Introduction
The National Cancer Institute of the USA estimates
that, based on current rates, 12.7% of females born today will be
diagnosed with breast cancer in their lifetime (1). Although primary breast cancer is the
most common malignancy of adult females, metastatic involvement of
the breast is rare, with a reported frequency of 0.4–1.3% in
clinical series (2–5). Despite its rarity, metastatic breast
disease is an significant diagnostic clinical problem, as its
treatment differs greatly from that of primary breast cancer. In
1907, Sitzentfrey was the first to publish a case of ovarian
carcinoma metastatic to the breast (6). To date, a wide variety of malignancies
have now been reported to metastasize to the breast and according
to the literature, the most common primary tumors are melanomas and
haematological malignancies (5–7). The
lung is the most common cancer site in terms of incidence and
mortality; however, there have only been a few published cases of
pulmonary carcinomas metastasizing to the breast (8–12).
Carcinomas with micropapillary components have been reported at
several anatomical sites, including the breast, urinary bladder,
ovary and major salivary glands (13). Micropapillary components are being
increasingly recognized as prognostic predictors in lung
adenocarcinomas and according to many studies, this may be a
manifestation of aggressive behaviour (14,15).
We report the case of a patient with breast metastasis from a
pulmonary adenocarcinoma characterized by a micropapillary pattern
and diagnosed in conjunction with the primary tumor. Written
informed consent was obtained from the patient for publication of
this case report and accompanying images.
Case report
A 43-year-old, non-smoking housewife presented to
the Emergency Department of St. Maria Hospital, Terni, Italy with
dyspnea and a dry cough of 3 weeks’ duration. A chest examination
revealed reduced breath sounds and a percussive dullness in the
left hemithorax. Physical examination revealed a painless, poorly
defined mass, with associated skin redness, in the upper outer
quadrant of the right breast. Palpable right axillary lymph nodes
