Introduction
Intraductal papillary mucinous neoplasm (IPMN) is a
rare intraductal epithelial neoplasm composed of mucin-producing
cells arising in the main pancreatic duct or its branches (1). IPMNs are estimated to account for 1–3%
of exocrine pancreatic neoplasms and 20% of all cystic neoplasms of
the pancreas, and the incidence of this disease is considered to be
increasing (1). The subtypes of
IPMNs are recognized as main-duct and branch-duct types by
macroscopic examination, and noninvasive IPMNs are classified into
three categories on the basis of cytoarchitectural atypia: low-,
intermediate- and high-grade dysplasia (1).
Pancreatic neuroendocrine neoplasms are uncommon and
represent 1–2% of all pancreatic neoplasms (2). According to the recent World Health
Organization Classification of the Digestive System, neuroendocrine
neoplasms are classified into three categories: neuroendocrine
tumor (NET) G1 and G2 and neuroendocrine carcinoma (NEC; NET G3)
(2).
IPMN and NET of the pancreas are rare tumors and
their association is not expected to be frequent. However, certain
studies have suggested that the concomitant occurrence of these
tumors may be more frequent than previously thought (3). In the current study, we describe a
case of concomitant occurrence of IPMN and NET of the pancreas, and
review the clinicopathological features of previously reported
cases and the current one. The study was approved by the Ethics
Committee of Shiga University of Medical Science, Shiga, Japan.
Informed consent was obtained from the patient.
Patient and methods
Case report
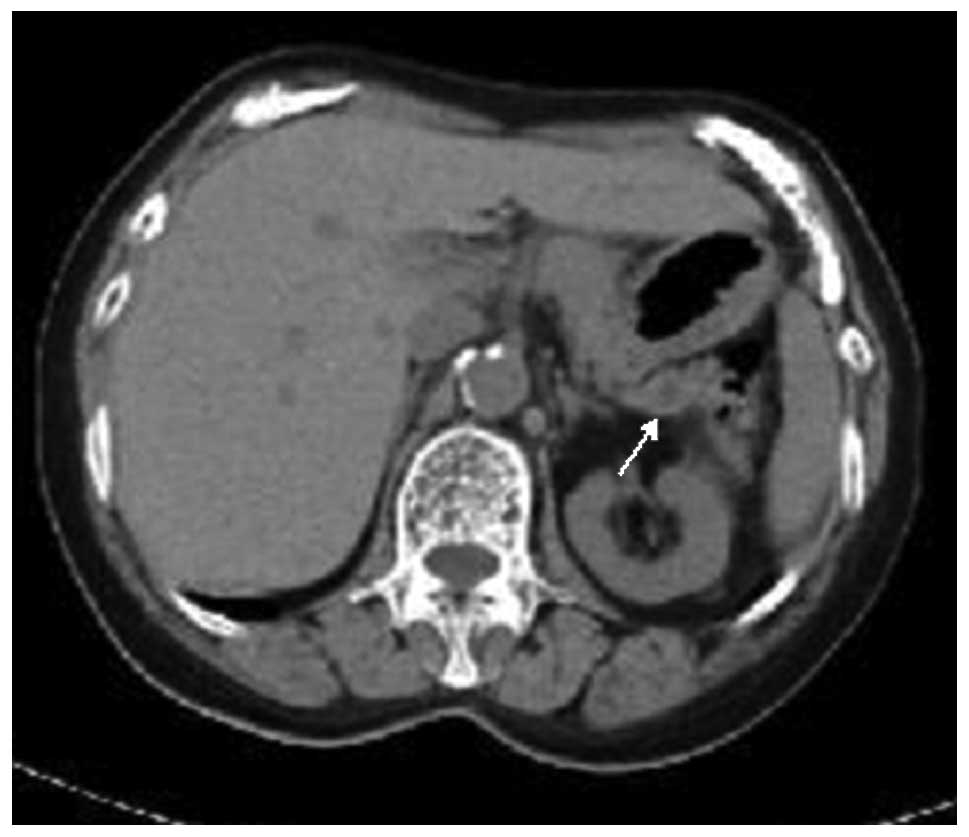
A 68-year-old Japanese female with a past history of
autoimmune hepatitis was incidentally found to have dilatation of
the main pancreatic duct, measuring ∼5 mm, in the pancreas tail by
computed tomography (CT; Fig. 1).
