Introduction
Neuroendocrine differentiation is a basic feature of
prostatic acinar cells. The identification of prostatic tumors with
a neuroendocrine component has been reported to range from 10 to
100% by immunohistochemical studies (1,2).
Neuroendocrine differentiation is characterized by the focal
neuroendocrine cells commonly observed in conventional prostatic
adenocarcinoma, but may also occur as rarer entities, including
small cell carcinoma (SCC), carcinoid-like tumors and Paneth-like
cells (3,4). Approximately 1% of prostate cancer in
biopsies is reported to be SCC or neuroendocrine carcinoma, which
is an aggressive form of cancer (5,6). The
clinical features of SCC of the prostate include a markedly
enlarged prostate, a disproportionately low prostate-specific
antigen (PSA) level in the presence of metastatic disease,
unresponsiveness to hormone therapy, visceral metastases and a high
proportion of lytic to blastic bone lesions (7–9). This
type of cancer may be identified at initial diagnosis or during
androgen deprivation therapy, with or without conventional
adenocarcinoma, and it is reported that SCC of the prostate was
found in 10–20% of autopsy cases with a hormone-refractory state
(10,11).
However, studies have indicated that radiation
therapy affects the neuroendocrine differentiation of prostate
cancer (12–14). Deng et al reported that the
serum chromogranin A (CgA) level was elevated in 4 out of 9
patients following radiotherapy (14). However, no previous study has
reported SCC of the prostate in a patient who underwent any type of
radiation therapy to the prostate. This is the first study to
report SCC of the prostate which arose following high-dose-rate
brachytherapy (HDR-BT) for low-risk prostate cancer. The study was
approved by the ethics committee of the University of Toyama,
Toyama-shi, Japan. Written informed consent for the patient’s
family.
Case report
The patient was an 80-year-old Japanese male with no
significant past medical history, with the exception of gastric
ulcers at the age of 58 years. The patient was referred to the
Department of Urology at Toyama University Hospital with elevated
serum PSA of 6.45 ng/ml in October 2007. No abnormal findings were
noted by a digital rectal examination. A transrectal 10-core
prostate needle biopsy revealed low grade adenocarcinoma of the
prostate in three cores. The patient’s Gleason score was 3+3=6.
Computed tomography, MRI, transrectal ultrasonography and a bone
scan revealed the clinical stage to be organ confined, T2aN0M0,
low-risk prostate cancer (15). In
January 2008, the patient received one implant of Ir-192 and 7
fractions of 6.5 Gy within 3.5 days, for a total prescribed dose of
45.5 Gy, and was treated without any significant adverse events.
The PSA nadir was 2.7 ng/ml at 6 months after HDR-BT.
During the follow-up at another hospital, the
patient complained of hip discomfort, numbness and difficulty
urinating 27 months after HDR-BT without PSA progression (Table I). Digital rectal examination,
urethroscopy, computed tomography and a bone scan revealed
enlargement of the prostate without induration, urethral stenosis,
swelling of multiple pelvic lymph nodes, multiple lung lesions and
multiple suspected bone metastases. His serum level of
neuron-specific enolase (NSE) was elevated to 120 ng/ml (normal
level, <10 ng/ml). The patient underwent a prostate needle
biopsy (4 cores) for a pathological examination in April 2010.