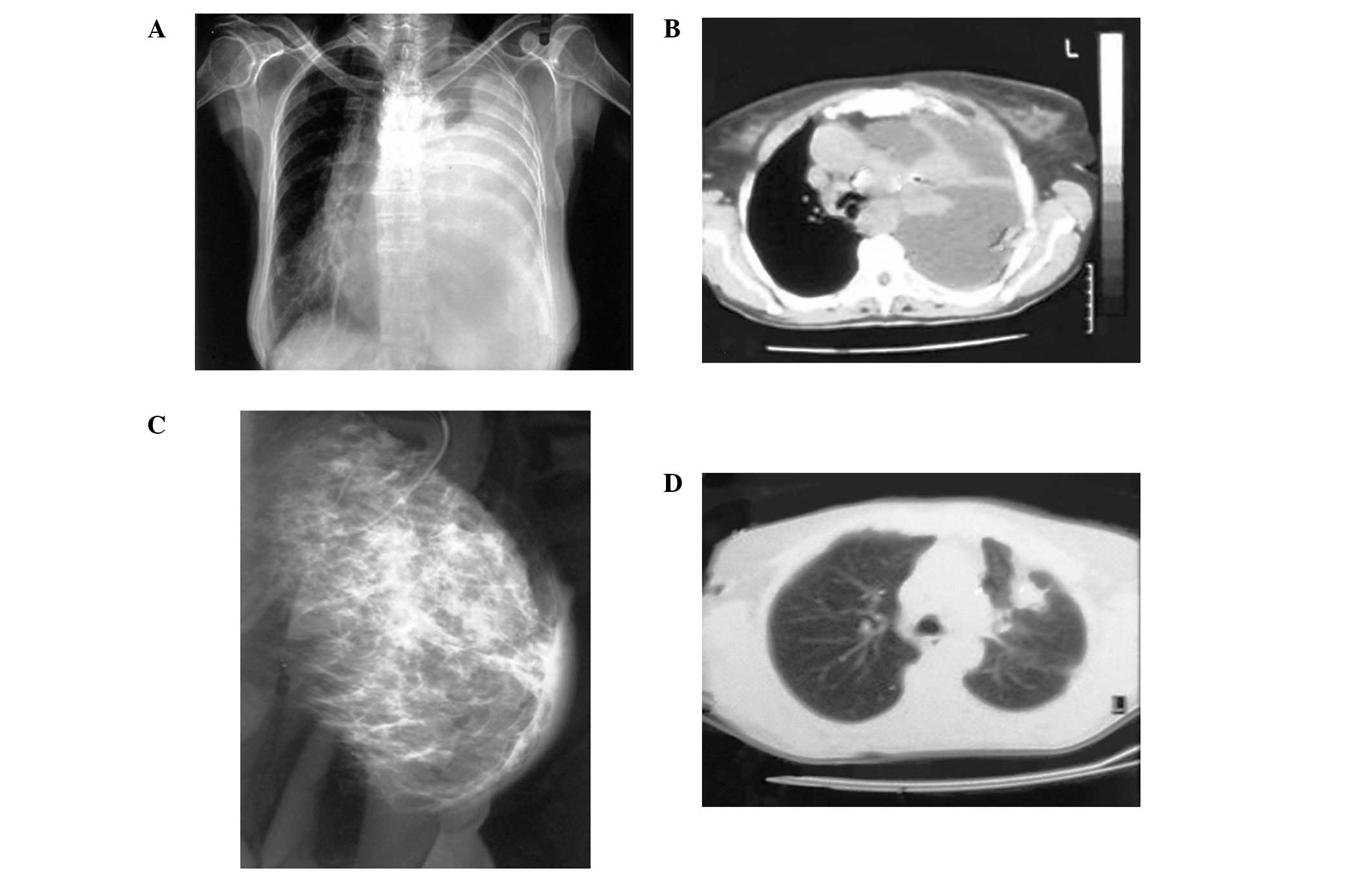
were also noted. A chest radiogram revealed a massive pleural
effusion occupying the majority of the left hemithorax (Fig. 1A). Chest computed tomography (CT)
(Fig. 1B) revealed the left lung to
be atelectasic and compressed by a massive pleural effusion. The
mediastinum and trachea were severely displaced to the right. A few
lymph nodes were identified deep in the left axilla and a number of
paratracheal lymph nodes were also observed. The clinical diagnosis
was considered to be either a primary breast tumor with lung and
pleural metastasis or two synchronous primary tumors. Mammography
revealed a diffuse asymmetrical density in the sub alveolar region
and the upper outer quadrant of the left breast (Fig. 1C). Additionally, skin thickening was
observed in the affected area. Calcifications were not observed.
The differential diagnosis included inflammation, lymphoma and
inflammatory breast carcinoma. The patient underwent bronchoscopy
which revealed submucosal infiltration causing widening of the
secondary carina and obstruction of the orifice of the lingula of
approximately 70%. Pleural effusion re-accumulated rapidly;
therefore, in order to perform pleural drainage and chemical
pleurodesis, medical thoracoscopy was carried out. During the
procedure, biopsies were obtained from the parietal pleura. A new
chest CT (Fig. 1D) followed and
revealed a 3.5×4.5 cm peripheral lesion on the left upper lobe,
with relatively abnormal contours and extension into the
surrounding parenchyma. The tumor was in contact with the
splanchnic pleura and approached the parietal pleura. A right
simple mastectomy was performed in order to remove the rapidly
growing breast lesion. Our patient received 4 courses of
bevacizumab, cisplatin and docetaxel with no clinical response. The
patient succumbed to her condition 8 months after the
diagnosis.
Cytological and immunocytochemical
findings
All cytological specimens were stained by the
Papanicolaou technique and evaluated by cytology. Following
examination of the pleural effusion, bronchial washing and
bronchial brush specimens, a diagnosis of adenocarcinoma was made.