No other tumorous lesions were detected in the pancreas and other
visceral organs by CT. No clinical symptoms of hormone
overproduction were present. Under the clinical diagnosis of
main-duct type IPMN, a distal pancreatectomy was performed.
The postoperative course has been uneventful, and no
tumor recurrence has been observed during three years of medical
follow-up.
Materials and methods
The formalin-fixed, paraffin-embedded tissue blocks
of the resected pancreas specimens were cut into 3-μm thick
sections, deparaffinized and rehydrated. Each section was stained
with hematoxylin and eosin and then used for immunostaining.
Immunohistochemical analyses were performed using an autostainer
(Benchmark XT system; Ventana Medical System, Tucson, AZ, USA)
according to the manufacturer’s instructions. The following primary
antibodies were used: a mouse monoclonal antibody against
α-internexin (2E3; Lab Vision, Freemont, CA, USA), a mouse
monoclonal antibody against chromogranin A (DAK-A3; Dako
Cytomation, Glostrup, Denmark), a rabbit polyclonal antibody
against gastrin (Dako Cytomation), a rabbit polyclonal antibody
against glucagon (Dako Cytomation), a mouse monoclonal antibody
against insulin (Z006; Nichirei Bioscience, Tokyo, Japan), a mouse
monoclonal antibody against Ki-67 (MM1; Novocastra Laboratories,
Ltd., Newcastle-upon-Tyne, UK), a mouse monoclonal antibody against
peripherin (PJM50; Novocastra Laboratories, Ltd.), a rabbit
polyclonal antibody against somatostatin receptor type 2a (SSTR2a;
Gramsch Laboratories, Schwabhausen, Germany) and a mouse monoclonal
antibody against synaptophysin (27G12; Novocastra Laboratories,
Ltd.).
Results
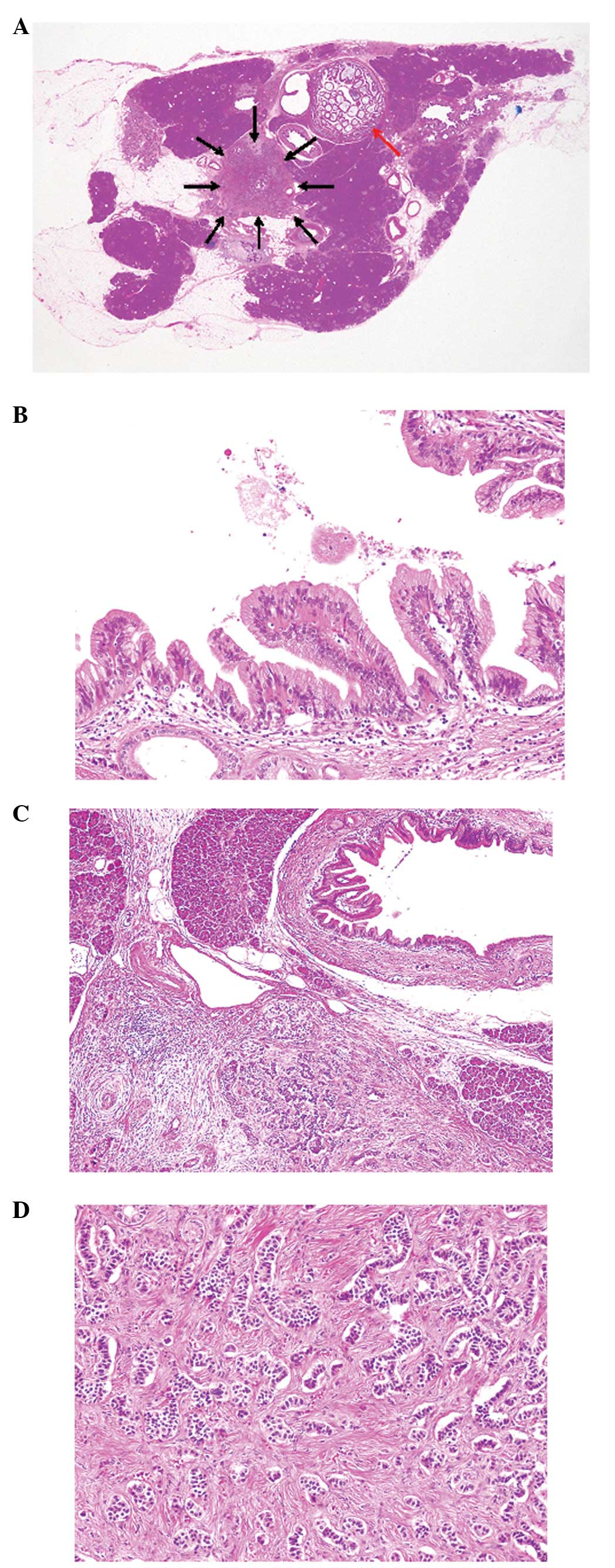
Histopathological study of the resected pancreas
tissue revealed dilatation and intraductal papillary proliferation
of the main pancreatic duct (Fig.
