Introduction
Pancreatic cancer, one of the most fatal types of
solid malignancy, is the fourth leading cause of cancer-related
mortality in the USA and other industrialized countries, leading to
an estimated 227,000 deaths per year worldwide (1,2).
Certain studies have demonstrated that the mortality rates of
pancreatic cancer in China have constantly increased over the past
decade. Presently, it is the eighth-leading cause of cancer-related
mortality in China (3). Due to
difficulties in early diagnosis and its highly aggressive malignant
behavior, only 10–20% of pancreatic cancers can be surgically
resected with curative intent at the time of diagnosis and the
majority of patients experience local recurrence and metastasis
(4). Despite therapeutic advances,
the prognosis of patients with pancreatic ductal adenocarcinoma is
extremely poor; the median survival time is 6 months and less than
5% survive 5 years following the initial diagnosis (1,5).
Gemcitabine is the current standard therapy for advanced pancreatic
cancer. However, gemcitabine treatment results in an objective
tumor response rate of less than 20%, and only a marginal survival
advantage is associated with multiple adverse events and drug
resistance (6,7). It is therefore of particular interest
to impose new therapeutic strategies and to improve the prognosis
of this potentially fatal disease.
The phosphoinositide 3-kinase (PI3K) pathway is a
key signal transduction system that links oncogenes and multiple
receptor classes to a number of essential cellular functions, and
is possibly the most commonly activated signaling pathway in human
cancer (8,9). The activation of the PI3K pathway is
relatively well understood; it is known to be a multi-step process
involving the PI3K-dependent phosphorylation of phospholipids
localized at the plasma membrane, and the subsequent membrane
localization of phosphoinositide-dependent kinase 1 (PDK1) and
Ser/Thr kinase Akt (also known as protein kinase B, PKB) via their
pleckstrin homology (PH) domains (9,10). The
activation of PI3K ultimately leads to Akt phosphorylation, and
activated Akt controls fundamental cellular processes including
cell survival by phosphorylating and inactivating several
downstream pro-apoptotic target molecules (9,11).
Another study has demonstrated that the PI3K/Akt pathway is
constitutively activated in a majority of human pancreatic cancer
cell lines (12). It has been
revealed that inhibition of the PI3K/Akt pathway results in
inhibition of tumor growth and apoptosis, and the PI3K/Akt pathway
is considered as a viable and effective target for pancreatic
cancer therapy (9,12,13).
Capsaicin is one of the major pungent ingredients
found in red peppers, which are among the most commonly and
frequently used spices in the world (14,15).
Due to its analgesic activity, topical application of capsaicin has
been used in clinical practice for the treatment of neuropathic
pain (16,17). Capsaicin has been revealed to
inhibit growth and induce apoptosis in various malignant cell lines
(18–21). Moreover, capsaicin treatment has
significantly suppressed the growth of tumors in athymic nude mice
transplanted with cancer cells (22,23).
Capsaicin was also revealed to inhibit the PI3K/Akt pathway in
B16-F10 melanoma cells (24).
However, data regarding the properties of capsaicin in pancreatic
cancer cells remain limited and the mechanisms of apoptosis have
not yet been fully elucidated. Based on these studies, we
hypothesized that capsaicin may exhibit an antitumor effect and
induce apoptosis in pancreatic cancer cells, via downregulation of
the PI3K/Akt pathway.
In the present study, we show that capsaicin
significantly inhibits the growth of PANC-1 cells and triggers
apoptosis in a dose-dependent manner. Treatment of PANC-1 cells
with capsaicin resulted in downregulation of phospho-PI3 Kinase p85
(Tyr458) and phospho-Akt (Ser473). Furthermore, capsaicin also
inhibited the growth of pancreatic cancer PANC-1 xenograft tumors
induced in athymic nude mice. These results suggest that capsaicin
may be an effective and promising antitumor agent against
pancreatic cancer.
Materials and methods
Reagents and antibodies
Capsaicin, dimethyl sulfoxide (DMSO), and propidium
iodide (PI) were obtained from Sigma-Aldrich (St. Louis, MO, USA).
RNase was obtained from Fermentas (St. Leon-Rot, Germany).
Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum
(FBS), penicillin-streptomycin and trypsin-EDTA were obtained from
Gibco BRL (Invitrogen, Grand Island, NY, USA). Rabbit cleaved
caspase-3 antibody, phospho-PI3 Kinase p85 (Tyr458) antibody,
phospho-Akt (Ser473) antibody and β-tubulin antibody were purchased
from Cell Signaling Technology, Inc. (Beverly, MA, USA).
Horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary
antibody was purchased from Beyotime Biotechnology (Haimen,
China).
Cell culture
The human pancreatic cancer cell line PANC-1 was
purchased from Shanghai Cell Bank (Shanghai, China). The cell line
was maintained in continuous exponential growth in DMEM
supplemented with 10% FBS, 100 U/ml penicillin and 100 μg/ml
streptomycin, at 37°C under a humidified 5% CO2
atmosphere.