Immunocytochemistry performed on the smears prepared from the
pleural effusion sample revealed the tumor cells to be strongly
immunoreactive for thyroid transcription factor-1 (TTF-1) and
monoclonal carcinoembryonic antigen (CEA). Tumor cells were
negative for cytokeratin (CK) 5/6, estrogen receptors (ER), cancer
antigen (CA)-125 and thyroglobulin.
Histopathological and immunohistochemical
findings
Hematoxylin and eosin (H&E)-stained paraffin
sections of the bronchoscopy biopsy revealed bronchial mucosal
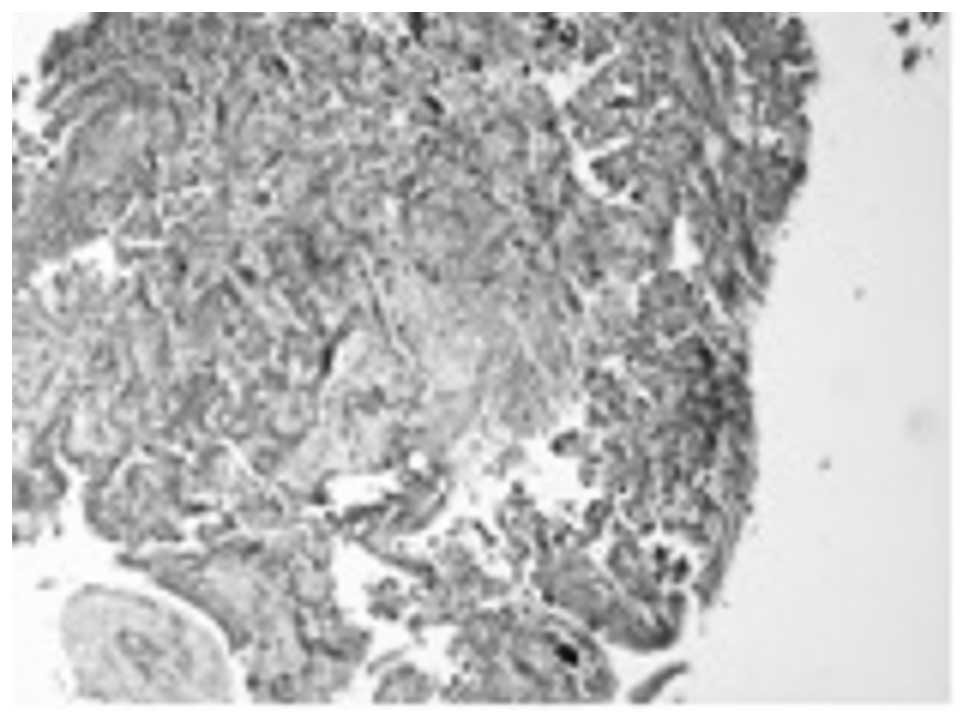
infiltration by a low differentiated adenocarcinoma. An extensive
micropapillary component was identified (Fig. 2). This was observed as papillary
structures with tufts that lacked a central fibrovascular core. In
addition, occasional psammoma bodies were noted. Our differential
diagnosis included primary lung adenocarcinoma, metastatic
adenocarcinoma from the thyroid, breast or ovary and finally
metastatic epithelioid (papillary) type-mesothelioma. The tumor