Histologically, the tumor cells with hyperchromatic nuclei and
scant cytoplasm showed a solid or trabecular growth pattern
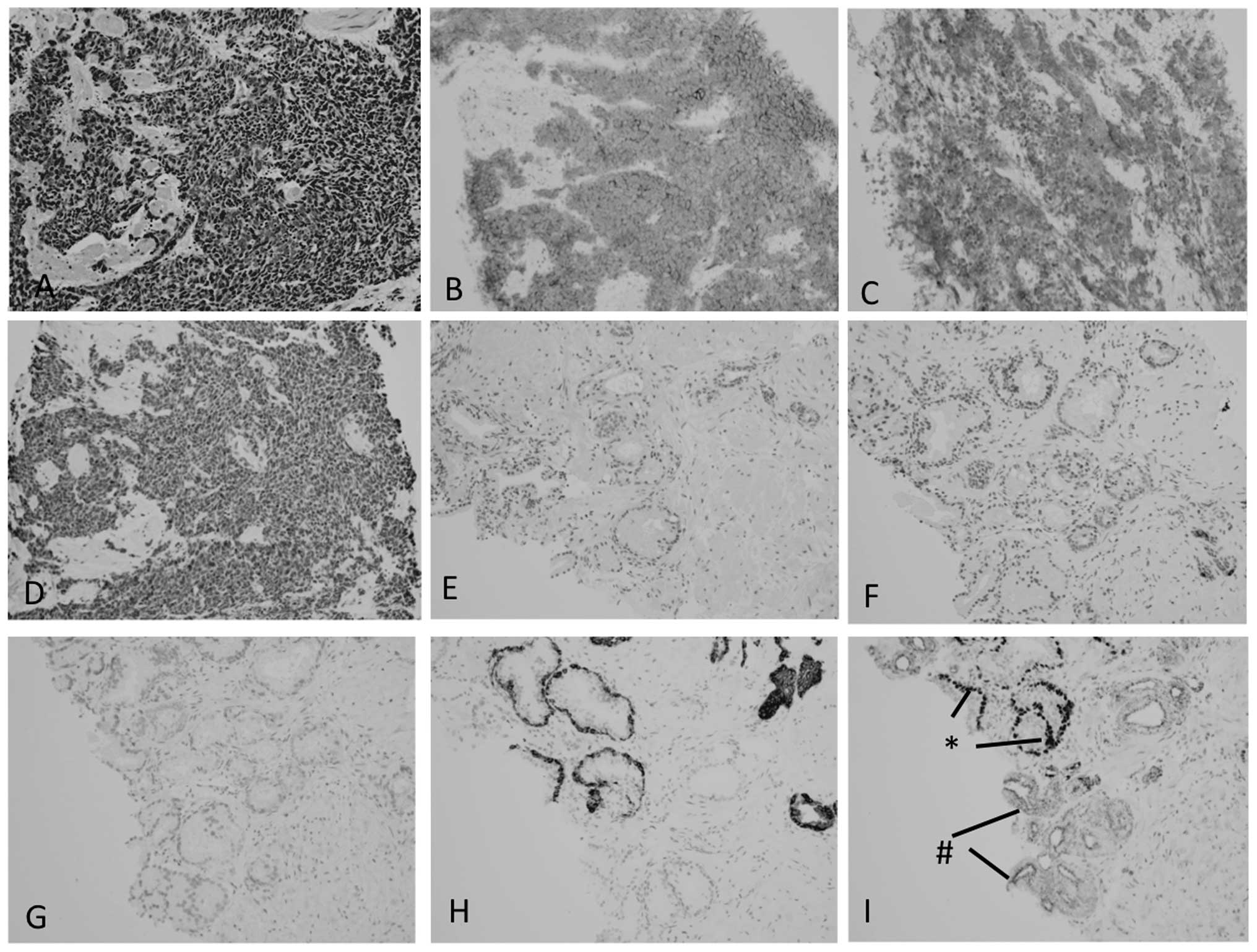
(Fig. 1A). Immunohistochemically,
these tumor cells were positive for AE1/3 (not shown), CD56
(Fig. 1B) and synaptophysin
(Fig. 1C), focally positive for CgA
(Fig. 1D) and TTF-1 (not shown) and
negative for PSA, PAP and CD57 (not shown). There was no component
of conventional prostatic adenocarcinoma noted. A review of the
prostate needle biopsy specimen obtained prior to HDR-BT did not
reveal the carcinoma to be positive for CgA (Fig. 1F), CD56 (Fig. 1G) nor synaptophysin (not shown),
whereas CK34bE12 (Fig. 1H) and p63
(Fig. 1I) were positive in benign
glands and P504S was positive in atypical glands without p63
expression (Fig. 1I). One week
after the second biopsy, the patient experienced acute urinary
retention and a Foley catheter was inserted.
 | Table IChanges in the serum PSA levels prior
to clinical progression. |
Table I
Changes in the serum PSA levels prior
to clinical progression.