2A). The epithelial cells that showed intraductal papillary
proliferation were columnar and had mucin in the cytoplasm and
small round nuclei with inconspicuous nucleoli (Fig. 2B). Mitotic figures were rarely
observed. No invasive growth was noted. These histopathological
features corresponded to IPMN with low-grade dysplasia.
Well-circumscribed neoplastic growth was present in the pancreas
adjacent to the IPMN (Fig. 2A and
C). Trabecular growth of the neoplastic cells with eosinophilic
cytoplasm and bland nuclei with inconspicuous nucleoli was observed
accompanied by fibrosis (Fig. 2C and
D). Mitotic figures were rarely identified (<1/10 high-power
fields). No vascular invasion was noted.
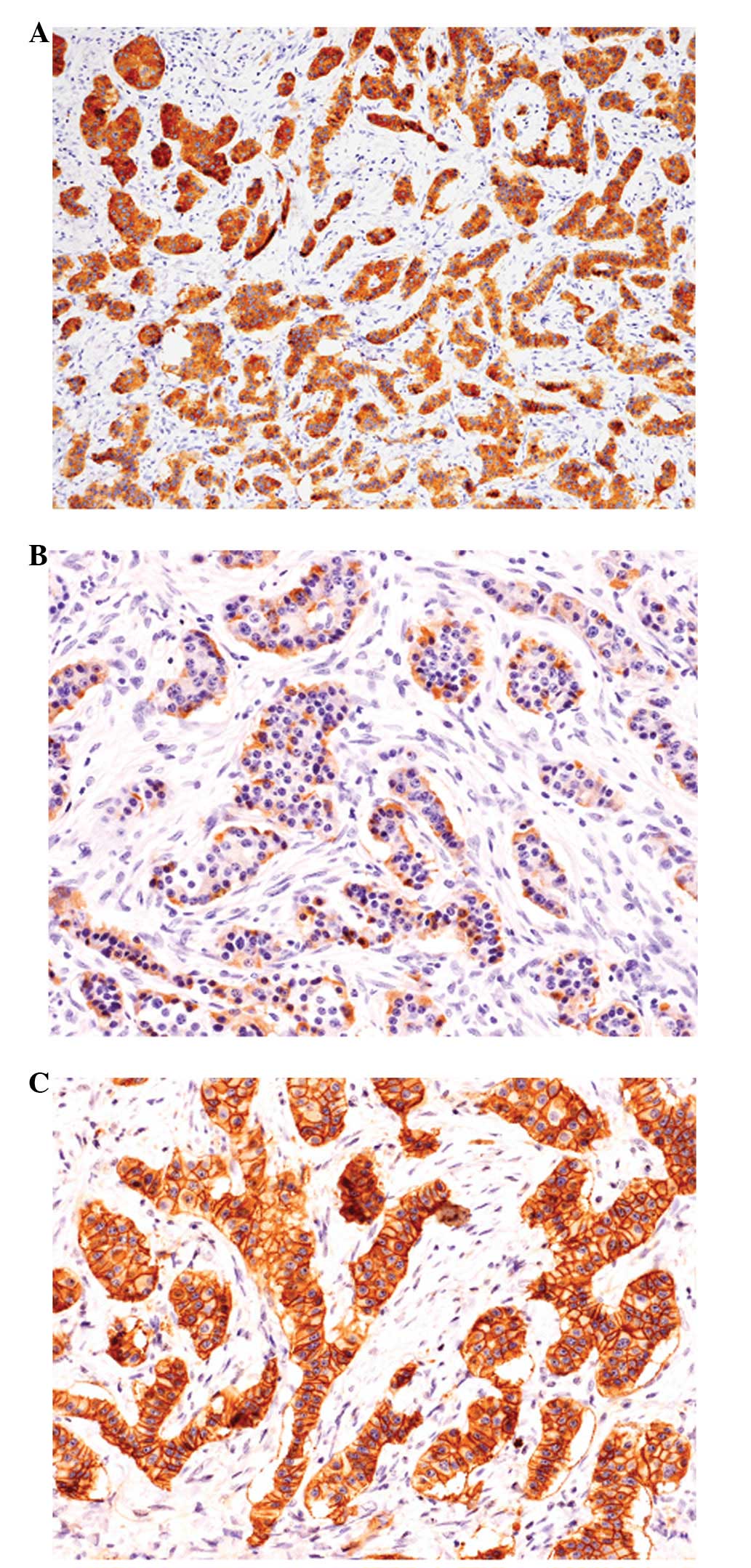
Immunohistochemical analyses revealed that the
neoplastic cells showing trabecular growth were diffusely positive
for synaptophysin and chromogranin A (Fig. 3A). Glucagon was expressed in
approximately half of the tumor cells (Fig. 3B), and a few insulin-positive tumor
cells were also observed. However, gastrin positivity was not
observed in any of the tumor cells. The Ki-67 labeling index was
<1%. SSTR2a immunostaining was diffusely positive in the cell
membrane of the tumor cells (Fig.
3C) and was score 3 according to the scoring system reported by
Volante et al(4). No
peripherin or α-internexin expression was observed in the tumor
cells.
According to these histopathological and
immunohistochemical findings, an ultimate diagnosis of concomitant