cells demonstrated immunoreactivity for CD 15 (Leu-M1), TTF-1,
surfactant protein A (SP-A) and monoclonal CEA. The neoplastic
cells lacked expression of gross cystic disease fluid protein-15
(GCDFP-15), ER, mammaglobin, CK 5/6, calretinin, CA-125 and
thyroglobulin. Based on the histology and the immunohistochemical
staining patterns, a diagnosis of primary lung adenocarcinoma with
a micropapillary component was made. H&E-stained paraffin
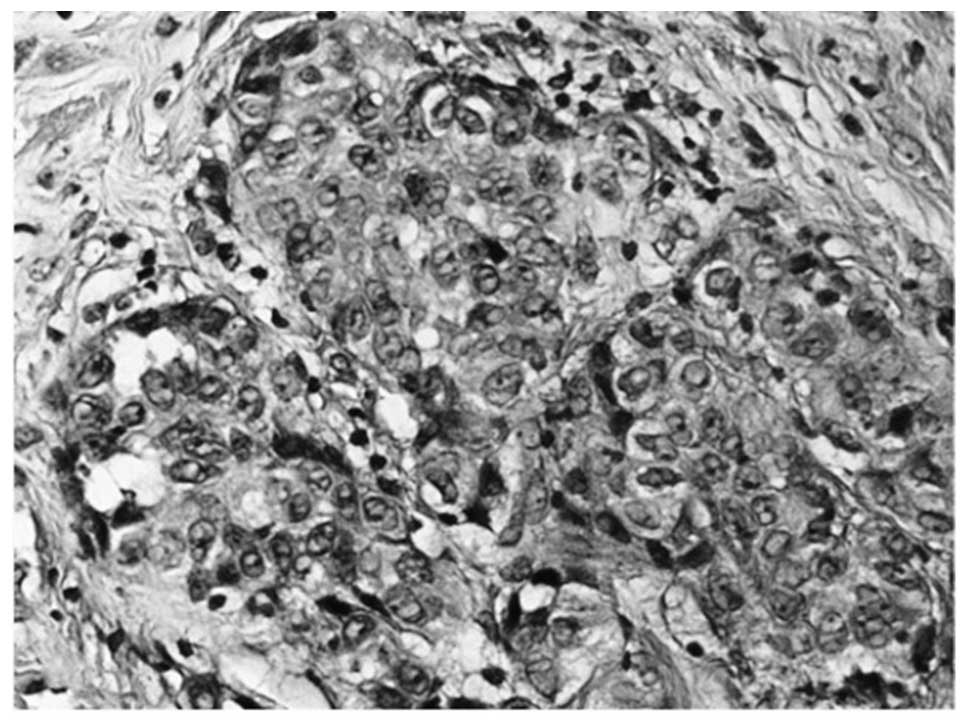
sections of the parietal pleura biopsies revealed diffuse
infiltration by malignant epithelioid type cells, which
demonstrated solid and micropapillary patterns. Additionally,
numerous psammoma bodies were observed (Fig. 3). The tumor cells revealed the same
immunoprofile as the lung biopsy. Finally, the breast biopsy
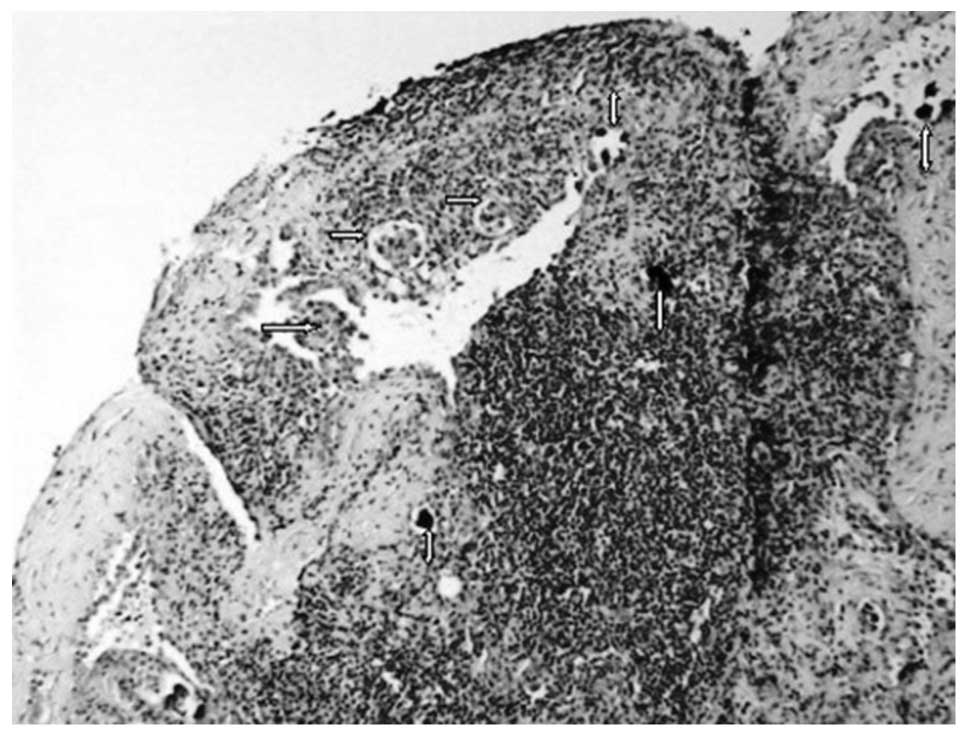
specimen revealed dense fibro-hyalinized stroma with atrophic
terminal ductal lobular units. Within the stroma, sharply
demarcated nodules of a high-grade adenocarcinoma with a solid and
micropapillary pattern were noted (Fig.