Cell viability
PANC-1 cells were seeded at a density of
5×103/well in 96-well plates. Following incubation
overnight, the medium was removed and replaced with fresh medium
containing different concentrations of capsaicin (50, 100, 150,
200, 250 or 300 μM) or DMSO (control) for 24 h. On
completion of incubation, cell viability was determined using Cell
Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Kumamoto,
Japan) according to the manufacturer’s instructions. CCK-8 reagent
(10 μl) was added to 100 μl media in each well and
incubation was continued for a further 3 h. The absorbance (A) of
each well was read at 450 nm using an enzyme-linked immunosorbant
assay (ELISA) reader (Bio-Tek ELx808, Winooski, VT, USA).
Percentage suvival rate was calculated using the following
equation: Survival rate (%) =
(Asample−Ablank)/(Acontrol−Ablank).
Cell cycle analysis
PANC-1 cells were seeded at a density of
approximately 5×105 cells/well into 6-well plates,
cultured overnight and then 150, 200 or 250 μM capsaicin or
DMSO (control) was added. Following 24 h of incubation, cells were
harvested, washed with PBS and then fixed with 70% ethanol
overnight at 4°C. Cells were stained with 20 μg/ml RNase and
20 μg/ml PI for 30 min at 37°C in the dark, and then
analyzed by flow cytometry (Becton-Dickinson, San Jose, CA,
USA).
Apoptosis assay
The measurement of phosphatidylserine redistribution
in a plasma membrane was conducted according to the manufacturer’s
instructions for the Annexin V-FITC/PI Apoptosis Detection kit
(BioVision, Mountain View, CA, USA). Following 150, 200 or 250
μM capsaicin or DMSO (control) treatment, harvested cells
were suspended in 500 μl Annexin V binding buffer. Then, 5
μl Annexin V-FITC and 10 μl PI were added and
incubated with the cells for 5 min in the dark. The stained cells
were analyzed directly by flow cytometry using the Cell Quest
program (Becton-Dickinson).
Western blot analysis
PANC-1 cells were treated with 150, 200 or 250
μM capsaicin or DMSO (control). Following incubation, the
cells were lysed in Cell Lysis buffer (20 mM Tris-HCl pH 7.5, 150
mM NaCl, 1 mM Na2 EDTA, 1 mM EGTA, 1% Triton, 2.5 mM
sodium pyrophosphate, 1 mM beta-glycerophosphate, 1 mM
Na3VO4, 1 μg/ml leupeptin and 1 mM
PMSF; Cell Signaling Technology, Beverly, MA, USA) for 5 min on ice
and then subjected to sonication for 20 sec. Protein concentrations
were measured using the BCA Protein Assay kit (Pierce
Biotechnology, Inc., Rockford, IL, USA). Equal amounts of protein
were separated on 8 or 12% SDS-PAGE, and transferred onto a
polyvinylidene difluoride membrane. Next, the membrane was blocked
with 5% BSA and then incubated overnight with cleaved caspase-3
antibody, phospho-PI3 Kinase p85 (Tyr458) antibody or phospho-Akt
(Ser473) antibody. Following extensive washing, the membrane was
incubated with appropriate secondary antibodies conjugated with
horseradish peroxidase for 1 h at room temperature. Following
washing, immunoblots were developed using the Enhanced
Chemiluminescence kit (Pierce).
Real-time polymerase cahin reaction
(PCR)
PANC-1 cells were treated with 200 μM
capsaicin or DMSO (control) for 24 h and then total RNA was
isolated from treated cells using TRIzol reagent (Invitrogen).
Total RNA (1 μg) was reverse transcribed in 20 μl
volume, using RevertAid™ First Strand cDNA Synthesis kit
(Fermentas). Reverse transcriptase reaction mixture (1 μl)
was then real-time PCR-amplified in Mastercycler® ep
Realplex (Eppendorf, Germany). The initial denaturation step was
95°C for 60 sec, followed by 40 cycles of amplification at 95°C for
15 sec, 60°C for 15 sec and 72°C for 45 sec. The primers used were:
Caspase-3 forward 5′-CAGTGGAGGCCGACTTCTTG-3′ and reverse
5′-TGGCACAAAGCGACTGGAT-3′; RPLP0 forward
5′-GAGACAAAGTGGGAGCCAGCGA-3′ and reverse
5′-ACCCTCCAGGAAGCGAGAATGC-3′. All samples were performed in
triplicate and the relative quantity of the target gene was
normalized with the housekeeping gene RPLP0.