IPMN (low-grade dysplasia) and NET G1 of the pancreas was made.
Discussion
In the current study, we describe a case of
concomitant IPMN and NET of the pancreas. Table I summarizes the clinicopathological
features of the 15 previously reported cases of concomitant IPMN
and NET (3,5–9) as
well as the present case. This condition mainly affects middle-aged
females (average age, 63.1 years; range, 40–76 years; male/female
ratio, 5:11). The main symptoms are abdominal or back pain (8
cases), no symptoms (5 cases) or weight loss (5 cases). A hormone
production symptom (hypoglycemia) was observed in only one case.
The most common degree of dysplasia of IPMN was low grade, however,
high-grade dysplasia was also present (2 cases). The size of the
NETs were not particularly large (average, 15.1 mm; range, 3–30),
however, the clinical behavior was not always indolent. Metastasis
of the NET was observed in 3 cases and one of these cases succumbed
to NET; the histopathology of this case was NEC (NET G3). The
preoperative clinical diagnosis was variable; IPMN in 7 cases and
concomitant in 6 cases. Therefore, detailed pathological analysis
of the resected pancreas tissue is required to indicate adequate
treatment since metastasis of the NET may occur in some patients
with concomitant IPMN and NET, even in those with small-sized
lesions.
 | Table IClinicopathological features of
concomitant IPMN and NET of the pancreas. |
Table I
Clinicopathological features of
concomitant IPMN and NET of the pancreas.