4). Lymphatic tumor emboli of micropapillary pattern
adenocarcinoma with multiple psammoma bodies were also identified.
The surrounding breast parenchyma demonstrated mild fibrocystic
changes. Finally, no evidence of in situ carcinoma or
elastosis was observed. Taking into account the diagnosis of the
lung and pleura biopsies, our differential diagnosis included a
second primary breast carcinoma and metastatic lung carcinoma. The
tumor cells demonstrated immunoreactivity for TTF-1 and SP-A, and
lacked expression of GCDFP-15, ER and mammaglobin.
Discussion
Worldwide, lung cancer is the most common cancer in
terms of both incidence and mortality (1.6 million new cases per
year and 1.378 million deaths) (8).
Approximately one fifth of newly diagnosed lung adenocarcinomas
present with distant metastases. The most common sites of
metastasis are the brain, bone, liver and adrenal glands, in
decreasing order. However, an autopsy series has demonstrated that
non-small cell lung cancer (NSCLC) may spread to virtually any
organ (16). Breast metastases from
extra-mammary malignancies are rare, accounting for 0.4 to 1.3% of
all breast malignancies (2–5). Approximately 700 cases have been
reported in small series and case reports (2–5,12,17,18).
According to the international literature, the most common sources
of primary tumors are hematological malignancies, malignant
melanoma, lung tumors, renal cell carcinoma, ovarian tumors,
thyroid carcinomas and small bowel carcinoids (3,7,18).
Williams et al published the largest series, which included
169 cases of metastases to the breast from extra mammary solid
tumors, and reported that the most common histological type was
malignant melanoma (7). A review of
the literature (1990–2010) revealed approximately 30 NSCLC case
reports or studies as part of a series of secondary breast tumors
(4,5,9–12,19–27).
Twelve of these cases were classified as adenocarcinomas (5,9,12,19,21–23,25).
Additionally, 53 cases of breast metastasis from lung tumors were
presented; however, no detailed histological classification was
provided (7,18,28–30).
The majority of breast metastases present as palpable, rapidly
growing, well-circumscribed and painless breast masses with
predilection to the upper outer quadrant (2,7,17,21,22).
Unlike primary tumors, the vast majority of metastases do not
demonstrate retraction of the skin or nipple, despite their
superficial location (5,22). However, in our patient, the lesion
was poorly defined and skin redness was observed. Similar findings
from other authors are rare (7,17,25,30).
Distinguishing a breast metastasis from a primary mammary
adenocarcinoma, based on mammographic findings, may be extremely
difficult due to the wide range of imaging manifestations of the
metastatic lesions (4,5,18).
Thus, metastasis can mimic a primary malignancy or even a benign
breast tumor (4,5,18). The
most commonly described mammographic presentation is usually single
but may sometimes present as multiple well-circumscribed lesions
with smooth margins (3,18,30).
Microcalcifications are very uncommon but have been reported in
patients with metastatic serous ovarian papillary carcinoma
(18,29,30).