| | Time following HDR-BT
(months)
|
|---|
| Prior to HDR-BT | 1 | 2 | 3 | 6 | 9 | 12 | 24 | 27 |
|---|
| PSA (ng/ml) | 6.50 | 6.75 | 3.49 | 3.03 | 2.71 | 2.82 | 3.05 | 3.20 | 3.23 |
The final diagnosis was SCC of the prostate with
local progression and lung, lymph node and bone metastases. Three
cycles of etoposide/cisplatin (EP) were administered. The
treatments were 28 days apart. The doses of etoposide and cisplatin
were 56 mg/m2 and 80 mg/m2, respectively. The
doses were reduced from the original regimen (7) due to the patient’s poor overall health
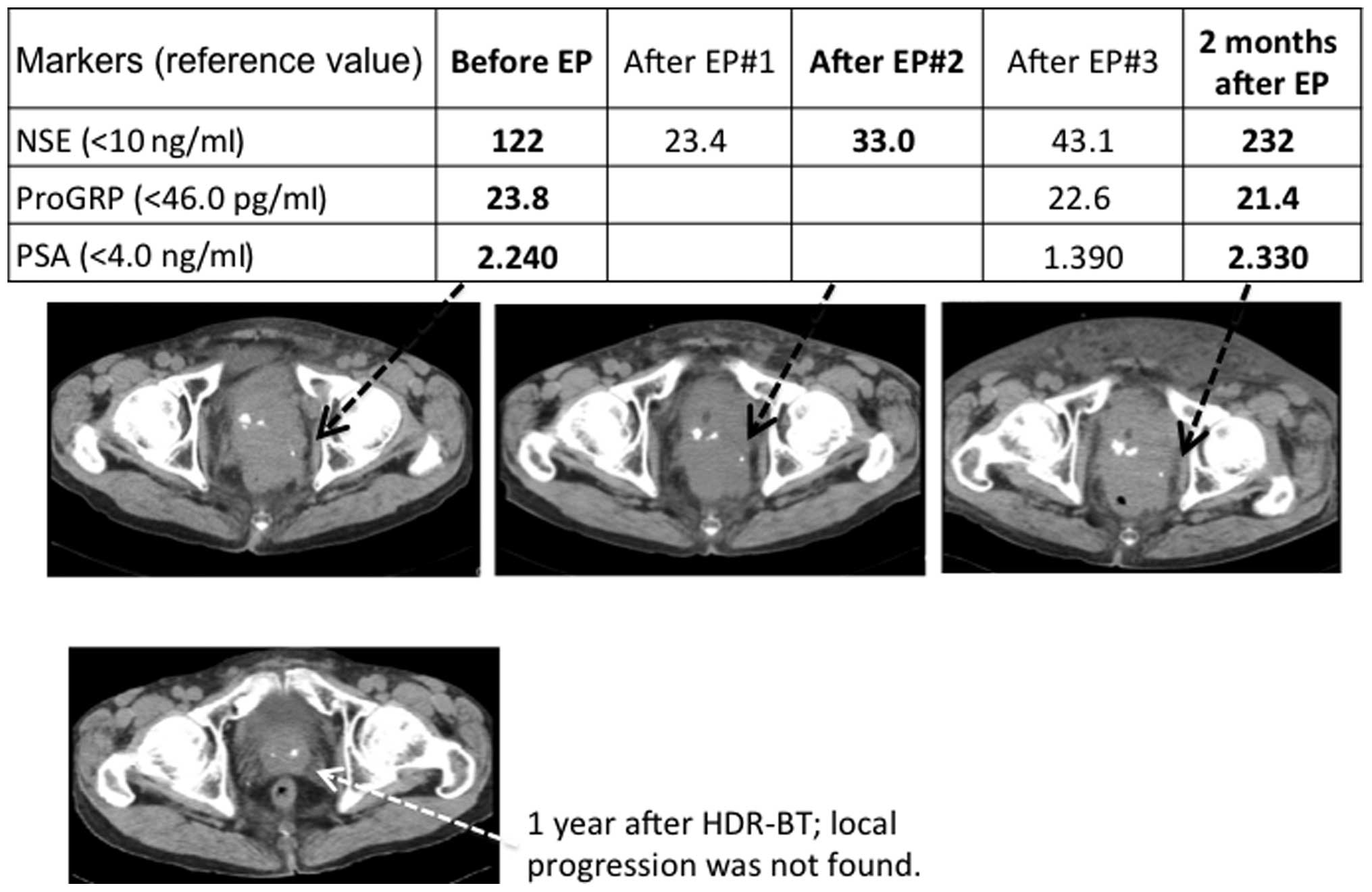
status. His serum NSE level showed a greater than 50% decrease
following EP therapy (Fig. 2), but
no definitive objective response was observed. The adverse events
(Common Terminology Criteria for Adverse Events v3.0, CTCAE)
associated with EP therapy were grade 3 leukocytopenia, grade 2
anemia, grade 3 hypocalcemia, grade 4 hyponatremia, grade 1
creatinine elevation and grade 3 anorexia. There was also
deterioration of his general condition, therefore, the patient
discontinued EP therapy and started to receive best supportive
care. One month later, the patient’s serum NSE showed a rapid
increase to 210 ng/ml with aggressive local progression and he
succumbed to the disease 5.5 months after the initiation of EP
therapy.
Discussion
SCC (neuroendocrine carcinoma) of the prostate
accounts for 10% of all extra-pulmonary SCCs (16). It is estimated that approximately 1%
of all prostate cancers are SCC (17). In approximately 50% of the cases,
the tumors are mixed SCC and adenocarcinoma of the prostate.