In vivo studies
BALB/c (nu/nu) four-week-old male mice were
purchased from Shanghai Laboratory Animals Center (Shanghai, China)
and maintained in specific pathogen-free conditions. All animal
studies were approved by the Animal Research and Ethical Committee
of Wenzhou Medical College (Zhejiang, China). Pancreatic cancer
xenograft tumor model was performed as described in our previous
studies (25,26). PANC-1 cells (5×106) in
200 μl complete culture medium were injected subcutaneously
into the right flank of each mouse. Four weeks after cell
inoculation, eight randomized animals for each experimental group
received capsaicin (5 mg/kg body weight in 100 μl of PBS
containing 0.3% ethanol) or vehicle (100 μl of PBS
containing 0.3% ethanol) by gavage three days a week (Monday,
Wednesday and Friday) for four weeks. One week after the last
treatment, the mice were sacrificed. The tumors were weighed with
an electronic balance and tumor volumes were calculated with a
vernier caliper using the following formula: (4π/3) ×
(width/2)2 × (length/2). Half of the tumor tissue in
each group was formalin-fixed and paraffin-embedded for TUNEL
assay. Remaining tumor tissue was stored in liquid nitrogen for
western blot analysis. Western blot analysis in tumor tissue was
performed as previously described in vitro.
In situ detection of apoptotic cells in
tumor tissues
Apoptotic cells in the tumor tissues were detected
by TUNEL assay, according to the manufacturer’s instructions for
the In Situ Cell Death Detection kit (Roche, Mannheim, Germany).
Sections were deparaffinized in xylene and then treated with a
graded series of alcohol (100, 95, 90, 80 and 70% ethanol in
double-distilled water) and rehydrated in PBS (pH 7.5). Tissues
were then treated with proteinase K solution for permeabilization
and treated with TUNEL reaction mixture, then incubated at 37°C for
1 h. Apoptotic cells were photographed under a fluorescence
microscope (Nikon, Tokyo, Japan).
Statistical analysis
Data are represented as mean ± standard deviation
for the absolute values or percentage of controls. SPSS13.0
software (SPSS, Inc., Chicago, IL, USA) was used for statistical
analysis. Differences between the capsaicin-treated and
DMSO-treated (control) groups were analyzed by an unpaired
Student’s t-test or ANOVA. P<0.05 was considered to indicate a
statistically significant difference.
Results
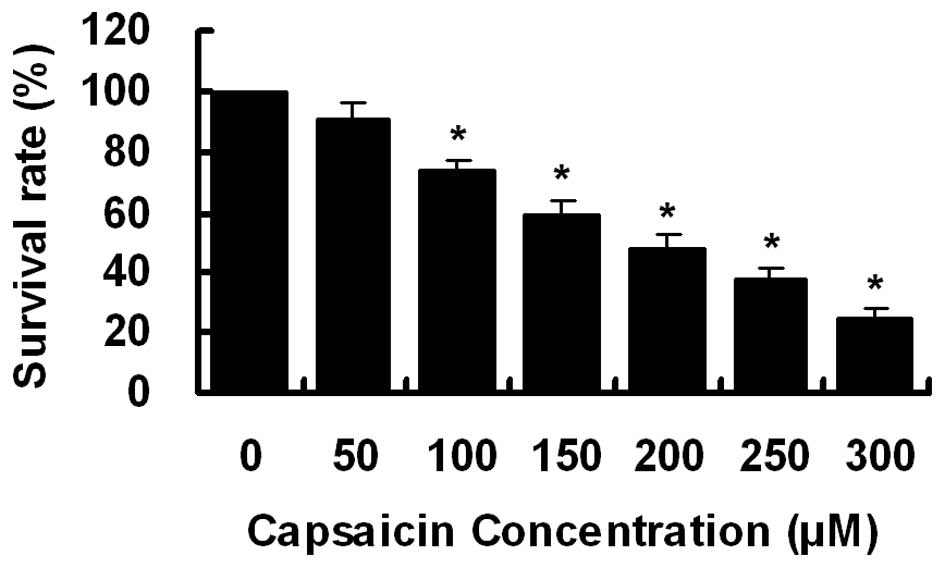
Effect of capsaicin on cell
proliferation
To investigate the effect of capsaicin on cell
growth, PANC-1 cells were treated with increasing concentrations of
capsaicin (0–300 μM) for 24 h. Cell viability was determined
by CCK-8 assay. As demonstrated in Fig.
1, cell growth was inhibited by capsaicin treatment in a
dose-dependent manner with an IC50 of ∼200
μM.