In the present case, mammography revealed diffuse asymmetrical
density and skin thickening. In similar cases, the differential
diagnosis includes inflammation, lymphoma and inflammatory breast
carcinoma. As cited in the literature, histological features that
may aid in the recognition of secondary tumors include the absence
of in situ carcinoma, which strongly supports a metastatic
tumor, although this may not be present in all primary invasive
carcinomas. Additionally, metastatic malignancies are often sharply
circumscribed from the surrounding breast tissue. Furthermore,
elastosis is common in primary tumors but rare in extra mammary
malignancies (2,4,5,12,19,21).
Occasionally, metastases to the breast demonstrate features that
lead pathologists to the correct diagnosis, such as the presence of
pigmentation and intranuclear inclusions in malignant melanomas.
Nevertheless, many extra mammary malignancies such as
adenocarcinoma of the lung lack specific histological features.
Carcinomas with a micropapillary component have been described in
many organs including the breast, urinary bladder, ovary and
salivary glands (13). In 2002,
Amin et al were the first to report lung adenocarcinomas
with micropapillary components (14). Histologically, the latter is
characterized by small papillary tufts lying freely within alveolar
spaces or encased within the thin walls of connective tissue. These
small, cohesive nests lack fibrovascular connective tissue cores
(14). In the present case, all
biopsies examined demonstrated an extensive micropapillary
component. Although psammoma bodies have not been observed in
invasive micropapillary pattern carcinoma of the urinary bladder
and salivary glands, they have occasionally been reported in cases
of lung adenocarcinoma with micropapillary morphology (13,14,30,31).
Multiple psammoma bodies were demonstrated in the tissue sections
of the samples examined. To the best of our knowledge, this is the
first report of breast metastasis from lung adenocarcinoma with a
micropapillary pattern, diagnosed concomitantly with the primary
tumor. The distinction between metastasis from lung adenocarcinoma,
particularly with an extensive micropapillary pattern, and primary
mammary adenocarcinoma may cause a significant diagnostic dilemma.
The contribution of immunohistochemistry to the correct diagnosis
is crucial. TTF-1 is expressed in 68–80% of lung adenocarcinomas,
and with the exception of a single case published by Klingen et
al(32), TTF-1 has not been
reported to stain positively in breast adenocarcinoma (32–34).
The sensitivity of SP-A is substantially less. It is expressed in
approximately 45% of lung adenocarcinomas (33,34). A
negative expression of thyroglobulin excludes the diagnosis of
papillary carcinoma of the thyroid, which stains positively for
both markers. ERs are expressed in 80% and GCDFP-15 in 45–53% of
breast carcinomas (33,35). Recent studies have revealed that ER
expression in lung adenocarcinoma is low (7.6–14.1%) by using the
monoclonal antibodies 1D5 and 6F11 (33,36).
Additionally, 5.2–15% of lung adenocarcinomas express GCDFP-15
(35,37). Finally, mammaglobin is expressed in
48–72.1% of mammary adenocarcinomas but stains negatively in
pulmonary adenocarcinomas (33,35,38).
Consequently, a panel of markers must be used as no single antibody
is 100% sensitive and false negative results do occur. In our case,
all the tumor specimens (lung, pleura and breast) showed positive
nuclear staining for TTF-1 and cytoplasmic staining for SP-A. The
neoplastic cells lacked expression of GCDFP-15, ER and mammaglobin.
Overall, metastasis to the breast has been associated with poor
prognosis with the majority of patients succumbing to the disease
within a year of diagnosis (7). Our
patient survived for 6 months following the diagnosis of both the
primary lung tumor and the breast metastasis.
Here, we reported a rare case of metastasis to the
breast from an adenocarcinoma of the lung with an extensive
micropapillary component. Metastatic disease to the breast,
although rare, should be considered in the differential diagnosis
of a primary mammary carcinoma as the treatment and prognosis
differ significantly. Furthermore, the distinction between
metastasis from lung adenocarcinoma, particularly with an extensive
micropapillary pattern, and primary breast adenocarcinoma may cause
a significant diagnostic dilemma. The contribution of
immunohistochemistry to the correct diagnosis is essential.