| | | | IPMN
| NET
| |
|---|
| Case | Age
(years)/gender | Symptom | Preoperative
diagnosis | Type | Dysplasia | Size (mm) | Location | Behavior | Ref. |
|---|
| 1 | 73/M | Left hypochondrium
pain | Concomitant | Branch | Low | 28 | Tail | Potential
malignant | 3 |
| 2 | 40/F | Epigastric pain | Concomitant | Branch | Low | 11 | Head | Benign | 3 |
| 3 | 61/F | Epigastric pain | IPMN | Branch/main | Intermediate | 12 | Tail | Benign | 3 |
| 4 | 55/F | Jaundice, weight
loss, epigastric pain | NET | Branch/main | Low | 30 | Head | Invasive, metastasis
(+) | 3 |
| 5 | 68/F | None | Concomitant | Branch/main | Low | 18 | Body | Benign | 3 |
| 6 | 62/M | Epigastric pain,
jaundice | IPMN | Branch/main | High | 20 | Head | Potential
malignant | 3 |
| 7 | 58/M | None | IPMN | Branch | Low | 8 | Tail | Benign | 4 |
| 8 | 51/M | Hypoglycemia | NET | NA | Low | 15 | Tail | Benign | 5 |
| 9 | 72/F | Back pain | NET | NA |
Intermediate-high | 25 | Head | Malignant, metastasis
(+)a | 6 |
| 10 | 59/F | Abdominal pain | Concomitant | Branch | NA | 7.8 | Body | Benign | 7 |
| 11 | 55/F | None | Concomitant | Branch | NA | 20 | Head | Low malignant
potential | 7 |
| 12 | 67/M | Weight loss | IPMN | Main | Low | 8 | Head |
Well-differentiated | 8 |
| 13 | 72/F | Abdominal pain | Concomitant | Branch | Low | 16 | Tail | Malignant, metastasis
(+) | 8 |
| 14 | 72/F | None | IPMN | Branch | Low | 9 | Body |
Well-differentiated | 8 |
| 15 | 76/F | Weight loss | IPMN | Branch | Low | 11 | Head |
Well-differentiated | 8 |
| Present case | 68/F | None | IPMN | Main | Low | 3 | Tail | NET G1 | |
Somatostatin is an acidic polypeptide that inhibits
cell proliferation and differentiation (10). The physiological action of
somatostatin is initiated by its interaction with a family of
receptors consisting of five different subtypes, SSTR1-5 (11). Somatostatin analogs (including
octreotide) bind to the SSTRs, particularly SSTR2a, which is the
most widely expressed subtype in NETs (4). A previous study revealed that
somatostatin analogs significantly lengthen the time to tumor
progression in patients with metastatic midgut NETs (12). Therefore, immunohistochemical
analysis of SSTR2a expression in NETs is required to examine the
suitability for somatostatin receptor analog treatment. Although
the metastatic rate is low in NET G1, the analysis of SSTR2a
expression is useful for identifying the utility of an optional
treatment for the unexpected metastasis of NETs.
Expression of the intermediate filaments, peripherin
and α-internexin, in NETs of the appendix and rectum has been
previously reported (13,14). We have previously characterized the
expression patterns of neuronal intermediate filament proteins in
the NETs of various organs (13,14).
While peripherin (a type III intermediate filament protein
expressed in normal peripheral nerves) is expressed in all NET G1
of the rectum, the frequency of its expression is low in NET G2 of
the rectum (13). By contrast, the
expression of α-internexin (a type IV intermediate filament protein
normally found in the central nervous system) is observed in all
NET G1 of the appendix and approximately half of rectal NET G1. All
appendiceal NET G1 co-express peripherin and α-internexin (14). Since neither peripherin nor
α-internexin expression was observed in this case of NET G1 of the
pancreas, it appears that intermediate filament protein expression
varies with NET origin.
It is well known that IPMNs are associated with a
high incidence of extrapancreatic malignancies, which proceed,
coexist with or succeed IPMN (approximately 25–30% of IPMN cases)
(15–17). Colorectal and gastric carcinomas are
the most common extrapancreatic carcinomas (15–17).
In conclusion, although patients with IPMN have a
favorable prognosis with a 5-year survival rate of almost 100%
(16), concomitant pancreatic NET
and extrapancreatic malignancies may occur, therefore, systemic
surveillance of extrapancreatic neoplasms and detection of
concomitant NETs of the pancreas are necessary for patients with
IPMN.
References
|
1
|
Adsay NV, Fukushima N, Furukawa T, et al:
Intraductal neoplasms of the pancreas. World Health Organization
Classification of Tumours of the Digestive System. Bosman FT,
Carneiro F, Hruban RH and Theise ND: IARC; Lyon: pp. 304–313.