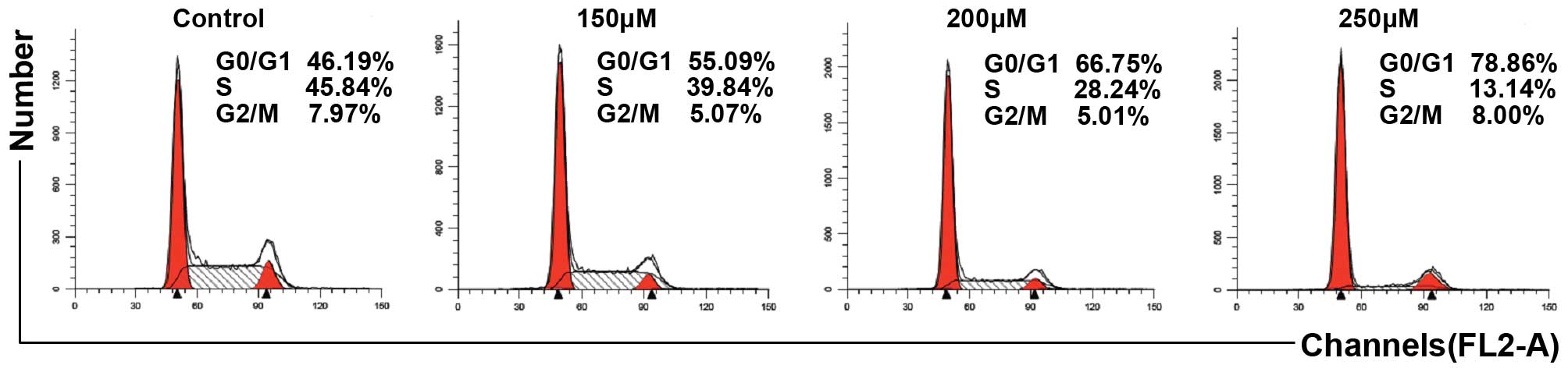
Capsaicin induces G0/G1 phase arrest in
PANC-1 cells
We investigated whether the antiproliferative
activity of capsaicin in PANC-1 cells was correlated with cell
cycle arrest. As demonstrated in Fig.
2, following capsaicin treatment, cell cycle analysis revealed
that capsaicin increased the number of cells in the G0/G1 phase in
a dose-dependent manner.
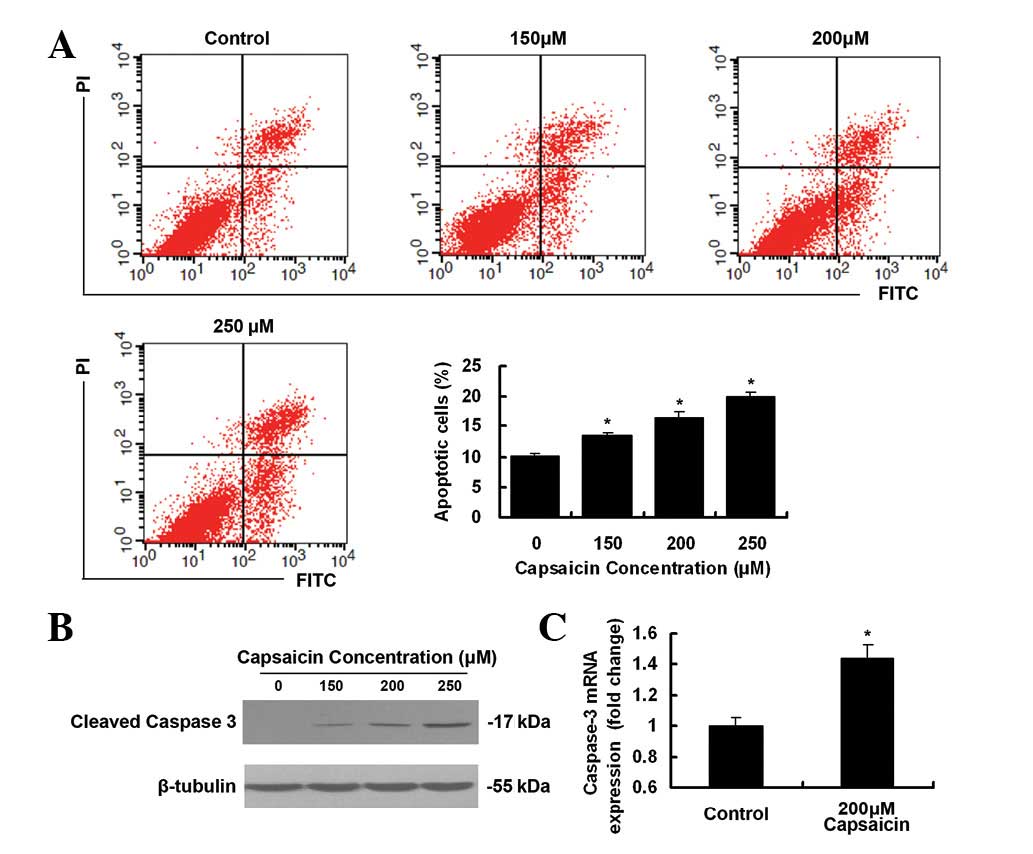
Capsaicin triggers apoptosis in PANC-1
cells
As demonstrated in Fig.
3A, treatment with capsaicin induced a greater level of
apoptosis in PANC-1 cells, as revealed by flow cytometric
assessment. Apoptotic rates in 250 μM capsaicin-treated
cells and control cells were 19.95±0.76% and 10.21±0.45%,
respectively. As demonstrated in Fig.
3B, a dose-dependent increase in the cleaved caspase-3 was
observed after the exposure of cells to increasing concentrations
of capsaicin. Compared with DMSO-treated cells, the level of
caspase-3 mRNA expression was higher (1.44-fold) following 200
μM capsaicin treatment (Fig.
3C).