References
|
1
|
National Cancer Institute: Probability of
breast cancer in American women. http://www.cancer.gov/cancertopics/factsheet/detection/probability-breastcancer.
Accessed March 9, 2012.
|
|
2
|
Hajdu SI and Urban JA: Cancers metastatic
to the breast. Cancer. 29:1691–1696. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vizcaino I, Torregrosa A, Higueras V, et
al: Metastasis to the breast from extramammary malignancies: a
report of four cases and a review of literature. Eur Radiol.
11:1659–1665. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Georgiannos SN, Chin J, Goode AW and
Sheaff M: Secondary neoplasms of the breast: a survey of the 20th
Century. Cancer. 92:2259–2266. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Klingen TA, Klaasen H, Aas H, Chen Y and
Akslen LA: Secondary breast cancer: a 5-year population-based study
with review of the literature. APMIS. 117:762–767. 2009.PubMed/NCBI
|
|
6
|
Sitzenfrey A: Mammakarzinom zwei jahre
nach abdominaler radikal operation wegen doppelseitigen carcinoma
ovarii. Prag Med Wochenschr. 221–235. 1907.(In German).
|
|
7
|
Williams SA, Ehlers RA II, Hunt KK, et al:
Metastases to the breast from nonbreast solid neoplasms:
presentation and determinants of survival. Cancer. 110:731–737.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: GLOBOCAN 2008 v12, Cancer Incidence and
Mortality Worldwide: IARC CancerBase No 10 [Internet]. Lyon,
France: International Agency for Research on Cancer; 2010,
http://globocan.iarc.fr.
Accessed March 9, 2012.
|
|
9
|
Masmoudi A, Mathieu MC and Soria JC:
Breast metastasis from lung adenocarcinoma: a case report.
Anticancer Res. 23:1825–1826. 2003.PubMed/NCBI
|
|
10
|
Ramar K, Pervez H, Potti A and Mehdi S:
Breast metastasis from non-small-cell lung carcinoma. Med Oncol.
20:181–184. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gomez-Caro A, Pinero A, Roca MJ, et al:
Surgical treatment of solitary metastasis in the male breast from
non-small cell lung cancer. Breast J. 12:366–367. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee AH: The histological diagnosis of
metastases to the breast from extramammary malignancies. J Clin
Pathol. 60:1333–1341. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nassar H: Carcinomas with micropapillary
morphology: clinical significance and current concepts. Adv Anat
Pathol. 11:297–303. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Amin MB, Tamboli P, Merchant SH, et al:
Micropapillary component in lung adenocarcinoma: a distinctive
histologic feature with possible prognostic significance. Am J Surg
Pathol. 26:358–364. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maeda R, Isowa N, Onuma H, et al: Lung
adenocarcinomas with micropapillary components. Gen Thorac
Cardiovasc Surg. 57:534–539. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matthews MJ: Problems in morphology and
behaviour of bronchopulmonary malignant disease. Lung Cancer:
Natural History, Prognosis and Therapy. Isreal L and Chahanian P:
Academic Press; New York: pp. 23–62. 1976
|
|
17
|
Toombs BD and Kalisher L: Metastatic
disease to the breast: clinical, pathologic, and radiographic
features. AJR Am J Roentgenol. 129:673–676. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Noguera JJ, Martinez-Miravete P, Idoate F,
et al: Metastases to the breast: a review of 33 cases. Australas
Radiol. 51:133–138. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Verger E, Conill C, Velasco M and Sole M:
Metastasis in the male breast from a lung adenocarcinoma. Acta
Oncol. 31:4791992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sadikot RT, Renwick DS, DaCosta P,
Chalmers AG and Pearson SB: Breast metastasis from non-small cell
lung cancer. South Med J. 90:1063–1064. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee SH, Park JM, Kook SH, Han BK and Moon
WK: Metastatic tumors to the breast: mammographic and
ultrasonographic findings. J Ultrasound Med. 19:257–262.