2010
|
|
2
|
Klimstra DS, Arnold R, Capella C, et al:
Neuroendocrine neoplasms of the pancreas. World Health Organization
Classification of Tumours of the Digestive System. Bosman FT,
Carneiro F, Hruban RH and Theise ND: IARC; Lyon: pp. 322–326.
2010
|
|
3
|
Marrache F, Cazals-Hatem D, Kianmanesh R,
et al: Endocrine tumor and intraductal papillary neoplasm of the
pancreas: a fortuitous association? Pancreas. 31:79–83. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Volante M, Brizzi MP, Faggiano A, et al:
Somatostatin receptor type 2A immunohistochemistry in
neuroendocrine tumors: a proposal of scoring system correlated with
somatostatin receptor scintigraphy. Mod Pathol. 20:1172–1182. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goh BK, Ooi LL, Kumarasinghe MP, et al:
Clinicopathological features of patients with concomitant
intraductal papillary mucinous neoplasm of the pancreas and
pancreatic endocrine neoplasm. Pancreatology. 6:520–526. 2006.
View Article : Google Scholar
|
|
6
|
Zhao X, Stabile BE, Mo J, Wang J and
French SW: Nesidioblastosis coexisting with islet cell tumor and
intraductal papillary mucinous hyperplasia. Arch Pathol Lab Med.
125:1344–1347. 2001.
|
|
7
|
Stukavec J, Jirasek T, Mandys V, et al:
Poorly differentiated endocrine carcinoma and intraductal
papillary-mucinous neoplasm of the pancreas: Description of an
unusual case. Pathol Res Pract. 203:879–884. 2007. View Article : Google Scholar
|
|
8
|
Larghi A, Stobinski M, Galasso D, Lecca PG
and Costamagna G: Concomitant intraductal papillary mucinous
neoplasm and pancreatic endocrine tumor: Report of two cases and
review of the literature. Dig Liver Dis. 41:759–761. 2009.
View Article : Google Scholar
|
|
9
|
Gill KR, Scimeca D, Stauffer J, et al:
Pancreatic neuroendocrine tumors among patients with intraductal
papillary mucinous neoplasms: real association or just a
coincidence? JOP. 10:515–517. 2009.
|
|
10
|
Lamberts SW, Krenning EP and Reubi JC: The
role of somatostatin and its analogs in the diagnosis and treatment
of tumors. Endocr Rev. 12:450–482. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reisine T and Bell GI: Molecular biology
of somatostatin receptors. Endocr Rev. 16:427–442. 1995.PubMed/NCBI
|
|
12
|
Rinke A, Müller H, Schade-Brittinger C, et
al: Placebo-controlled, double-blind, prospective, randomized study
on the effect of octreotide LAR in the control of tumor growth in
patients with metastatic neuroendocrine midgut tumors: A report
from the PROMID Study Group. J Clin Oncol. 27:4656–4663. 2009.
View Article : Google Scholar
|
|
13
|
Ishida M, Kushima R, Chano T and Okabe H:
Immunohistochemical demonstration of the type III intermediate
filament peripherin in human rectal mucosae and well-differentiated
endocrine neoplasms. Oncol Rep. 18:633–637. 2007.
|
|
14
|
Ishida M, Kushima R, Brevet M, Chatelain D
and Okabe H: Co-expression of neuronal intermediate filaments,
peripherin and α-internexin in human well-differentiated endocrine
neoplasms (carcinoid tumors) of the appendix. Mol Med Rep.
1:191–195. 2008.
|
|
15
|
Sugiyama M and Atomi Y: Extrapancreatic
neoplasms occur with unusual frequency in patients with intraductal
papillary mucinous tumors of the pancreas. Am J Gastroenterol.
94:470–473. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sugiyama M, Suzuki Y, Abe N, Mori T and
Atomi Y: Management of intraductal papillary mucinous neoplasm of
the pancreas. J Gastroenterol. 43:181–185. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ishida M, Egawa S, Kawaguchi K, et al:
Synchronous and metachronous extrapancreatic malignant neoplasms in
patients with intraductal papillary-mucinous neoplasm of the
pancreas. Pancreatology. 8:577–582. 2008. View Article : Google Scholar : PubMed/NCBI
|