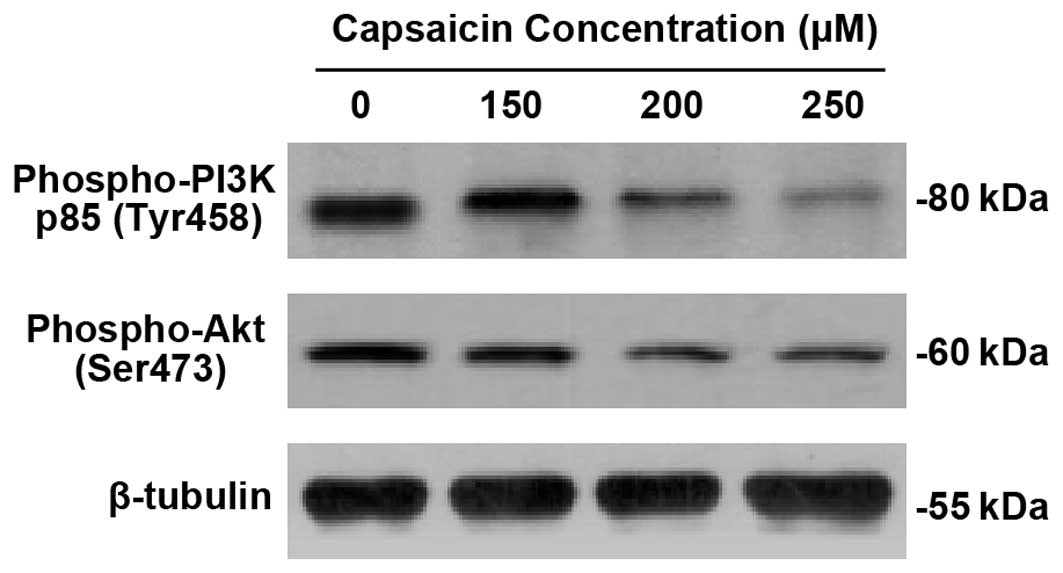
Downregulation of PI3K/Akt pathway by
capsaicin in PANC-1 cells
To elucidate the mechanism of antiproliferation and
apoptosis of capsaicin in PANC-1 cells, we employed western blot
analysis for phospho-PI3 Kinase p85 (Tyr458) and phospho-Akt
(Ser473). As demonstrated in Fig.
4, capsaicin significantly downregulated the expression of
phospho-PI3 Kinase p85 (Tyr458) and phospho-Akt (Ser473) in a
dose-dependent manner.
Antitumoral effect of capsaicin in
vivo
To investigate the antitumoral effect of capsaicin
on pancreatic cancer cells in vivo, we first generated
pancreatic cancer xenograft tumors in athymic nude mice. One week
after the last treatment, the mice were sacrificed and the tumors
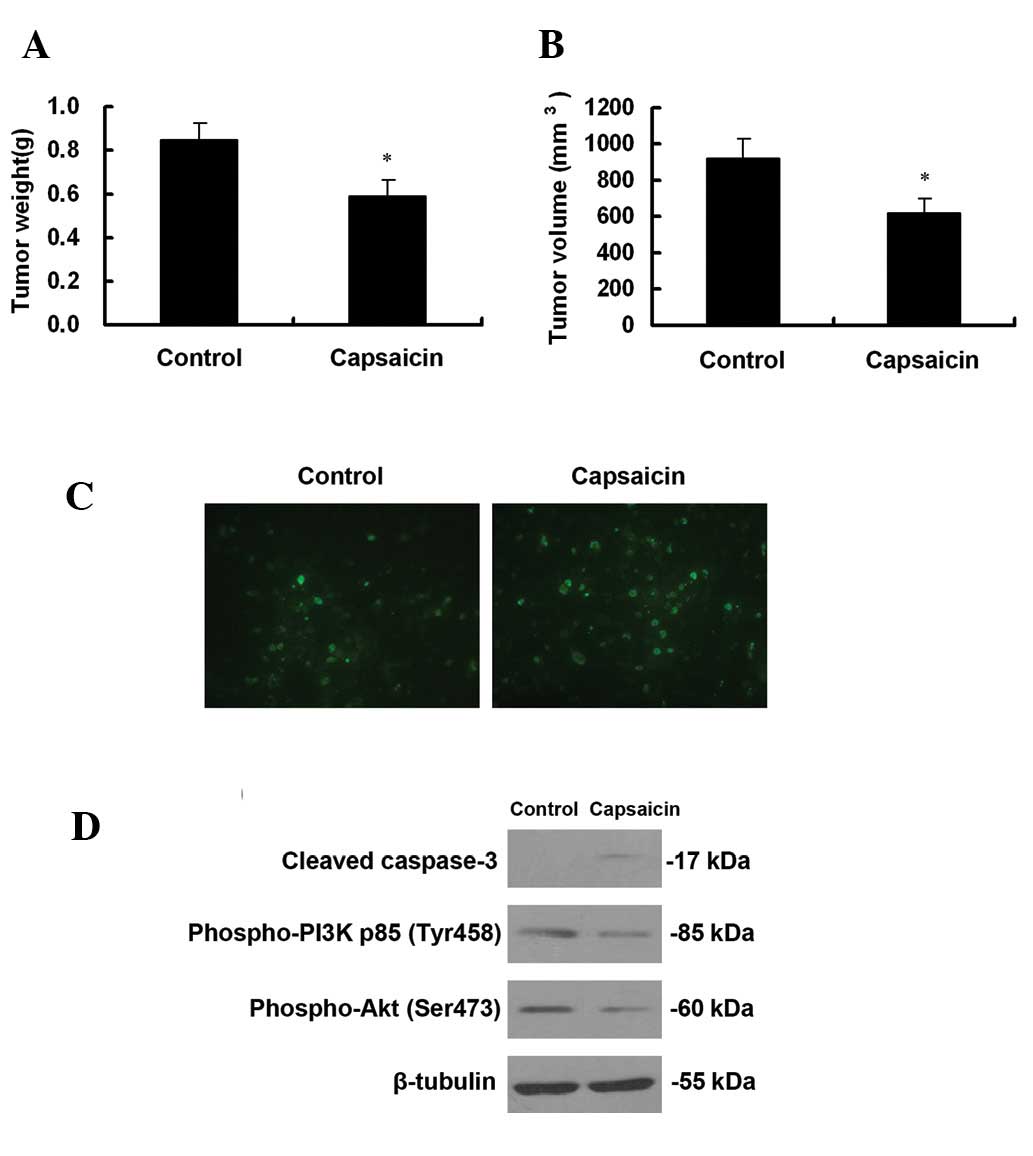
were weighed. Tumor volumes were also measured. As demonstrated in
Fig. 5A, the weights of tumors in
vehicle-treated mice were ∼1.43-fold greater than that of
capsaicin-treated mice. Tumor volumes in capsaicin-treated mice and
vehicle-treated mice were 617.25±85.07 mm3 and
921.16±110.29 mm3, respectively (Fig. 5B). As demonstrated in Fig. 5C, increased TUNEL-positive cells
were observed in capsaicin-treated mice. Consistent with our
studies in vitro, cleaved caspase-3 was observed in
capsaicin-treated mice and capsaicin downregulated the expression
of phospho-PI3 Kinase p85 (Tyr458) and phospho-Akt (Ser473)
(Fig. 5D).
Discussion
Epidemiologic studies have revealed that several
dietary agents modulate diverse biochemical processes involved in
carcinogenesis (27). These include
inhibition of carcinogen activation, cellular proliferation and
tumor metastasis, blockading tumor cell cycle progression and
induction of apoptosis (27). In
vitro and in vivo studies have shown that dietary
chemopreventive agents may serve as potent agents for enhancing the
therapeutic effects of chemotherapy, radiotherapy or other standard
therapeutics for the treatment of human cancers (28). Capsaicin is the principal pungent
ingredient present in red peppers, which are among the most
frequently consumed spices worldwide (14,15).
Capsaicin has been revealed to possess inhibitory effects in