2000.PubMed/NCBI
|
|
22
|
Yeh CN, Lin CH and Chen MF: Clinical and
ultrasonographic characteristics of breast metastases from
extramammary malignancies. Am Surg. 70:287–290. 2004.PubMed/NCBI
|
|
23
|
Komorowski AL, Wysocki WM and Mitus J:
Metastasis to the breast - a clinical challenge in outpatient. Acta
Chir Belg. 105:59–61. 2005.PubMed/NCBI
|
|
24
|
Ucar N, Kurt OK, Alpar S, Orsel O, Demirag
F and Kurt B: Breast metastasis in a male patient with nonsmall
cell lung carcinoma. South Med J. 100:850–851. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fulciniti F, Losito S, Botti G, et al:
Metastases to the breast: role of fine needle cytology samples. Our
experience with nine cases in 2 years. Ann Oncol. 19:682–687.
2008.PubMed/NCBI
|
|
26
|
Hsu W, Sheen-Chen SM, Wang JL, Huang CC
and Ko SF: Squamous cell lung carcinoma metastatic to the breast.
Anticancer Res. 28:1299–1301. 2008.PubMed/NCBI
|
|
27
|
Wood B, Sterrett G, Frost F and Swarbrick
N: Diagnosis of extra-mammary malignancy metastatic to the breast
by fine needle biopsy. Pathology. 40:345–351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nielsen M, Andersen JA, Henriksen FW, et
al: Metastases to the breast from extramammary carcinomas. Acta
Pathol Microbiol Scand A. 89:251–256. 1981.PubMed/NCBI
|
|
29
|
Muttarak M, Nimmonrat A and Chaiwun B:
Metastatic carcinoma to the male and female breast. Australas
Radiol. 42:16–19. 1998. View Article : Google Scholar
|
|
30
|
Lee SK, Kim WW, Kim SH, et al:
Characteristics of metastasis in the breast from extramammary
malignancies. J Surg Oncol. 101:137–140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kuroda N, Hamaguchi N, Takeuchi E, Ohara
M, Hirouchi T and Mizuno K: Lung adenocarcinoma with a
micropapillary pattern: a clinicopathological study of 25 cases.
APMIS. 114:381–385. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Klingen TA, Chen Y, Gundersen MD, Aas H,
Westre B and Sauer T: Thyroid transcription factor-1 positive
primary breast cancer: a case report with review of the literature.
Diagn Pathol. 5:372010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang M and Nonaka D: A study of
immunohistochemical differential expression in pulmonary and
mammary carcinomas. Mod Pathol. 23:654–661. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zamecnik J and Kodet R: Value of thyroid
transcription factor-1 and surfactant apoprotein A in the
differential diagnosis of pulmonary carcinomas: a study of 109
cases. Virchows Arch. 440:353–361. 2002. View Article : Google Scholar
|
|
35
|
Takeda Y, Tsuta K, Shibuki Y, et al:
Analysis of expression patterns of breast cancer-specific markers
(mammaglobin and gross cystic disease fluid protein 15) in lung and
pleural tumors. Arch Pathol Lab Med. 132:239–243. 2008.PubMed/NCBI
|
|
36
|
Gomez-Fernandez C, Mejias A, Walker G and
Nadji M: Immunohistochemical expression of estrogen receptor in
adenocarcinomas of the lung: the antibody factor. Appl
Immunohistochem Mol Morphol. 18:137–141. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Striebel JM, Dacic S and Yousem SA: Gross
cystic disease fluid protein-(GCDFP-15): expression in primary lung
adenocarcinoma. Am J Surg Pathol. 32:426–432. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bhargava R, Beriwal S and Dabbs DJ:
Mammaglobin vs GCDFP-15: an immunohistologic validation survey for
sensitivity and specificity. Am J Clin Pathol. 127:103–113. 2007.
View Article : Google Scholar : PubMed/NCBI
|