However, no study has reported this type of cancer arising
following radiation therapy to the prostate in the literature. In
the present patient, the HDR-BT itself may have caused the SCC in
the prostate. It is also possible that the SCC existed at the
initial diagnostic prostate biopsy, but was too small to be
identified, or that the SCC developed by chance following the
HDR-BT.
Previous studies have revealed that radiation
therapy induces the neuroendocrine differentiation of prostate
cancer. Changes in the levels of CgA and NSE were shown among
hormone-refractory patients undergoing palliative radiation therapy
for bone metastases by Hvamstad et al(12). The serum NSE value was decreased,
whereas CgA and PSA were increased, after radiation. NSE and CgA
have been used as serum neuroendocrine markers for neuroendocrine
differentiation (18). Deng et
al(13,14), reported that ionizing radiation (IR)
induced neuroendocrine differentiation in prostate cancer cells
through the interaction between CREB and ATF2. IR-induced
neuroendocrine-like cells were resistant to docetaxel and androgen
depletion-induced growth inhibition. The authors’ pilot study in
prostate cancer patients showed that the serum CgA level was
elevated in 4 out of 9 patients following radiotherapy (14). Taken together, these findings
provide evidence that radiation-induced neuroendocrine
differentiation is a general therapeutic response in a subset of
prostate cancer patients. However, radiation-induced SCC has never
been reported in any specific organ.
However, certain studies have reported that
co-existing adenocarcinoma and SCC share the same clonal origin.
Hansel et al(19) reported
that the same TP53 mutation was shared in morphologically and
phenotypically distinct concurrent primary small cell
neuroendocrine carcinoma and adenocarcinoma of the prostate
(Gleason score 4+3=7). Williamson et al(20) demonstrated that ERG-TMPRSS2
rearrangement, the most frequent molecular alteration in prostate
cancer, was shared by concurrent prostatic adenocarcinoma and
prostatic SCC, and was absent in SCC of the urinary bladder as
evidence supporting the monoclonal origin of the prostate cancers.
Therefore, the SCC in this patient may have had the same origin as
the Gleason 3+3 adenocarcinoma.
SCCs of the prostate are thought to be identical to
SCCs of the lung. Therefore, cisplatin-based cytotoxic agents that
are similar to those used for SCC of the lung are usually applied,
such as irinotecan + cisplatin or etoposide + cisplatin (2,21,22).
In the present patient, treatment with etoposide and cisplatin was
effective in terms of the serum marker response; however, it was
impossible to continue this therapy due to deterioration of the
patient’s general condition. Recently, Deorah et al showed
that the median survival in 191 subjects with SCC of the prostate
was 11 months, which falls within the range 5–17.5 months reported
previously (23). The 12-, 24-,
36-, 48- and 60-month observed survival rates were 47.9, 27.5, 19,
17 and 14.3%, respectively, in patients with primary SCC of the
prostate (23). Age, concomitant
low-grade prostatic adenocarcinoma and the stage of the disease
were the strongest predictors of survival for patients with
prostatic SCC. The survival of the present patient following EP
therapy was 5.5 months, which was shorter than the results in the
literature. This may be due to the patient’s characteristics, such
as his older age (80 years), metastatic disease and pure SCC.
In conclusion, we present a case of SCC of the
prostate with local progression and metastases to lung, pelvic
lymph nodes and bones. This disease arose 27 months following
HDR-BT monotherapy without PSA progression. This report alerts
physicians to the possibility of neuroendocrine progression after
radiotherapy, and confirms the importance of objective diagnostic
imaging and physical examination, in addition to PSA testing, in
the management of prostate cancer, even in patients who have
localized and low-risk disease. If disease progression is unusual,
as the case presented in this study, a prostate biopsy should be
considered for pathological exploration.
References
|
1
|
Abrahamsson PA, Wadström LB, Alumets J, et
al: Peptide-hormone- and serotonin-immunoreactive tumour cells in
carcinoma of the prostate. Pathol Res Pract. 182:298–307. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Komiya A, Suzuki H, Imamoto T, et al:
Neuroendocrine differentiation in the progression of prostate
cancer. Int J Urol. 16:37–44. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cussenot O, Villette JM, Cochand-Priollet
B and Berthon P: Evaluation and clinical value of neuroendocrine
differentiation in human prostatic tumors. Prostate Suppl. 8:43–51.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weaver MG, Abdul-Karim FW and Srigley JR:
Paneth cell-like change and small cell carcinoma of the prostate.