various cancer cells (18–20,22,24).
However, the precise molecular mechanisms have not been well
elucidated in pancreatic cancer cells. In this study, we first used
a CCK-8 assay to detect cell viability following capsaicin
treatment. The results revealed that capsaicin significantly
inhibited PANC-1 cell proliferation in a dose-dependent manner.
Furthermore, capsaicin gavage significantly inhibited the growth of
pancreatic cancer PANC-1 cell xenografts in athymic nude mice.
Next, we investigated whether the antiproliferative
activity of capsaicin in PANC-1 cells was due to cell cycle arrest
and apoptosis. In the present study, capsaicin treatment in PANC-1
cells induced G0/G1 phase arrest and apoptosis in a dose-dependent
manner. The results of the TUNEL assay revealed that increased
numbers of apoptotic cells were observed in capsaicin-treated mice.
Previously, accumulated evidence indicated that caspases, a family
of cysteine proteases, play a pivotal role in the apoptotic
process; caspase-3 is an apoptosis executioner and is activated by
other activated caspases, including caspase-8 and 9 (29,30).
Activated caspase-3 subsequently cleaves certain specific
substrates, including poly (ADP-ribosyl) polymerase (PARP) and
D4-GDI proteins, which are important for the occurrence of typical
biochemical and morphological changes in apoptotic cells (29,31).
To further confirm that the antiproliferative activity of capsaicin
was due to apoptosis, we examined caspase-3 activation, an event
that is commonly used as a hallmark of apoptosis. In this study,
caspase-3 was activated after capsaicin treatment in vitro
and in vivo. These results suggest that apoptosis may be a
potential general mechanism and provide a mechanistic basis for the
antiproliferative as well as anti-neoplastic effects of capsaicin
in PANC-1 cells.
To further elucidate the mechanism of
antiproliferation and apoptosis of capsaicin in PANC-1 cells, we
investigated the PI3K/Akt pathway. It has been demonstrated that
the PI3K/Akt signaling pathway components are frequently altered in
human cancers and inappropriately activated (9,11).
