the
correlation between the BCSG1 levels and the long-term prognosis of
the TNBC patients also requires further investigation in the
future.
In summary, the results of the present study
revealed a correlation between the BCSG1 level after NAC and the
effect of NAC, indicating that BCSG1 may act as a target for
chemotherapy and be used in the screening of new agents for TNBC
treatment.
Acknowledgements
This study was supported by grants
from the Medical Science and Technology Research Foundation of
Guangdong Province (A2008605) and the Science and Technology
Research Foundation of Shenzhen (no. 200802030 and no.
201101005).
References
|
1.
|
G CuriglianoA GoldhirschThe
triple-negative subtype: new ideas for the poorest prognosis breast
cancerJ Natl Cancer Inst
Monogr2011108110201110.1093/jncimonographs/lgr03822043054
|
|
2.
|
H JiYE LiuT JiaIdentification of a breast
cancer-specific gene, BCSG1, by direct differential cDNA
sequencingCancer Res5775976419979044857
|
|
3.
|
K WuZ WengQ TaoStage-specific expression
of breast cancer-specific gene gamma-synucleinCancer Epidemiol
Biomarkers Prev12920925200314504205
|
|
4.
|
T JiaYE LiuJ LiuYE ShiStimulation of
breast cancer invasion and metastasis by synuclein gammaCancer
Res5974274719999973226
|
|
5.
|
J GuoC ShouL MengNeuronal protein
synuclein gamma predicts poor clinical outcome in breast cancerInt
J Cancer12112961305200710.1002/ijc.2276317534899
|
|
6.
|
K WuZ QuanZ WengExpression of neuronal
protein synuclein gamma gene as a novel marker for breast cancer
prognosisBreast Cancer Res
Treat101259267200710.1007/s10549-006-9296-716821081
|
|
7.
|
A BoschP ErolesR ZaragozaJR ViñaA
LluchTriple-negative breast cancer: molecular features,
pathogenesis, treatment and current lines of researchCancer Treat
Rev36206215201010.1016/j.ctrv.2009.12.00220060649
|
|
8.
|
SK MohsinM ZhangGM ClarkD Craig
AllredMaspin expression in invasive breast cancer: association with
other prognostic factorsJ
Pathol199432435200310.1002/path.131912635133
|
|
9.
|
Z NahlehNeoadjuvant chemotherapy for
‘triple negative’ breast cancer: a review of current practice and
future outlookMed Oncol275315392010
|
|
10.
|
S CleatorW HellerRC CoombesTriple-negative
breast cancer: therapeutic optionsLancet
Oncol8235244200710.1016/S1470-2045(07)70074-817329194
|
|
11.
|
H FarmerN McCabeCJ LordTargeting the DNA
repair defect in BRCA mutant cells as a therapeutic
strategyNature434917921200510.1038/nature0344515829967
|
|
12.
|
K RatnamJA LowCurrent development of
clinical inhibitors of poly(ADP-ribose) polymerase in oncologyClin
Cancer Res1313831388200710.1158/1078-0432.CCR-06-226017332279
|