Two divergent forms of prostatic neuroendocrine differentiation. Am
J Surg Pathol. 16:1013–1016. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Helpap B and Köllermann J:
Undifferentiated carcinoma of the prostate with small cell
features: immunohistochemical subtyping and reflections on
histogenesis. Virchows Arch. 434:385–391. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
di Sant’Agnese PA: Neuroendocrine
differentiation in carcinoma of the prostate. Diagnostic,
prognostic, and therapeutic implications. Cancer. 70(1 Suppl):
254–268. 1992.
|
|
7
|
Amato RJ, Logothetis CJ, Hallinan R, Ro
JY, Sella A and Dexeus FH: Chemotherapy for small cell carcinoma of
prostatic origin. J Urol. 147:935–937. 1992.PubMed/NCBI
|
|
8
|
Sella A, Konichezky M, Flex D, Sulkes A
and Baniel J: Low PSA metastatic androgen- independent prostate
cancer. Eur Urol. 38:250–254. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oesterling JE, Hauzeur CG and Farrow GM:
Small cell anaplastic carcinoma of the prostate: A clinical,
pathological and immunohistological study of 27 patients. J Urol.
147:804–807. 1992.PubMed/NCBI
|
|
10
|
Miyoshi Y, Uemura H, Kitami K, Satomi Y,
Kubota Y and Hosaka M: Neuroendocrine differentiated small cell
carcinoma presenting as recurrent prostate cancer after androgen
deprivation therapy. BJU Int. 88:982–983. 2001. View Article : Google Scholar
|
|
11
|
Tanaka M, Suzuki Y, Takaoka K, et al:
Progression of prostate cancer to neuroendocrine cell tumor. Int J
Urol. 8:431–436. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hvamstad T, Jordal A, Hekmat N, Paus E and
Fosså SD: Neuroendocrine serum tumour markers in hormone-resistant
prostate cancer. Eur Urol. 44:215–221. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deng X, Liu H, Huang J, et al: Ionizing
radiation induces prostate cancer neuroendocrine differentiation
through interplay of CREB and ATF2: implications for disease
progression. Cancer Res. 68:9663–9670. 2008. View Article : Google Scholar
|
|
14
|
Deng X, Elzey BD, Poulson JM, et al:
Ionizing radiation induces neuroendocrine differentiation of
prostate cancer cells in vitro, in vivo and in prostate cancer
patients. Am J Cancer Res. 1:834–844. 2011.PubMed/NCBI
|
|
15
|
D’Amico AV, Whittington R, Malkowicz SB,
et al: Biochemical outcome after radical prostatectomy, external
beam radiation therapy, or interstitial radiation therapy for
clinically localized prostate cancer. JAMA. 280:969–974. 1998.
|
|
16
|
Asmis TR, Reaume MN, Dahrouge S and Malone
S: Genitourinary small cell carcinoma: a retrospective review of
treatment and survival patterns at The Ottawa Hospital Regional
Cancer Center. BJU Int. 97:711–715. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yao JL, Huang J and di Sant’Agnese PA:
Small cell carcinoma of the prostate. Diagn Histopathol.
14:117–121. 2008. View Article : Google Scholar
|
|
18
|
Sasaki T, Komiya A, Suzuki H, et al:
Changes in chromogranin a serum levels during endocrine therapy in
metastatic prostate cancer patients. Eur Urol. 48:224–229. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hansel DE, Nakayama M, Luo J, et al:
Shared TP53 gene mutation in morphologically and phenotypically
distinct concurrent primary small cell neuroendocrine carcinoma and
adenocarcinoma of the prostate. Prostate. 69:603–609. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Williamson SR, Zhang S, Yao JL, et al:
ERG-TMPRSS2 rearrangement is shared by concurrent prostatic
adenocarcinoma and prostatic small cell carcinoma and absent in
small cell carcinoma of the urinary bladder: evidence supporting
monoclonal origin. Mod Pathol. 24:1120–1127. 2011. View Article : Google Scholar
|
|
21
|
Têtu B, Ro JY, Ayala AG, Johnson DE,
Logothetis CJ and Ordonez NG: Small cell carcinoma of the prostate.
Part I A clinicopathologic study of 20 cases. Cancer. 59:1803–1809.
1987.PubMed/NCBI
|
|
22
|
Noda K, Nishiwaki Y, Kawahara M, et al
Japan Clinical Oncology Group: Irinotecan plus cisplatin compared
with etoposide plus cisplatin for extensive small-cell lung cancer.
N Engl J Med. 346:85–91. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Deorah S, Rao MB, Raman R, Gaitonde K and
Donovan JF: Survival of patients with small cell carcinoma of the
prostate during 1973–2003: a population-based study. BJU Int.
109:824–830. 2012.
|