PI3Ks are divided into three classes according to their structural
characteristics and substrate specificity. Of these, the most
commonly studied are the class I enzymes that are activated
directly by cell surface receptors. Class I PI3Ks are further
divided into class IA enzymes, which are activated by RTKs, GPCRs
and certain oncogenes including the small G protein RAS, and class
IB enzymes, which are regulated exclusively by GPCRs. Class IA
PI3Ks are heterodimers consisting of a p110 catalytic subunit and a
p85 regulatory subunit (8,9). In the present study, the
phosphorylation level of PI3K at Tyr458 of the p85 regulatory
subunit was significantly reduced in capsaicin-treated cells as
compared with the control experiment. Akt, a serinethreonine kinase
that is directly activated in response to PI3Ks, is a major
effector of PI3Ks in cancer (9). In
this study, the phosphorylation level of Akt at Ser473 (one of the
two target amino acids whose phosphorylation upregulates Akt kinase
activity) was significantly downregulated in response to capsaicin
treatment. Furthermore, downregulated expression of phospho-PI3
Kinase p85 (Tyr458) and phospho-Akt (Ser473) were observed in
capsaicin-treated mice. These results suggest that downregulation
of the PI3K/Akt signaling pathway may be involved in
capsaicin-induced apoptosis in PANC-1 cells.
Activated Akt phosphorylates several cellular
proteins, including glycogen synthase kinase-3α (GSK-3α), GSK-3β,
forkhead box O transcription factors (FoxO), murine double minute 2
(MDM2), B-cell lymphoma-2 (BCL2)-interacting mediator of cell death
(BIM) and BCL2-associated agonist of cell death (BAD), to
facilitate cell survival and cell cycle entry (8,9).
However, it remains unknown how activation of the PI3K/Akt
signaling pathway promotes cell survival and suppresses apoptosis.
It has been demonstrated that inhibition of GSK3β, one of the
effectors downstream of Akt, leads to G0/G1 phase arrest (32,33).
In this study, capsaicin treatment induced G0/G1 phase arrest and
downregulation of the PI3K/Akt pathway in PANC-1 cells. Therefore,
downregulation of the PI3K/Akt/GSK3β pathway may be involved in
capsaicin-induced G0/G1 phase arrest and apoptosis, which indeed
needs further investigation.
Together, our studies suggest that capsaicin-induced
apoptosis may correlate with downregulation of the PI3K/Akt
pathway. Thus, the present study provides novel insights into the
molecular mechanisms of capsaicin in panreatic cancer cells. These
findings strengthen the idea that capsaicin may be used as an
anti-neoplastic medicine and that the PI3K/Akt pathway is a
promising target for therapeutic intervention in pancreatic
cancer.
Acknowledgements
The authors are grateful for funding
support from the Administration of Traditional Chinese Medicine of
Zhengjing Province, China (Grant No. 2011ZZ010), Zhejiang
Provincial Science Fund for Distinguished Young Scholars (Grant No.
LR12H280001) and The National Natural Science Foundation of China
(Grant No. 81173606).
References
|
1
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar
|
|
2
|
Raimondi S, Maisonneuve P and Lowenfels
AB: Epidemiology of pancreatic cancer: an overview. Nat Rev
Gastroenterol Hepatol. 6:699–708. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang L, Yang GH, Lu XH, Huang ZJ and Li H:
Pancreatic cancer mortality in China (1991–2000). World J
Gastroenterol. 9:1819–1823. 2003.
|
|
4
|
Li D, Xie K, Wolff R and Abbruzzese JL:
Pancreatic cancer. Lancet. 363:1049–1057. 2004. View Article : Google Scholar
|
|
5
|
Saif MW: Pancreatic neoplasm in 2011: an
update. JOP. 12:316–321. 2011.PubMed/NCBI
|
|
6
|
Stathis A and Moore MJ: Advanced
pancreatic carcinoma: current treatment and future challenges. Nat
Rev Clin Oncol. 7:163–172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arends JJ, Sleeboom HP, Leys MB, et al: A
phase II study of raltitrexed and gemcitabine in patients with
advanced pancreatic carcinoma. Br J Cancer. 92:445–448.
2005.PubMed/NCBI
|
|
8
|
Liu P, Cheng H, Roberts TM and Zhao JJ:
Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev
Drug Discov. 8:627–644. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Engelman JA: Targeting PI3K signalling in
cancer: opportunities, challenges and limitations. Nat Rev Cancer.
9:550–562. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Datta SR, Dudek H, Tao X, Masters S, Fu H,
Gotoh Y and Greenberg ME: Akt phosphorylation of BAD couples
survival signals to the cell-intrinsic death machinery. Cell.
91:231–241. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and Gonzalez-Baron M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004.PubMed/NCBI
|
|
12
|
Bondar VM, Sweeney-Gotsch B, Andreeff M,
Mills GB and McConkey DJ: Inhibition of the phosphatidylinositol
3′-kinase-AKT pathway induces apoptosis in pancreatic carcinoma
cells in vitro and in vivo. Mol Cancer Ther.
1:989–997. 2002.
|
|
13
|
Roy SK, Srivastava RK and Shankar S:
Inhibition of PI3K/AKT and MAPK/ERK pathways causes activation of
FOXO transcription factor, leading to cell cycle arrest and
apoptosis in pancreatic cancer. J Mol Signal. 5:102010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Surh YJ: More than spice: capsaicin in hot
chili peppers makes tumor cells commit suicide. J Natl Cancer Inst.
94:1263–1265. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Surh YJ, Lee E and Lee JM: Chemoprotective
properties of some pungent ingredients present in red pepper and
ginger. Mutat Res. 402:259–267. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Caterina MJ, Schumacher MA, Tominaga M,
Rosen TA, Levine JD and Julius D: The capsaicin receptor: a
heat-activated ion channel in the pain pathway. Nature.
389:816–824. 1997. View
Article : Google Scholar
|
|
17
|
Hartel M, di Mola FF, Selvaggi F, et al:
Vanilloids in pancreatic cancer: potential for chemotherapy and
pain management. Gut. 55:519–528. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pramanik KC, Boreddy SR and Srivastava SK:
Role of mitochondrial electron transport chain complexes in
capsaicin mediated oxidative stress leading to apoptosis in
pancreatic cancer cells. PLoS One. 6:e201512011. View Article : Google Scholar
|
|
19
|
Amantini C, Ballarini P, Caprodossi S, et
al: Triggering of transient receptor potential vanilloid type 1
(TRPV1) by capsaicin induces Fas/CD95-mediated apoptosis of
urothelial cancer cells in an ATM-dependent manner. Carcinogenesis.
30:1320–1329. 2009. View Article : Google Scholar
|
|
20
|
Kim JY, Kim EH, Kim SU, Kwon TK and Choi
KS: Capsaicin sensitizes malignant glioma cells to TRAIL-mediated
apoptosis via DR5 upregulation and survivin downregulation.
Carcinogenesis. 31:367–375. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ito K, Nakazato T, Yamato K, et al:
Induction of apoptosis in leukemic cells by homovanillic acid
derivative, capsaicin, through oxidative stress: implication of
phosphorylation of p53 at Ser-15 residue by reactive oxygen
species. Cancer Res. 64:1071–1078. 2004. View Article : Google Scholar
|
|
22
|
Sanchez AM, Sanchez MG, Malagarie-Cazenave
S, Olea N and Diaz-Laviada I: Induction of apoptosis in prostate
tumor PC-3 cells and inhibition of xenograft prostate tumor growth
by the vanilloid capsaicin. Apoptosis. 11:89–99. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang R, Humphreys I, Sahu RP, Shi Y and
Srivastava SK: In vitro and in vivo induction of
apoptosis by capsaicin in pancreatic cancer cells is mediated
through ROS generation and mitochondrial death pathway. Apoptosis.
13:1465–1478. 2008. View Article : Google Scholar
|
|
24
|
Shin DH, Kim OH, Jun HS and Kang MK:
Inhibitory effect of capsaicin on B16-F10 melanoma cell migration
via the phosphatidylinositol 3-kinase/Akt/Rac1 signal pathway. Exp
Mol Med. 40:486–494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wei WT, Chen H, Wang ZH, et al: Enhanced
antitumor efficacy of gemcitabine by evodiamine on pancreatic
cancer via regulating PI3K/Akt pathway. Int J Biol Sci. 8:1–14.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei WT, Chen H, Ni ZL, et al: Antitumor
and apoptosis-promoting properties of emodin, an anthraquinone
derivative from Rheum officinale Baill, against pancreatic cancer
in mice via inhibition of Akt activation. Int J Oncol.
39:1381–1390. 2011.
|
|
27
|
Shanmugam MK, Kannaiyan R and Sethi G:
Targeting cell signaling and apoptotic pathways by dietary agents:
role in the prevention and treatment of cancer. Nutr Cancer.
63:161–173. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sarkar FH and Li Y: Using chemopreventive
agents to enhance the efficacy of cancer therapy. Cancer Res.
66:3347–3350. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Degterev A, Boyce M and Yuan J: A decade
of caspases. Oncogene. 22:8543–8567. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hamacher R, Schmid RM, Saur D and
Schneider G: Apoptotic pathways in pancreatic ductal
adenocarcinoma. Mol Cancer. 7:642008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kasibhatla S and Tseng B: Why target
apoptosis in cancer treatment? Mol Cancer Ther. 2:573–580.
2003.PubMed/NCBI
|
|
32
|
Hashimoto T, He Z, Ma WY, Schmid PC, Bode
AM, Yang CS and Dong Z: Caffeine inhibits cell proliferation by
G0/G1 phase arrest in JB6 cells. Cancer Res. 64:3344–3349. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liang J and Slingerland JM: Multiple roles
of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell
Cycle. 2:339–345. 2003. View Article : Google Scholar : PubMed/NCBI
|